Abstract
Background
Increasing rates of prosthetic joint infection (PJI) have presented challenges for general practitioners, orthopedic surgeons and the health care system in the recent years. The diagnosis of PJI is complex; multiple diagnostic tools are used in the attempt to correctly diagnose PJI. Evidence-based algorithms can help to identify PJI using standardized diagnostic steps.
Methods
We reviewed relevant publications between 1990 and 2015 using a systematic literature search in MEDLINE and PUBMED. The selected search results were then classified into levels of evidence. The keywords were prosthetic joint infection, biofilm, diagnosis, sonication, antibiotic treatment, implant-associated infection, Staph. aureus, rifampicin, implant retention, pcr, maldi-tof, serology, synovial fluid, c-reactive protein level, total hip arthroplasty (THA), total knee arthroplasty (TKA) and combinations of these terms.
Results
From an initial 768 publications, 156 publications were stringently reviewed. Publications with class I–III recommendations (EAST) were considered. We developed an algorithm for the diagnostic approach to display the complex diagnosis of PJI in a clear and logically structured process according to ISO 5807.
Conclusions
The evidence-based standardized algorithm combines modern clinical requirements and evidence-based treatment principles. The algorithm provides a detailed transparent standard operating procedure (SOP) for diagnosing PJI. Thus, consistently high, examiner-independent process quality is assured to meet the demands of modern quality management in PJI diagnosis.
Keywords: Prosthetic joint infection, Algorithm, Total joint replacement, Revision surgery, THA, TKA
Background
The total number of hip and knee arthroplasties performed in the US is constantly increasing, with an expected increase in total knee arthroplasties (TKAs) of approximately 600% by 2030. Similarly, the number total hip arthroplasties (THAs) performed is estimated to triple during this period, leading to a significant increase in revision surgeries [1].
One major reason for revision arthroplasty is prosthetic joint infection (PJI). The incidence of PJI after primary surgery is 0.2–1.1%; in cases of revision surgery, it can reach 5% [2]. In contrast with acute PJI, low-grade infections are characterized by unspecific symptoms, such as pain and early implant loosening. Typical low-grade infections are often a result of infection with less virulent bacterial strains of the dermal flora, including coagulase-negative Staphylococci and Propionibacterium acnes, often lacking severe inflammatory symptoms [3].
Although it is of fundamental importance for further treatment, diagnosing a low-grade PJI prior to revision surgery can be challenging.
In addition to clinical findings, conventional radiographs, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), the percutaneous aspiration of synovial fluid for evaluating cell counts and differentials, and microbiology workups are routinely employed diagnostic tools (3).
Moreover, arthroscopically or fluoroscopically controlled biopsy of periprosthetic tissue can be performed. Some authors suggest the use of synovial biomarkers, leukocyte esterase tests or radionuclide imaging to support the diagnosis of a low-grade PJI [4, 5].
All tests have a role in the workup of PJI; however, the diagnostic values reported in the recent literature vary greatly [4–6].
To safely rule out or verify the presence of PJI prior to arthroplasty revision surgery, a customized combination of different diagnostic tests must be employed for every single case. Although the composition of an appropriate set of diagnostic tests may be self-evident for senior orthopedic surgeons who specialize in treating PJI, it can be challenging for attending orthopedic surgeons and general practitioners. The correct preoperative diagnosis is, however, of major significance because treatment strategies differ greatly between septic and aseptic revision surgery and have far-reaching consequences for the patient (20). Whereas false-positive findings lead to unnecessary two-stage revisions, one-stage revision surgery without the essential implementation of antibiotic therapy can result from false-negative results, inevitably causing new prosthetic failure and PJI persistence [6].
We, thus, developed an evidence-based diagnostic algorithm with examiner-independent diagnostic reliability for identifying PJI, and we prospectively observed its use in daily clinical routine according to the requirements of modern quality management in our institution.
Methods
A systematic literature search was conducted in the databases of PubMed and Medline using the following search terms: prosthetic joint infection, implant-associated infection, biofilm, diagnosis, sonication, antibiotic treatment, microcalorimetry, Staph. aureus, coagulase-negative staphylococci, Propionibacterium rifampicin, implant retention, pcr, maldi-tof, serology, synovial fluid, C-reactive protein level, THA, TKA leucocyte esterase test, alpha-defensin test. All relevant publications between January 1990 and January 2015 were screened according to methodological aspects using QUADAS, STARD and PRISMA criteria [7–11] and classified according to the Grade system (The Grading of Recommendation Assessment, Development and Evaluation). Referring to the Grade system, the included studies were grouped according to the EAST classification, shown in Table 1 and Fig. 1, which allows the evaluation of medical publications, reviews and recommendations in terms of their level of evidence (LoE) and class of recommendation (CoR) [12–21].
Table 1.
EAST level of evidence (LoE) and class of recommendation (CoR)
| LoE | |
| I | Prospective randomized controlled trials (ORCTs) |
| II | Clinical studies in which the data was collected prospectively, and retrospective analyses which were based on clearly reliable data Types of studies so classified: observational studies, cohort studies, prevalence studies and case control studies |
| III | Studies based on retrospectively collected data. Evidence used in this class indicate clinical series, database or registry review, large series of case reviews and expert opinion |
| CoR | |
| I | The recommendation is convincingly justifiable based on the available scientific information alone. This recommendation is usually based on Class I data; however, strong Class II evidence may form the basis for a level I recommendation, especially if the issue does not lead itself to testing in a randomized trial. |
| II | The recommendation is reasonably justifiable by available scientific evidence and strongly supported by expert opinion This recommendation is usually supported by Class II data or a preponderance of Class II evidence |
| III | The recommendation is supported by available data but adequate scientific evidence is lacking This recommendation is generally supported by Class III data. This type of recommendation is useful for educational purposes and in guiding future clinical research |
Fig. 1.
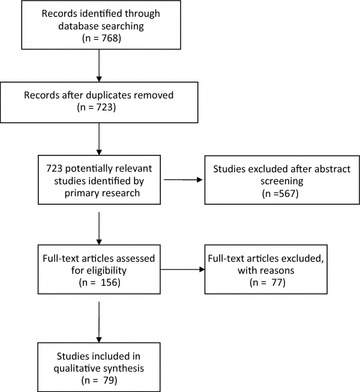
Flow chart of selection process
The literature review exclusively included studies of adults and publications in the English or German language. All references from the publications used in this study were examined for additionally relevant publications.
We identified 723 studies that met our search criteria. To perform statistically valid analyses, we only included studies with a minimum of 26 patients (Fig. 1).
Furthermore, only studies that used the definition criteria [22] of the Musculoskeletal Infection Society (MSIS) [23], Infectious Diseases Society of America (IDSA) [24] and International Consensus Meeting [25] (Table 2) were included.
Table 2.
Definition of prosthetic joint infection
| MSIS | IDSA | International consensus | ||||
|---|---|---|---|---|---|---|
| Main criteria | Supportive criteria | Main criteria | Supportive criteria | Main criteria | Supportive criteria | |
| Sinus tract | o | o | o | |||
| Identical microorganisms isolated form 2 or more cultures | o | o | o | |||
| Purulence surrounding the prosthesis | o | o | ||||
| Inflammation in histological examination of prosthetic tissue | o | o | o | |||
| Single positive culture | o | o | ||||
| Single positive culture with virulent microorganism | o | |||||
| Elevated synovial fluid leukocyte count | o | o | ||||
| Elevated synovial fluid neutrophil percentage | o | o | ||||
| Elevated serum ESR and CRP values | o | o | ||||
A total of 79 studies were included, and the data were extracted from the studies. Subsequently, sensitivities, specificities, positive and negative likelihood ratios and positive and negative predictive values were calculated from the extracted data if they were not stated in the publication.
The evidence levels of the selected studies were taken into account, fulfilling the formal requirement of the International Organization for Standardization (ISO) for development of algorithms. The creation of the algorithm was performed according to ISO norm 5807 Modification ITU-I, initially designed for telecommunication defaults, to ensure explicit decision-making criteria for a logical and standardized procedure. The ISO 5807 norm defines the use of a different symbol for the single operation to create an operation plan that has only one input and output [26]. An algorithm that meets the ISO 5807 criteria is composed of process and decision symbols that differ from the symbols for the initial criteria and endpoints. Checklists are introduced to reduce the number of decision symbols. For more practical reasons, the algorithm should not exceed a single page in length.
