Abstract
Context:
Rapid diagnosis and expeditious cooling of individuals with exertional heat stroke is paramount for survival.
Objective:
To evaluate the efficacy of various cooling systems after exercise-induced hyperthermia.
Design:
Crossover study.
Setting:
Laboratory.
Patients or Other Participants:
Twenty-two men (age = 24 ± 2 years, height = 1.76 ± 0.07 m, mass = 70.7 ± 9.5 kg) participated.
Intervention(s):
Each participant completed a treadmill walk until body core temperature reached 39.50°C. The treadmill walk was performed at 5.3 km/h on an 8.5% incline for 50 minutes and then at 5.0 km/h until the end of exercise. Each participant experienced 4 cooling phases in a randomized, repeated-crossover design: (1) no cooling (CON), (2) body-cooling unit (BCU), (3) EMCOOLS Flex.Pad (EC), and (4) ThermoSuit (TS). Cooling continued for 30 minutes or until body core temperature reached 38.00°C, whichever occurred earlier.
Main Outcome Measure(s):
Body core temperature (obtained via an ingestible telemetric temperature sensor) and heart rate were measured continuously during the exercise and cooling phases. Rating of perceived exertion was monitored every 5 minutes during the exercise phase and thermal sensation every minute during the cooling phase.
Results:
The absolute cooling rate was greatest with TS (0.16°C/min ± 0.06°C/min) followed by EC (0.12°C/min ± 0.04°C/min), BCU (0.09°C/min ± 0.06°C/min), and CON (0.06°C/min ± 0.02°C/min; P < .001). The TS offered a greater cooling rate than all other cooling modalities in this study, whereas EC offered a greater cooling rate than both CON and BCU (P < .0083 for all). Effect-size calculations, however, showed that EC and BCU were not clinically different.
Conclusion:
These findings provide objective evidence for selecting the most effective cooling system of those we evaluated for cooling individuals with exercise-induced hyperthermia. Nevertheless, factors other than cooling efficacy need to be considered when selecting an appropriate cooling system.
Key Words: exertional heat stroke, core temperature, safety
Athletes training and competing in high ambient temperatures face considerable heat strain and may develop exertional heat stroke (EHS).1–3 Rapid diagnosis and prompt cooling of an individual with suspected heat stroke is crucial.4–6 Body core temperature must be lowered immediately to prevent organ and tissue damage.4 Among many others,7,8 cooling techniques that have been used to treat EHS include ice-water or cold-water immersion,6,9 a body-cooling unit (BCU),10,11 and ice packs.12
The most effective mode of cooling offers the highest cooling rate, calculated by the change in body core temperature over time. McDermott et al8 defined the effectiveness of cooling modalities based on the cooling rate: ideal cooling modalities provide a cooling rate equal to or greater than 0.155°C/min, acceptable cooling modalities provide a cooling rate greater than 0.078°C/min and less than 0.155°C/min, and unacceptable cooling modalities provide a cooling rate equal to or less than 0.078°C/min. Authors of several reviews and studies have identified cold-water immersion as the most effective strategy for rapidly lowering body core temperature,6–8 with the coldest water offering the greatest cooling rates in the laboratory.13
The BCU is commonly used in health care, sporting, and military settings to treat individuals with EHS (Figure 1).4,10,11,14 It functions via evaporative cooling and convection with wind: fine mists of water sprayed on the body and evaporated by fanning. The BCU is similar to a misting fan except that mists of water are dispensed from the bed rather than from the fan itself. Weiner and Khogali11 reported a cooling rate of 0.31°C/min to reduce 6 participants' body core temperature from 39.5°C to 37.5°C after exercise-induced hyperthermia. The effectiveness of the BCU was supported by the successful treatment of 19 of 21 patients with heat stroke during the Mecca pilgrimages in 1978 and 1979.11 The Israel Defense Forces14 have also consistently used this cooling method for patients with EHS and reported an average cooling rate of 0.14°C/min ± 0.11°C/min (7-year average, 1996–2003). However, critics of this method have argued that the high relative humidity during a BCU trial may limit heat dissipation by evaporation during the cooling phase.15
Figure 1.
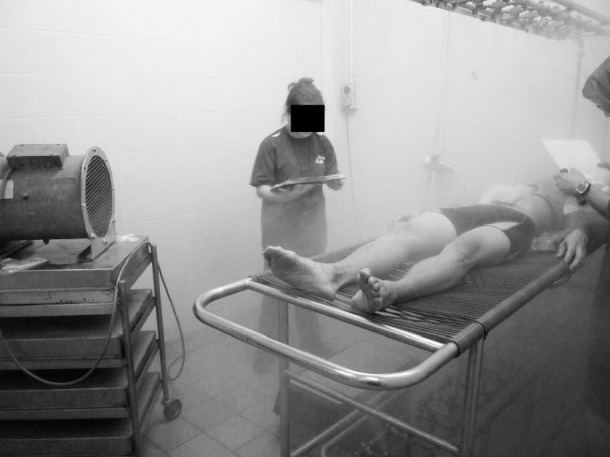
Body-cooling unit. Ambient-temperature water dispensed as a fine mist from pressure valves above and below the participant with a fan blowing across participants from feet to head. The unit is similar to a misting fan except that it dispenses mists of water from the bed rather than from the fan.
The EMCOOLS Flex.Pad (EC; EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria) uses HypoCarbon technology, which is a patented material comprising graphite and water and possessing 58- and 15-fold greater thermal conductivity than water and ice, respectively. The latex pads are nontoxic and easy to apply to the skin. The EC works in a similar way to ice packs, extracting heat from the body via conduction. It has primarily been used for therapeutic hypothermia in patients after cardiac arrest16,17 and myocardial infarction18 and for treating malignant hyperthermia.19 Uray et al17 cooled 15 patients resuscitated after cardiac arrest from an esophageal temperature of 36.6°C to 33.0°C within 70 minutes (range, 55–106 minutes), with 12 ECs providing cooling rates of approximately 0.06°C/min.
