Abstract
This study reports the distribution coefficient between phospholipid bilayer membranes and phosphate buffered saline (PBS) medium (DMW,PBS) for 19 cationic surfactants. The method used a sorbent dilution series with solid supported lipid membranes (SSLMs). The existing SSLM protocol, applying a 96 well plate setup, was adapted to use 1.5 mL glass autosampler vials instead, which facilitated sampling and circumvented several confounding loss processes for some of the cationic surfactants. About 1% of the phospholipids were found to be detached from the SSLM beads, resulting in nonlinear sorption isotherms for compounds with log DMW values above 4. Renewal of the medium resulted in linear sorption isotherms. DMW values determined at pH 5.4 demonstrated that cationic surfactant species account for the observed DMW,PBS. Log DMW,PBS values above 5.5 are only experimentally feasible with lower LC-MS/MS detection limits and/or concentrated extracts of the aqueous samples. Based on the number of carbon atoms, dialkylamines showed a considerably lower sorption affinity than linear alkylamine analogues. These SSLM results closely overlapped with measurements on a chromatographic tool based on immobilized artificial membranes (IAM-HPLC) and with quantum-chemistry based calculations with COSMOmic. The SSLM data suggest that IAM-HPLC underestimates the DMW of ionized primary and secondary alkylamines by 0.8 and 0.5 log units, respectively.
Introduction
Phospholipid membranes separate (sub)cellular compartments from surrounding conditions and play an important role in the uptake, distribution, and toxicity of xenobiotics in multicellular organisms. Traditionally, the octanol–water partitioning coefficient (KOW) is used as a predictor for the uptake, distribution, and accumulation of organic chemicals in various organisms and their tissues. However, KOW does not adequately represent the partitioning of ionogenic organic chemicals (IOCs) between water and phospholipid membranes, because the ionic solute’s interactions with octanol do not include ionic bonds that occur with the anionic phosphate groups and cationic choline groups in phosphatidylcholine phospholipids.1−3 Cell membranes are expected to be the dominant sorption site for organic cations in tissue.4,5 Just like sorption coefficients to individual soil components are much more relevant for IOCs than KOW,6−10 the membrane–water distribution coefficient (DMW) is a logical alternative for KOW as the main model parameter to predict, for example, the tissue distribution and critical membrane burden in organisms.4,11 Many IOCs are highly relevant from an ecotoxicological perspective because of designed bioactive properties and/or continuous input via wastewater streams.12,13 Cationic surfactants are hydrophobic IOCs with a relatively high potential to disrupt cell membranes.14 Cationic surfactants are commonly used down-the-drain ingredients in personal care products, because of antistatic properties (hair conditioner, fabric softener). A variety of quaternary ammonium salt cationic surfactants are specifically used as biocide or disinfectants.15,16 Several cationic surfactants are regularly detected in various aquatic environments, particularly sediments.16−20 Quaternary dialkylamines are highly adsorptive and therefore less accessible for biodegradation processes under certain conditions, and the longest chain structures such as DODMAC have subsequently been prohibited in several countries for certain uses.21 This study aims to measure and model the DMW for cationic surfactants with different alkyl chain lengths and head groups, in order to improve environmental and toxicological hazard/risk assessment for this class of IOCs. Since most common protocols to determine KOW are considered to be impractible for surfactants, the assessment of DMW would provide a representative alternative hydrophobicity parameter.