Results
The algorithm was a composition of evidence-based procedures developed in our clinic that fulfilled the ISO 5807. Studies were integrated dependent of their LoE in a logical, structured, priority-orientated way in the algorithm. Checklists are located on the left side, the vertical flow represents the main diagnostic criteria and treatment, and the horizontal flow represents the supportive criteria [27] (Fig. 2). For practical reasons, the decisions symbol has been modified to a binary-decision hexagon.
Fig. 2.
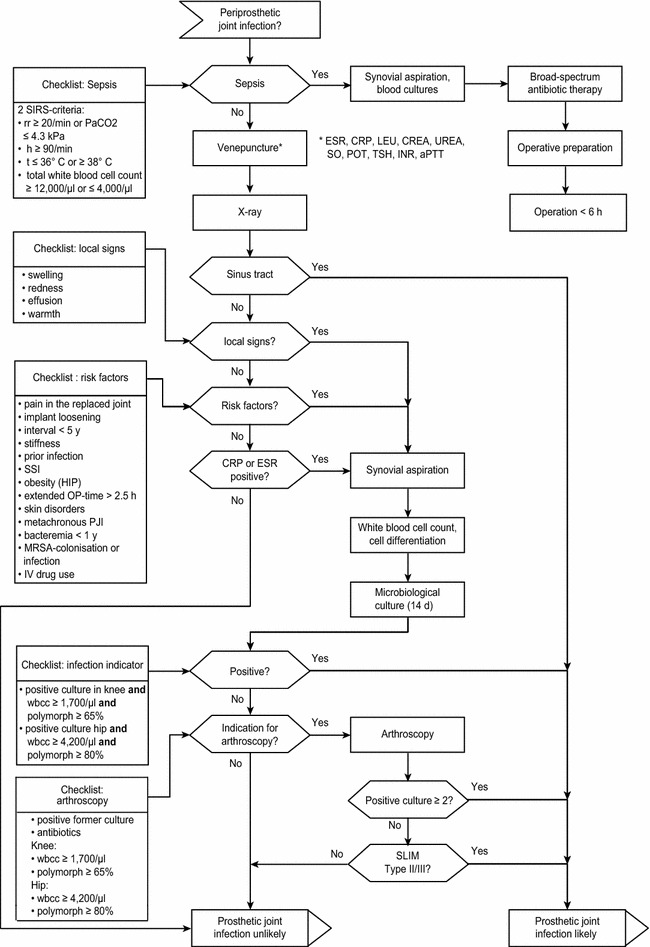
Diagnostic algorithm
All diagnostic aspects of the algorithm and the underlying literature are specified below.
Risk factors checklist
In total, 31 included studies (patients: n = 312.946; LoE I: n = 2, LoE II: n = 13, LoE III: n = 16) discussed the risk factors for a PJI (Table 3). According to a study by Virolainen, pain in the index joint exhibits a specificity of 100% in patients with PJI. Various consensus recommendations and expert opinions consider a limited range of motion in the total joint an indicator for PJI [25]. The risk factors according to studies with a class of recommendation of I (CoR I) are an extended operation time (n = 142.120) [28–32], obesity (n = 116,682) [33–41], malnutrition (n = 678) [42], diabetes (n = 72,778) [35, 39, 40, 43], immunosuppression (n = 86,675) [32, 34, 35, 37, 41, 44, 45], Prior infection of the joint [44], prior infection [44], early implant failure [46], early implant loosening [46, 47] and superficial surgical site infections [32, 48, 49]. Other risk factors have been associated with PJI, although the related studies do not provide strong evidence (CoR II–III); these factors include asymptomatic bacteriuria [50] and tooth interventions, oral surgery and colonoscopies, which provide a crucial risk factor for PJI because of relevant bacteraemia [51, 52]. Likewise, skin disorders in the surgical area during implantation (psoriasis, chronic venous stasis, skin ulcers, lymphedema) increase the risk of implant-associated infection [41, 53].
Table 3.
Riskfactors for prosthetic joint infections
| Author | Year | LoE | (n = x) | Risk factor | CoR |
|---|---|---|---|---|---|
| Huotari et al. | 2007 | LoE II | n = 8201 | ||
| Smarbrekke et al. | 2004 | LoE II | n = 31,750 | ||
| Kurtz et al. | 2010 | LoE II | n = 69,663 | Extended time | CoR I |
| Uckay et al. | 2009 | LoE I | n = 6001 | ||
| Berbari et al. | 1998 | LoE II | n = 26,505 | ||
| Dowsey et al. | 2009 | LoE III | n = 1214 | ||
| Peersman et al. | 2001 | LoE III | n = 6120 | ||
| Lübbeke et al. | 2007 | LoE II | n = 2495 | ||
| Dowsey et al. | 2008 | LoE III | n = 1207 | ||
| Pulido et al. | 2008 | LoE III | n = 9245 | Obesity | CoR I |
| Namba et al. | 2012 | LoE II | n = 30,491 | ||
| Namba et al. | 2013 | LoE II | n = 56,216 | ||
| Malinzak et al. | 2009 | LoE III | n = 8494 | ||
| Peel et al. | 2011 | LoE III | n = 1200 | ||
| Berbari et al. | 2012 | LoE III | n = 678 | Malnutrition | CoR III |
| Namba et al. | 2013 | LoE II | n = 56,216 | ||
| Malinzak et al. | 2009 | LoE III | n = 8494 | Diabetes | CoR I |
| Peersman et al. | 2001 | LoE III | n = 6120 | ||
| Mraovic et al. | 2011 | LoE III | n = 1948 | ||
| Dowsey et al. | 2008 | LoE III | n = 1207 | ||
| Peersman et al. | 2001 | LoE III | n = 6120 | ||
| Jämsen et al. | 2009 | LoE II | n = 43,149 | Immunsuppression | CoR I |
| Pulido et al. | 2008 | LoE II | n = 9245 | ||
| Peel et al. | 2011 | LoE III | n = 63 | ||
| Berbari et al. | 1998 | LoE II | n = 26,505 | ||
| Bongratz et al. | 2008 | LoE III | n = 462 | ||
| Jämsen et al. | 2009 | LoE II | n = 43,149 | Prior infection | CoR II |
| Aslam et al. | 2010 | LoE III | n = 126 | ||
| Coelho-Prabhu et al. | 2013 | LoE III | n = 678 | Bacteremia | CoR III |
| Murdoch et al. | 2001 | LoE III | n = 80 | ||
| Murray et al. | 1991 | Level III | n = 159 | Metachronous infections | CoR III |
| Luessenhop et al. | 1996 | Level III | n = 145 | ||
| Portillo et al. | 2013 | LoE I | n = 116 | Implant loosening | CoR I |
| Sousa et al. | 2013 | LoE III | n = 2497 | Bacteriuria | CoR III |
| Berbari et al. | 2010 | Level III | n = 678 | Dental | CoR III |
Sepsis checklist
As a first step, sepsis and septic shock are ruled out. Concerning sepsis, we included 9 studies discussing diagnostic parameters and treatment options for highly acute PJIs. Two LoE I studies, three LoE II studies and six LoE III studies were included. To identify sepsis, we used the diagnostic criteria published by Llewelyn and Dellinger (CoR III) [54–56]. Prior to the initiation of a calculated antibiotic therapy, a synovial aspiration (CoR III) for subsequent examination of cell counts and differentials and a microbiologic workup to identify the causative pathogen should be performed [55, 57, 58]. Additionally, blood cultures should be obtained (CoR I) [59, 60]. Subsequently, early surgical focus management can significantly reduce the mortality rate (CoR II) [61, 62].
Physical exam
The highest LoE (CoR III) for physical examination is found in a study by Teller et al. [63]. They report a sensitivity of 18.95% (CI 0.05–0.4) and a specificity of 100% (CI 0.98–1.0) for the identification of PJI based on local signs of inflammation, such as warmth, effusion, redness and swelling of the corresponding joint [63]. For fever, a sensitivity of 9% (CI 95 0.03–0.21) and a specificity of 99% (CI 95 0.98–1.00) were reported. These data contribute to a negative predictive value of 0.89 and a negative likelihood ratio of 0.82 (CI 0.66–0.98) for local signs of inflammation (Table 4). Studies with higher levels of evidence for clinical signs of inflammation have not yet been published.