The ThermoSuit (TS; Life Recovery Systems HD, LLC, Kinnelon, NJ) is a whole-body suit designed to cool the body via convective-immersion surface cooling. The system comprises an inflatable mold that holds a patient lying supine. A pump controller circulates ice water through the suit at a rate of 14 L/min. This cooling modality has been tested clinically in 22 patients after cardiac arrest and provided a 0.06°C/min cooling rate to reduce esophageal temperature to less than 34°C.20
Therefore, the purpose of our study was to evaluate the efficacy of various cooling systems after exercise-induced hyperthermia. To our knowledge, we are the first to evaluate the efficacy of 2 commercial, off-the-shelf cooling systems (EC, TS) for treating exercise-induced hyperthermia. Both cooling systems are practical for administration in the laboratory or on the field. We hypothesized that, given its similarity to cold-water immersion, the TS would provide the greatest cooling rate of the cooling systems being evaluated. A secondary objective was to evaluate individual responses to the various cooling modalities. We hypothesized that we would observe interindividual differences in the response to the various cooling modalities, and if we found these differences, we would investigate if any anthropometric factors could have led to these deviations.
METHODS
Participants
Twenty-two healthy men participated (age = 24 ± 2 years, height = 1.76 ± 0.07 m, mass = 70.7 ± 9.5 kg, body mass index = 22.9 ± 2.8 kg/m, body surface area-to-lean body mass ratio [AD/LBM] = 349 ± 6 cm2/kg). One participant was white, and 21 were of Chinese ethnicity. We performed a power analysis and determined that a sample size of 15 would provide 80% power at an α level of .05 (2 tailed) to reject the null hypothesis. Individuals were recruited only if they met the following inclusion criteria: (1) no history of heat illness or gastrointestinal surgery; (2) no chronic health conditions; (3) no cardiovascular, metabolic, or respiratory disease; (4) participation in exercise for at least 30 minutes, 3 times per week; and (5) no use of any form of medication. All participants provided written informed consent, and the study was approved by the DSO National Laboratories (Singapore) and Singapore Armed Forces Institutional Review Board.
Control of Pretrial Status
Trials were separated by a minimum of 6 days to allow adequate recovery and to minimize any training effects. To control for circadian variations in body core temperature, participants reported at the same shift time (0900 hours or 1300 hours) for all experimental trials. Participants were instructed to avoid physical activity and to refrain from consuming alcohol for 24 hours before each trial. We instructed them to record their diet and any physical activity 24 hours before the familiarization trial and to repeat these procedures before the subsequent experimental trials. Participants were also instructed to consume their breakfast or lunch 90 minutes before arriving at the laboratory. The pretrial breakfast or lunch was a meal of their own preference and was repeated for all subsequent trials. These procedures ensured that participants began each trial in a similar state of euglycemia. Dietary record sheets were given to each participant to facilitate compliance. We also instructed participants to sleep at least 8 hours the night before each trial.
Experimental Design
All participants performed a series of 5 trials. The first was a familiarization trial in which they were acquainted with the trial procedures and measurements. No cooling was conducted during the familiarization trial. This trial was followed by 4 experimental trials performed in a randomized counterbalanced order: (1) no cooling (CON), (2) BCU, (3) EC, and (4) TS.
Each trial comprised an exercise and a cooling phase. Dry-bulb temperature, wet-bulb temperature, globe temperature, and relative humidity were measured using a climate logger (Questemp-15 Area Heat Stress Monitor; Quest Technologies, Oconomowoc, WI) placed in the gymnasium and a cooling station (Squirrel 2020 Series Data Logger; Grant Instruments, Cambridge, United Kingdom). Wet-bulb globe temperature was calculated as 0.1 × (dry-bulb temperature) + 0.7 × (wet-bulb temperature) + 0.2 × (globe temperature).
After arriving at the trial site, participants provided a midstream urine sample to indicate hydration status. Urine specific gravity was measured using a digital refractometer (model PAL-10S; Atago Co, Ltd, Tokyo, Japan). If urine specific gravity was less than 1.029, participants were considered slightly dehydrated but allowed to continue with the experimental trial.21 Nude body mass was measured to the nearest 0.01 kg (model BBA211; Mettler-Toledo International Inc, Greifensee, Switzerland). Body surface area was calculated according to the equation of Du Bois and Du Bois,22 and lean body mass was calculated according to the equation of Hume.23 At least 8 hours before each trial began, the participant ingested a telemetric temperature sensor (Mini Mitter Co, Inc, Bend, OR) to measure body core temperature (Tc). A telemetric check was performed using a Tc data-recording system (Mini Mitter Co, Inc) before each trial to ensure the sensor was still located within the participant. The Tc logger was sealed in a waterproof bag and placed in the left-side pocket of the participant's attire. Body core temperature was measured continuously and averaged over 1 minute. Participants were fitted with a chest band and a wristwatch heart-rate monitor (model S810i; Polar Electro Oy, Kempele, Finland). We measured heart rate continuously throughout the trial and averaged it over 1 minute. Subjective ratings of perceived exertion24 (6 = no exertion at all, 20 = maximal exertion) and ratings on a 15-point thermal-sensation scale (−7 = unbearably cold, 0 = neutral, 7 = unbearably hot) were reported every 5 minutes during the exercise phase. Thermal sensation was reported every minute during the cooling phase.
Exercise Phase.
Participants wore swimming trunks and physical training attire (t-shirt and exercise shorts) under a standard military uniform, a raincoat with hood over the uniform, socks, and running shoes. They were seated for 5 minutes before the trial began, during which time we recorded baseline measurements of Tc, heart rate, and ratings of perceived exertion and thermal sensation. Next, participants proceeded to the treadmill (model 500 MD; Technogym, Cesena, Italy) for 5 minutes of standing rest. After baseline measurements, participants performed an exercise phase.
The exercise phase comprised walking on a treadmill at 5.3 km/h on an 8.5% incline for 50 minutes and then at 5.0 km/h for 40 minutes to complete the exercise. It was performed in thermoneutral conditions (temperature = 29.0°C, relative humidity = 71%). Exercise was terminated if any of the following criteria were met: (1) the participant wanted to discontinue the trial; (2) Tc reached 39.50°C and was maintained for 1 minute; (3) the participant completed 90 minutes of the exercise phase; (4) the participant was unable to continue exercise voluntarily; (5) the participant reported symptoms of nausea, headache, dizziness, or cold, clammy skin; or (6) researchers observed signs of lethargy. Water at temperatures close to 37°C was provided ad libitum during the exercise phase of the familiarization trial, and the volume consumed was repeated for subsequent trials. All urine excreted throughout the trial was collected and weighed to the nearest 1 g (model EK3250; Zhongshan Camry Electronic Co Ltd, Zhongshan, Guangdong, China).