Several experimental methods exist to determine the DMW of IOCs at physiological pH, such as liposome dispersions, specialized HPLC columns with phospholipid coatings, and solid supported phospholipid membranes (SSLM). Methods employing dispersions of freshly prepared liposomes are most realistic and accurate, but equilibrium dialysis requires considerable effort and long equilibration times.2,22 Potentiometric titrations need substantial concentrations of chemical and phospholipids.23 Recently, immobilized artificial membrane HPLC columns (IAM-HPLC) have been used to determine (relative) measures of lipophilicity in a number of frameworks.24−27 Confounding pH-dependent surface charges in IAM-HPLC have recently been recorded in detail.28 These surface charges can considerably influence the retention capacity factors of IOCs on IAM-HPLC physiological pH.28−30 At least for cationic compounds, confounding surface charges can be avoided by testing at low pH and highly saline eluent medium, and therewith one can specifically determine the IAM phospholipid–water distribution coefficient for the ionic form (KIAM,ion).28 IAM-HPLC consists of an ordered monolayer of phospholipids covalently linked to a silica support,31 instead of a dispersed double layer of phospholipids. This might reduce its relevance as a surrogate for the lipid bilayer cellular membrane. Solid supported phospholipid membranes (SSLMs) are available with macroporous spherical supports (e.g., silica beads) which are readily separated from the aqueous phase by mild centrifugation.32
Recently, IAM-HPLC was used to determine intrinsic sorption affinities to the IAM phospholipid monolayer KIAM,intr for 80 different hydrocarbon-based monoprotic cations (CxHyN+).24 Remarkably, these KIAM,ion values did not differ between analogue structures of primary, secondary, and tertiary alkylamines with the same alkyl chain length, and were marginally lower for quaternary ammonium chloride (QAC) analogues (∼0.2 log units). Quantum-chemistry based molecular calculations with a model DMPC bilayer (using the COSMOmic module within COSMOtherm software) of KDMPC-W values for the ionic species closely aligned the full set of KIAM,ion values but predicted a stepwise decrease in KDMPC-W with each methylation of the charged N, with primary amines at a log unit higher affinity than analogous QACs. Droge et al.24 stated that only measurements on phospholipid bilayers would clarify if either the effect of N-methylation is overpredicted by molecular simulations, or underpredicted by the IAM monolayer.
The main goal of the present study was to measure partitioning of several series of cationic surfactants with the molecular formula CxHyN+ to phospholipid bilayers using a commercially available SSLM assay, for comparison with IAM-HPLC results and COSMOmic simulations. We thereby focused on the influence of the alkyl chain length and different types of charged head groups but also on the difference between linear single chain alkylamines and dialkylamines. The applied SSLM assay (trademark TRANSIL) is sold as a standardized sorbent dilution series assay in a 96 well plate format33 but was improved to facilitate measurements with hydrophobic organic cations.
Materials and Methods
Chemicals, Sorbent, and Solutions
Nineteen amine based cationic surfactants compounds were selected. Their molecular structures, physicochemical properties, purities, and suppliers are listed in the Supporting Information (SI, Table S1). Two secondary amines contained two linear alkyl chains of equal length (dihexylamine “S2–C6”, dioctylamine “S2–C8”). Other moieties besides linear alkyl chains include benzyl in three benzalkonium chloride compounds (BAC), and dodecylpyridinium (C12-PYR) has the permanently charged nitrogen as part of an aromatic ring. All stock solutions were prepared as 100 ± 10 mM solutions in methanol, further diluted with methanol as necessary. All solutions in methanol were stored at −20 °C until use.
TRANSILXL Intestinal Absorption kits and TRANSILXL Intestinal Absorption kits for low affinity compounds were purchased from Sovicell GmbH (Leipzig, Germany). These kits consist of a 96 well plate made up of 12 strips with 8 wells each, individual strips containing two reference wells with phosphate buffered saline (PBS), and six wells with decreasing amounts (serial dilution factor of 1.8) of phosphatidylcholine coated macroporous silica beads (“beads”) suspended in phosphate buffered solution (PBS). The pore diameter of the silica beads has been specified as 4000 Å in the literature.47 A 2012 paper by Hou et al. provides SEM images that give more insight into the three-dimensional structure of the beads.48 PBS (Lonza BioWhittaker, Walkersville, USA; pH 7.4 ± 0.05, without Ca2+ and Mg2+) was used as the test medium for all experiments, unless noted otherwise. To assess the contribution to the observed DMW of the small neutral fraction present at pH 7.4 for the ionizable amines (<1%), the DMW was also determined at pH 5.2 (<0.01% neutral) for several alkylamines. A 10 mM acetate buffer was used with analytical grade acetic acid (2.0 mM) and sodium acetate (8.0 mM), dissolved in Milli-Q pure water (>18.2 MΩ·cm–1, Millipore, Amsterdam, The Netherlands), and addition of 140 mM NaCl. Additions of all liquids were checked gravimetrically to record actual volumes.
