Abstract
Stem cells therapy has been suggested as a promising option for the treatment of acute kidney injury (AKI). This study was performed to compare the abilities of xenogenic transplantation of human adipose stromal vascular fraction (SVF) and adipose-derived mesenchymal stem cells (AdMSCs) to facilitate the recovery of renal function and structure in a rat model of ischemia/reperfusion (IR) induced AKI. SVF or AdMSCs were transplanted to the injured kidney through intra-parenchymal injection. Significantly improved renal function and reduced tubular injury were observed in SVF and AdMSCs groups. Administration of SVF or AdMSCs contributed to significantly improved cell proliferation and markedly reduced cell apoptosis in parallel with reduced microvascular rarefaction in injured kidney. IR injury resulted in higher levels of inflammatory cytokines, whereas xenogenic transplantation of SVF or AdMSCs reduced but not induced inflammatory cytokines expression. Additionally, in vitro study showed that administration of SVF or AdMSCs could also significantly promote the proliferation and survival of renal tubular epithelial cells underwent hypoxia/reoxygenation injury through secreting various growth factors. However, cell proliferation was significantly promoted in SVF group than in AdMSCs group. In conclusion, our study demonstrated that administration of SVF or AdMSCs was equally effective in attenuating acute renal IR injury.
Acute kidney injury (AKI), which is a common clinical syndrome, is considered to be an increasing global concern due to the increased patient morbidity and mortality, as well as the high risk for subsequent development of chronic kidney disease (CKD), despite the current advances in medical treatment1,2,3. Ischemia/reperfusion (IR) induced injury is a leading cause of AKI, which is associated with acute tubular-epithelial damage, loss of peritubular capillary, and inflammation4. Since the currently main therapies for AKI, including dialysis and renal transplantation, are often limited by the high expense and sever shortage of donor organs5, a new and effective method is in urgent need for the repair of AKI.
Recently, stem cell transplantation has become new candidate for the treatment of AKI. Simultaneously, adipose tissue has gained considerable attention as a cell source for tissue regeneration6. Studies have shown that adipose derived mesenchymal stem cells (AdMSCs) could effectively rescue IR induced kidney injury due to various mechanisms7,8,9,10. However, during the in vitro culture of AdMSCs to gain adequate cell numbers for in vivo administration, there are several concerns regarding the transplantation of cultured stem cells into human subjects, including the potential risks of xenogenic nutritional sources, microbial contamination, and so on11. In addition, the possible loss of the optimal opportunity for the repair of AKI is also a non-negligible issue due to the long-time cell culture in vitro. Fortunately, recent studies have suggested that uncultured adipose stromal vascular fraction (SVF) might be an attractive cell source for cell-based therapy on account of the real time isolation in an adequate quantity without in vitro expansion, which could avoid the risks described above11. In previous studies, both our team and other groups reported that autologous uncultured SVF could protect kidney from both IR and cisplatin induced acute injury12,13,14.
Despite the superiority of SVF compared to AdMSCs described above, no systematic study has compared the application of such two different cell types derived from adipose tissue for the treatment of AKI, as well as the efficacy and safety of them after being transplanted in vivo12,13,14. Moreover, though adipose tissue can be obtained through a minimally invasive method, the harvest of adipose tissue from a patient with serious illness, such as AKI, is still an invasive procedure, which may limit the application of autologous SVF therapy in the clinic. Due to the immunosuppressive property of AdMSCs, allogeneic or xenogeneic transplantation of AdMSCs into immunocompetent recipients is feasible and effective for the improvement of targeted diseases15. In addition, a recent study has demonstrated that xenogenic transplantation of human SVF successfully improved erectile function in diabetic mice without inducing systemic inflammation16. Therefore, to test the feasibility of xenogenic transplantation of human SVF to treat AKI, this study was performed to investigate the effects of xenogenic transplantation of uncultured human SVF on the repair of IR induced AKI in a rat model. Furthermore, we also compared the efficacy of human SVF and AdMSCs on the recovery of renal function and structure after AKI.
