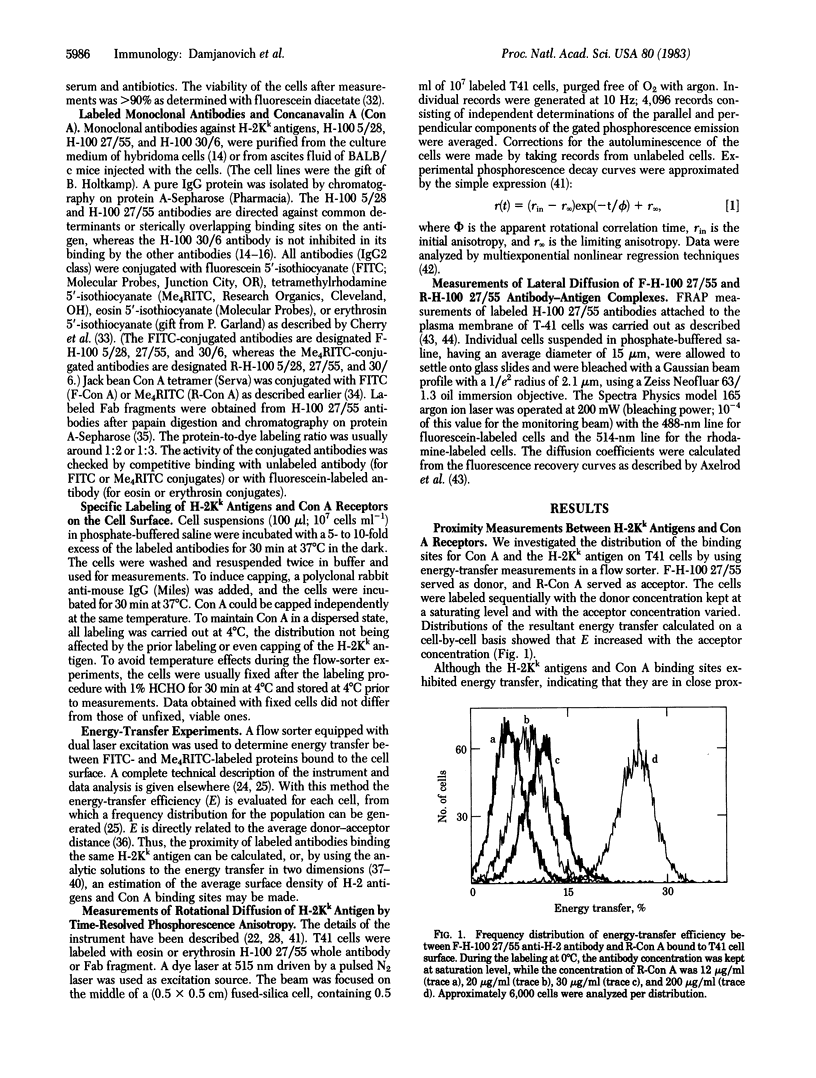
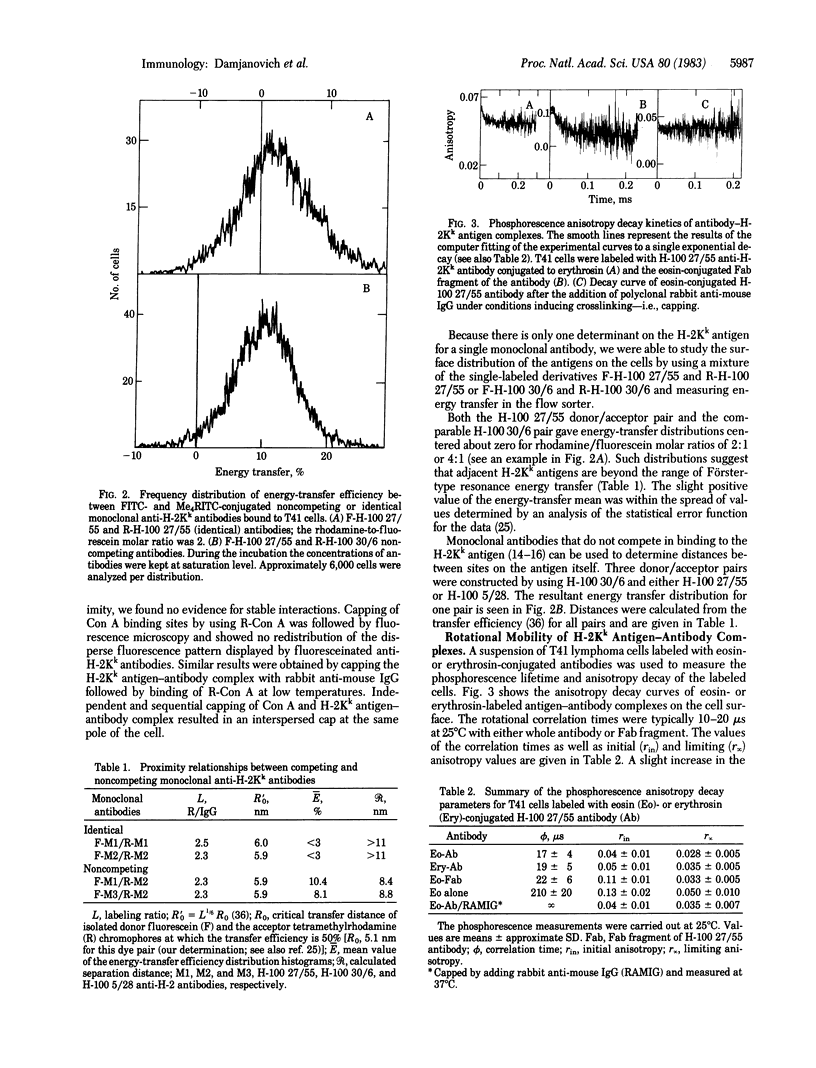
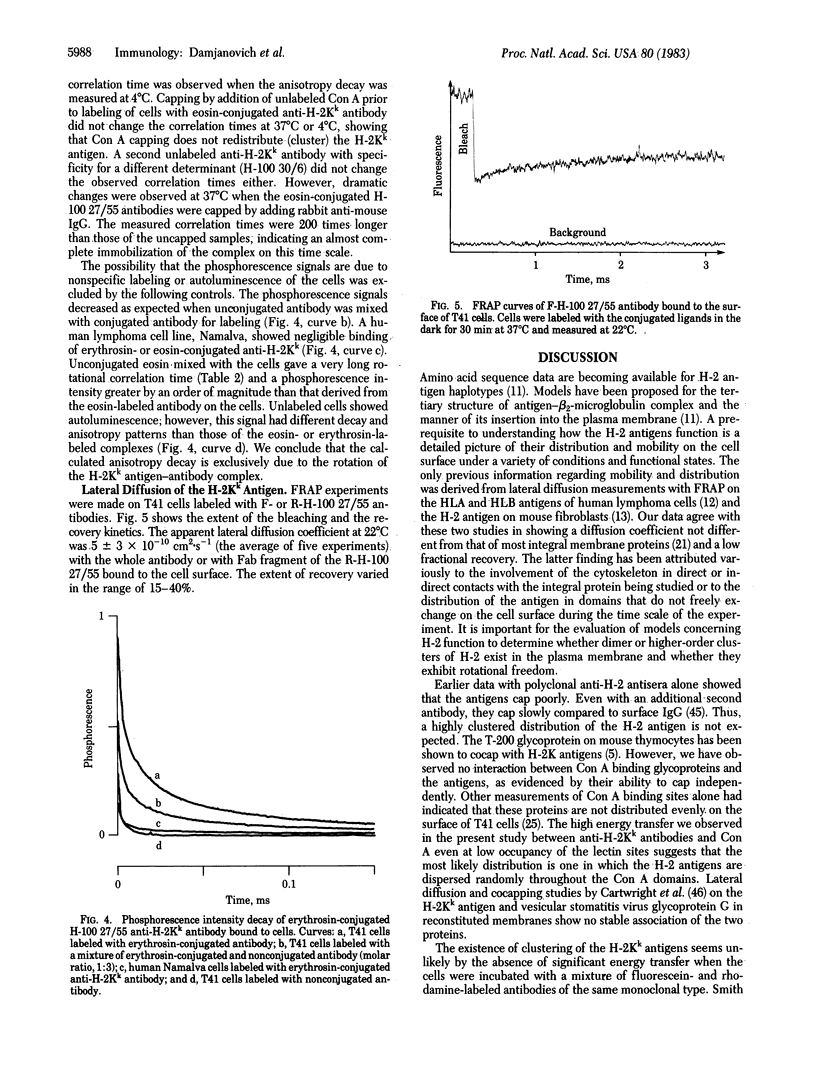
Abstract
The topographical distributions and mobilities of the murine histocompatibility antigen H-2Kk and of concanavalin A (Con A) binding sites have been studied on a murine lymphoma cell line. The spatial distribution of H-2Kk antigens, the average distance between H-2Kk antigens and Con A binding sites, and the separation of different determinants on the H-2Kk antigen itself were determined by using fluorescence resonance energy-transfer measurements with a dual-laser flow sorter. From the lack of energy transfer between bound monoclonal anti-H-2Kk antibodies conjugated with fluorescein (donor) and rhodamine (acceptor), we conclude that the H-2Kk antigen exists without appreciable clustering on the cell surface. Substantial energy transfer between appropriately labeled Con A and antibodies bound to the H-2Kk antigen shows that the two populations are interspersed. Donor/acceptor pairs of monoclonal antibodies binding to different determinants on the same H-2Kk antigen exhibited a degree of energy transfer indicative of a mean separation of 8.6 nm between the sites. Time-resolved phosphorescence anisotropy measurements with anti-H-2Kk antibodies labeled with eosin or erythrosin yielded rotational mobility information for the antigen-antibody complexes on the cell membrane. The rotational correlation time of 10-20 mus and the finite residual anisotropy are compatible with an uniaxial mode of rotation of monomeric antigen around its transmembrane portion and, thus, provide additional evidence for an unclustered distribution. Capping by rabbit anti-mouse IgG immobilized the antigen-antibody complex. Fluorescence recovery after photobleaching was used to calculate an apparent lateral diffusion coefficient of 5 +/- 3 X 10(-10) cm2 . s-1 for the H-2Kk antigen labeled with fluoresceinated IgG or its corresponding Fab fragment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Chan S. S., Jovin T. M. Rotational diffusion of cell surface components by time-resolved phosphorescence anisotropy. