Abstract
Folate receptor-targeted PET radiotracers can potentially serve as versatile imaging agents for the diagnosis, staging, and prediction of response to therapy of patients with folate receptor (FR)-expressing cancers. Because current FR-targeted PET reagents can be compromised by complex labeling procedures, low specific activities, poor radiochemical yields or unwanted accumulation in FR negative tissues, we have undertaken to design an improved folate-PET agent that might be more amenable for clinical development. For this purpose, we have synthesized a folate-NOTAAl18F radiotracer and examined its properties both in vitro and in vivo.
Methods
Radiochemical synthesis of folate-NOTA-Al18F was achieved by incubating 18F− with AlCl3 for 2 min followed by heating in the presence of folate-NOTA for 15 min at 100 °C. Binding of folate-NOTA-Al18F to FR was quantitated in homogenates of KB and Cal51 tumor xenografts in the presence and absence of excess folic acid as a competitor. In vivo imaging was performed on nu/nu mice bearing either FR+ve (KB cell) or FR−ve (A549 cell) tumor xenografts, and specific accumulation of the radiotracer in tumor and other tissues was assessed by high-resolution micro-PET and ex vivo biodistribution in the presence and absence of excess folic acid. Image quality of folate-NOTA-Al18F was compared with that of 99mTc-EC20, a clinically established folate-targeted SPECT imaging agent.
Results
Total radiochemical synthesis and purification of folate-NOTA-Al18F was completed within 37 min, yielding a specific activity of 68.82±18.5 GBq/μmol, radiochemical yield of 18.6±4.5%, and radiochemical purity of 98.3±2.9%. Analysis of FR binding revealed a Kd of ~1.0 nM, and micro-PET imaging together with ex vivo biodistribution analyses demonstrated high FR-mediated uptake in an FR+ tumor and the kidneys.
Conclusions
Folate-NOTA-Al18F constitutes an easily prepared FR-targeted PET imaging agent with improved radiopharmaceutical properties and high specificity for folate receptor expressing tumors. Given its improved properties over 99mTc-EC20 (i.e. higher resolution, shorter image acquisition time, etc.), we conclude that folate-NOTA-Al18F constitutes a viable alternative to 99mTc-EC20 for use in identification, diagnosis, and staging of patients with FR-expressing cancers.
Keywords: folate receptor, Al18F-NOTA chelate, 18F-PET imaging, cancer imaging, imaging autoimmune disease
Graphical abstract
INTRODUCTION
Uptake of physiological folates is mediated in most cells by either the reduced folate carrier (Km ~ 1–5 × 10−6 M)1 or proton coupled folate transporter (Km ~ 0.5–1.0 × 10−6 M),2 however, a few cells (a subset of epithelial cells and activated pro-inflammatory macrophages) may internalize the vitamin via folate receptor-mediated endocytosis.3, 4 Although the reduced folate carrier and protein coupled folate transporter do not facilitate influx of folate conjugates (i.e. folate-linked drugs and imaging agents), the folate receptor does catalyze internalization of folate and folate conjugates with roughly equal affinity. For this reason, folic acid has been frequently exploited to deliver imaging and therapeutic agents selectively to pathologic cells that over-express FR.4–7
A variety of folate receptor-targeted imaging agents have been developed for optical,8–14 nuclear,15–19 and magnetic resonance imaging of cancers (ovarian cancer, non-small lung cancer, kidney cancer, and endometrial cancer etc.) and sites of inflammation (e.g. rheumatoid arthritis, atherosclerosis, and pulmonary fibrosis etc.).20–26 For whole body imaging, positron emission tomography (PET) has attracted the greatest interest because of its higher sensitivity, better spatial resolution, greater signal to noise ratio, and superior tracer quantification.27, 28 Most PET imaging protocols also require only low doses of radiotracer, thereby minimizing radiation exposure and allowing for repeated imaging of the same patient.29 When pharmacokinetic and/or pharmacodynamic data on drug candidates are required, PET methodologies also allow for quantitative dynamic imaging, facilitating acquisition of kinetic data on target engagement and retention.
While multiple radionuclides have been successfully employed for PET imaging (e.g. 18F, 89Zr, 66Ga, 68Ga, 64Cu, and I124), 18F is often preferred because of its commercial availability and optimal decay characteristics, including: i) relatively long half-life (109.8 min; enabling distribution to clinics following centralized syntheses), ii) ideal positron energy (Emax = 0.635 MeV; Eaverage energy is 0.25 MeV), and iii) high probability of β+ emission (97 %), permitting high resolution images and relatively low tissue energy deposition. Although several 18F-labeled folate-targeted PET agents have already been developed and evaluated in preclinical studies, a need still exists for improved folate-targeted 18F-PET agents with shorter preparation times, higher radiochemical yields, or increased specific activities.30–32 In this investigation, we report the chemical synthesis and one-step radiochemical labeling of a folate-NOTA-Al18F PET imaging agent and describe its radiopharmaceutical properties both in vitro and in vivo.
EXPERIMENTAL SECTION
General
Water was distilled and then deionized (18 MΩ/cm2) by passing through a Milli-Q water filtration system (Millipore Corp., Milford, MA). All chemicals and solvents, unless otherwise specified, were purchased from Sigma (St. Louis, MO) and were used without further purification. 2,2′-(7-(2-((2,5-dioxopyrrolidin-1-yl)oxy)-2-oxoethyl)-1,4,7-triazonane-1,4-diyl)diacetic acid (NOTA-NHS) was purchased from CheMatech (France). N10-TFA-pteroic acid was kindly provided by Endocyte, Inc. The diammonium salt of 3H-folic acid (37 MBq/mL, 1483.7 GBq/mmol) was purchased from Moravek Biochemicals. The folate-NOTA PET precursor and all other intermediates were synthesized as described below. High-performance liquid chromatography (HPLC) analysis and purification of the folate-NOTA precursor were performed on an Agilent 1200. Carrier-free 18F-fluoride was obtained from PETNET. The scintillation solution, Ultima Gold high-flash-point liquid scintillation cocktail, was purchased from Packard Co. Folic acid deficient RPMI cell culture medium (lacking folic acid, vitamin B12, and phenol red) was obtained from Cell Culture Technologies GmbH. Raw data acquired in vitro were analyzed using Prism software (GraphPad Prism 4), and PET imaging files were evaluated using dedicated software PMOD 2.95 (PMOD Technologies, Adliswil, Switzerland).