Results
Cell characterization
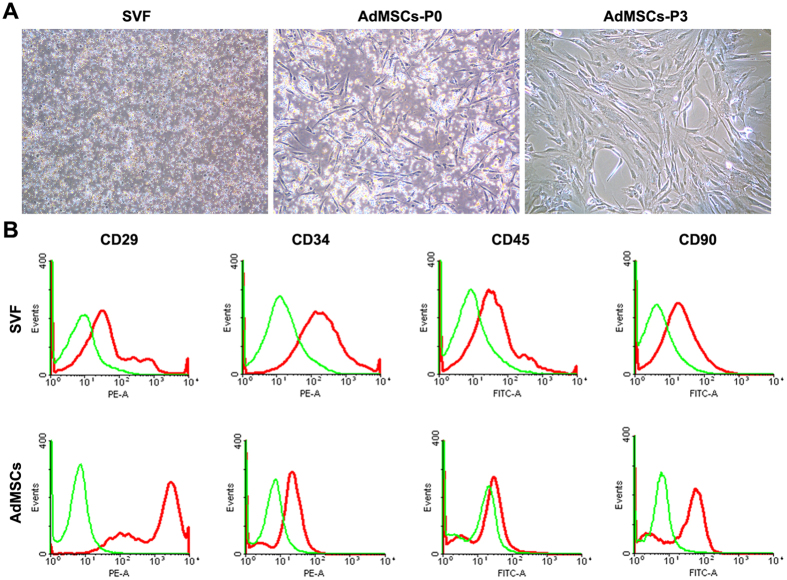
SVF cells were successfully isolated from perinephric adipose tissue. After two days of culture, AdMSCs emerged with spindle-like morphologies. Cells were passaged when they reached over 80% confluence after about one week of culture. At passage 3, AdMSCs became fusiform and exhibited typical spindle shaped morphologies (Fig. 1A). Flow cytometry was performed to characterized SVF and AdMSCs by determining CD markers expression. Flow cytometric analysis demonstrated that SVF was positive for CD29 (29.7%), CD34 (7.9%), CD45 (15.8%) and CD90 (25.1%). AdMSCs were positive for CD29(99.8%), CD90(92.8%) and CD34(11.2%), but negative for CD45 (1.4%) (Fig. 1B).
Figure 1. Characterization of SVF and AdMSCs.
(A) Cell morphologies of freshly isolated SVF and cultured AdMSCs at passge 0 and 3. (B) Flow cytometric analysis of freshly isolated SVF and cultured AdMSCs at passge 3. Scale bars = 100 mm
Effects of SVF and AdMSCs on renal tubular epithelial cells in vitro cultured in hypoxic condition
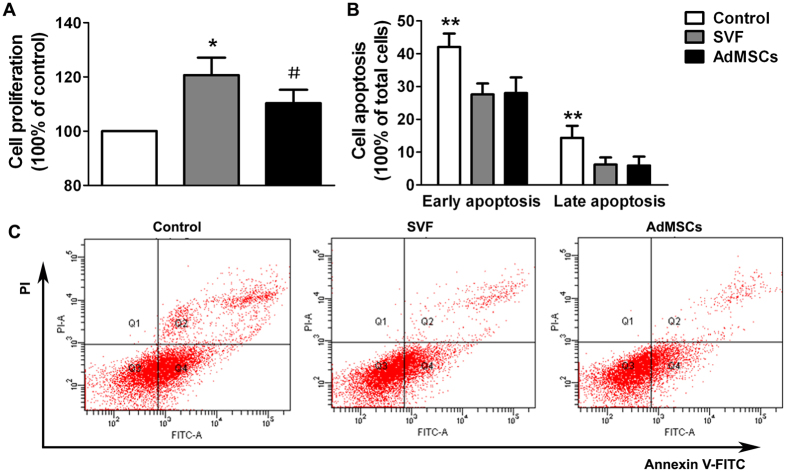
Cell proliferation assay showed that both SVF and AdMSCs could significantly enhance the proliferation activity of HK-2 cells underwent H/R injury. In addition, the proliferation of HK-2 cells was significantly promoted in SVF group than that in AdMSCs group (Fig. 2A). Annexin V/propidiumiodide (PI) apoptosis assay was performed to assess H/R injury induced cell apoptosis of HK-2 cells, including early apoptotic cells (Annexin V positive, PI negative) and late apoptotic cells (Annexin V positive, PI positive). Results showed that both early and late apoptotic cells were increased after H/R injury. However, SVF or AdMSCs treatment could significantly reduce the proportion of apoptotic cells. Furthermore, no significant difference on the proportion of apoptotic cells could be found between SVF and AdMSCs groups (Fig. 2B,C).
Figure 2. In vitro effects of SVF or AdMSCs on renal tubular epithelial cells in hypoxic environment.
(A) SVF or AdMSCs administration could significantly promote the proliferation of hypoxic HK-2 cells, compared with control group. *p < 0.05, vs. Control and AdMSCs; #p < 0.05, vs. Control. (B) SVF or AdMSCs administration could significantly reduce the apoptosis of hypoxic HK-2 cells, compared with control group. **p < 0.05, vs. SVF and AdMSCs. (C) Representative flow cytometry histograms of Annexin V/PI apoptosis assay in different groups.
ELISA showed that culture medium from SVF and AdMSCs contained significantly higher hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF) and stromal cell derived factor-1α (SDF-1α) than control medium, which indicated that both SVF and AdMSCs could secret HGF, VEGF and SDF-1α. However, HGF secretion from SVF was significantly higher than from AdMSCs (supplemental Fig. 1).