Cooling Phase.
When exercise ceased, participants were immediately transferred to a cooling station with a mean dry-bulb temperature of 25.6°C; 70% relative humidity in the CON, EC, and TS trials; and 90% relative humidity in the BCU trial for the cooling phase. Participants removed all footwear and clothing except their swimming trunks. A cooling modality was administered 5 minutes after the end of exercise. The cooling phase was terminated if any of the following criteria were met: (1) the participant wanted to discontinue the trial, (2) Tc decreased to less than 38.00°C, (3) the participant completed 30 minutes of the cooling phase, or (4) researchers observed signs of substantial discomfort (eg, intense shivering or inability to maintain a supine position). A safe cooling limit of 38.00°C was chosen in line with the recommendations of Makranz et al25 to prevent hypothermia due to excessive cooling of an individual with EHS.26,27
Cooling Modalities
No Cooling.
Participants lay supine on an elevated, vinyl-meshed bed with no cooling modalities administered. This trial was used to determine their natural cooling rate.
Body-Cooling Unit.
Participants lay supine on an elevated, vinyl-meshed bed. Ambient-temperature water was dispensed as fine mist from pressure valves above and below the participant (Figure 1). A fan that had a wind speed of approximately 2.0 m/s and was placed 120 cm away from the torso blew across participants from feet to head.
EMCOOLS Flex.Pad.
Participants lay supine on an elevated, vinyl-meshed bed. A total of 6 single-use ECs were placed on the participant's skin: 2 pads on the chest, 2 pads on the back, and 1 pad on each thigh. The thigh pad was torn into 3 equal pieces, with 2 pieces applied to the front of the thigh and 1 piece applied to the back of the thigh. The pads were precooled in a freezer box (model Freezer SUPERPOLO; C.F. di Ciro Fiocchetti & C. s.n.c., Luzaara RE, Italy) at approximately −11°C for a minimum of 24 hours before placement according to the manufacturer's instructions. The pads were not replaced for any participant.
Key Points
The ThermoSuit offered the greatest mean absolute cooling rate.
Effect-size calculations showed no clinical difference between the EMCOOLS Flex.Pad and a body cooling unit.
Factors other than cooling efficacy, such as cost and the manpower required to operate these cooling systems, need to be considered when selecting an appropriate cooling system.
ThermoSuit.
Participants lay supine in a TS tub on an elevated, vinyl-meshed bed. The suit was automatically inflated, and ice water (2°C ± 2°C) flowed into the inflated tub via a pump. A top sheet was placed above the participant. The water was returned to the reservoir pump and continuously circulated. The temperature of the water in the reservoir was continuously monitored throughout the cooling phase, and ice cubes were replenished when the water temperature increased above 4°C. The TS was used according to the manufacturer's instructions.
Statistical Analysis
Normality of data was assessed using the Shapiro-Wilk test. Data that were not normally distributed were analyzed using the Wilcoxon matched-pair signed rank or Kruskal-Wallis test. Post hoc Bonferroni corrections were used to isolate differences between trials at an α level of .0083. Normally distributed data were analyzed using a paired t test or a 1-factor analysis of variance to evaluate differences in variables at a single time. We used a 2-factor (group, time) repeated-measures analysis of variance to evaluate changes in the stepwise cooling rate over time and a Greenhouse-Geisser adjustment for violations of sphericity. Independent t tests were used to identify differences between participants who did and did not respond to the cooling modalities. Figures are illustrated as mean ± standard error of the mean (SEM) for clarity of presentation, and all other data are presented as mean ± standard deviation (SD). The α level was set at .05.
The effect size (Hedges g) and 95% confidence intervals (CIs) of the absolute cooling rate were calculated as a quantitative measure of the strength of the differences among cooling modalities. Hedges g was derived using the mean differences in variables divided by the pooled SD. The magnitude of Hedges g was classified according to the scale of Hopkins et al28: 0–0.2 (trivial), 0.2–0.6 (small), 0.6–1.2 (moderate), 1.2–2.0 (large), 2.0–4.0 (very large), and greater than 4.0 (extremely large). All statistical analyses were performed using SPSS (version 15.0; SPSS, Inc, Chicago, IL).
RESULTS
Pretrial Physiological Status
Baseline hydration status was similar among trials, as demonstrated by body mass (CON = 70.7 ± 9.8 kg, BCU = 70.7 ± 9.9 kg, EC = 70.7 ± 9.7 kg, TS = 70.8 ± 9.9 kg; P > .99) and urine specific gravity (CON = 1.018 ± 0.006, BCU = 1.016 ± 0.007, EC = 1.017 ± 0.008, TS = 1.016 ± 0.007; P = .69). The other baseline physiological measures, including Tc (CON = 37.19°C ± 0.28°C, BCU = 37.21°C ± 0.29°C, EC = 37.23°C ± 0.34°C, TS = 37.19°C ± 0.32°C; F3,84 = 0.07, P = .98) and heart rate (CON = 78 ± 11 beats/min, BCU = 79 ± 8 beats/min, EC = 77 ± 10 beats/min, TS = 80 ± 11 beats/min; F3,84 = 0.29, P = .83), were similar between trials.
Environmental Conditions
We observed no difference in wet-bulb globe temperature across the 4 trials during the exercise phase (CON = 27.0°C ± 1.3°C, BCU = 27.1°C ± 0.9°C, EC = 26.9°C ± 1.3°C, TS = 26.9°C ± 1.2°C; P = .80).
During the cooling phase, wet-bulb globe temperature was higher during the BCU trial (25.3°C ± 0.7°C) than during the CON (23.8°C ± 0.9°C), EC (22.8°C ± 1.7°C), and TS trials (22.9°C ± 1.4°C; P < .001). The higher wet-bulb globe temperatures during the BCU trial can be attributed to the nature of the cooling modality; fine mists of water are sprayed over the participant during cooling, enveloping the cooling station with water vapor and resulting in a higher wet-bulb temperature.