Synthesis of Folate-NOTA Conjugate (1)
A detailed description of the chemical synthesis of the PET precursor, folate-NOTA (1), and and the reference compound, folate-NOTA-Al19F, is available in the Supporting Information. Briefly, a protected derivative of folic acid 4 was prepared via PyBOP-mediated coupling of N10-TFA-pteroic acid to H2N-Glu(OtBu)-OMe, followed by deprotection of the tert-butyl ester with trifluoroacetic acid (TFA). Deprotected 4 was then conjugated with 2,2′-[1,2-ethanediylbis(oxy)] bisethanamine using PyBOP, followed by Boc-removal with TFA to produce 6. Incubation of 6 with NOTA-NHS ester in DMSO then yielded the protected folate-NOTA 7, which upon deprotection in 1 M NaOH (aq.) led to the folate-NOTA labeling precursor 1 (Figure 1 and Supporting Information). The crude product was purified by preparative reverse-phase HPLC using a gradient mobile phase of A = 10 mM ammonium acetate buffer and B= acetonitrile; solvent gradient 5% B to 100% B in 30 minutes (XBridge Prep C18, 5 μm; 19 × 150 mm). Elution of the conjugate was monitored at λ = 280 nm and the identities of the eluted compounds were analyzed by LC-MS.
FIGURE 1.
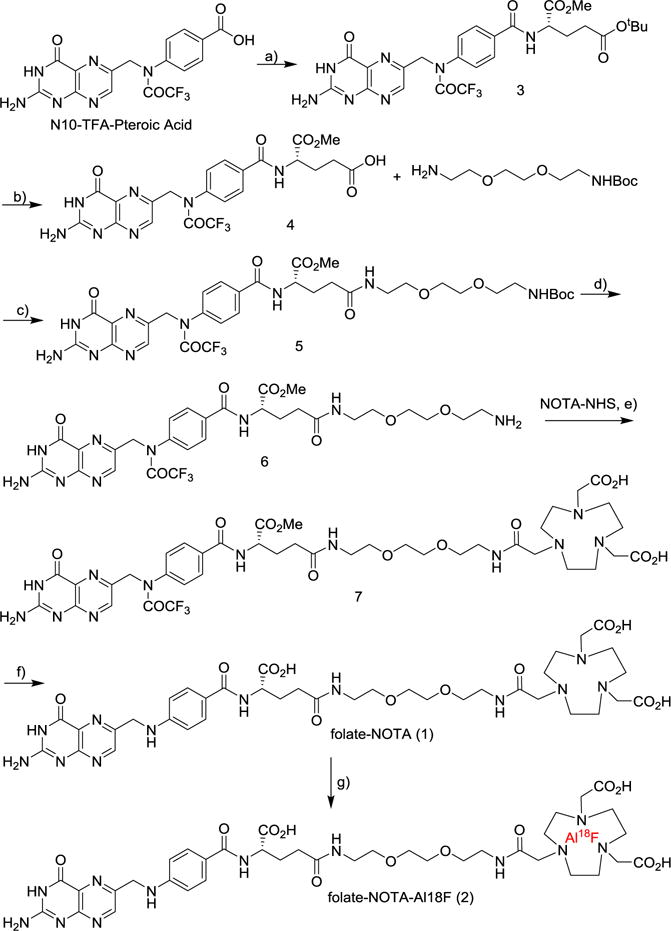
Synthesis of folate-NOTA precursor (1) and radiochemical synthesis of folate-NOTA-Al18F PET radiotracer (2). All reactions were conducted under N2 or Ar at room temperature. Reagents and conditions of reactions (a–e) are: a) HCl.H2N-Glu(OtBu)-OMe, PyBOP, DIPEA, DMSO, 23 °C; b) TFA/DCM, 1/3, 23 °C, 83% for 2 steps; c) PyBOP, BocNH-PEG-NH2, DIPEA, DMSO, 23 °C; d) TFA/DCM, 1/3, 23 °C, 90% for 2 steps; e) DIPEA, DMSO, 23 °C, 59%; f) 1M NaOH, 23 °C, 5 min, 60% after HPLC; g) AlCl3-K18F, NaOAc buffer, pH 4, 100 °C
Radiochemistry
A cartridge containing 18F-fluoride was first washed with 1.5 mL of ultrapure water and then 18F-fluoride was eluted with 1.0 mL of 0.4 M KHCO3. The eluted 18F-fluoride solution (100 μL) was added to a stem vial charged with 10 μL acetic acid, 25 μL AlCl3 (2 mM in 0.1 M NaOAc buffer, pH 4) and 125 μL 0.1 M NaOAc pH 4. The solution was incubated for 2 min at r.t., mixed with 0.25 mg folate-NOTA precursor (1) in 125 μL of 0.1 M NaOAc, pH 4, and heated immediately to 100 °C for 15 min. After cooling to room temperature, the crude material was mixed with 0.7 Ml 0.1% formic acid and purified by semi-preparative radioactive HPLC equipped with a γ-counter (Wizard, PerkinElmer) by applying a linear gradient starting from 90% A to 15% B over 15 min at a flow rate of 4 mL/min (A = acetonitrile and B = 0.1% formic acid), and the fraction at 11.5 min was collected. Corresponding UV absorption was recorded at 254 nm wavelength. Volatile solvents were removed from the purified product (folate-NOTA-Al18F (2)) by heating under a nitrogen stream, and the resulting aqueous solution was sterile filtered and diluted in isotonic saline to the desired radioactivity.
Cell Culture and IC50 evaluation
Analysis of 3H-folate binding to FR was conducted on KB cells cultured in folate deficient RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (HIFBS), 1% L-glutamine and 1% penicillin at 37°C in a humidified atmosphere containing 5% CO2. Spent medium in each well was replaced with 10 nM [3H]-folate in the presence of increasing concentrations of either folate-NOTA or free folic acid. After incubating for 1 h at 37 °C, cells were rinsed with PBS (2 × 0.5 mL) and 1 M trichloroacetic acid (1 × 0.5 mL) to remove any unbound radioactive material. Cells were dissolved in 1% sodium dodecylsulfate (0.5 mL) and transferred to Ecolume scintillation cocktail for scintillation counting. Experiments were performed in triplicate for each concentration and relative binding affinities (IC50) were calculated using a plot of cell bound radioactivity versus concentration of test compound using GraphPad Prism 4.
Kd evaluation
For analysis of folate-NOTA-Al18F binding, KB tumors were collected, weighed, added to 9 volumes of lysis buffer (50 mM Tris-HCL containing 2 mM EDTA, pH 7.4), and homogenized at 4 °C using a Potter-Elvehjem homogenizer attached to a variable-speed drill and a tissuemizer. The homogenized tissue was centrifuged for 5 minutes in a microfuge at 1000 rpm, and the cell pellet was resuspended in folate deficient 1640 RPMI medium, seeded in 24-well (100,000 cells/well) Falcon plates and allowed to form monolayers over a period of 24 h. To each well were added increasing concentrations of folate-NOTA-Al18F (2) in fresh medium (0.5 mL). After incubating for 1 h at 37 °C, cells were rinsed with PBS (2 × 0.5 mL) and 1 M trichloroacetic acid (1 × 0.5 mL) to remove any unbound radioactive materials. 1% sodium dodecylsulfate in PBS (0.5 mL) was then added and solubilized cells were transferred into individual test tubes and bound folate-NOTA-Al18F was measured in a γ-counter (Wizard; PerkinElmer) using an energy window of 300–700 keV. Experiments were performed in triplicate at each concentration. The Bmax and Kd values were calculated using GraphPad Prism 4.