Adapting the SSLM Test Protocol for Cationic Surfactants
Cationic surfactants are notoriously difficult to work with due to relatively high adsorptive properties and accumulation at the water–air interface. Concentration dependent sorption to polystyrene well plate material was expected for several of the tested surfactants, as well as sorption to pipet tips when transferring supernatant to autosampler vials.34 To partially avoid these adsorptive challenges, the SSLM beads were transferred quantitatively to 2 mL glass autosampler vials. After equilibration of the chemicals with the beads on a roller bank, the vials were centrifuged and the supernatant in the autosampler vials could be directly injected by the stainless steel needle of the autosampler. This reduces pipetting steps and allows for testing in a larger aqueous volume, of which the composition can be customized. Whereas the 96 well plate assay applies two PBS reference wells (without SSLM) serving as 100% references of dissolved concentrations, it was expected that for several cationic surfactants substantial binding to the walls of the test vials would compromise a mass balance approach. We therefore applied quadruplicate methanol solutions as 100% reference (as calibration standards) and assume that losses due to sorption to the wall of the test vials in the vials that contain the lipids does not affect the estimated membrane sorption coefficients. The validity of this assumption has been validated.
A Rainin Pipet-Lite XLS electronic multichannel pipet with adjustable spacer (Mettler Toledo BV, Tiel, The Netherlands) was used to transfer the contents of six wells from one strip with decreasing amounts of phospholipid coated beads to 2 mL autosampler vials. Wells were flushed six times with 50 μL of PBS, to transfer all beads to the respective autosampler vials. Initially, autosampler vials were then filled with additional 400 μL PBS, placed on a Stuart SRT9 roller mixer (Boom BV, Meppel, The Netherlands) for 15 min at 33 rpm, centrifuged at 750 g (20 °C) for 10 min. To these test vials, as well as to the four 500 μL methanol references and four additional 500 μL PBS controls (to verify the extent of glass binding), 20 μL of spike solution was added. Overall, our tests were performed in the 1–1000 nM concentration range. We tried to cover isotherms with concentrations spanning at least a factor of 10, ideally a factor of 100. After addition of the spike, vials were transferred to a roller mixer for 15 min, centrifuged at 750 g (20 °C) for 10 min, and stored at 4 °C until LC-MS/MS analysis.
Pilot experiments with longer chain surfactants showed distinct nonlinear sorption isotherms, while the shorter chain surfactants showed linear isotherms. Several tests were performed to evaluate effects of spiking with solvent and bead density. Since the phospholipid bilayer is not covalently bound to the beads, we considered that minute—but significant—fractions of phospholipids might leak from the beads into the test medium during storage and handling (e.g., thawing) and form small suspended liposomes. Especially for compounds with high DMW, these liposomes could impact the DMW measurements by artificially increasing measured concentrations in the aqueous phase being sampled. After transfer of the beads to the autosampler vials, we therefore added fresh PBS up to 1.8 mL, centrifuged, and carefully pipetted off 1.7 mL of supernatant to remove the majority of the detached phospholipids and added 400 μL of PBS and 20 μL spike solution. Equilibration times of 5, 30, 60, and 240 min were compared with N-methyldodecylamine (“S12”). A test with dodecylpyridinium (“PYR12”) was performed to assess the tendency of phospholipids to leak from centrifuged beads while in the autosampler at room temperature, by injecting the same test series of six vials with washed beads 3, 12, and 21 h after centrifugation.
LC-MS/MS Quantification and SSLM Data Analysis
All samples were analyzed using a PerkinElmer (Norwalk, USA) HPLC system with autosampler, coupled to an MDS Sciex API3000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, USA). Retention of test compounds from the saline test medium was achieved on a Kinetex 5 μm XB-C18 column (50 × 2.1 mm; 100 Å) with a C18 guard column. The mobile phase consisted of Milli-Q (pump A) and methanol (pump B), both containing 0.1% formic acid by volume. A solvent switch was employed to flush PBS salts into waste, at 10% B for 6 min, before eluting the surfactants from the column with 90% B. The autosampler needle depth was adjusted to prevent accidental injection of beads. External calibration standards in methanol had concentrations ranging from ∼2 nM up to ∼35 μM. Detailed LC and MS/MS parameter settings for each compound can be found in Table S2.