Effects of SVF and AdMSCs on renal function and tubular injury
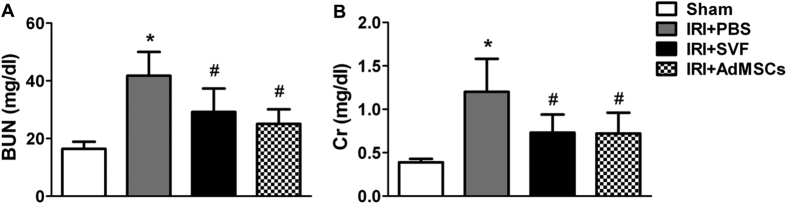
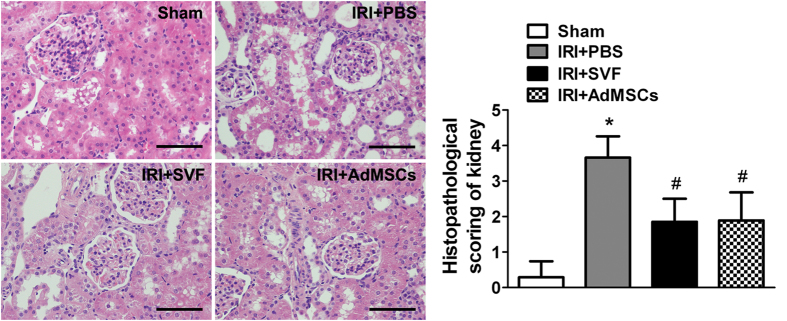
We compared the effects of AdMSCs and SVF on renal function and tubular injury in renal IR injury model. Animals in the control group showed a significant rise in serum levels of SCr and BUN compared with the sham group. However, a significant decrease in SCr and BUN was detected in both AdMSCs and SVF groups. Furthermore, there was no significant difference in renal function between the SVF and AdMSCs groups (Fig. 3). Histological examination showed that animals in control group subjected marked tubular injury, including cast formation, vacuolization, tubular necrosis, and so on, compared to sham group. SVF and AdMSCs treatment contributed to a significant reduction of tubular injury after IR injury. Furthermore, there were no significant differences in tubular injury between the SVF and AdMSCs groups (Fig. 4).
Figure 3. In vivo protective effect of SVF or AdMSCs on renal function in acute IR injuried kidney.
Rats received SVF or AdMSCs administration showed significantly lower BUN (A) and SCr (B) values at 72 h after IR compared with control ones. There were no significant differences on the level of BUN and SCr between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs; #p < 0.05, vs. Control.
Figure 4. In vivo protective effect of SVF or AdMSCs administration on renal morphology in acute IR injuried kidney.
Administration of SVF or AdMSCs contributed to reduced tubular injury compared with control group. There were no significant differences on tubular injury between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs; #p < 0.05, vs. Control. Scale bars = 100 mm.
Effects of SVF and AdMSCs on cell proliferation and apoptosis in injured kidney
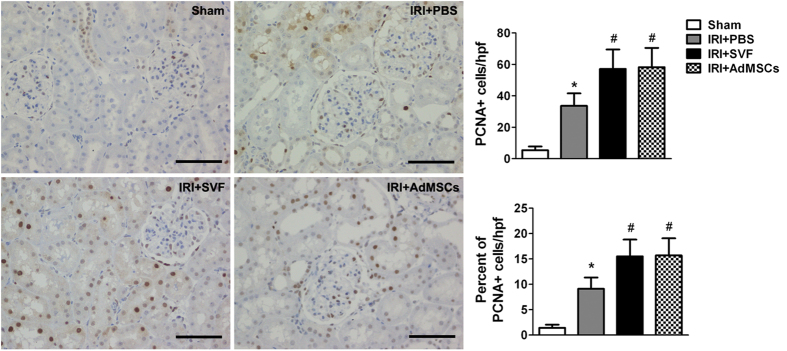
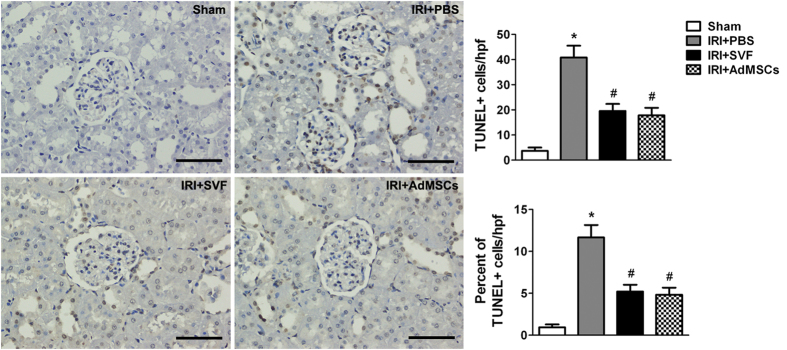
The effects of SVF and AdMSCs on tubular cell proliferation were observed by evaluating PCNA expression in the kidney sections after IR injury. The enhancement of proliferation activity of renal tubular cells was demonstrated by the detection of increased number of PCNA positive cells in kidney sections. The number and proportion of PCNA positive cells was significantly increased after the treatment of SVF or AdMSCs. However, there was no significant difference on cell proliferation between the SVF and AdMSCs groups (Fig. 5). Additionally, the effect of SVF and AdMSCs on cell apoptosis was observed by evaluating TUNEL-positive cells in the kidney sections after IR injury. The number and proportion of TUNEL-positive cells were significantly increased in animals suffered from renal IR injury. SVF or AdMSCs administration could significantly reduce the number and proportion of TUNEL-positive cells. However, no significant difference on cell apoptosis was found between SVF and AdMSCs groups (Fig. 6).