Tumor implantation in mice
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Merck, West Point, PA. KB (high FR expression) or Cal-51 (moderate FR expression) cells were cultured in complete growth medium containing folate free RPMI 1640 medium with 5% fetal bovine serum, while A549 cells (low FR expression) were cultured in complete growth medium containing F-12K medium with 10% fetal bovine serum at 37°C with 5% CO2. The growth media was changed 2 or 3 times per week and the cells were subcultured at a ratio of 1:10 when needed.
Female CD1 nu/nu (KB) and nu/nu (Cal51 or A549), 6–8 week old, were ordered from Charles River Laboratories (Stone Ridge, NY, USA) and housed in a temperature and humidity controlled room and maintained on a folate-deficient rodent diet. After 7 to 14 days of acclimatization, tumors were implanted at the right shoulder with subcutaneously injection of 1×106 KB cells in 100 uL folate free RPMI, 5×106 Cal51 or A549 cells in 100 uL PBS + Growth Factor Reduced Matrigel (1:1). Animal experiments were then performed 2–3 weeks after tumor cell injection, when the tumors had grown to a mass of 200 to 400 mg.
MicroPET Imaging
In order to evaluate the in vivo imaging performance of folate-NOTA-Al18F (2) to FR+ tumors, microPET imaging was performed on nude mice bearing KB tumor xenografts both in the presence and absence of excess free folate to block all accessible FR. PET experiments were performed with a dedicated small-animal PET system (Focus220, Siemens Medical Solution, Hoffman Estate, IL). Anesthesia was induced with 4–5% isoflurane and maintained with 1–3% isofluorane delivered with a mixed air/oxygen through a closed nose cone throughout the PET imaging session. Mice bearing KB tumor xenografts on their left shoulders were injected with 8.5–12.9 MBq (0.12–0.24 nmol) of folate-NOTA-Al18F (2). The competition group (n = 3) received an intravenous injection of 100 μg folic acid (FA) 2 min before radiotracer administration, and the baseline control (n = 4 per tumor type) was injected with the same radiotracer plus the corresponding volume of isotonic saline.
PET data were collected for 90 minutes following radiotracer administration, and reconstructed using maximum a posteriori reconstruction (MAP, β = 0.2, 256 × 256 × 95 voxels, 0.9 × 0.9 ×0.80 mm3, 57Co transmission-based photon attenuation and scatter correction). PET images were converted to percent injected dose per gram (%ID/g tissue) for evaluation of tumor specificity, Image analysis was performed using the software program MATLAB (MathWorks, Natick, Massachusetts, USA).
Biodistribution Study
The binding specificity of folate-NOTA-Al18F (2) to FR was further evaluated through ex vivo biodistribution studies in mice in comparison with 99mTc-EC20 under both baseline and competition conditions. Immediately after PET imaging, mice were euthanized via CO2 inhalation. Tissues (blood, plasma, heart, lung, liver, spleen, kidney, muscle and tumor) were collected, cleaned and weighed, then counted in a gamma counter (Wizard 3, PerkinElmer). All mice that underwent PET imaging with folate-NOTA-Al18F (2) including mice with A549 tumors, were included in the biodistribution study. For 99mTc-EC20 studies, 0.37–0.74 MBq (0.036 – 0.084 nmol) was administered, and mice were sacrificed by decapitation and dissected 120 min post injection (p.i.) of radiotracer. Competition with free folic acid was achieved using the same methodology as in folate-NOTA-Al18F (2) studies. Radiotracer accumulation for all biodistribution studies was calculated as %ID/g.
Radioactive Saturation Binding Assay
KB and Cal51 xenografts were harvested and stored at −80°C freezer before use. Crude homogenates of the xenografts were prepared by homogenizing them separately in ice cold buffer (50 mM Tris pH 7.5, 120 mM NaCl, 2mM KCl, 1 mM MgCl2, 1 mM CaCl2) for 30 sec. at 4°C on setting 5 of Polytron. Crude homogenates of KB and Cal51 xenografts (1 mg/mL of wet tissue weight) were used in the assay. Tissue homogenates were first pre-incubated at room temperature for 2 hours in assay buffer (50 mM Tris-HCl pH 7.5, 120 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 1:1000 Protease Inhibitor (P-8340) with 2% DMSO or 5 μM self-block using Skatron tube strips (SK15776) covered with aluminum foil. Non-displaceable binding of folate-NOTAAl18F was defined using 5 μM self-block. Following pre-incubation, twelve concentrations of folate-NOTA-Al18F from 0.1 nM to 30 nM were added to the assay tubes in duplicates with final assay volume 0.25 mL per tube. The assay tubes were mixed by brief vortex, and then incubated at room temperature for 60 minutes. After completion of incubation, each assay tube mixtures were transferred onto Skatron GF/C filters (SK11731), which was pre-soaked in 0.2% PEI for 30 minutes at RT before use, using a Skatron Combi cell 12-well harvester. The filters were promptly washed 3 times on setting 3-3-3 with ice cold wash buffer (50 mM Tris, pH 7.5, 120 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2). The filters were punched into Pico Pro vials, and then counted in Gamma counter (Wizard 3, PerkinElmer). The data were analyzed using nonlinear fit (one site binding) model with Prism software (GraphPad, CA)
RESULTS
Radiochemistry
Radiochemical synthesis of folate-NOTA-Al18F (2) was performed by reacting the folate-NOTA precursor 1 with Al18F followed by HPLC purification (Rt=11.5 min) to afford compound 2 (Figure 2A). Starting with 99.3±25.4 mCi (n=3) of [18F]fluoride, compound 2 was isolated in a decay corrected radiochemical yield of 18.6±4.5% (17.7±0.1 mCi) with a radiochemical purity of 98.3±2.9% (Figure 2B) and a specific activity of 68.82±18.5 GBq/μmol in a total synthesis time of 37 min.
FIGURE 2.