The total spiked amount (Atotal) of surfactant in the autosampler will distribute between the aqueous phase (Awater), the phospholipid coating on the beads (Alipid), and the glass/cap surfaces (Aglass). Atotal is obtained from the average concentrations in the methanol controls (CMeOH), and DMW can then be calculated for each sample:
 |
1 |
Vlipid is the volume of phospholipids on the beads, as provided by the supplier. Because Aglass is in equilibrium with Awater, then Aglass can be considered negligible for the calculation of both Alipid and DMW if Alipid is >90% of Atotal and if Aglass < Awater. Aglass was determined using the PBS reference samples, which demonstrated the level of equilibrium between glass sorbed fractions and dissolved fractions (at the spiked concentration). Samples for decylbenzyldimethylammonium (“BAC10”) were emptied after analysis of Cwater and flushed once with Milli-Q, and glass walls were extracted with 90% B/10% A eluent mixture. Concentration independent log DMW values were obtained by fitting a linear curve on a double logarithmic plot with a forced slope of 1.
IAM-HPLC Measurements of the Phospholipid Monolayer–Water Distribution Coefficient KIAM,intr for Cationic Surfactants
The IAM-HPLC procedure described for strongly sorbing CxHyN+ cationic amines without UV-absorbing moieties was followed as described previously.24 Briefly, a solvent dilution series at pH 5 (10 mM acetate buffer) was tested in triplicate with LC-MS/MS detection. From a ∼1–5 mg/L surfactant sample in 10% acetonitrile, 5 μL was injected into an eluent mixtures of ≤30% acetonitrile, at flow rates of 1.0 mL/min. Multiplying the retention capacity factor kIAM with the column’s phase ratio of 18.9 gives the apparent distribution coefficient to the IAM phospholipid phase (KIAM,app) in each tested eluent mixture.35 Extrapolation of the KIAM,app values to fully aqueous medium buffered at pH 5 gives the intrinsic KIAM,intr. For each surfactant at least six measurements were made.
COSMOmic Calculations of the KDMPC-W for Cationic Surfactants
COSMOmic was run within COSMOtherm Version C30_1501, as described in the previous comparison between IAM-HPLC and COSMOmic.24 However, instead of using COSMOmic’s DMPC example micelle (1,2-dimyristoyl-sn-glycero-3-phosphocholine), we now used the same time averaged DMPC micelle file and TZVP-optimized structure of DMPC as used by Bittermann et al.36 Briefly, the input file to represent a hydrated phospholipid bilayer is obtained with a molecular dynamics (MD) run, using 128 DMPC molecules equilibrated with thousands of water molecules. COSMOmic divides the average atomic distribution in the MD simulated DMPC bilayer into 30 layers for one-half of the hydrated bilayer and uses the lowest free energy for each surfactant structure at 162 orientations at each layer to calculate the weighted DMPC–water partition coefficient (KDMPC-W). The three-dimensional input structures for each cationic surfactant were quantum-chemically optimized for calculations at TZVP level with COSMOmic, including different conformers (see Table S3 for information on conformers). COSMOconf version 3.0 was used to create up to six of the most relevant conformers for all charged surfactants.
Results and Discussion
Measurements of DMW with adapted SSLM Assays
Measured concentrations of the quadruplicate methanol control samples differed by less than 3.9% for all compounds tested. For C8-alkylamines and C10-alkylamines, the PBS controls showed 0–30% lower concentrations than the methanol controls, with exception of the larger C10-benzalkonium (“C10-BAC” 39%). For C12 surfactants, losses to autosampler surfaces were between 20 and 60% in PBS. If PBS references would have been used for C10-BAC as if they represented 100% of the available compound DMW would have been 0.2 log units lower than with methanol control samples. Using methanol controls, measured extracts of the glass walls in vials with beads showed that the residual impact of glass binding on DMW calculations was insignificant (0.011 log units).
Concentrations of test compound were aimed at keeping the phospholipid/sorbed compound molar ratio above 60 to prevent possible electrostatic effects due to the accumulated charge in the membranes.37,38 As shown in the full matrix of the final sorption isotherms for all tested compounds in the SI Figure S8, for nearly all of the selected surfactants, we have tested up to this maximum sorbed concentration in the membranes of ∼40 mmol/kg to avoid electrostatic effects. Although the corresponding dissolved concentrations are orders of magnitude below the critical micelle concentrations (CMC, Table S1), the tested concentrations are most likely still well above highest environmental concentrations but may be in the range of adverse effect concentrations. Concentrations of phospholipid in the test vial should result in sorbed fractions of at least 30%, to minimize effects of analytical uncertainties of Cwater on the mass balance calculations. The adapted TRANSILXL Intestinal Absorption kits allows for measuring DMW values above 1000 with buffer volumes of ∼525 μL in the autosampler vials. The TRANSILXL Intestinal Absorption kits for low affinity compounds contain a factor of ∼20 higher levels of beads per well, thereby allowing for measurements of DMW ≥ 50. The results from these two kits show overlapping sorption isotherms and concentration independent DMW, as shown for dihexylamine (“S2-C6”) in Figure 1. The sorption isotherms for C8- and C10-alkylamines also showed concentration independent DMW values, and overlapping sorption data with and without PBS renewal (Figure S1).