Figure 5. Immunohistochemical staining of PCNA in the kidney after acute renal IR injury.
Administration of SVF or AdMSCs contributed to enhanced cell proliferation in injured kidney compared with control group. There were no significant differences on cell proliferation between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs; #p < 0.05, vs. Control. Scale bars = 100 mm.
Figure 6. TUNEL staining of the kidney after acute renal IR injury.
Administration of SVF or AdMSCs contributed to reduced cell apoptosis in injured kidney compared with control group. There were no significant differences on cell apoptosis between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs; #p < 0.05, vs. Control. Scale bars = 100 mm.
Effects of SVF and AdMSCs on microvasculature in injured kidney
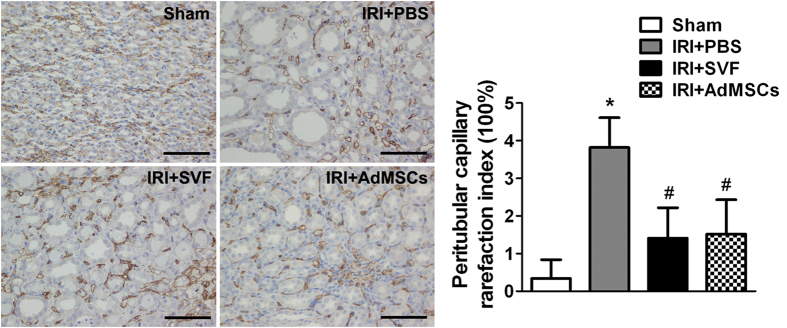
To evaluate the effects of SVF and AdMSCs on microvasculature, we detected the CD34 expression in the kidney sections after IR injury. PCRI was analyzed by detecting CD34 positive cells for the evaluation of peritubular capillary densities. The reduction of peritubular capillary densities was observed in animals suffered from IR injury. Treatment of SVF or AdMSCs could significantly increase the densities in injured kidney. The PCRI in SVF and AdMSCs groups was 1.41 ± 0.81% and 1.52 ± 0.91%, respectively, which were significantly lower than 3.82 ± 0.79% in control group (Fig. 7).
Figure 7. Immunohistochemical staining of CD34 in the kidney after acute renal IR injury.
Administration of SVF or AdMSCs contributed to lower PCRI in injured kidney compared with control group. There were no significant differences on CD34 expression between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs; #p < 0.05, vs. Control. Scale bars = 100 mm.
Effects of SVF and AdMSCs on the expression of TNF-α and IL-10
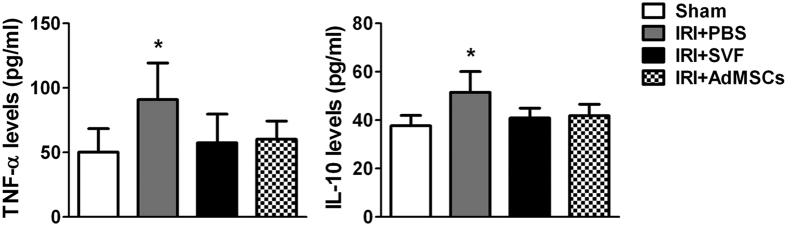
Serum levels of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10), were detected by ELISA to evaluate the effect of xenogenic transplantation of SVF or AdMSCs on systemic inflammatory response. The levels of TNF-α and IL-10 were significantly increased in the rats underwent IR injury. After the treatment of SVF or AdMSCs, the levels of TNF-α and IL-10 were significantly reduced, while no significant difference could be found in both groups. (Fig. 8)
Figure 8. Expression of TNF-α and IL-10 in the serum of animals after acute renal IR injury.
SVF or AdMSCs treatment contributed to lower expression of TNF-α and IL-10 in the serum of rats compared with control group. There were no significant differences on the expression of TNF-α and IL-10 between the SVF and AdMSCs groups. *p < 0.05, vs. Control, IRI + SVF and IRI + AdMSCs.