Exercise Phase
We observed no differences in the time to reach the end-exercise Tc of 39.50°C (CON = 52.4 ± 9.1 minutes, BCU = 52.5 ± 10.4 minutes, EC = 54.9 ± 13.3 minutes, TS = 53.5 ± 12.8 minutes; P = .99). Physiological measures of Tc and heart rate and subjective ratings of perceived exertion and thermal sensation reached similar levels at the end of the exercise phase (Table 1). These observations suggest that the degree of exercise-induced hyperthermia was identical in all trials.
Table 1.
Physiological and Subjective Measures at the End of the Exercise Phase
| Descriptor |
Cooling System, Mean ± SD |
P Value |
|||
| No Cooling |
Body-Cooling Unit |
EMCOOLS Flex.Pada |
ThermoSuitb |
||
| Body core temperature, °C | 39.53 ± 0.07 | 39.54 ± 0.05 | 39.55 ± 0.02 | 39.55 ± 0.05 | .89 |
| Heart rate, beats/min | 180 ± 12 | 181 ± 13 | 180 ± 11 | 179 ± 11 | .95 |
| Rating of perceived exertion | 15 ± 3 | 15 ± 3 | 15 ± 3 | 15 ± 3 | >.99 |
| Rating of thermal sensation | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | .83 |
EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria.
Life Recovery Systems HD, LLC, Kinnelon, NJ.
Absolute Cooling Rate
The number of participants who terminated cooling after 30 minutes was 7, 4, and 1 in the CON, BCU, and EC trials, respectively. All other participants terminated cooling when Tc was less than 38.00°C. Body core temperature at the start of cooling had increased slightly and was still similar among trials (CON = 39.55°C ± 0.18°C, BCU = 39.56°C ± 0.20°C, EC = 39.64°C ± 0.14°C, TS = 39.63°C ± 0.16°C; P = .21).
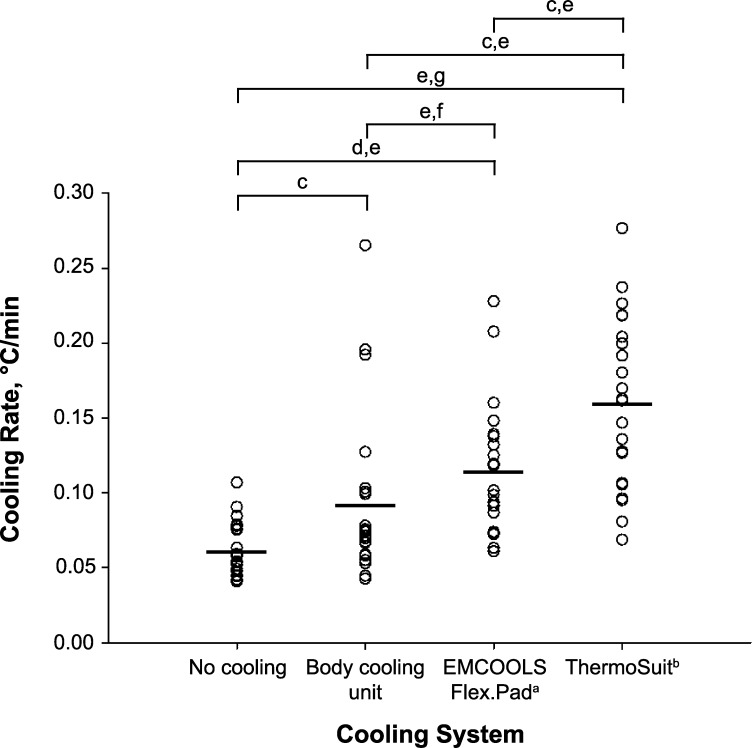
The mean absolute cooling rate was greatest with the TS (0.16°C/min ± 0.06°C/min), followed by the EC (0.12°C/min ± 0.04°C/min), BCU (0.09°C/min ± 0.06°C/min), and CON (0.06°C/min ± 0.02°C/min; P < .001; Figure 2) conditions. The cooling rate was greater during the TS trial than during the CON (Z = 4.02, P < .001), BCU (Z = 3.15, P = .002), and EC (Z = 2.87, P = .004) trials. The cooling rate was also greater during the EC trial than during the CON (Z = 3.86, P < .001) or BCU (Z = 2.64, P = .008) trial. We observed no difference between the CON and BCU trials (Z = 2.56, P = .01). The Hedges g effect size of the mean absolute cooling rates was also analyzed (Figure 2). We found a moderate effect for CON versus BCU (P = .01, Hedges g = 0.73, 95% CI = 0.13, 1.33), BCU versus TS (P = .002, Hedges g = 1.18, 95% CI = 0.55, 1.81), and EC versus TS (P = .004, Hedges g = 0.88, 95% CI = 0.27, 1.49). The effect for CON versus EC was large (P < .001, Hedges g = 1.57, 95% CI = 0.90, 2.23) and for CON versus TS was very large (P < .001, Hedges g = 2.31, 95% CI = 1.55, 3.06). We observed an uncertain effect that was not different for BCU versus EC (P = .008, Hedges g = 0.44, 95% CI = −0.15, 1.03).
Figure 2.
Absolute cooling rate of the cooling modalities. Each circle represents an individual's cooling rate. The lines represent the mean cooling rate of each cooling modality. a EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria. b Life Recovery Systems HD, LLC, Kinnelon, NJ. c Moderate effect. d Large effect. e Indicates difference between trials (P < .0083). f Uncertain effect. g Very large effect.