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5650–5654. doi: 10.1073/pnas.76.11.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdi M., Barrantes F. J., Jovin T. M. Rotational molecular dynamics of the membrane-bound acetylcholine receptor revealed by phosphorescence spectroscopy. Eur J Biochem. 1981 Nov;120(2):389–397. doi: 10.1111/j.1432-1033.1981.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y. Biochemical analysis of ligand-induced receptor patching and capping using a novel immunolactoperoxidase iodination technique. J Cell Biol. 1979 Dec;83(3):649–656. doi: 10.1083/jcb.83.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright G. S., Smith L. M., Heinzelmann E. W., Ruebush M. J., Parce J. W., McConnell H. M. H-2Kk and vesicular stomatitis virus G proteins are not extensively associated in reconstituted membranes recognized by T cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1506–1510. doi: 10.1073/pnas.79.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Arndt-Jovin D. J., Jovin T. M. Proximity of lectin receptors on the cell surface measured by fluorescence energy transfer in a flow system. J Histochem Cytochem. 1979 Jan;27(1):56–64. doi: 10.1177/27.1.374620. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Cogoli A., Oppliger M., Schneider G., Semenza G. A spectroscopic technique for measuring slow rotational diffusion of macromolecules. 1: Preparation and properties of a triplet probe. Biochemistry. 1976 Aug 24;15(17):3653–3656. doi: 10.1021/bi00662a001. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Criado M., Vaz W. L., Barrantes F. J., Jovin T. M. Translational diffusion of acetylcholine receptor (monomeric and dimeric forms) of Torpedo marmorata reconstituted into phospholipid bilayers studied by fluorescence recovery after photobleaching. Biochemistry. 1982 Nov 9;21(23):5750–5755. doi: 10.1021/bi00266a004. [DOI] [PubMed] [Google Scholar]