Discussion
Stem cells therapy has been suggested as a promising option to repair AKI. SVF and AdMSCs are two different types of adipose tissue-derived cells, and both of them are able to facilitate tissue repair and regeneration via a variety of mechanisms17. However, compare with AdMSCs, SVF can be real time obtained in a sufficient quantity without in vitro culture, which can avoid the potential risk of microbial contamination during culturing procedures11. Therefore, uncultured SVF has been considered as more attractive cell source than cultured AdMSCs for tissue repair and regeneration, especially for the repair of acute organ injury including AKI. Despite both AdMSCs and SVF have been reported to be able to protect the kidney from IR injury7,8,9,12, no study has compared the effects of them on AKI. The present study was conducted to compare the therapeutic efficacy of intra-parenchymal injection of SVF or AdMSCs on renal IR injury. Additionally, the possible mechanism of their effects on AKI was investigated through an in vitro co-culture system that was applied to investigate the effects of SVF or AdMSCs on H/R induced injury of renal tubular cell.
Similar to a previous study, which compared SVF and adipose derived stem cells treatments in a rat model of cavernous nerve injury and showed that both cell types are equally effective in recovering penile erection18, the present study demonstrated that transplantation of SVF or AdMSCs also showed equally effective attenuation of acute renal IR injury. Both SVF and AdMSCs could improve renal function and structure after IR injury through enhancing tubular cell proliferation, increasing peritubular capillary densities, and inhibiting cell apoptosis, by acting in a paracrine way. We have also demonstrated that SVF or AdMSCs could secret various growth factors, including HGF, VEGF and SDF-1α, which might be responsible for the paracrine mechanism of SVF or AdMSCs attenuating renal IR injury. Yasuda et al.13 reported that subcapsular injection of SVF protected the kidney from cisplatin-induced acute injury by secreting renoprotective molecules, such as HGF and VEGF. SDF-1α is considered to be a regulator of angiogenesis and plays an important role in kidney repair19,20. In the present study, SVF or AdMSCs influenced the activity of renal tubular cell by secreting those growth factors transferred through a co-culture system. SVF showed stronger enhancement of renal tubular cell proliferation to AdMSCs in vitro but not in vivo, which might be attributed to the more secretion of HGF by SVF, because HGF is able to enhance the proliferation activity of renal tubular cell21.
Previously, studies mainly focused on the effect of cultured stem cells including AdMSCs on the repair of AKI, and proposed that they could facilitate renal repair through direct or indirect approaches. Li et al.9 reported that human AdMSCs promoted kidney tissue repair via direct differentiating into renal tubular epithelial-like cells. However, numerous studies have shown that the renoprotective effect of AdMSCs was attributable to their paracrine actions on the injured kidney by releasing soluble factors21,22,23. The application of uncultured SVF for the repair of kidney injury was firstly reported by Feng et al., who named it as uncultured adipose tissue-derived stem and regenerative cells, in a rat model of renal IR injury12. Subsequently, Yasuda et al.13 reported that SVF could ameliorate rat AKI induced by cisplatin through paracrine mechanism. In previous study, we had concluded that both pre-ischemic and post-ischemic administration of SVF could protect the kidney from IR induced injury via paracrine effect14. However, it remains unclear whether SVF cells could differentiate into renal tubular cells when transplanted in vivo. As is well known, SVF is a heterogeneous cell population in adipose tissue and contains plenty of stem/progenitor cells, including AdMSCs, endothelial progenitor cells (EPCs), and so on24. A previous study had shown that transplantation of SVF contributed to the regeneration of ischemic tissue in a rat model of chronic myocardial infarction through both differentiation and paracrine activity25. The direct differentiation of SVF cells into renal tubular cells may also play an important role in kidney tissue repair. Further study is needed to investigate whether direct differentiation activity of SVF taking part in kidney tissue regeneration by using cell tracking technique.
It has been confirmed that AdMSCs had immunosuppressive property due to the lack of major histocompatibility complex-II expression, which may be mediated by prostaglandin E215,26. Studies have shown that xenotransplantation of human AdMSCs into different animal models was safe and effective for tissue repair and regeneration15,27,28. In a rat model of cisplatin-induced kidney injury, xenotransplantation of human AdMSCs led to decreased expression level of proinflammatory mediators22. Because SVF is a rich source of AdMSCs, it may also possess immunosuppressive property. Das et al.16 reported that xenotransplantation of human SVF into a diabetic mice model of erectile function did not induce systemic inflammation. In this study, decreased serum level of proinflammatory cytokine was detected after xenotransplantation of human SVF in a rat model of renal IR injury, which demonstrated immunosuppressive property of SVF. However, we have also found that xenotransplantation of human SVF or AdMSCs contributed to decreased serum level of IL-10, an anti-inflammatory cytokine. To further investigate immunosuppressive property of SVF, future study is needed to measure other anti-inflammatory cytokines, such as transforming growth factor-β1, which was reported to downregulate the secretion of IL-10 in vitro29. In addition, it is also needed to evaluate the possible rejection after xenotransplantation of human SVF or AdMSCs. Although we found decreased expression of TNF-α, which was reported to play an important role in allograft rejection30, more rejection markers should be examined in the future study.