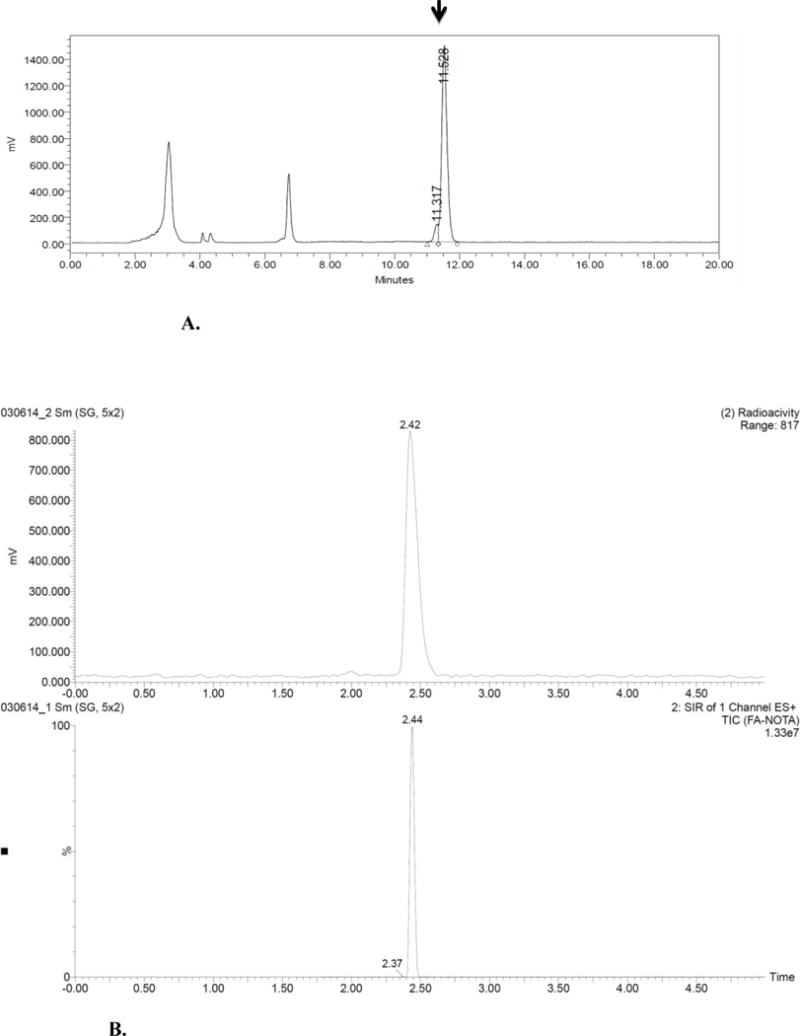
A. HPLC Chromatogram Purification of folate-NOTA-Al18F (2)
B. Representative analytical HPLC chromatogram of purified folate-NOTA-Al18F (2).
In Vitro Characterization
Relative Binding Affinity
To investigate the potential influence of conjugation of NOTA to folate on the binding affinity of the folate conjugate to FR, a relative binding affinity assay was performed by evaluating the concentration of conjugate required to block binding of 3H-folic acid. As shown in figure 3, folate-NOTA conjugate (1) displayed high affinity for FR with a mean IC50 of 18.7 nM at the concentration of 3H-folic acid used, which is only slightly weaker than the affinity of free folic acid (4.6 nM),suggesting that attachment of the NOTA chelating moiety has only a minor effect on FR binding affinity.
FIGURE 3.
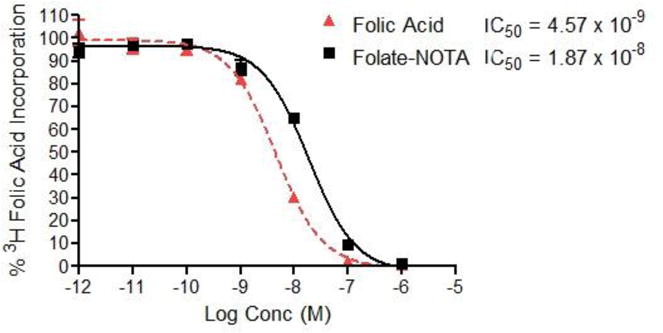
Relative binding of folate-NOTA to cultured KB cells: Inhibition of 3H-folate with folic acid and folate-NOTA (1)
Radioactive Saturation Binding Assay
In saturation binding assay, folate-NOTA-Al18F (2) was found to bind to crude homogenates of KB and Cal51 xenografts (1 mg/mL wet weight) in a monophasic manner, consistent with a single class of binding sites (Figure 4). The Kd values estimated for folate-NOTA-Al18F were 0.7 nM (KB) and 1.1 nM (Cal51) respectively. Non-displaceable binding (assessed in the presence of 5 μM self-block) was low, approximately < 1% (KB) and 11% (Cal51) of total binding at concentrations near the Kd value. Bmax values of folate-NOTA-Al18F were found to vary 14-fold between KB (511 nM) and Cal51 (36 nM) xenografts. Folate-NOTA-Al18F binding potentials (Bmax/Kd) in xenografts were 730 (KB) and 33 (Cal51) respectively, resulting in 22-fold greater binding potential of folate-NOTA-Al18F in KB xenografts than in Cal51 xenografts. The lower expression of FR in Cal51 xenografts could account for the reduced retention of folate-NOTAAl18F in the Cal51 samples.
FIGURE 4. Specific binding affinity assays of folate-NOTA-Al18F (2) to FR in.
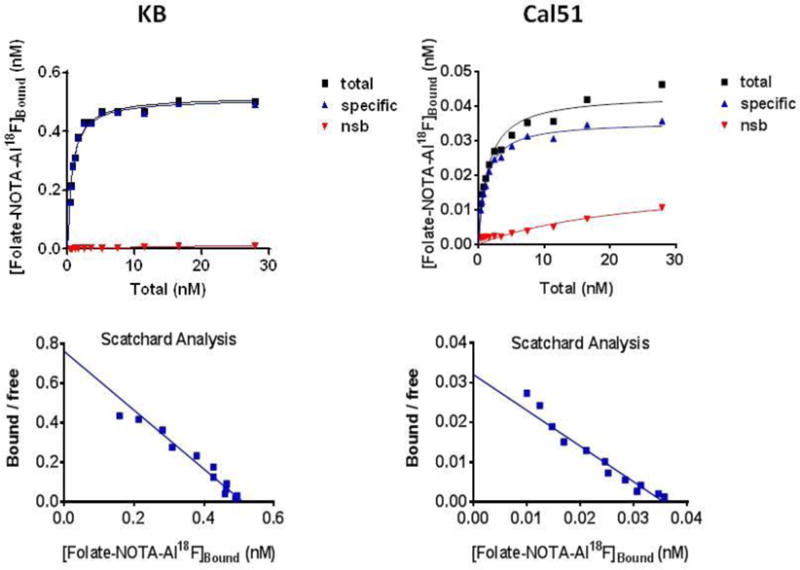
KB (upper graphs) and Cal51 (lower graphs) tumor crude homogenate (1 mg/mL)
In Vivo microPET imaging
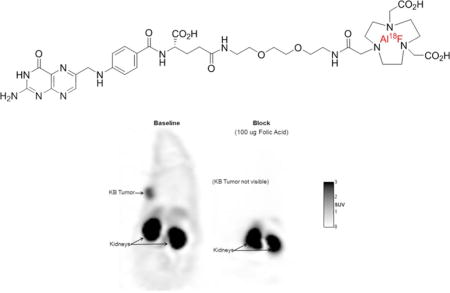
As shown in figure 5, tumors were readily visible under baseline conditions (injection of radiotracer with no competing folic acid), whereas the uptake of folate-NOTA-Al18F (2) was completely blocked with excess free folic acid, supporting a high specificity of folate-NOTA-Al18F binding to FR in vivo. The high radioactivity found in kidneys was due both to FR-mediated uptake by folate receptors expressed on the proximal tubule cells of the kidneys and the presence of excreted folate-NOTA-Al18F flowing through the urine concentrating ducts and tubules of the kidneys. No significant uptake in other organs was found except limited accumulation in liver.