Individual Cooling Rates
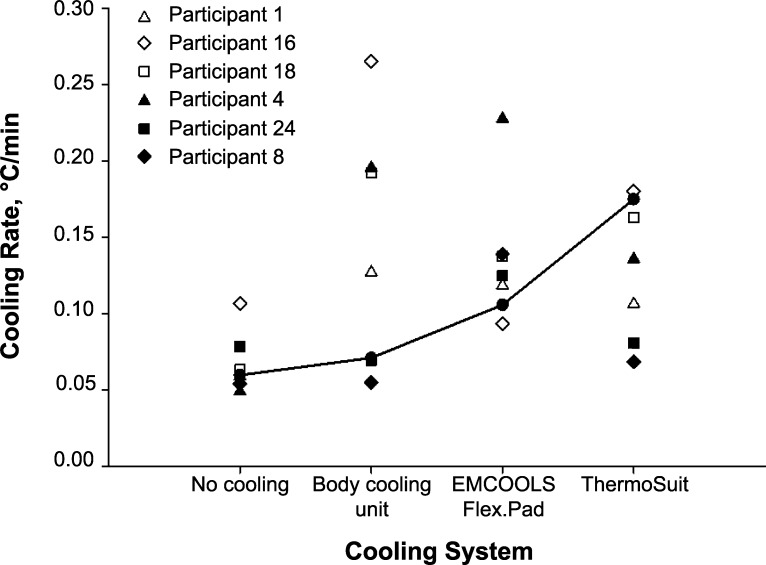
The individual cooling rates of 6 participants whose cooling rates did not follow the general trend of CON < BCU < EC < TS are summarized in Figure 3. Participants 1, 16, and 18 had higher cooling rates with the BCU than with the other cooling modalities. They had a generally higher AD/LBM than average (participant 1 = 354 cm2/kg, participant 16 = 354 cm2/kg, participant 18 = 360 cm2/kg versus the mean of the other participants = 347 cm2/kg; t20 = 2.71, P = .01).
Figure 3.
Individual cooling rates of 6 participants compared with the general trend. Six participants did not follow the general trend of no cooling < body-cooling unit < EMCOOLS Flex.Pad (EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria) < ThermoSuit (Life Recovery Systems HD, LLC, Kinnelon, NJ) in their rates of cooling. The individual cooling-rate profiles are indicated with triangles, diamonds, and squares. The solid line represents the general trend (mean of 16 participants excluding the 6 outliers).
Participants 4, 8, and 24 (Figure 3) did not seem to respond to the TS trial, with poorer cooling rates during the TS than the EC trial. Participants 4 and 8 had among the highest body mass (participant 4 = 80.7 kg, participant 8 = 89.0 kg; mean of the other participants = 69.7 kg; t19 = 2.38, P = .03) and body mass index (participant 4 = 25.5 kg/m2, participant 8 = 30.0 kg/m2; mean of the other participants = 22.5 kg/m2; t19 = 2.93, P = .01). The AD/LBM for participants 4, 8, and 24 was 346, 347, and 355 cm2/kg, respectively, and the mean of the other participants was 348 cm2/kg (t20 = 0.23, P = .82).
Stepwise Cooling Rate
The cooling rate for every 0.5°C decrease in Tc is presented in Table 2.
Table 2.
Stepwise Cooling Rate at 0.50°C Intervals
| Temperature Interval, °C |
Cooling System, °C/min (Mean ± SD) |
|||
| No Cooling |
Body-Cooling Unit |
EMCOOLS Flex.Pada |
ThermoSuitb |
|
| 39.50–39.00 | 0.08 ± 0.05 | 0.14 ± 0.11c | 0.11 ± 0.04c | 0.11 ± 0.03c |
| 39.00–38.50 | 0.08 ± 0.03 | 0.15 ± 0.08c | 0.16 ± 0.08c | 0.23 ± 0.10c,e |
| 38.50–38.00 | 0.05 ± 0.02 | 0.07 ± 0.07 | 0.12 ± 0.07c,d | 0.24 ± 0.12c–e |
EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria.
Life Recovery Systems HD, LLC, Kinnelon, NJ.
Indicates different from no cooling (P < .0083).
Indicates different from body cooling unit (P < .0083).
Indicates different from EMCOOLS Flex.Pad (P < .0083).
Heart Rate
Heart rate at the beginning of the cooling phase was similar among trials (CON = 116 ± 12 beats/min, BCU = 119 ± 12 beats/min, EC = 118 ± 12 beats/min, TS = 125 ± 13 beats/min; F3,84 = 2.30, P = .08). We observed no differences in heart rate among trials at the end of the cooling phase (CON = 89 ± 9 beats/min, BCU = 85 ± 10 beats/min, EC = 87 ± 9 beats/min, TS = 93 ± 13 beats/min; F3,84 = 2.39, P = .07).
Rating of Thermal Sensation
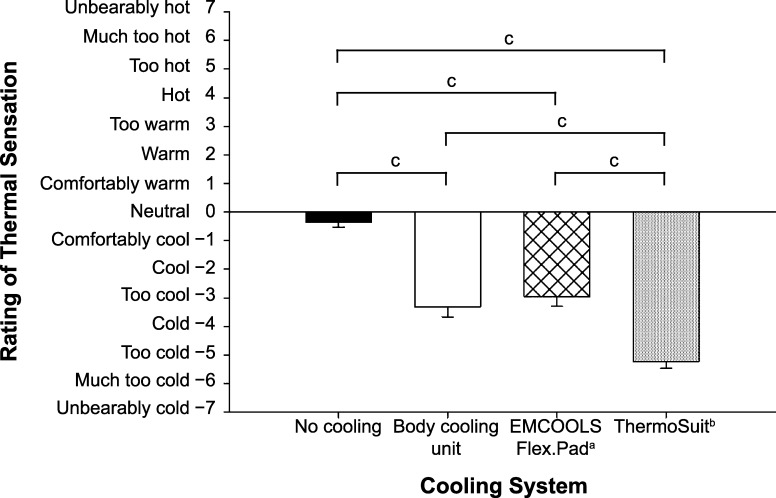
Subjective ratings of thermal sensation were different among all trial combinations at the end of the cooling phase (P < .0083; Figure 4) except between the BCU and EC trials (Z = 0.95, P = .34). On average, participants reported being “too cold” during the cooling phase of the TS trial. This observation is aligned with the results from the stepwise cooling rate, as the TS was still cooling at a relatively high rate between 38.00°C and 38.50°C.
Figure 4.
Rating of thermal sensation at the end of the cooling phase. a EMCOOLS Medical Cooling Systems GmbH, Vienna, Austria. b Life Recovery Systems HD, LLC, Kinnelon, NJ. c Indicates difference between trials (P < .0083).