One limitation of this study is that long-term effect of SVF and AdMSCs treatment on renal IR injury is not investigated, because even small change in kidney function can lead to both short-term and long-term complications2. Secondly, since SVF is a heterogeneous cell population and contains numerous cell types, the respective contributions of these cell types to the protective effect on renal IR injury should be evaluated in the future. At last, even though xenogenic transplantation of SVF or AdMSCs did not induce systemic inflammation, the systemic safety of xenogenic transplantation of the two cell types needs further investigated.
In conclusion, this study has confirmed that administration of SVF or AdMSCs contributed to the attenuation of IR induced tubular injury and improvement of renal function. Both SVF and AdMSCs are equally effective in improving renal function and structure after IR injury. However, SVF may be superior to AdMSCs when used for acute organ injury including AKI, because it can be obtained instantly in a sufficient quantity without in vitro expansion.
Materials and Methods
Cell isolation and culture
Perinephric adipose tissue was collected from eight patients (24 to 54 years of old) underwent abdominal operation and ruled out cancer, autoimmune diseases, etc. All the procedures involving humans were approved by the ethics committee of Nanjing First Hospital, Nanjing Medical University and informed consent was obtained from the patients. The procedures of SVF isolation were performed according to our previous protocol31. Briefly, the adipose tissue was rinsed with phosphate-buffered saline (PBS) and cut into small pieces, followed by incubation with 0.075% type I collagenase at 37 °C for 40 min in a shaking water bath. After being filtered with 200 μm nylon mesh and centrifuged at 400 g for 5 min to eliminate the mature adipocytes, the pellet was collected and treated with Red Blood Cell Lysis Buffer for 10 min, followed by being washed twice with ice-cold PBS. The nucleated cells was resuspended with PBS, counted with an automated cell counter, and then separated for the following experiments.
The SVF cells were plated in tissue culture flasks with Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) to in vitro expand AdMSCs. The culture flasks were maintained at 37 °C humidified atmosphere with 5% CO2. The medium was changed every 2 or 3 days until subconfluence for subculture.
Flow cytometric analysis
Freshly isolated SVF cells and AdMSCs at passage 3 were collected, washed thrice with PBS containing 1% BSA, and then pelleted by centrifugation at 400 g for 5 min. Cell surface markers were examined by immunostaining with the following antibodies: phycoerythrin (PE) conjugated anti-CD29 (BioLegend), PE conjugated anti-CD34 (Bioss Inc), fluorescein isothiocyanate (FITC) conjugated anti-CD45 (BioLegend), and FITC conjugated anti-CD90 (BioLegend). The labeled cells were washed twice, resuspended and then analyzed with FACSCaliber (BD Biosciences). An isotype-matched IgG was set as the negative control for each primary antibody.
Cell proliferation assay
Cell proliferation assay was performed as our previous protocol but with some modifications14. Briefly, HK-2 cells (1.5 × 103/well) were seeded in the bottom chambers of HTS Transwell-96 system (Corning) and cultured in Thermo 3131 incubator (Thermo Fisher Scientific) for 24 h set at 37 °C, 1% O2, and 5% CO2. Then the plates were transferred into the normoxic humidified incubator at 37 °C with 5% CO2 and cultured for 2 h to induce hypoxia/reoxygenation (H/R) injury of HK-2 cells. Cells were divided into three groups: HK-2 cells cultured independently (control group), HK-2 cells co-cultured with SVF (SVF group), and HK-2 cells co-cultured with AdMSCs (AdMSCs group). SVF or AdMSCs (105 cells for each) were seeded in the upper chambers. After 24 h of culture in normoxic condition, 10 μL of Cell Counting Kit-8 (CCK-8, Dojindo) was added into each well, and then the plates were incubated for 3 h. Finally, the absorbance of each well was measured at 450/620 nm on a microplate reader (Tecan).
Annexin V/propidiumiodide apoptosis assay
HK-2 cells (50%–60% confluence) cultured in 24-well plates (Corning) were used for this assay. After H/R injury of HK-2 cells was induced, cells were divided into three groups as described above. The MilllicellTM hanging Cell Culture Inserts (0.4 μm pore size, Millipore) were used for the co-culture of HK-2 and SVF cells (105 cells for each). The plates were placed into the normoxic humidified incubator and cultured for 24 h. HK-2 cells were collected by using trypsin and stained with Annexin V Apoptosis Detection Kit FITC (eBioscience) according to the manufacturer’s instruction. At last, the proportion of apoptotic cells was measured by flow cytometry.