- Dewey T. G., Hammes G. G. Calculation on fluorescence resonance energy transfer on surfaces. Biophys J. 1980 Dec;32(3):1023–1035. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edidin M., Wei T. Lateral diffusion of H-2 antigens on mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):458–462. doi: 10.1083/jcb.95.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Use of nonspecific dye labeling for singlet energy-transfer measurements in complex systems. A simple model. Biochemistry. 1972 Jun 20;11(13):2509–2517. doi: 10.1021/bi00763a020. [DOI] [PubMed] [Google Scholar]
- Holtkamp B., Cramer M., Lemke H., Rajewsky K. Isolation of a cloned cell line expressing variant H-2Kk using fluorescence-activated cell sorting. Nature. 1981 Jan 1;289(5793):66–68. doi: 10.1038/289066a0. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Bartholdi M., Vaz W. L., Austin R. H. Rotational diffusion of biological macromolecules by time-resolved delayed luminescence (phosphorescence, fluorescence) anisotropy. Ann N Y Acad Sci. 1981;366:176–196. doi: 10.1111/j.1749-6632.1981.tb20753.x. [DOI] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Moore C., Boxer D., Garland P. Phosphorescence depolarization and the measurement of rotational motion of proteins in membranes. FEBS Lett. 1979 Dec 1;108(1):161–166. doi: 10.1016/0014-5793(79)81200-4. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C., De Petris S. Movement of lymphocyte surface antigens and receptors: the fluid nature of the lymphocyte plasma membrane and its immunological significance. Fed Proc. 1973 Jan;32(1):48–54. [PubMed] [Google Scholar]