Table 4.
Physical Exam
| Author | Year | LoE | CoR | (n = x) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | LR for a positive result | LR for a negative result |
|---|---|---|---|---|---|---|---|---|---|---|
| Teller et al. | 2000 | III | III | 166 | ||||||
| Local signs | 0.18 (0.02–0.34) | 1 | 1 | 0.5 (0.34–0.66) | n.p. | 0.82 (0.66–0.98) | ||||
| Fever | 0.9 (0.03–0.21) | 0.99 (0.98–1.00) | 0.67 (0.13–1.2) | 0.88 (0.83–0.93) | 13.09 (1.42–31.88) | 0.92 (0.78–1.05) |
Inflammatory markers
Decision paths for the CRP and ESR were derived from six studies with a LoE of I, resulting in a CoR I. In this context, Bottner et al. showed significantly increased preoperative CRP and ESR levels in patients with PJI compared with cases with aseptic knee and hip revisions. Considering a cut-off value of 1.5 mg/dl for the CRP, the authors reported a sensitivity of 0.95 (95% CI 0.86–1.0) and a specificity of 0.91 (0.84–0.99). The ESR showed a lower sensitivity of 0.81 (0.64–0.98) and a lower specificity of 0.89 (0.82–0.97) compared with the CRP given a cut-off value of 32 mm/h [64]. In their series of total knee arthroplasties, Valle Della et al. reported sensitivities of 0.95 (0.89–1.0) and 0.9 (0.81–0.99) and specificities of 0.75 (0.64–0.87) and 0.66 (0.53–0.79) for the CRP and ESR, respectively [65]. Greidanus et al. observed a lower sensitivity of 0.82 (0.71–0.95) and a higher specificity of 0.88 (0.76–0.9) for the ESR, with a cut-off value of 30 mm/h. The CRP showed a sensitivity of 0.93 (0.86–1.0) and a specificity of 0.83 (0.76–0.9) given a cut-off value of 1.0 mg/dl [66]. In the 1980s, Kamme et al. determined the ESR and reported a sensitivity of 0.89 (0.8–0.95) and a specificity of 0.73 (0.54–0.9) [67]. Schinsky et al. showed high sensitivity [ESR: 0.96 (0.91–1.0); CRP: 0.95 (0.89–1.0)] and a low specificity [ESR: 0.39 (0.31–0.47); CRP: 0.71 (0.94–1.0)] for the ESR and CRP [68]. In contrast, Savorino et al. reported a low sensitivity [ESR: 0.6 (0.3–0.9); CRP 0.38 (0.14–0.61)] but a higher specificity [ESR: 0.94 (0.82–1.0); CRP: 0.7(0.42–0.98)] [69]. Recently, Fink et al. calculated a sensitivity of 0.73 (0.59–0.86) and a specificity of 0.81 (0.73–0.88) for the CRP in their series of total knee arthroplasties [70]. Positive and negative predictive values and positive and negative likelihoods were calculated and are shown in Table 5. Inflammatory markers such as IL-6, PCT and TNF-alpha have also been the focus of clinical trials; however, there is no Level I or Level II study indicating their superior diagnostic value [71–73].
Table 5.
Value of serological analysis of c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)
| Author | Year | LoE | CoR | (n = x) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | LR for a positive result | LR for a negative result |
|---|---|---|---|---|---|---|---|---|---|---|
| Bottner et al. | 2007 | I | I | 78 | ||||||
| CRP (1.5 mg/dl) | 0.95 (0.86–1.0) | 0.91 (0.94–0.99) | 0.8 (0.64–0.96) | 0.98 (0.94–1.0) | 10.86 (5.3–73.07) | 0.05 (0.0–0.17) | ||||
| ESR (32 mm/h) | 0.81 (0.64–0.98) | 0.89 (0.82–0.97) | 0.74 (0.56–0.92) | 0.93 (0.8–1.0) | 7.69 (3.47–38.2) | 0.21 (0.02–0.44) | ||||
| Savarino et al. | 2004 | I | I | 26 | ||||||
| ESR (50 mm/h) | 0.6 (0.3–0.9) | 0.94 (0.82–1.0) | 0.86 (0.71–1.0) | 0.79 (0.61–0.97) | 9.6 (1.64–16.1) | 0.43 (0.09–0.86) | ||||
| CRP (2 mg/dl) | 0.38 (0.14–0.61) | 0.7 (0.42–0.98) | 0.67 (0.36–0.97) | 0.42 (0.18–0.65) | 1.25 (0.24–38.34) | 0.89 (0.39–2.07) | ||||
| Kamme et al. | 1980 | I | I | 63 | ||||||
| ESR(30 mm/h) | 0.89 (0.8–0.99) | 0.72 (0.54–0.9) | 0.83 (0.71–0.94) | 0.82 (0.66–0.98) | 3.2 (1.75–9.54) | 0.15 (0.01–0.37) | ||||
| Greidanus et al. | 2007 | I | I | 151 | ||||||
| ESR (30 mm/h) | 0.82 (0.71–0.93) | 0.88 (0.81–0.94) | 0.74 (0.62–0.86) | 0.92 (0.87–0.97) | 6.7 (3.84–15.52) | 0.2 (0.07–0.36) | ||||
| CRP (1.0 mg/dl) | 0.93 (0.86–1.0) | 0.83 (0.76–0.9) | 0.7 (0.58–0.82) | 0.97 (0.93–1.0) | 5.5 (3.57–10.23 | 0.08 (0.01–0.18) | ||||
| Della Valle et al. | 2007 | I | I | 94 | ||||||
| ESR (30 mm/h) | 0.9 (0.81–0.99) | 0.66 (0.53–0.79) | 0.67 (0.55–0.8) | 0.9 (0.8–0.99) | 2.66 (1.74–4.68) | 0.15 (0.01–0.35) | ||||
| CRP (1 mg/dl) | 0.95 (0.89–1.00) | 0.75 (0.64–0.87) | 0.75 (0.63–0.87) | 0.95 (0.89–1.0) | 3.88 (2.45–7.86) | 0.06 (0.02–0.18) | ||||
| Schinsky et al. | 2008 | I | I | 201 | ||||||
| ESR (30 mm/h) | 0.96 (0.91–1.0) | 0.39 (0.31–0.47) | 0.37 (0.29–0.45) | 0.97 (0.92–1.0) | 1.58 (1.33–1.91) | 0.09 (0.0–0.28) | ||||
| CRP (1 mg/dl) | 0.95 (0.89–1.0) | 0.71 (0.94–1.0) | 0.55 (0.45–0.65) | 0.97 (0.94–1.0) | 3.29 (2.45–4.69) | 0.08 (0.0–0.18) | ||||
| Fink et al. | 2007 | I | I | 145 | ||||||
| CRP (1.35 mg/dl) | 0.73 (0.59–0.86) | 0.81 (0.73–0.88) | 0.59 (0.45–0.73) | 0.89 (0.82–0.95) | 3.81 (2.21–7.48) | 0.34 (0.15–0.56) | ||||
| Bottner et al. | 2007 | I | I | 78 | 0.95 | 0.87 | 0.74 | 0.98 | n.p. | n.p. |
| IL-6 (>12 pg/ml) | 0.43 | 0.94 | 0.75 | 0.85 | n.p. | n.p. | ||||
| TNF-a (>40 nl/ml) | 0.33 | 0.98 | 0.87 | 0.8 | n.p. | n.p. | ||||
| PCT (>0.3 ng/ml) | ||||||||||
| Di Cesare et al. | 2005 | III | III | 58 | ||||||
| Il-6 (>10 pg/ml) | 1 | 0.95 (0.89–1.0) | 0.88 (0.73–1.0) | 1 | 21.5 (9.14–60.85) | 0 | ||||
Sinus tract
According to the criteria of the MSIS, the ISDA and the International Consensus Meeting, a sinus tract communicating with the prosthesis is a criterion for the presence of a PJI [23, 25, 74]. Two studies dealing with microbiological cultures of patients with a sinus tracts were included; however, a pathogen was not identified in all cases (Table 4). Bogut et al. calculated a sensitivity of 0.82 (0.66–0.98) and a specificity of 1.0 (1.0–1.0; LoE II) [75]. Trampus et al. identified a positive microbiological culture in all cases after sonication and described a sensitivity of 1.0 and a specificity of 1.0 (LoE I) [76].