DISCUSSION
The purpose of our study was to evaluate the efficacy of various commercial, off-the-shelf cooling systems after exercise-induced hyperthermia. In accordance with our hypothesis, the TS offered the highest cooling rate (0.16°C/min) after exercise-induced hyperthermia, followed by EC (0.12°C/min), BCU (0.09°C/min), and CON (0.06°C/min). Effect-size calculations showed that the TS was more effective than the EC (moderate effect), BCU (moderate effect), and CON (very large effect). The EC was more effective than the CON (large effect). The effect-size calculation showed the EC and BCU were not clinically different. We observed that BCU was more effective than CON (moderate effect). We also found interindividual variations in the responses to each cooling modality. Three participants had poorer cooling rates with the TS than with the EC, and another 3 participants had higher cooling rates with the BCU than with other cooling modalities.
Environmental temperature and relative humidity are important factors in the effectiveness of a CON condition.8 The dry-bulb temperature during the CON trial was 26.0°C, indicating a generally large gradient between the core and environmental temperatures, thereby promoting greater cooling. Wyndham et al29 reported that passive cooling, which was similar to our CON trial, performed at a dry-bulb temperature of 21°C led to a cooling rate of 0.06°C/min, whereas passive cooling performed at a dry-bulb temperature of 36°C led to a cooling rate of 0.02°C/min. Given that sweat evaporating off the skin's surface is a key contributor to heat dissipation,30 lower wet-bulb temperatures (ie, low water-vapor pressure) could lead to more effective cooling rates during passive cooling. A wider vapor-pressure gradient allows for more effective heat dissipation. Passive cooling in wet-bulb temperatures of 22.5°C in our study led to a cooling rate of 0.06°C/min, whereas Wyndham et al29 observed that passive cooling in wet-bulb temperatures of 31°C led to a cooling rate of 0.04°C/min. Regardless of these observations and with the goal of expeditious cooling in mind, efforts should be taken to quickly reduce the Tc of patients with suspected heat stroke, and applying any of the cooling modalities we evaluated is highly recommended. Based on the recommendations of McDermott et al,8 using cooling modalities that can provide cooling rates greater than 0.155°C/min, such as ice- and cold-water immersion, should be the priority. In the absence of these methods, modalities with cooling rates greater than 0.078°C/min should be used.8
The mean cooling rate provided by the BCU (0.09°C/min ± 0.06°C/min) in our study is consistent with that from an unpublished study conducted by our research group (0.10°C/min ± 0.06°C/min; Lim et al, written communication, 2002). However, these rates are lower than the average cooling rates reported by Weiner and Khogali11 (0.31°C/min) and for the Israel Defense Forces14 (0.14°C/min ± 0.11°C/min). The latter cooling rates14 were from a 7-year average of 52 patients with EHS, with their starting Tc exceeding 40°C. In contrast, we induced hyperthermia at only 39.50°C. Therefore, the cooling rates must be compared with the different levels of thermal strain in mind. The cooling modality that was closest to using a misting fan sprayed room-temperature water and compressed air from separate plastic hoses at low pressure, providing a cooling rate of 0.07°C/min.29 In addition, despite the BCU having the lowest cooling rate among the various modalities in our study, it fell within the acceptable range of greater than 0.078°C/min, as defined by McDermott et al.8 Effect-size calculations also indicated a moderate effect of the BCU over CON.
In our study, the individuals who had the highest cooling rates (Figure 3) with the BCU had a higher AD/LBM than average. This implies that, apart from its role in cold-water immersion,31 AD/LBM may also contribute to the effectiveness of a cooling modality via combined conductive and convective cooling. This observation, however, is not conclusive, and more work should be done in this area. Participant 16, whose cooling rates deviated the most from the general trend (CON = 0.11°C/min, BCU = 0.27°C/min, EC = 0.09°C/min, TS = 0.18°C/min), was the only white participant in our study. Farnell et al32 found that whites had a higher rectal temperature than African Americans during recovery from acute cold exposure, suggesting that individuals of different ethnicities may exhibit different Tc responses to various cooling modalities. Inherent factors, such as ethnic cardiovascular differences, may lead to such differences in thermoregulatory responses.33
The EC provided a mean cooling rate of 0.12°C/min, approximately 4 times higher than the 0.03°C/min rate reported with ice packs.12 Based on a Web-based survey administered to athletic trainers, Mazerolle et al34 found that ice bags and towels were the most commonly used cooling methods. The EC presents an effective alternative for using ice packs in the field because the cooling pads require only precooling for a minimum of 24 hours and are easy to apply to the skin.
Using a TS is similar to the method of whole-body ice-water immersion commonly reported in the literature. Cold- and ice-water immersion have been advocated as the criterion standard for treating EHS.4,6 The mean cooling rate (0.16°C/min) in our study was lower than the rates reported for ice-water immersion at 2°C (0.35°C/min13 and 0.20°C/min9). A possible reason for these differences is that the initial rectal temperatures reported in these studies were 40°C13 (controlled laboratory study) and 41.2°C9 (cohort study), respectively. These temperatures were much higher than the initial Tc of 39.50°C in our study. The environmental temperatures that we recorded during the cooling phase were similar to those reported by Armstrong et al9 and Proulx et al.13
Cold-water immersion has been challenged due to speculation that shivering and vasoconstriction may lead to an increase in Tc.12,29 However, participants have not displayed these negative consequences in laboratory trials,13,35 and cold-water immersion has been shown to be superior to all other cooling modalities for treating EHS.6,8,9 Whereas heart rate was similar among trials at the end of cooling, we observed a generally higher heart rate with the TS. Heart rate can be used to indicate shivering during cooling,13 and indeed, all participants were observed to shiver during the TS trials. One participant also experienced cold shock (gasping, hyperventilation, disorientation) and was uncomfortable during the TS trial, but his data were not included in the analysis, as he did not complete all 4 experimental trials.
Participants 4, 8, and 24 (Figure 3) had poorer cooling rates during the TS than during the EC trial. White et al36 found a correlation between total body mass and the cooling rate of rectal temperature during temperate-water immersion (31°C). Similarly, participants 4 and 8 had the highest body mass and body mass index among the 22 participants, but participant 24 had a body mass and body mass index that were similar to those of the rest of the participants. This suggests that anthropometry may affect cooling via water immersion. In addition, Friesen et al31 reported that during cold-water immersion, individuals with a low AD/LBM took longer to cool than those with a high AD/LBM. This distinction, however, was not evident in our study.