FIGURE 5.
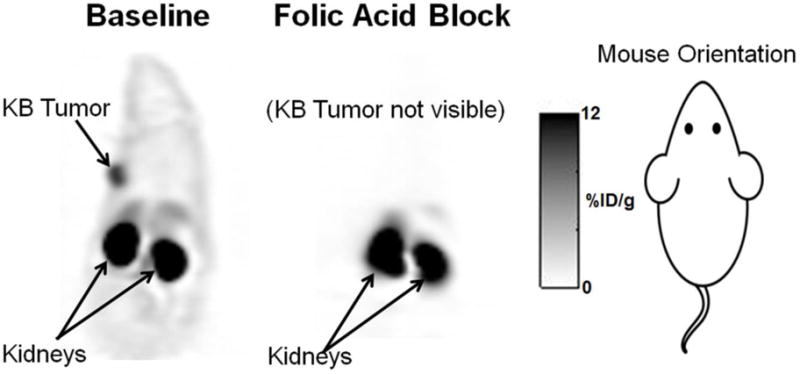
PET images with folate-NOTA-Al18F (2) are shown in mice bearing KB tumors on left shoulders (arrows) under baseline and blocking conditions. A blocking dose of 100 μg Folic acid was administered i.v. immediately before the radiotracer. PET images are corresponding coronal slices through the tumor and kidneys, with PET data summed for 90 min post-folate-NOTA-Al18F (2) administration.
Ex Vivo Biodistribution Studies
As shown in table 1 and figure 6, uptake of folate-NOTA-Al18F (2) in KB tumors was 10.9 ± 2.7 %ID/g in baseline animals, whereas the radioactive accumulation in the animals subjected to competition with excess free folica acid was substantially reduced to 1.3 ± 0.1 %ID/g, indicating a highly specific uptake mediated by FR. In contrast, the uptake of folate-NOTA-Al18F (2) in the very low FR expressing A549 tumors was 2.8 ± 0.7 %ID/g, i.e., substantially lower than that in KB tumors (Figure 6). The high accumulation of folate-NOTA-Al18F (2) in kidneys, along with much lower uptake in liver shown in figure 7 suggests that renal elimination is the predominant route for excretion. Moreover, a measurable blocking effect of excess folic acid was also found in heart, lung, muscle and spleen, suggesting low levels of FR present in these organs. Overall, the biodistribution of folate-NOTA-Al18F (2) is comparable but perhaps slightly better than that of 99mTc-EC20, the clinically established folate-based SPECT tracer.
TABLE 1.
Ex Vivo biodistribution study of folate-NOTA-Al18F (2) at 90 minutes p.i. in nude mice bearing KB tumor xenografts in comparison to the SPECT Tracer 99mTc-EC20 evaluated at 120 minutes post-injection. All values are in units of %ID/g ± SEM (except ratios).
| Organ or tissue | Control (N=8) KB Tumor (N = 4) |
FA Block (N=6) KB Tumor (N = 3) |
Ratio control:block |
99mTc-EC20 Control (N=26) KB Tumor (N = 20) |
99mTc-EC20 FA Block (N=6) KB Tumor (N = 3) |
|---|---|---|---|---|---|
| Blood | 0.2 ± 0.02 | 0.3 ± 0.1 | 0.6 | 0.3 ± 0.01 | 0.3 ± 0.1 |
| Heart | 0.8 ± 0.03 | 0.1 ± 0.03 | 11.5 | 1.9 ± 0.1 | 0.1 ± 0.02 |
| Kidney | 78.6 ± 5.1 | 16.6 ± 12.8 | 4.7 | 113.0 ± 3.6 | 6.7 ± 1.6 |
| Liver | 5.3 ± 0.5 | 3.5 ± 0.5 | 1.5 | 4.1 ± 0.3 | 2.5 ± 0.5 |
| Lung | 0.9 ± 0.1 | 0.6 ± 0.4 | 1.5 | 2.1 ± 0.1 | 0.8 ± 0.2 |
| Muscle | 1.0 ± 0.1 | 0.1 ± 0.1 | 8.4 | 1.2 ± 0.04 | 0.1 ± 0.02 |
| Plasma | 0.3 ± 0.04 | 0.5 ± 0.2 | 0.5 | 0.5 ± 0.03 | 0.5 ± 0.1 |
| Spleen | 0.6 ± 0.1 | 0.3 ± 0.1 | 2.0 | 0.8 ± 0.1 | 0.5 ± 0.2 |
| KB Tumor | 10.9 ± 2.7 | 1.3 ± 0.1 | 8.3 | 10.9 ± 0.6 | 0.9 ± 0.1 |
| KB Tumor/Liver | 2.0 ± 0.4 | 0.4 ± 0.2 | 4.5 | 3.1 ± 0.3 | 0.4 ± 0.1 |
| KB Tumor/Kidney | 0.1 ± 0.03 | 0.4 ± 0.3 | 0.3 | 0.1 ± 0.01 | 0.1 ± 0.02 |
| KB Tumor/Blood | 55.8 ± 11.5 | 14.0 ± 12.1 | 4.0 | 41.5 ± 2.2 | 2.9 ± 0.4 |
In the competition group, each animal received 100 μg of folic acid 10 min before radiotracer injection.
FIGURE 6.
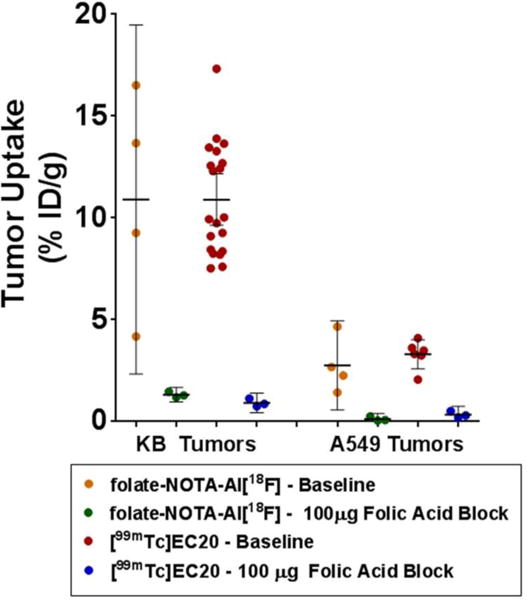
Ex Vivo tumor accumulation of folate-NOTA-Al18F (2) at 90 min post injection in nude mice bearing KB tumor xenografts or A549 tumor xenografts in comparison to the SPECT tracer 99mTc-EC20 at 120 min. Horizontal lines indicate arithmetic mean, and error bars represent 95% confidence intervals.