Renal IR injury model and cell transplantation
Renal IR injury model was conducted according to previously described protocol but with some modifications14. A total of 32 male Sprague-Dawley rats (220 ~ 250 g) were used in this study. The animals were anesthetized by inducting with isoflurane and intraperitoneal administration of ketamine (100 mg/kg). The right kidney was removed by a midline abdominal incision. To establish the IR injury model, the left renal pedicle was blocked using a non-traumatic vascular clamp for 45 min, followed by removal of the clamp to initiate the left kidney reperfusion. PBS, SVF or AdMSCs were transplanted to the left kidney through intra-parenchymal injection. Animals were divided randomly into four groups: sham group (sham-operated); control group (injection with PBS); SVF group (injection with SVF); and AdMSCs group (injection with AdMSCs). 2 × 106 SVF cells or AdMSCs suspended in 100 μL of PBS were used for cell transplantation. Three injections (poles and middle areas) were performed by using a 28-gauge needle.
Animals were housed in a standard room with constant temperature and humidity, on a 12 h light/dark cycle, and accessing to food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Nanjing First Hospital, Nanjing Medical University. The investigation was performed in strict conformity with the institutional and national guidelines for laboratory animals.
Renal function
Blood samples were collected for measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) values at 72 h after IR injury in all four groups of rats (n = 8 for each group). Quantification of BUN and SCr concentrations was conducted by using standard laboratory equipment in our hospital.
Histological analysis
The kidney specimens retrieved at 72 h after IR injury were fixed in 10% buffered formalin, gradually dehydrated, embedded in paraffin, cut into 5 μm sections, and stained with Hematoxylin and eosin (H&E). The degree of tubular injury was scored, based on HE staining in a blinded fashion, range from Grade 0 to 5 as previously described32. The scores reflected the severity of tubular damage as follows: 0, normal kidney; 1, ≤ 10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; 5, ≥ 76%.
Immunohistochemical analysis
Tissue sections (5 μm) were prepared as described above and used for immunohistochemical staining. After deparaffinizing and blocking, the sections were incubated at 4 °C overnight with rabbit anti-proliferating cell nuclear antigen (anti-PCNA) and rabbit anti-CD34 antibodies, respectively. Immunohistochemical assay for the staining of anti-PCNA and anti-CD34 was performed according to our previous protocol33. Peritubular capillary rarefaction index (PCRI) was analyzed by staining with anti-CD34 antibody and determined using the previously described procedures19,34. PCRI was determined by calculating the numbers of squares in 10 × 10 grids that was negative for CD34 staining. At least 10 nonoverlapping sequential fields at 400 x magnification were counted. The minimal PCRI score is 0, which indicates that no square in the grids is negative for CD34 staining, whereas the maximal score is 100, which means that all the squares are negative for CD34 staining.
Enzymes linked immunosorbent assay
Levels of TNF-α and IL-10 in the serum of rats at 72 h after IR injury in the four groups were determined by Enzyme-linked immunosorbent assay (ELISA) kit (Uscn Life Science Inc) according to the manufacturer’s instruction. Levels of HGF, VEGF and SDF-1α in the culture medium of three groups after H/R in the section of Annexin V/propidiumiodide apoptosis assay were also determined by ELISA kit (RayBiotech). The absorbance was evaluated on a microplate reader (Tecan) and the concentration was calculated on the basis of standard curve.
Statistical analysis
All data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) was used to evaluate statistical comparisons of the data among groups. If ANOVA showed a significant difference, post hoc Tukey test was used to further evaluate the comparison between groups. Statistical significance was set at p < 0.05.
Additional Information
How to cite this article: Zhou, L. et al. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci. Rep. 7, 44058; doi: 10.1038/srep44058 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81570613, 31500785). We thank Dr. Chen Yu (Tongji University, China) for kindly providing HK-2 cell line.
Footnotes
The authors declare no competing financial interests.
Author Contributions Experiment designed: L.H.Z. and R.P.J. Experiment Performed: L.H.Z., Q.S., J.W.S., L.W.X., Z.X., R.W. and Y.Z.G. Data Analyzed: L.H.Z., J.G.Z., J.P.W. and Q.L.D. Manuscript Written: L.H.Z and R.P.J.