Joint aspiration (knee)
For tentative PJI of the knee, five studies (5× LoE I) addressing percutaneous aspiration of synovial fluid were included. Based on an exclusive bacteriologic culture analysis, Fink et al. reported a sensitivity of 0.73 (0.59–0.86) and a specificity of 0.95 (0.91–0.99) [70]. Della Valle et al. reported a similar result for microbiological cultures (sensitivity 0.8 (95% CI n.p.); specificity 0.93 (95% CI n.p.) [65]. Four authors examined the synovial fluid, considering white blood cell counts (WBC) and cell differentiation (Neutrophil-%). Trampuz et al. observed a sensitivity of 0.94 (0.86–1.0) for WBC and of 0.97 (0.91–1.0) for Neutrophil-%, with specificities of 0.88 (0.81–0.94) and 0.98 (0.95–1.0), respectively [77]. Della Valle et al. showed comparable results: WBC sensitivity 0.91 (0.86–0.95)/specificity 1; Neutrophil-% sensitivity 0.98 (0.93–1.0) and specificity 0.85 (0.75–0.95). Ghanem et al. showed a sensitivity for WBC of 0.91 (0.86–0.95) and a specificity of 0.88 (0.84–0.92); for Neutrophil-%, they found a sensitivity of 0.95 (0.92–0.98) and a specificity of 0.95 (0.92–0.97). Zmistowski et al. confirmed the results in their series, reporting a sensitivities of 0.93 (0.87–0.99) for WBC and Neutrophil-% and specificities of 0.94 (0.88–0.99) and 0.83 (0.75–0.91), respectively. Positive and negative predictive values and positive and negative likelihoods were calculated for all studies and are shown in Table 6.
Table 6.
Value of joint aspiration in the diagnosis of infected total knee arthroplasty
| Author | Year | LoE | CoR | (n = x) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | LR for a positive result | LR for a negative result |
|---|---|---|---|---|---|---|---|---|---|---|
| Della Valle et al. | 2007 | I | I | 94 | ||||||
| Aspiration culture | 0.8 | 0.93 | 0.94 | 0.84 | n.p. | n.p. | ||||
| WBC (3.0 × 103) | 0.98 (0.93–1.9) | 1 | 1 | 0.98 (0.94–1.0) | X | 0.02 | ||||
| Neutrophile-% | 0.98 (0.93–1.0) | 0.85 (0.75–0.95) | 0.83 (0.73–0.94) | 0.98 (0.94–1.0) | 6.46 (3.75–18.75) | 0.03 (0–0.1) | ||||
| Fink et al. | 2007 | I | I | 145 | ||||||
| Aspiration culture | 0.73 (0.59–0.86) | 0.95 (0.91–0.99) | 0.85 (0.73–0.97) | 0.9 (0.85–0.96) | 15.23 (6.64–125.4) | 0.29 (0.14–0.45) | ||||
| Ghanem et al. | 2008 | I | I | 429 | ||||||
| WBC (3.0 × 103) | 0.91 (0.86–0.95) | 0.88 (0.84–0.92) | 0.82 (0.76–0.88) | 0.94 (0.91–0.97) | 7.59 (5.45–11.81) | 0.11 (0.05–0.16) | ||||
| Neutorphile (64%) | 0.95 (0.92–0.98) | 0.95 (0.92–0.97) | 0.92 (0.87–0.96) | 0.97 (0.95–0.99) | 18.19 (11.62–38.43) | 0.05 (0.02–0.09) | ||||
| Trampuz et al. | 2007 | I | I | |||||||
| WBC (1.7 × 103) | 0.94 (0.86–1.0) | 0.88 (0.81–0.94) | 0.73 (0.60–0.86) | 0.98 (0.95–1.0) | 7.76 (4.65–17.92) | 0.07 (0.0–0.17) | ||||
| Neutrophi (65%) | 0.97 (0.91–1.0) | 0.98 (0.95–1.0) | 0.94 (0.87–1.0) | 0.99 (0.97–1.0) | 48.04 (19.07–136.76) | 0.03 (0.0–0.09) | ||||
| Zmistowski et al. | 2012 | I | I | 150 | ||||||
| WBC (3.0 × 103) | 0.93 (0.87–0.99) | 0.94 (0.88–0.99) | 0.93 (0.87–0.99 | 0.94(0.88–0.99) | 14.35 (7.28–99) | 0.07 (0.01–0.14) | ||||
| Neutrophile (75%) | 0.93 (0.87–0.99) | 0.83 (0.75–0.91) | 0.84 (0.76–0.92) | 0.93 (0.87–0.99) | 5.52(3.46–11.62) | 0.08 (0.01–0.17) |
Joint aspiration (hip)
The diagnostic value of percutaneous synovial aspiration in total hip arthroplasties was addressed in six studies corresponding to a LoE of I. Four studies addressed microbiological cultures, and two studies addressed the WBC and Neutrophil-% of the synovial aspiration (Table 7).
Table 7.
Value of joint aspiration in the diagnosis of infected total hip arthroplasty
| Author | Year | LoE | CoR | (n = x) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | LR for a positive result | LR for a negative result |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrack et al. | 1993 | I | I | 291 | ||||||
| Aspiration culture | 0.6 (0.3–0.9) | 0.88 (0.84–0.92) | 0.15 (0.04–0.27) | 0.98 (0.97–1.0) | 5.11 (1.91–11.32) | 0.45 (0.1–0.83) | ||||
| Mulcahy et al. | 1996 | I | I | 71 | ||||||
| Aspiration culture | 0.69 (0.46–0.91) | 0.91 (0.83–0.99) | 0.69 (0.46–0.91) | 0.91 (0.83–0.99) | 7.56 (2.76–61.25) | 0.34 (0.09–0.65) | ||||
| Williams et al. | 2004 | I | I | 273 | ||||||
| Aspiration culture | 0.8 (0.71–0.9) | 0.94 (0.9–0.97) | 0.81 (0.72–0.91) | 0.93 (0.90–0.97) | 12.47 (7.23–29.34) | 0.21 (0.11–0.32) | ||||
| Malhotra et al. | 2004 | I | I | 41 | ||||||
| Aspiration culture | 0.44 (0.12–0.77) | 0.91 (0.81–1.0) | 0.57 (0.2–0.94) | 0.85 (0.73–0.97) | 4.74 (0.62–106.19) | 0.61 (0.23–1.09) | ||||
| Schinsky et al. | 2008 | I | I | 201 | ||||||
| Wbc (4.2 × 103) | 0.84 (0.74–0.93) | 0.93 (0.89–0.97) | 0.82 (0.72–0.92) | 0.94 (0.90–0.98) | 12.21 (6.75–33.94) | 0.18 (0.07–0.29) | ||||
| Neutrophile (80%) | 0.82 (0.72–0.92) | 0.83 (0.77–0.89) | 0.64 (0.53–0.76) | 0.92 (0.88–0.97) | 4.78 (3.08–8.36) | 0.22 (0.09–0.37) | ||||
| Spanghel et al. | 1999 | I | I | 183 | ||||||
| WBC (5.0 × 103) | 0.36 (0.18–0.53) | 0.99 (0.98–1.0) | 0.91 (0.74–1.0) | 0.9 (0.85–0.94) | 55.36 (9.43–86.89) | 0.65 (0.46–0.84) | ||||
| Neutrophile (80%) | 0.89 (0.78–1.0) | 0.85 (079–0.91) | 0.52 (0.38–0.66) | 0.98 (0.95–1.0) | 5.94 (3.76–10.75) | 0.13 (0.0–0.28) | ||||
| Dinneen et al. | 2013 | I | I | 75 | ||||||
| WBC (1.58 × 103) | 0.895 (0.783–0.997) | 0.913 (0.827–0.999) | n.p. | n.p. | n.p. | n.p. | ||||
| Neutrophile (65%) | 0.897 (0.795–0.999) | 0.866 (0.761–0.971) | n.p. | n.p. | n.p. | n.p. |
Mulcahy et al. reported a sensitivity of 0.69 (0.46–0.91) and a specificity of 0.91 (0.83–0.99) for microbiological culture [78]. Similar results with a sensitivity of 0.44 (0.12–0.77) and a specificity of 0.91 (0.81–1.0) were published by Malhotra et al. [79], and Barrack et al. reported a sensitivity of 0.6 (0.3–0.9) and a specificity of 0.88 (0.84–0.92) [80]. Williams et al. reported a higher sensitivity of 0.8 (0.71–0.9) and a specificity of 0.94 (0.9–0.97) [81], while Schinsky et al. published a sensitivity of 0.84 (0.74–0.93) and specificity of 0.93 (0.89–0.97) for cell count analysis and a sensitivity of 0.82 (0.72–0.92) and specificity 0.83 (0.77–0.89) [68]. The results confirm the series of Dinneen et al., who reported a sensitivity of 0.89 (0.783–0.997) and specificity of 0.91 (0.827–0.99); for WBC, the values were 0.89 (0.79–0.99) and 0.86 (0.76–0.97), respectively [82].