FIGURE 7.
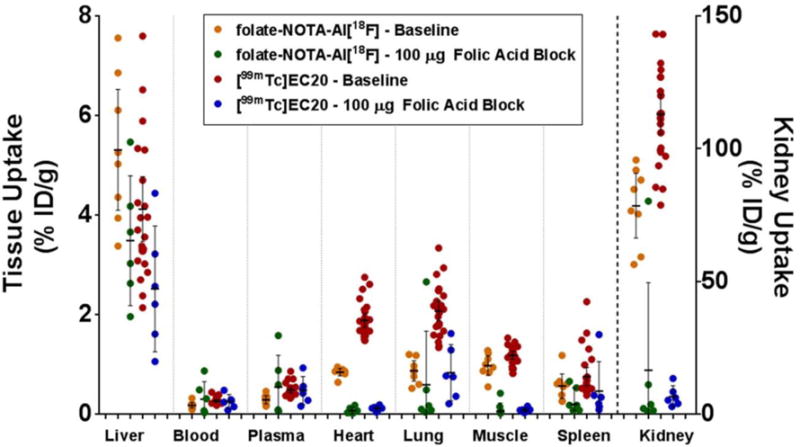
Biodistribution of folate-NOTA-Al18F and 99mTc-EC20 in athymic tumor bearing mice at 90 min and at 120 min post injection, respectively. Folate receptor specific binding is evaluated by competition with 100 ug excess folic acid. Data on tumor retention for the same animals were shown in Figure 6. Horizontal lines indicate arithmetic mean, and error bars represent 95% confidence interval.
DISCUSSION
In this study, we have shown that a rationally-designed folate-NOTA-Al18F (2) can be used as a PET imaging agent for targeting FR+ tumor xenografts in mice. The highly specific binding of 2 suggests that attachment of a chelating group like NOTA to folic acid has little effect on the conjugate’s affinity for FR. Radiochemical synthesis of folate-NOTA-Al18F was achieved in one step by reaction with Al18F3, yielding folate-NOTA-Al18F in good radiochemical radiochemical yield (18.6±4.5%), high radiochemical purity (98.3±2.9%, after HPLC purification), and a total synthesis time of 37 min, making the entire procedure compatible with a standard automated production system.
Biodistribution data and PET imaging experiments demonstrated that the uptake of folate-NOTA-Al18F (2) was concentrated primarily in FR+ KB tumors and kidneys. As displayed in table 1 and figure 6, uptake of radiotracer 2 in FR+ KB tumors was ~10.9 %ID/g at 90 minutes after injection. Accumulation of this radiotracer was also shown to be FR-mediated, as the administration of excess free folic acid blocked its retention in tumor. As expected, a high uptake of radioactivity was found in the kidneys (78.6 ± 5.1 %ID/g, 90 min p.i.), because of elevated expression of FR in proximal tubule cells. Ongoing renal excretion of this radiotracer may also contribute to the high accumulation in kidneys, especially at early time points. The more limited radioactivity in the liver (5.3 ± 0.5 %ID/g, 90 min p.i.) suggests that only a minor fraction of the conjugate undergoes hepatobiliary excretion. In contrast, the biodistribution study in A549 xenografts revealed a very limited accumulation of 2 in FR- tumor, which further confirmed that the highly specific uptake of 2 is mediated by FR.
Folate-NOTA-Al18F and 99mTc-EC20 showed largely similar distribution in mice, with the folate targeted PET agent displaying somewhat lower retention in normal tissues than the 99mTc- EC20. In a clinical setting, PET may show approximately thirty-fold better sensitivity and eightfold better volumetric resolution than SPECT. Quantitation of tracer concentration is inherent in all clinical PET and PET/CT scanners, whereas for SPECT and SPECT/CT it is still an area of development. These advantages for PET translate into potentially shorter whole-body acquisition times, improved sensitivity of tracer uptake in lesions smaller than ~20 mm, and the ability to quantify the signal in individual lesions with the possibility of establishing a correlation tracer uptake and lesion response to therapy.
It should be emphasized that the folate-NOTA-Al18F conjugate described here is not the first folate-targeted PET agent to be described in the literature. Indeed, a variety of folate-conjugated 68Ga and 18F PET agents have already been shown to produce highly defined images of FR positive tumors in animal tumor models.33–56 Our motivation for seeking yet another folate-targeted PET agent was prompted by the need to create an imaging agent that could meet the economic requirements for translation of a PET agent into the clinic. 18F was selected ahead of 68Ga not only because of the higher resolution images that it yields,28, 57–59 but also because of the extensive production and distribution networks that already exists for 18F.58 Folate-NOTA-Al18F was preferred over earlier folate-18F conjugates for reasons that differ for each previous conjugate, including its higher specific activity,39, 45, 47, 55 comparable or in some cases better radiochemical yield,39, 40, 47, 49 and faster or simpler synthesis.39, 40, 47, 49, 51 As a consequence, we propose that folate-NOTA-Al18F meets most of the requirements for eventual translation into human clinical trials.
Supplementary Material
Acknowledgments
This work was supported by a research grant from Endocyte, Inc. The authors gratefully acknowledge the Mass Spectrometry, NMR, Flow cytometry and support from the Purdue University Center for Cancer Research, P30CA023168.