- Robertson M. The evolutionary past of the major histocompatibility complex and the future of cellular immunology. Nature. 1982 Jun 24;297(5868):629–632. doi: 10.1038/297629a0. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Sherman L. A. Recognition of conformational determinants on H-2 by cytolytic T lymphocytes. Nature. 1982 Jun 10;297(5866):511–513. doi: 10.1038/297511a0. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Petty H. R., Parham P., McConnell H. M. Cell surface properties of HLA antigens on Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982 Jan;79(2):608–612. doi: 10.1073/pnas.79.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder B., Freire E. Fluorescence energy transfer in two dimensions. A numeric solution for random and nonrandom distributions. Biophys J. 1982 Nov;40(2):137–148. doi: 10.1016/S0006-3495(82)84468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I., Lagenaur C., Schachner M. Recognition of Bergmann glial and ependymal cells in the mouse nervous system by monoclonal antibody. J Cell Biol. 1981 Aug;90(2):448–458. doi: 10.1083/jcb.90.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariasse K. A., Vaz W. L., Sotomayor C., Kühnle W. Investigation of human erythrocyte ghost membranes with intramolecular excimer probes. Biochim Biophys Acta. 1982 Jun 14;688(2):323–332. doi: 10.1016/0005-2736(82)90343-1. [DOI] [PubMed] [Google Scholar]
- Zarling D. A., Miskimen J. A., Fan D. P., Fujimoto E. K., Smith P. K. Association of Sendai virion envelope and a mouse surface membrane polypeptide on newly infected cells: lack of association with H-2K/D or alteration of viral immunogenicity. J Immunol. 1982 Jan;128(1):251–257. [PubMed] [Google Scholar]
- Zidovetzki R., Yarden Y., Schlessinger J., Jovin T. M. Rotational diffusion of epidermal growth factor complexed to cell surface receptors reflects rapid microaggregation and endocytosis of occupied receptors. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6981–6985. doi: 10.1073/pnas.78.11.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