Other synovial fluid markers, such as synovial CRP and synovial IL-6, and antimicrobial peptides, such as alpha-defensin, are undergoing clinical trials [6, 83–85]. However, there is no Level I or Level II study indicating their superior diagnostic value.
Synovial biopsy histological workup
Overall, seven LoE I studies including 822 patients (5× frozen sections/2× fixed sections) addressing synovial biopsy and histological workup and one LoE III study establishing a histopathological classification of the periprosthetic membrane were included (Table 8). Banit et al. showed a sensitivity of 0.45 (0.16–0.75) and specificity of 0.92 (0.85–1.0) in their cohort. Borrego et al. reported a sensitivity of 0.5 (0.15–0.85), and a specificity of 1.00 in their series of 83 patients with THA and a sensitivity of 0.67 (0.48–0.86) and a specificity of 0.8 (0.7–0.93) in their series of 63 patients with TKA [86].
Table 8.
Value of synovial biopsy
| Author | Year | LoE | CoR | (n = x) | Sensitivity | Specifity | Positive predictive value | Negative predictive value | LR for a positive result | LR for a negative result |
|---|---|---|---|---|---|---|---|---|---|---|
| Banit et al. | 2002 | I | I | |||||||
| Frozen section (Hip) | 63 | 0.45 (0.16–0.75) | 0.92 (0.85–1.0) | 0.56 (0.23–0.88) | 0.89 (0.81–0.97) | 5.91 (1.07–166.5) | 0.59 (0.25–0.99) | |||
| Frozen section (knee) | 55 | 1 | 0.96 (0.9–1.0) | 0.82 (0.59–1.0) | 1 | 23.00 (9.76–64.7) | 0 | |||
| (Knee + hip.shoulder) | 121 | 0.67 (0.48–0.86) | 0.95 (0.91–1.0) | 0.8 (0.62–0.98) | 0.91 (0.86–0.97) | 14.67 (5.37–442) | 0.35 (0.15–0.57) | |||
| Borrego et al. | 2007 | I | I | |||||||
| Frozen sectio (Hip) | 83 | 0.5 (0.15–0.85) | 1 | 1 | 0.95 (0.9–1.0) | X | 0.5 (0.15–0.85) | |||
| Frozen section (knee) | 63 | 0.67 (0.48–0.86) | 0.9 (0.8–0.99) | 0.8 (0.62–0.98) | 0.81 (0.7–0.93) | 6.5 (2.42–116.4) | 0.37 (0.15–0.65) | |||
| Schinsky et al. | 2008 | I | I | 201 | ||||||
| Frozen section | 0.73 (0.61–0.84) | 0.94 (0.9–0.98) | 0.82 (0.71–0.92) | 0.9 (0.85–0.95) | 11.8 (6.06–37.34) | 0.29 (0.16–0.43) | ||||
| Fink et al. | 2008 | I | I | 145 | ||||||
| Fixed section (knee) | 1 | 0.98 (0.95–1.0) | 0.95 (0.89–1.0) | 1 | 52.5 (22.13–140.88) | X | ||||
| Tissue culture (knee) | 0.78 (0.65–0.9) | 0.98 (0.95–1.0) | 0.94 (0.86–1.0) | 0.92 (0.87–0.97) | 40.69 (14.28–127.41) | 0.23 (0.09–0.37) | ||||
| Both | 0.78 (0.65–0.9) | 0.98 (0.95–1.0) | 0.94 (0.86–1.0) | 0.92 (0.87–0.97) | 40.69 (14.28–127.41) | 0.23 (0.09–0.37) | ||||
| Della Valle et al. | 2007 | I | I | 94 | ||||||
| Forzen section | 0.88 (0.78–0.98) | 0.96 (0.91–1.0) | 0.95 (0.88–1.0) | 0.91 (0.84–0.99) | 23.27 (8.74–72.1) | 0.13 (0.02–0.24) | ||||
| Fink et al. | 2013 | I | I | 100 | ||||||
| Fixed section (hip) | 0.62 (0.48–0.76) | 1 | 1 | 0.76 (0.67–0.86) | X | 0.38 (0.24–0.52) | ||||
| Tissue culture (hip) | 0.73 (0.6–0.86) | 0.98 (0.95–1.0) | 0.97 (0.91–1.0) | 0.82 (0.73–0.91) | 40.33 (11.29–50.36) | 0.27 (0.14–0.42) | ||||
| Both | 0.82 (0.71–0.93) | 0.98 (0.95–1.0) | 0.97 (0.92–1.0) | 0.87 (0.06–0.31) | 45.22 (13.28–54.52) | 0.18 (0.06–0.31) |
Nunez et al. calculated a sensitivity of 0.86 (0.76–0.96) and a specificity of 0.87 (0.8–0.94) [87].
Banit et al. observed a sensitivity of 1.0 and a specificity of 0.96 (0.9–1.0) for TKA and a sensitivity of 0.45 (0.16–0.75) and a specificity of 0.92 (0.85–1.0) for THA. Overall, the authors reported a sensitivity of 0.67 (0.48–0.86) and a specificity of 0.95 (0.91–1.0) [88]. In their series of 105 patients with painful TKA, Valle Della et al. observed a sensitivity of 0.88 (0.78–0.98) and a specificity of 0.96 (0.91–1.0). For THA, Schinsky et al. reported a sensitivity of 0.73 (0.61–0.84) and a specificity of 0.94 (0.9–0.98) [65, 68].
Regarding fixed sections, Fink et al. showed a sensitivity of 1.0 and a specificity of 0.98 (0.95–1.0) for TKA (145) and a sensitivity of 0.62 (0.48–0.76) and a specificity of 1.0 for THA (100) [70, 89].
Synovial biopsy microbiological workup
In their series of THAs, Fink et al. showed a sensitivity of 0.73 (0.6–0.86) and a specificity of 0.98 for microbiological culture of biopsies (0.95–1.0). The combination of histological biopsy and microbiological culture increased the sensitivity to 0.82 (0.71–0.93) [90]. In their series of TKAs, they reported a sensitivity of 0.78 (0.65–0.9) and a specificity of 0.98 (0.95–1.0); when combined with histological analysis, the sensitivity increased to 1, with equal specificity. Regarding the recommended number of microbiological cultures, the mathematical model of Atkins et al. was used: at least 5 or 6 biopsy cultures should be taken [91]. Marin et al. showed a sensitivity of 0.87 (0.66–0.94) and a specificity of 0.67 (0.56–0.76) for one positive microbiological culture using that model; when three positive cultures were used, the sensitivity decreased to 0.46 (0.32–0.61), while the specificity increased to 0.98 (0.93–0.99) [92].