Footnotes
SUPPORTING INFORMATION
- The revised manuscript copy with tracked changes (for review only)
- The revised supporting information file (for publication)
- Materials and Methods
- Detailed Experimental section
- References
References
- 1.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The Proton-Coupled Folate Transporter: Impact on Pemetrexed Transport and on Antifolates Activities Compared with the Reduced Folate Carrier. Mol Pharmacol. 2008;74(3):854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao R, Goldman ID. The molecular identity and characterization of a Proton-Coupled Folate Transporter—PCFT; biological ramifications and impact on the activity of pemetrexed—12 06 06. Cancer Metastasis Rev. 2007;26(1):129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- 3.Spinella MJ, Brigle KE, Sierra EE, Goldman ID. Distinguishing between Folate Receptor-α-mediated Transport and Reduced Folate Carrier-mediated Transport in L1210 Leukemia Cells. J Biol Chem. 1995;270(14):7842–7849. doi: 10.1074/jbc.270.14.7842. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Low PS. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J Controlled Release. 1998;53(1–3):39–48. doi: 10.1016/s0168-3659(97)00236-8. [DOI] [PubMed] [Google Scholar]
- 5.Antony AC. Folate Receptors. Annu Rev Nutr. 1996;16(1):501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- 6.Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J Pharm Sci. 2005;94(10):2135–2146. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 7.Low PS, Henne WA, Doorneweerd DD. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc Chem Res. 2008;41(1):120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 8.Tung CH, Lin Y, Moon WK, Weissleder R. A Receptor-Targeted Near-Infrared Fluorescence Probe for In Vivo Tumor Imaging. ChemBioChem. 2002;3(8):784–786. doi: 10.1002/1439-7633(20020802)3:8<784::AID-CBIC784>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MD, Jallad KN, Thompson DH, Ben-Amotz D, Low PS. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J Biomed Opt. 2003;8(4):636–641. doi: 10.1117/1.1609453. [DOI] [PubMed] [Google Scholar]
- 10.Moon WK, Lin Y, O’Loughlin T, Tang Y, Kim D-E, Weissleder R, Tung C-H. Enhanced Tumor Detection Using a Folate Receptor-Targeted Near-Infrared Fluorochrome Conjugate. Bioconjug Chem. 2003;14(3):539–545. doi: 10.1021/bc0340114. [DOI] [PubMed] [Google Scholar]
- 11.Chen WT, Mahmood U, Weissleder R, Tung CH. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther. 2005;7(2):R310–R317. doi: 10.1186/ar1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. Folate-Receptor-Mediated Delivery of InP Quantum Dots for Bioimaging Using Confocal and Two-Photon Microscopy. J Am Chem Soc. 2005;127(32):11364–11371. doi: 10.1021/ja051455x. [DOI] [PubMed] [Google Scholar]
- 13.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104(28):11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mindt TL, Muller C, Stuker F, Salazar JF, Hohn A, Mueggler T, Rudin M, Schibli R. A “click chemistry” approach to the efficient synthesis of multiple imaging probes derived from a single precursor. Bioconjug Chem. 2009;20(10):1940–9. doi: 10.1021/bc900276b. [DOI] [PubMed] [Google Scholar]
- 15.Ke CY, Mathias CJ, Green MA. Folate-receptor-targeted radionuclide imaging agents. Adv Drug Delivery Rev. 2004;56(8):1143–1160. doi: 10.1016/j.addr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Sega E, Low P. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008;27(4):655–664. doi: 10.1007/s10555-008-9155-6. [DOI] [PubMed] [Google Scholar]
- 17.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(3):256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Muller C. Folate based radiopharmaceuticals for imaging and therapy of cancer and inflammation. Curr Pharm Des. 2012;18(8):1058–83. doi: 10.2174/138161212799315777. [DOI] [PubMed] [Google Scholar]
- 19.Müller C, Schibli R. Folic Acid Conjugates for Nuclear Imaging of Folate Receptor–Positive Cancer. J Nucl Med. 2011;52(1):1–4. doi: 10.2967/jnumed.110.076018. [DOI] [PubMed] [Google Scholar]
- 20.Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000;35(1):50–7. doi: 10.1097/00004424-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. MAGMA. 2001;12(2–3):104–13. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 22.Konda SD, Wang S, Brechbiel M, Wiener EC. Biodistribution of a 153 Gd-folate dendrimer, generation = 4, in mice with folate-receptor positive and negative ovarian tumor xenografts. Invest Radiol. 2002;37(4):199–204. doi: 10.1097/00004424-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Choi H, Choi SR, Zhou R, Kung HF, Chen IW. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery1. Acad Radiol. 2004;11(9):996–1004. doi: 10.1016/j.acra.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Sun C, Sze R, Zhang M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res, Part A. 2006;78A(3):550–557. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 25.Saborowski O, Simon GH, Raatschen HJ, Wendland MF, Fu Y, Henning T, Baehner R, Corot C, Chen MH, Daldrup-Link HE. MR imaging of antigen-induced arthritis with a new, folate receptor-targeted contrast agent. Contrast Media Mol Imaging. 2007;2(2):72–81. doi: 10.1002/cmmi.128. [DOI] [PubMed] [Google Scholar]
- 26.Swanson SD, Kukowska-Latallo JF, Patri AK, Chen C, Ge S, Cao Z, Kotlyar A, East AT, Baker JR. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomedicine. 2008;3(2):201–10. [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen FP, Vanderheyden JL. The future of SPECT in a time of PET. Nucl Med Biol. 2007;34(7):733–735. doi: 10.1016/j.nucmedbio.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Muller C. Folate-Based Radiotracers for PET Imaging-Update and Perspectives. Molecules. 2013;18(5):5005–31. doi: 10.3390/molecules18055005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29(3):193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 30.McBride WJ, D’Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang C-H, Goldenberg DM. Improved 18F Labeling of Peptides with a Fluoride-Aluminum-Chelate Complex. Bioconjug Chem. 2010;21(7):1331–1340. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Park R, Conti PS, Li Z. “Kit like” (18)F labeling method for synthesis of RGD peptide-based PET probes. Am J Nucl Med Mol Imaging. 2013;3(1):97–101. [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson O, Kiesewetter DO, Chen X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug Chem. 2014 doi: 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang ZF, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl Med Biol. 2003;30(7):725–731. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- 34.Fani M, Wang X, Nicolas G, Medina C, Raynal I, Port M, Maecke H. Development of new folate-based PET radiotracers: preclinical evaluation of 68Ga-DOTA-folate conjugates. Eur J Nucl Med Mol Imaging. 2011;38(1):108–119. doi: 10.1007/s00259-010-1597-8. [DOI] [PubMed] [Google Scholar]