The decline in Tc over time due to external cooling modalities may not be constant, as the rate of cooling may change at different levels of heat strain.13,31,35 The general trend observed in the stepwise cooling rate over the three 0.50°C intervals is consistent with the physiology of heat dissipation and with results reported in the literature.13,31 The cooling rate in the first interval (39.50°C–39.00°C) was influenced by the initial cooling when the cooling modalities were applied and was typically lower than that in the second interval (39.00°C–38.50°C). This may be a result of acute peripheral vasoconstriction in the initial moments after cooling was applied.6,13,35 The cooling modalities typically reached their peak effectiveness during the second interval. The cooling rate during the third interval (38.50°C–38.00°C) generally decreased as Tc approached homeostasis. In general, the stepwise cooling-rate trends demonstrated that cooling between 39.50°C and 38.50°C is critically important for effective cooling6,8 and that cooling Tc should be performed as quickly as possible during EHS.
The cooling rate of the TS continued to increase in the third interval (38.50°C–38.00°C), which implied that the TS promoted rapid convective-immersion cooling of the surface, regardless of Tc. This rapid cooling rate would be useful when hypothermia of greater than 34°C must be induced in patients with cardiac arrest to improve neurologic outcomes.37,38 However, it may induce hypothermia in patients with EHS, particularly if Tc is not regularly monitored during cooling.13,39 This can be potentially dangerous, especially with an added Tc afterdrop, which is a sustained reduction in core temperature after cold-water immersion ends.40,41 Therefore, Tc must be closely monitored during treatment and recovery from EHS to avoid overcooling. Cooling should be terminated at a safe Tc, ideally between 38.0°C and 38.6°C.26,27
In a Web-based survey conducted on high school and collegiate athletic trainers, most participants reported using cooling modalities with cooling rates inferior to the cooling rate of cold-water immersion due to limited resources and manpower, improper facilities, and safety concerns.34 In addition, cold-water immersion is cumbersome and impractical in some instances, such as in an ambulance, where water spilling from an open tub can be dangerous. A practical system for administering various cooling modalities in the laboratory or field is needed; the EC and TS are commercial, off-the-shelf systems that fulfill this purpose.
Our study had some limitations. All participants were healthy men who participated in physical activity at least 3 times each week and did not have any predisposing factors for EHS (ie, illness, medication use, sleep deprivation, sedentary lifestyle).42 However, the profile of patients with heat stroke is largely heterogeneous. Given that it is unethical to induce EHS in healthy individuals, the Tc of participants was induced to a maximum of 39.50°C through exercise. In actual EHS cases, Tc may exceed 40°C. Therefore, the cooling profiles and responses to cooling of our participants may differ from those of patients with suspected heat stroke. Yet the experimental design of our study still serves as an adequate and viable means of testing the efficacy of these cooling modalities. We also did not assess other considerations (eg, cost, manpower required to operate each cooling system) for selecting and implementing each cooling system.
CONCLUSIONS
Relative to CON, TS offered the greatest mean absolute cooling rate compared with the EC and BCU. The EC offered the next best cooling rate. The difference between the EC and BCU was not clinically significant. These findings provide objective evidence for selecting the most effective cooling system of those we evaluated for cooling individuals after exercise-induced hyperthermia. Nevertheless, factors other than cooling efficacy, such as cost and the manpower required to operate these cooling systems, need to be considered when selecting an appropriate cooling system.
ACKNOWLEDGMENTS
This study was funded by the Ministry of Defence, Republic of Singapore. We thank Dr Goh Jor Ming for his invaluable technical input in study design and data collection; Mr Morgan Lim, Mr Darren Lim, Ms Germaine Ng, Mr David Fun, and Ms Margaret Yap for their role in data collection; and Dr Lim Tian Zhi for his help with preparing this manuscript for publication. We also thank all participants for their commitment to this study.
REFERENCES
- 1. Dematte JE O'Mara K Buescher J, et al. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998; 129 3: 173– 181. [DOI] [PubMed] [Google Scholar]
- 2. National Centre for Catastrophic Injury Research. Catastrophic Sport Injury 31st Annual Report. https://nccsir.unc.edu/files/2015/02/NCCSIR-31st-Annual-All-Sport-Report-1982_2013.pdf. Accessed October 14, 2015. [Google Scholar]
- 3. Racinais S Alonso JM Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015; 25 suppl 1: 6– 19. [DOI] [PubMed] [Google Scholar]
- 4. American College of Sports Medicine, LE, Armstrong Casa DJ, et al. American College of Sports Medicine position stand: exertional heat illnesses during training and competition. Med Sci Sports Exerc. 2007; 39 3: 556– 572. [DOI] [PubMed] [Google Scholar]
- 5. Casa DJ Armstrong LE Kenny GP O'Connor FG Huggins RA. . Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012; 11 3: 115– 123. [DOI] [PubMed] [Google Scholar]
- 6. Casa DJ McDermott BP Lee EC Yeargin SW Armstrong LE Maresh CM. . Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007; 35 3: 141– 149. [DOI] [PubMed] [Google Scholar]
- 7. Hadad E Rav-Acha M Heled Y Epstein Y Moran DS. . Heat stroke: a review of cooling methods. Sports Med. 2004; 34 8: 501– 511. [DOI] [PubMed] [Google Scholar]
- 8. McDermott BP Casa DJ Ganio MS, et al. Acute whole-body cooling for exercise-induced hyperthermia: a systematic review. J Athl Train. 2009; 44 1: 84– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong LE Crago AE Adams R Roberts WO Maresh CM. . Whole-body cooling of hyperthermic runners: comparison of two field therapies. Am J Emerg Med. 1996; 14 4: 355– 358. [DOI] [PubMed] [Google Scholar]