Discussion
Although the diagnosis of PJIs prior to revision surgery is of paramount importance for further treatment, it can be challenging, and a well-structured diagnostic approach is necessary. A PJI diagnosis results in substantial changes in the therapeutic procedure [3]. Thus, an evidence-based and priority-orientated algorithm (Fig. 2) can provide an incremental and easy-to-use guideline for non-specialists and less-experienced orthopedic surgeons.
AAOS guidelines strongly recommend determining the ESR and CRP [93]. According to the included studies, sensitivities vary from 81 to 93% for the ESR and from 73 to 95% for the CRP [93]. In a recent meta-analysis by Berbari and colleagues that included 30 studies with a total of 3909 patients, pooled sensitivities of 75% for the ESR and 88% for the CRP were reported [94]. The specificities were 70 and 74%, respectively. Despite the relatively high sensitivity of the CRP, its specificity remains unsatisfactory, confirming the observations of McArthur et al. who identified a considerable subset of patients with PJI and negative serology within their series of 414 infected THAs. In contrast, the AAOS guidelines recommend percutaneous aspiration only in case of altered ESR and CRP levels and thus exclude seronegative patients from this procedure [93]. In these cases, one-stage revision surgery without adequate antibiotic treatment may be performed, inevitably resulting in new prosthetic failure and PJI persistence. In our algorithm, the decision to perform joint aspiration is based on ESR and CRP levels and on the radiological findings and medical history (risk factors) of the patient.
Large multicenter LoE I studies were able to define some risk factors. In particular, potential intraoperative contamination and the immune system of the patients were determined to have an important role. Namba et al. showed in a large multicenter study that an extended operation time leads to an increase of PJIs. An additional 15 min of operation time was determined to increase the risk by up to 9% [39]. This relationship is explained by increased time for potential intraoperative microbial contamination. However, the increased risk of PJI in immunocompromised patients, such as those with rheumatoid arthritis and/or diabetes, has also been proven. The most recent studies suggest that even asymptomatic bacteriuria is an independent risk factor for PJI; the authors indicated that an immunocompromised status puts patients at risk for colonization with Gram-negative microorganisms [50]. Early implant loosening (<5 years) without evidence of mechanical failure or progressive radiolucency adjacent to the implant must be considered a decisive risk factor for a low-grade PJI. As proposed by Lachiewicz et al., premature implant loosening and the presence of the previously described risk factors require further diagnostic procedures [47]. In this context, Portillo et al. were able to demonstrate a significantly longer period between primary implantation and diagnosed aseptic loosening (7.8 years) compared with septic implant loosening (2 years; CoR I) [46].
Considering the aforementioned evidence-based risk factors in our algorithm prior to joint aspiration permits a benefit-risk assessment for post-interventional complications and economic issues. Although iatrogenic complications in the context of synovial aspiration are considered rare, Murray et al. reported a complication rate of 5.1% (0.2–10%), including hematoma, infections and lesions on nerve structures after synovial aspiration of the hip [95]. Barrack et al. showed a 1% (0.1–2.2%) rate of infections after synovial aspiration of the hip [96]. This benefit–risk assessment is of major importance to minimize the risk of infection for the patient and thus avoid false-positive results leading to overtreatment [97].
However, the diagnostic value of synovial aspiration and subsequent microbiological workup is controversial according to recent literature. Sensitivities vary between 12 and 89%, with specificities between 50 and 100% for synovial aspiration of hip joints [68, 78, 79, 81, 82, 96, 98, 99]. Similar results are available for TKA [65, 70, 77, 100, 101]. However, extended synovial analysis combining microbiological culture with WBC and neutrophil-% is the gold standard for synovial aspiration investigation. The sensitivity, specificity, positive/negative predictive value and positive/negative likelihood ratio for the WBC and neutrophil-% are given in Tables 6 and 7. Several studies have examined the optimal cut-off values for WBC and neutrophil-%. Trampuz et al. suggest 1.7 × 103/µl (WBC) and 65% (neutrophil-%), and Zmistowski et al. and Della Valle report quite similar results, using higher cut-off values of 3.0 × 103 for WBC and 75% neutrophils [65, 101]. However, cut-off values calculated using receiver-operating characteristics are linked to the microbiological strains that cause the PJI. In our algorithm, we used the lower cut-off values that Trampuz et al. and Schinsky et al. identified to ensure that we detected the low-grade infections caused by slow-growing and low virulence strains, such as coagulase-negative staphylococci or Proprionibacterium acnes, which generate a low immune reaction. Considering the defining criteria for PJI (Table 2), 2 or more separate synovial fluid samples should be obtained from the index joint. However, this main criterion is usually not met with routine synovial aspiration. In the daily clinical routine, the additional use of blood culture bottles, as proposed by Minassian et al. [102], to obtain two separate microbiological cultures should thus be encouraged. Although the data on diagnostic value are discordant, the causative pathogen and its antibiotic sensitivity pattern can be identified via synovial aspiration and microbiological examination. This information, in turn, is of great importance for preoperatively planning the surgical strategy and the antibiotic regimen.
Unfortunately, a causative pathogen can only be identified in approximately 44% [79]–80% [81] of cases, reflecting the heterogeneous diagnostic value of synovial aspiration. Among the factors influencing microbiological results, the length of the incubation period is crucial because the bacteria that cause PJIs occur only in a very low number in the biofilm and often are in a sessile form that is very slow growing [103, 104]. Accordingly, in many of the aforementioned studies, the length of microbiological incubation was only 48 h or was not specifically disclosed. Furthermore, the omission of antibiotic treatment termination at least 2 weeks prior to the joint aspiration can lead to false-negative microbiological results and thus to maltreatment [105].
Other synovial fluid markers, such as alpha-defensin, show promising results, with a sensitivity of 100% [4, 85], but they lack the evidence and independent studies to support their use. Similarly, the leukocyte esterase test requires further evidence to support its role in diagnosing PJI [5, 106]. According to the Plan-Do-Check-Act principle, constant improvement of the algorithm by reintegrating actual evidence-based literature at half-year intervals is intended. If new diagnostic procedures fulfill the LoR I criteria, they will be included in the algorithm.
As a further, more invasive diagnostic step, arthroscopic synovial biopsy has been implemented in our algorithm. As itemized in the “Arthroscopy” checkbox (Fig. 2), increased WBC or neutrophil percentage but negative microbiological assessment of the aspirate, continued antibiotic treatment and history of PJI are indications for synovial biopsy according to our algorithm.
In this context, recent studies by Williams et al. showed equal results for aspiration and tissue biopsy with sole microbiological examination [81]. These results underline the importance of concurrent histological and microbiological workup of the biopsy specimens, as confirmed by Malhotra and Morgan in their series of 41 THAs [79]. The authors reported a sensitivity of 80% and a specificity of 100% for synovial biopsy compared with a sensitivity of 44% and a specificity of 91% for synovial fluid aspiration. Likewise, Fink et al. reported synovial biopsy sensitivities of 100 and 87% for TKA and THA, respectively [70, 89] Specificity was 98% for both TKA and THA. According to the authors, the underlying hypothesis for the discrepancy of results between hip and knee joints was that biopsy samples can be obtained at many more places adjacent to the prosthesis in the knee compared with hip joints, where only the head and neck of the prosthesis and the inlay of the acetabular cup are easily accessible [89].
Despite its excellent diagnostic value, synovial biopsy should only be applied in selected cases, as stated above. Although the procedure can be considered a minor operation, potential risks such as neuro-vascular injury or surgical site infection should not be underestimated [107]. Furthermore, arthroscopic instruments can damage the hip or knee replacement, particularly in ceramic implants. Thus, similar to percutaneous joint aspiration, a benefit–risk assessment prior to the intervention is essential to maximize the diagnostic yield within the diagnostic cascade and while minimizing the potentially harmful effects for the patient.
Conclusions
The diagnostic algorithm presented in this study is derived from high-quality studies in the field of PJI and provides a well-structured diagnostic approach in form of a detailed and transparent SOP. These incremental and easy-to-use guidelines facilitate consistently high and examiner-independent process quality in terms of PJI treatment and provide a basis for scientific analyses.
Authors’ contributions
Conception and designs: HM, FP, JS,RvER, KGK, UL. Generation, collection assembly, analysis and interpretation: HM, FP, KGK, UL, FL, AT, SK, NH. Drafting and revising the manuscript: HM, FP, KGK, JS. Approval of the final version of the manuscript: HM, FP, RvER, JS, KGK. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
All authors declare that they have no competing interests.