- 35.Kühle B, Müller C, Ross T. A Novel 68Ga-Labeled Pteroic Acid-Based PET Tracer for Tumor Imaging via the Folate Receptor. In: Baum RP, Rösch F, editors. Theranostics, Gallium-68, and Other Radionuclides. Vol. 194. Springer Berlin Heidelberg; 2013. pp. 257–267. [DOI] [PubMed] [Google Scholar]
- 36.Fani M, Tamma ML, Nicolas GP, Lasri E, Medina C, Raynal I, Port M, Weber WA, Maecke HR. In Vivo Imaging of Folate Receptor Positive Tumor Xenografts Using Novel 68Ga-NODAGA-Folate Conjugates. Mol Pharmaceutics. 2012;9(5):1136–1145. doi: 10.1021/mp200418f. [DOI] [PubMed] [Google Scholar]
- 37.Aljammaz I, Al-Otaibi B, Al-Hokbany N, Amer S, Okarvi S. Development and Pre-clinical Evaluation of New 68Ga-NOTA-folate Conjugates for PET Imaging of Folate Receptor-positive Tumors. Anticancer Res. 2014;34(11):6547–6556. [PubMed] [Google Scholar]
- 38.AlJammaz I, Alotabi B, Bin Amer S, AlHokbani N, Okarvi S. Synthesis and in-vitro and in-vivo evaluation of new 68Ga-NOTA-folates for folate receptor-positive tumor imaging. J Nucl Med. 2013;54(supplement 2):1136. [Google Scholar]
- 39.Bettio A, Honer M, Müller C, Brühlmeier M, Müller U, Schibli R, Groehn V, Schubiger AP, Ametamey SM. Synthesis and Preclinical Evaluation of a Folic Acid Derivative Labeled with 18F for PET Imaging of Folate Receptor–Positive Tumors. J Nucl Med. 2006;47(7):1153–1160. [PubMed] [Google Scholar]
- 40.Ross TL, Honer M, Lam PYH, Mindt TL, Groehn V, Schibli R, Schubiger PA, Ametamey SM. Fluorine-18 Click Radiosynthesis and Preclinical Evaluation of a New 18F-Labeled Folic Acid Derivative. Bioconjug Chem. 2008;19(12):2462–2470. doi: 10.1021/bc800356r. [DOI] [PubMed] [Google Scholar]
- 41.Schieferstein H, Betzel T, Haller S, Cindy F, Muller C, Ross TL. Total evaluation of a new polar F-18-labeled PEG-click-folate. J Labelled Compd Rad. 2013;56:S183–S183. [Google Scholar]
- 42.Amartey JK, Al-Jammaz I, Al-Otaibi B, Esguerra C. Novel synthesis of 2-[18F]-fluoroisonicotinic acid hydrazide and initial biological evaluation. Nucl Med Biol. 2002;29(8):817–823. doi: 10.1016/s0969-8051(02)00340-2. [DOI] [PubMed] [Google Scholar]
- 43.Okarvi SM, Jammaz IA. Preparation and in vitro and in vivo evaluation of technetium-99m-labeled folate and methotrexate conjugates as tumor imaging agents. Cancer Biother Radiopharm. 2006;21(1):49–60. doi: 10.1089/cbr.2006.21.49. [DOI] [PubMed] [Google Scholar]
- 44.Al Jammaz I, Al-Otaibi B, Okarvi S, Amartey J. Novel synthesis of [18F]-fluorobenzene and pyridinecarbohydrazide-folates as potential PET radiopharmaceuticals. J Labelled Compd Radiopharm. 2006;49(2):125–137. [Google Scholar]
- 45.Al Jammaz I, Al-Otaibi B, Amer S, Okarvi SM. Rapid synthesis and in vitro and in vivo evaluation of folic acid derivatives labeled with fluorine-18 for PET imaging of folate receptor-positive tumors. Nucl Med Biol. 2011;38(7):1019–28. doi: 10.1016/j.nucmedbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Schibli R, Moser R, Mueller CM, Ametamey SM, Ross TL, Groehn V. WO2010040854A1. Preparation of 18F-labeled folate derivatives for use as PET radiotracers. 2010
- 47.Ross TL, Honer M, Müller C, Groehn V, Schibli R, Ametamey SM. A New 18F-Labeled Folic Acid Derivative with Improved Properties for the PET Imaging of Folate Receptor–Positive Tumors. J Nucl Med. 2010;51(11):1756–1762. doi: 10.2967/jnumed.110.079756. [DOI] [PubMed] [Google Scholar]
- 48.Groehn V, Moser R, Ross TL, Betzel T, Muller C, Schibli R, Ametamey S. Synthesis of precursors for 18F-labeling of folic acid for PET application. Synthesis. 2011;(22):3639–3648. [Google Scholar]
- 49.Betzel T, Muller C, Groehn V, Muller A, Reber J, Fischer CR, Kramer SD, Schibli R, Ametamey SM. Radiosynthesis and Preclinical Evaluation of 3′-Aza-2′-[(18)F]fluorofolic Acid: A Novel PET Radiotracer for Folate Receptor Targeting. Bioconjug Chem. 2013;24(2):205–14. doi: 10.1021/bc300483a. [DOI] [PubMed] [Google Scholar]
- 50.Shreve PD, Anzai Y, Wahl RL. Pitfalls in Oncologic Diagnosis with FDG PET Imaging: Physiologic and Benign Variants. Radiographics. 1999;19(1):61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 51.Fischer CR, Müller C, Reber J, Müller A, Krämer SD, Ametamey SM, Schibli R. [18F]Fluoro-Deoxy-Glucose Folate: A Novel PET Radiotracer with Improved in Vivo Properties for Folate Receptor Targeting. Bioconjug Chem. 2012;23(4):805–813. doi: 10.1021/bc200660z. [DOI] [PubMed] [Google Scholar]
- 52.Schibli R, Moser R, Mueller CM, Ametamey SM, Fischer CR, Groehn V. WO2013026842A1. Preparation of 18F-labeled glycoside folates as imaging and diagnosis agents of cancer, inflammatory, and autoimmune diseases. 2013
- 53.Maschauer S, Prante O. A series of 2-O-trifluoromethylsulfonyl-d-mannopyranosides as precursors for concomitant 18F-labeling and glycosylation by click chemistry. Carbohydr Res. 2009;344(6):753–761. doi: 10.1016/j.carres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Maschauer S, Einsiedel J, Haubner R, Hocke C, Ocker M, Hübner H, Kuwert T, Gmeiner P, Prante O. Labeling and Glycosylation of Peptides Using Click Chemistry: A General Approach to 18F-Glycopeptides as Effective Imaging Probes for Positron Emission Tomography. Angew Chem Int Ed. 2010;49(5):976–979. doi: 10.1002/anie.200904137. [DOI] [PubMed] [Google Scholar]
- 55.Al Jammaz I, Al-Otaibi B, Amer S, Al-Hokbany N, Okarvi S. Novel synthesis and preclinical evaluation of folic acid derivatives labeled with 18F-[FDG] for PET imaging of folate receptor-positive tumors. Nucl Med Biol. 2012;39(6):864–870. doi: 10.1016/j.nucmedbio.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Boss SD, Betzel T, Müller C, Fischer CR, Haller S, Reber J, Groehn V, Schibli R, Ametamey SM. Comparative Studies of Three Pairs of α- and γ-Conjugated Folic Acid Derivatives Labeled with Fluorine-18. Bioconjug Chem. 2015 doi: 10.1021/acs.bioconjchem.5b00644. [DOI] [PubMed] [Google Scholar]
- 57.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew Chem Int Ed. 2008;47(47):8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson M, Steichen R, Peller P. PET Radiochemistry and Radiopharmacy. In: Peller P, Subramaniam R, Guermazi A, editors. PET-CT and PET-MRI in Oncology. Springer Berlin Heidelberg; 2012. pp. 19–30. [Google Scholar]
- 59.Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJG, Weber WA. Imaging the Folate Receptor on Cancer Cells with 99mTc-Etarfolatide: Properties, Clinical Use, and Future Potential of Folate Receptor Imaging. J Nucl Med. 2014;55(5):701–704. doi: 10.2967/jnumed.113.133074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