References
- Kam Tao Li, P., Burdmann E. A. & Mehta R. L. Acute kidney injury: Global health alert. Journal of nephropathology 2, 90–97, doi: 10.12860/JNP.2013.15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameire N. H. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179, doi: 10.1016/S0140-6736(13)60647-9 (2013). [DOI] [PubMed] [Google Scholar]
- Ali T. et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18, 1292–1298, doi: 10.1681/ASN.2006070756 (2007). [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. & Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121, 4210–4221, doi: 10.1172/JCI45161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleniceanu O., Harari-Steinberg O. & Dekel B. Concise review: Kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells 28, 1649–1660, doi: 10.1002/stem.486 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279–4295, doi: 10.1091/mbc.E02-02-0105 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T. et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. Journal of translational medicine 9, 51, doi: 10.1186/1479-5876-9-51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y. C. et al. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plastic and reconstructive surgery 132, 940e–951e, doi: 10.1097/PRS.0b013e3182a806ce (2013). [DOI] [PubMed] [Google Scholar]
- Li K., Han Q., Yan X., Liao L. & Zhao R. C. Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem cells and development 19, 1267–1275, doi: 10.1089/scd.2009.0196 (2010). [DOI] [PubMed] [Google Scholar]
- Zhao X. et al. Three-Dimensional Aggregates Enhance the Therapeutic Effects of Adipose Mesenchymal Stem Cells for Ischemia-Reperfusion Induced Kidney Injury in Rats. Stem cells international 2016, 9062638, doi: 10.1155/2016/9062638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Bunnell B. A., Chiu E. S. & Guilak F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells 29, 749–754, doi: 10.1002/stem.629 (2011). [DOI] [PubMed] [Google Scholar]
- Feng Z. et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 25, 3874–3884, doi: 10.1093/ndt/gfq603 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K. et al. Autologous cell therapy for cisplatin-induced acute kidney injury by using non-expanded adipose tissue-derived cells. Cytotherapy 14, 1089–1100, doi: 10.3109/14653249.2012.693157 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou L. et al. Preischemic Administration of Nonexpanded Adipose Stromal Vascular Fraction Attenuates Acute Renal Ischemia/Reperfusion Injury and Fibrosis. Stem cells translational medicine 5, 1277–1288, doi: 10.5966/sctm.2015-0223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Lin G. & Lue T. F. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem cells and development 21, 2770–2778, doi: 10.1089/scd.2012.0176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N. D. et al. Xenogenic transplantation of human breast adipose-derived stromal vascular fraction enhances recovery of erectile function in diabetic mice. Biology of reproduction 90, 66, doi: 10.1095/biolreprod.113.115113 (2014). [DOI] [PubMed] [Google Scholar]
- Kokai L. E., Marra K. & Rubin J. P. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Translational research: the journal of laboratory and clinical medicine 163, 399–408, doi: 10.1016/j.trsl.2013.11.009 (2014). [DOI] [PubMed] [Google Scholar]
- You D. et al. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem cells translational medicine 4, 351–358, doi: 10.5966/sctm.2014-0161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. et al. Ischemic preconditioning increases endothelial progenitor cell number to attenuate partial nephrectomy-induced ischemia/reperfusion injury. PloS one 8, e55389, doi: 10.1371/journal.pone.0055389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S. & Huang S. The signaling pathway of stromal cell-derived factor-1 and its role in kidney diseases. Journal of receptor and signal transduction research 34, 85–91, doi: 10.3109/10799893.2013.865746 (2014). [DOI] [PubMed] [Google Scholar]
- Katsuno T. et al. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell transplantation 22, 287–297, doi: 10.3727/096368912X655019 (2013). [DOI] [PubMed] [Google Scholar]
- Kim J. H. et al. Human adipose tissue-derived mesenchymal stem cells protect kidneys from cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol 302, F1141–1150, doi: 10.1152/ajprenal.00060.2011 (2012). [DOI] [PubMed] [Google Scholar]
- Furuichi K. et al. Effects of adipose-derived mesenchymal cells on ischemia-reperfusion injury in kidney. Clinical and experimental nephrology 16, 679–689, doi: 10.1007/s10157-012-0614-6 (2012). [DOI] [PubMed] [Google Scholar]
- Bourin P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641–648, doi: 10.1016/j.jcyt.2013.02.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo M. et al. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell transplantation 21, 1023–1037, doi: 10.3727/096368911X623862 (2012). [DOI] [PubMed] [Google Scholar]
- Puissant B. et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. British journal of haematology 129, 118–129, doi: 10.1111/j.1365-2141.2005.05409.x (2005). [DOI] [PubMed] [Google Scholar]
- Zhu W. et al. Effects of xenogeneic adipose-derived stem cell transplantation on acute-on-chronic liver failure. Hepatobiliary & pancreatic diseases international: HBPD INT 12, 60–67 (2013). [DOI] [PubMed] [Google Scholar]
- Lasso J. M. et al. Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. Journal of plastic, reconstructive & aesthetic surgery: JPRAS 68, e189–197, doi: 10.1016/j.bjps.2015.07.005 (2015). [DOI] [PubMed] [Google Scholar]
- Rodriguez T. M. et al. Effect of TGF-beta1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells. Stem cells translational medicine 4, 894–898, doi: 10.5966/sctm.2015-0012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha I. L. et al. In situ expression of tumor necrosis factor-alpha, interferon-gamma, and interleukin-2 receptors in renal allograft biopsies. Transplantation 54, 1017–1024 (1992). [DOI] [PubMed] [Google Scholar]
- Zhou L. et al. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PloS one 10, e0117644, doi: 10.1371/journal.pone.0117644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney international 84, 521–531, doi: 10.1038/ki.2013.114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A 19, 264–276, doi: 10.1089/ten.TEA.2011.0609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. H. et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12, 1434–1447 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.