Availability of data and materials
All data are available in the included figures, tables and bibliography.
Ethics approval and consent to participate
No approval by an institutional review board and no consent to participate were required.
Funding
The present study was funded by the authors institution.
Abbreviations
- PJI
prosthetic joint infection
- THA
total hip arthroplasty
- TKA
total knee arthroplasty
- EAST
Eastern Association of the Surgery of Trauma
- SOP
standard operating procedure
- ESR
erythrocyte sedimentation rate
- CRP
c-reactive protein
- GRADE
The Grading of Recommendation Assessment, Development and Evalutation
- STARD
Standards for Reporting of Diagnostic Accuracy
- LoE
level of evidence
- CoR
class of recommendation
- IDSA
Infectious Deceases Society of America
- MSIS
Musculoskeletal Infection Society
- ISO
International Organization for Standardization
- IL-6
interleukin-6
- PCT
procalcitonin
- AAOS
American Academy of Orthopaedic Surgeons
Contributor Information
Heinrich M. L. Mühlhofer, Phone: 0049-89-41405490, Email: heinrich.muehlhofer@mri.tum.de
Florian Pohlig, Email: Florian.Pohlig@mri.tum.de.
Karl-Georg Kanz, Email: Karl-Georg.Kanz@mri.tum.de.
Ulrich Lenze, Email: uli_lenze@web.de.
Florian Lenze, Email: flo_lenz@yahoo.de.
Andreas Toepfer, Email: andreastoepfer@t-online.de.
Sarah Kelch, Email: sarah.kelch@mri.tum.de.
Norbert Harrasser, Email: Norbert.harrasser@gmx.net.
Rüdiger von Eisenhart-Rothe, Email: eisenhart@tum.de.
Johannes Schauwecker, Email: j.schauwecker@tum.de.
References
- 1.Kurtz S. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Urquhart DM, Hanna FS, Brennan SL, Wluka AE, Leder K, Cameron PA, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty Elsevier. 2010;25:1216–1222. doi: 10.1016/j.arth.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerli W, Trampuz A. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 4.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254–3262. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplast. 2012;27(8):8–11. doi: 10.1016/j.arth.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95:644. doi: 10.2106/JBJS.L.00205. [DOI] [PubMed] [Google Scholar]
- 7.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49:7–18. doi: 10.1373/49.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem Lab Med. 2003;41:68–73. doi: 10.1515/CCLM.2003.012. [DOI] [PubMed] [Google Scholar]
- 10.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence-publication bias. J Clin Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. J Clin Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence-indirectness. J Clin Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Infectious diseases Society of America. Clin Infect Dis. 1994;18:422–427. doi: 10.1093/clinids/18.3.422. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Oussedik S, Gould K, Stockley I. Defining peri-prosthetic infection. J Bone Joint Surg Br. 2012;94:1455–1456. doi: 10.1302/0301-620X.94B11.30244. [DOI] [PubMed] [Google Scholar]
- 23.Society TWCBTMI New definition for periprosthetic joint infection. J Arthroplast. 2011;26:1136–1138. doi: 10.1016/j.arth.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. Oxford University Press; 2013. pp. e1–e25. [DOI] [PubMed]
- 25.Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J. 2013;95:1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 26.ISO G. International Organisation for Standarisation (1985) ISO 5807. 1985 Aug.
- 27.Khalil PN, Kleespies A, Angele MK, Thasler WE, Siebeck M, Bruns CJ, et al. The formal requirements of algorithms and their implications in clinical medicine and quality management. Langenbecks Arch Surg. 2010;396:31–40. doi: 10.1007/s00423-010-0713-3. [DOI] [PubMed] [Google Scholar]
- 28.Huotari K, Lyytikäinen O, Seitsalo S. Patient outcomes after simultaneous bilateral total hip and knee joint replacements. J Hosp Infect. 2007;65:219–225. doi: 10.1016/j.jhin.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Småbrekke A, Espehaug B, Havelin LI, Furnes O. Operating time and survival of primary total hip replacements: an analysis of 31,745 primary cemented and uncemented total hip replacements from local hospitals reported to the Norwegian Arthroplasty Register 1987–2001. Acta Orthop Scand. 2004;75:524–532. doi: 10.1080/00016470410001376. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the medicare population. Clin Orthop Relat Res. 2009;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uçkay I, Lübbeke A, Emonet S, Tovmirzaeva L, Stern R, Ferry T, et al. Low incidence of haematogenous seeding to total hip and knee prostheses in patients with remote infections. J Infect. 2009;59:337–345. doi: 10.1016/j.jinf.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 33.Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2008;467:1577–1581. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowsey MM, Choong PFM. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res. 2008;466:153–158. doi: 10.1007/s11999-007-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57:327–334. doi: 10.1002/art.22542. [DOI] [PubMed] [Google Scholar]
- 37.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namba RS, Inacio M, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br. 2012;94:1330–1338. doi: 10.1302/0301-620X.94B10.29184. [DOI] [PubMed] [Google Scholar]
- 39.Namba RS, Inacio MCS, Paxton EW. risk factors associated with deep surgical site infections after primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95:775–778. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 40.Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplast. 2009;24(6):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Peel TN, Dowsey MM, Daffy JR, Stanley PA, Choong PFM, Buising KL. Risk factors for prosthetic hip and knee infections according to arthroplasty site. J Hosp Infect. 2011;79:129–133. doi: 10.1016/j.jhin.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Berbari EF, Osmon DR, Lahr B, Eckel-Passow JE, Tsaras G, Hanssen AD, et al. The mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol. 2012;33:774–781. doi: 10.1086/666641. [DOI] [PubMed] [Google Scholar]
- 43.Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5:412–418. doi: 10.1177/193229681100500231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jämsen E. Risk factors for infection after knee arthroplasty. J Bone Joint Surg Am. 2009;91:38. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 45.Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1713–1720. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Portillo ME, Salvadó M, Alier A, Sorli L, Martínez S, Horcajada JP, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471:3672–3678. doi: 10.1007/s11999-013-3200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lachiewicz PF, Rogers GD, Thomason HC. Aspiration of the hip joint before revision total hip arthroplasty. Clinical and laboratory factors influencing attainment of a positive culture. J Bone Joint Surg Am. 1996;78:749–754. doi: 10.2106/00004623-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2014;20:130–135. doi: 10.1111/1469-0691.12209. [DOI] [PubMed] [Google Scholar]
- 49.Wymenga AB, van Horn JR, Theeuwes A, Muytjens HL, Slooff TJ. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2651 hip operations. Acta Orthop Scand. 1992;63:665–671. doi: 10.1080/17453679209169732. [DOI] [PubMed] [Google Scholar]
- 50.Sousa R, Muñoz-Mahamud E, Quayle J, Dias da Costa L, Casals C, Scott P, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis. 2014;59:41–47. doi: 10.1093/cid/ciu235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murdoch DR, Roberts SA, Fowler VG, Jr, Shah MA, Taylor SL, Morris AJ, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647–649. doi: 10.1086/318704. [DOI] [PubMed] [Google Scholar]
- 52.Coelho-Prabhu N, Oxentenko AS, Osmon DR, Baron TH, Hanssen AD, Wilson WR, et al. Increased risk of prosthetic joint infection associated with esophago-gastro-duodenoscopy with biopsy. SORT. 2013;84:82–86. doi: 10.3109/17453674.2013.769079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishriki SF, Law D, Jeffery PJ. Factors affecting the incidence of postoperative wound infection. J Hosp Infect. 1990;16:223–230. doi: 10.1016/0195-6701(90)90110-A. [DOI] [PubMed] [Google Scholar]
- 54.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2014. Crit Care Med. 2012;2013:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 55.Llewelyn M, Cohen J. International sepsis forum. Diagnosis of infection in sepsis. Intensive Care Med. 2001;27:S10–S32. doi: 10.1007/PL00003792. [DOI] [PubMed] [Google Scholar]
- 56.Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Kern W, et al. Prävention, diagnose, therapie und Nachsorge der Sepsis. Der Anaesthesist. 2010;59:1–68. doi: 10.1007/s00101-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 57.Brook I, Frazier EH. Aerobic and anaerobic microbiology of retroperitoneal abscesses. Clin Infect Dis. 1998;26:938–941. doi: 10.1086/513947. [DOI] [PubMed] [Google Scholar]