- 10. Hee-Nee P Rupeng M Lee VJ Chua WC Seet B. . Treatment of exertional heat injuries with portable cooling unit in a mass endurance event. Am J Emerg Med. 2010; 28 2: 246– 248. [DOI] [PubMed] [Google Scholar]
- 11. Weiner JS Khogali M. . A physiological body-cooling unit for treatment of heat stroke. Lancet. 1980; 1 8167: 507– 509. [DOI] [PubMed] [Google Scholar]
- 12. Kielblock AJ Van Rensburg JP Franz RM. . Body cooling as a method for reducing hyperthermia. S Afr Med J. 1986; 69 6: 378– 380. [PubMed] [Google Scholar]
- 13. Proulx CI Ducharme MB Kenny GP. . Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol (1985). 2003; 94 4: 1317– 1323. [DOI] [PubMed] [Google Scholar]
- 14. Hadad E Moran DS Epstein Y. . Cooling heat stroke patients by available field measures. Intensive Care Med. 2004; 30 2: 338. [DOI] [PubMed] [Google Scholar]
- 15. Maughan RJ Otani H Watson P. . Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2012; 112 6: 2313– 2321. [DOI] [PubMed] [Google Scholar]
- 16. Holzer M. . Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010; 363 13: 1256– 1264. [DOI] [PubMed] [Google Scholar]
- 17. Uray T Malzer R, . Vienna Hypothermia After Cardiac Arrest (HACA) Study Group. Out-of-hospital surface cooling to induce mild hypothermia in human cardiac arrest: a feasibility trial. Resuscitation. 2008; 77 3: 331– 338. [DOI] [PubMed] [Google Scholar]
- 18. Testori C Sterz F Delle-Karth G, et al. Strategic target temperature management in myocardial infarction: a feasibility trial. Heart. 2013; 99 22: 1663– 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhaese HL Martens PR Müller NH Casier IM Mulier JP Heytens L. . The use of emergency medical cooling system pads in the treatment of malignant hyperthermia. Eur J Anaesthesiol. 2010; 27 1: 83– 85. [DOI] [PubMed] [Google Scholar]
- 20. Howes D Ohley W Dorian P, et al. Rapid induction of therapeutic hypothermia using convective-immersion surface cooling: safety, efficacy and outcomes. Resuscitation. 2010; 81 4: 388– 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong LE Pumerantz AC Fiala KA, et al. Human hydration indices: acute and longitudinal reference values. Int J Sport Nutr Exerc Metab. 2010; 20 2: 145– 153. [DOI] [PubMed] [Google Scholar]
- 22. Du Bois D Du Bois EF. . A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989; 5 5: 303– 311. [PubMed] [Google Scholar]
- 23. Hume R. . Prediction of lean body mass from height and weight. J Clin Pathol. 1966; 19 4: 389– 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borg GA. . Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14 5: 377– 381. [PubMed] [Google Scholar]
- 25. Makranz C Heled Y Moran DS. . Hypothermia following exertional heat stroke treatment. Eur J Appl Physiol. 2011; 111 9: 2359– 2362. [DOI] [PubMed] [Google Scholar]
- 26. Gagnon D Lemire BB Casa DJ Kenny GP. . Cold-water immersion and the treatment of hyperthermia: using 38.6°C as a safe rectal temperature cooling limit. J Athl Train. 2010; 45 5: 439– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Proulx CI Ducharme MB Kenny GP. . Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol. 2006; 96 4: 434– 445. [DOI] [PubMed] [Google Scholar]
- 28. Hopkins WG Marshall SW Batterham AM Hanin J. . Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009; 41 1: 3– 13. [DOI] [PubMed] [Google Scholar]
- 29. Wyndham CH Strydom NB Cooke HM, et al. Methods of cooling subjects with hyperpyrexia. J Appl Physiol. 1959; 14: 771– 776. [DOI] [PubMed] [Google Scholar]
- 30. Kenny GP Webb P Ducharme MB Reardon FD Jay O. . Calorimetric measurement of postexercise net heat loss and residual body heat storage. Med Sci Sports Exerc. 2008; 40 9: 1629– 1636. [DOI] [PubMed] [Google Scholar]
- 31. Friesen BJ Carter MR Poirier MP Kenny GP. . Water immersion in the treatment of exertional hyperthermia: physical determinants. Med Sci Sports Exerc. 2014; 46 9: 1727– 1735. [DOI] [PubMed] [Google Scholar]
- 32. Farnell GS Pierce KE Collinsworth TA, et al. The influence of ethnicity on thermoregulation after acute cold exposure. Wilderness Environ Med. 2008; 19 4: 238– 244. [DOI] [PubMed] [Google Scholar]
- 33. Barnes VA Treiber FA Musant L Turner JR Davis H Strong WB. . Ethnicity and socioeconomic status: impact on cardiovascular activity at rest and during stress in youth with a family history of hypertension. Ethn Dis. 2000; 10 1: 4– 16. [PubMed] [Google Scholar]
- 34. Mazerolle SM Scruggs IC Casa DJ, et al. Current knowledge, attitudes, and practices of certified athletic trainers regarding recognition and treatment of exertional heat stroke. J Athl Train. 2010; 45 2: 170– 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clements JM Casa DJ Knight J, et al. Ice-water immersion and cold-water immersion provide similar cooling rates in runners with exercise-induced hyperthermia. J Athl Train. 2002; 37 2: 146– 150. [PMC free article] [PubMed] [Google Scholar]
- 36. White MD Ross WD Mekjavić IB. . Relationship between physique and rectal temperature cooling rate. Undersea Biomed Res. 1992; 19 2: 121– 130. [PubMed] [Google Scholar]
- 37. Bernard SA Gray TW Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002; 346 8: 557– 563. [DOI] [PubMed] [Google Scholar]
- 38. Holzer M Bernard SA Hachimi-Idrissi S, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005; 33 2: 414– 418. [DOI] [PubMed] [Google Scholar]
- 39. Moran DS Heled Y Shani Y Epstein Y. . Hypothermia and local cold injuries in combat and non-combat situations: the Israeli experience. Aviat Space Environ Med. 2003; 74 3: 281– 284. [PubMed] [Google Scholar]
- 40. Mittleman KD Mekjavić IB. . Effect of occluded venous return on core temperature during cold water immersion. J Appl Physiol (1985). 1988; 65 6: 2709– 2713. [DOI] [PubMed] [Google Scholar]
- 41. Romet TT. . Mechanism of afterdrop after cold water immersion. J Appl Physiol (1985). 1988; 65 4: 1535– 1538. [DOI] [PubMed] [Google Scholar]
- 42. Casa DJ Armstrong LE Kenny GP O'Connor FG Huggins RA. . Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012; 11 3: 115– 123. [DOI] [PubMed] [Google Scholar]