- 58.Nichols RL, Smith JW. Wound and intraabdominal infections: microbiological considerations and approaches to treatment. Clin Infect Dis. 1993;16(Suppl 4):S266–S272. doi: 10.1093/clinids/16.Supplement_4.S266. [DOI] [PubMed] [Google Scholar]
- 59.Bates DW, Cook EF, Goldman L, Lee TH. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113:495–500. doi: 10.7326/0003-4819-113-7-495. [DOI] [PubMed] [Google Scholar]
- 60.Smith-Elekes S, Weinstein MP. Blood cultures. Infect Dis Clin North Am. 1993;7:221–234. [PubMed] [Google Scholar]
- 61.Koperna T, Schulz F. Relaparotomy in peritonitis: prognosis and treatment of patients with persisting intraabdominal infection. World J Surg. 2000;24:32–37. doi: 10.1007/s002689910007. [DOI] [PubMed] [Google Scholar]
- 62.Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am. 2005;19:885–914. doi: 10.1016/j.idc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Teller RE, Christie MJ, Martin W, Nance EP, Haas DW. Sequential indium-labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin Orthop Relat Res. 2000;373:241–247. doi: 10.1097/00003086-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 64.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–99. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 65.Valle Della CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplast. 2007;22:90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Greidanus NV, Masri BA, Garbuz DS, Wilson SD, McAlinden MG, Xu M, et al. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am. 2007;89:1409–1416. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- 67.Kamme C, Lindberg L. Aerobic and anaerobic bacteria in deep infections after total hip arthroplasty: differential diagnosis between infectious and non-infectious loosening. Clin Orthop Relat Res. 1981;154:201–207. [PubMed] [Google Scholar]
- 68.Schinsky MF. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 69.Savarino L, Baldini N, Tarabusi C, Pellacani A, Giunti A. Diagnosis of infection after total hip replacement. J Biomed Mater Res. 2004;70:139–145. doi: 10.1002/jbm.b.30030. [DOI] [PubMed] [Google Scholar]
- 70.Fink B, Makowiak C, Fuerst M, Berger I, Schäfer P, Frommelt L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90:874–878. doi: 10.1302/0301-620X.90B7.20417. [DOI] [PubMed] [Google Scholar]
- 71.Di Cesare PE, Chang E, Preston CF, Liu C-J. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87:1921–1927. doi: 10.2106/JBJS.D.01803. [DOI] [PubMed] [Google Scholar]
- 72.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop SICO. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Worthington T, Dunlop D, Casey A. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to shortchain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci. 2010;67:71–76. doi: 10.1080/09674845.2010.11730294. [DOI] [PubMed] [Google Scholar]
- 74.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. 2012;56:1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 75.Bogut A, Niedźwiadek J, Kozioł-Montewka M, Strzelec-Nowak D, Blacha J, Mazurkiewicz T, et al. Sonication as a diagnostic approach used to investigate the infectious etiology of prosthetic hip joint loosening. Pol J Microbiol. 2014;63:299–306. [PubMed] [Google Scholar]
- 76.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 77.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. AJM. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Mulcahy DM, Fenelon GC, McInerney DP. Aspiration arthrography of the hip joint. Its uses and limitations in revision hip surgery. J Arthroplast. 1996;11:64–68. doi: 10.1016/S0883-5403(96)80162-8. [DOI] [PubMed] [Google Scholar]
- 79.Malhotra R, Morgan D. Role of core biopsy in diagnosing infection before revision hip arthroplasty. J Arthroplast. 2004;19:78–87. doi: 10.1016/S0883-5403(03)00453-4. [DOI] [PubMed] [Google Scholar]
- 80.Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. The coventry award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res. 1997;345:8–16. doi: 10.1097/00003086-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplast. 2004;19:582–586. doi: 10.1016/j.arth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Dinneen A, Guyot A, Clements J. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2013;95:554–557. doi: 10.1302/0301-620X.95B4.30388. [DOI] [PubMed] [Google Scholar]
- 83.Jacovides CL, Parvizi J, Adeli B, Am Jung K. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplast. 2011;26:99–103. doi: 10.1016/j.arth.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 84.Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplast. 2012;27:12–16. doi: 10.1016/j.arth.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439–1445. doi: 10.2106/JBJS.M.01316. [DOI] [PubMed] [Google Scholar]
- 86.Francés Borrego A, Martínez FM, Cebrian Parra JL, Grañeda DS, Crespo RG, López-Durán Stern L. Diagnosis of infection in hip and knee revision surgery: intraoperative frozen section analysis. Int Orthop SICO. 2006;31:33–37. doi: 10.1007/s00264-005-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nuñez LV, Buttaro MA, Morandi A, Pusso R, Piccaluga F. Frozen sections of samples taken intraoperatively for diagnosis of infection in revision hip surgery. SORT. 2007;78:226–230. doi: 10.1080/17453670710013726. [DOI] [PubMed] [Google Scholar]
- 88.Banit DM, Kaufer H, Hartford JM. Intraoperative frozen section analysis in revision total joint arthroplasty. Clin Orthop Relat Res. 2002;401:230–238. doi: 10.1097/00003086-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 89.Fink B, Gebhard A, Fuerst M, Berger I, Schäfer P. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Relat Res. 2013;471:956–964. doi: 10.1007/s11999-012-2474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fink B, Gebhard A, Fuerst M, Berger I, Schäfer P. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Relat Res. 2012;471:956–964. doi: 10.1007/s11999-012-2474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marin M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcala L, Gimeno M, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic Joint Infection. J Clin Microbiol. 2012;50:583–589. doi: 10.1128/JCM.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, III, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:760–70. [DOI] [PubMed]
- 94.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg. 2010;92:2102–2109. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 95.Murray RP, Bourne MH, Fitzgerald RH. Metachronous infections in patients who have had more than one total joint arthroplasty. J Bone Joint Surg Am. 1991;73:1469–1474. doi: 10.2106/00004623-199173100-00004. [DOI] [PubMed] [Google Scholar]
- 96.Barrack RL, Harris WH. The value of aspiration of the hip joint before revision total hip arthroplasty. J Bone Joint Surg Am. 1993;75:66–76. doi: 10.2106/00004623-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Yee DKH, Chiu KY, Yan CH, Ng FY. Review article: joint aspiration for diagnosis of periprosthetic infection. J Orthop Surg (Hong Kong). 2013;21:236–240. doi: 10.1177/230949901302100225. [DOI] [PubMed] [Google Scholar]
- 98.Detection of occult infection following total joint arthroplasty using sequential technetium-99 m HDP bone scintigraphy and indium-111 WBC imaging. 1988;29:1347–53. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=3404252&retmode=ref&cmd=prlinks. [PubMed]
- 99.Spangehl MJ, Masri BA, O’connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81(5):672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Ghanem E, Parvizi J, Burnett RSJ, Sharkey PF, Keshavarzi N, Aggarwal A, et al. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 101.Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis. J Arthroplast. 2012;27:1589–1593. doi: 10.1016/j.arth.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 102.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC. Use of an automated blood culture system (BD BACTEC™) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis. 2014;14:1–7. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Oxford University Press; 2004;39:1599–603. http://cid.oxfordjournals.org/lookup/doi/10.1086/425303. [DOI] [PubMed]
- 104.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 105.Saleh KJ, Clark CR, Sharkey PF, Goldberg VM, Rand JA, Brown GA. Modes of failure and preoperative evaluation. J Bone Joint Surg Am. 2003;85(Suppl 1):S21–S25. doi: 10.2106/00004623-200300001-00006. [DOI] [PubMed] [Google Scholar]
- 106.Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. Am Orthop Assoc; 2011;93:2242–8. http://jbjs.org/cgi/doi/10.2106/JBJS.J.01413. [DOI] [PubMed]
- 107.Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84–88. doi: 10.1097/00003086-200301000-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the included figures, tables and bibliography.