Figure 1.
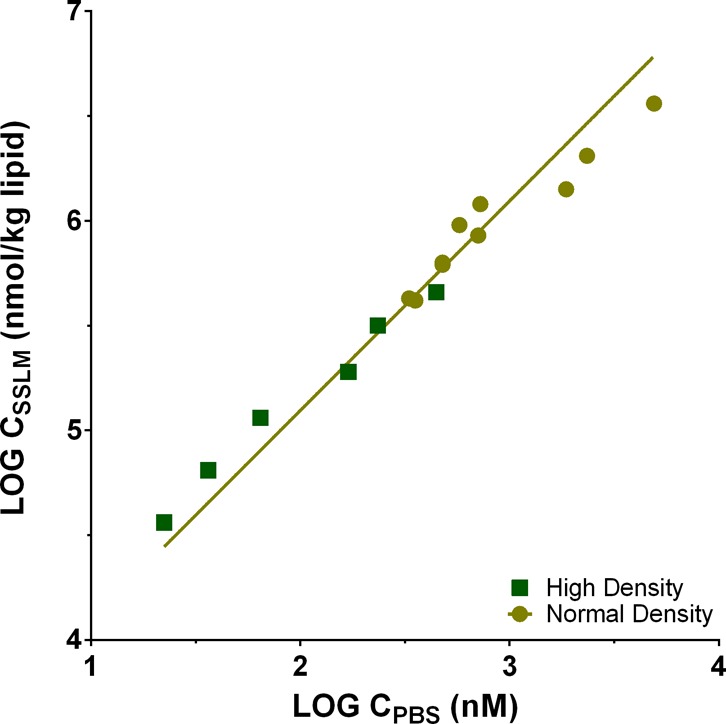
Sorption data for dihexylamine (S2-C6) obtained with two different sorbent dilution series and fit of a linear sorption isotherm (slope of 1), resulting in a log DMW,PBS of 3.15 (95% CI 3.10–3.20).
However, for the C12-chain surfactants series measured without flushing off leaked phospholipids showed distinctly nonlinear isotherms, with higher DMW values for the highest concentrations in a series (samples with lowest amounts of SSLM beads), and no correspondence between two series spiked at different initial concentrations (Figure S1). For each individual series, the slope of a linear isotherms on the double logarithmic plots was >1. This result would cause doubt on the resulting KMW from the SSLM assay, and an apparent concentration dependent sorption affinity over such a narrow tested concentration range would have considerable deviations of the KMW at considerably lower (e.g., most environmental) and possibly higher concentrations (e.g., as applied, or at adverse effect levels). We found no evidence of influence of solvent from spiking solution, as data for secondary N-methyldodecylamine (“S12”) from methanol stock solutions overlapped with nonlinear results from stocks dissolved in water (Figure S2). Instead of a sorbent dilution series, we then tested primary dodecylamine (“P12”) with two series with constant concentration of SSLM material, spiked at six different concentrations (accompanied by six sets of methanol controls). Now, each series indicated concentration independent DMW (slope of 1 on logarithmic plot), but again the series with higher amount of SSLM material showed a lower sorption affinity (Figure S3). Evidently, higher SSLM material resulted in a higher detached amount of phospholipids from the beads leaking into the aqueous phase. If the sorbed amount of cationic surfactants to this phase significantly increases the measured Cwater, this leads to underestimation of the DMW. Using a common extension of eq 1 for third phase systems,39 we modeled this effect by assuming a constantly leaked fraction (fleak) of the total amount of lipids coated on the beads, i.e., where the amount of lipids dispersed in the medium equals Vlipid·fleak:
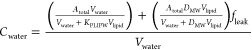 |
2 |
Experimental and modeled results are plotted for dodecylpyridinium (PYR12) in Figure 2, combining data for “unwashed” samples (still including medium from the well plate, thus with fleak still present) with “washed” samples (with fleak mostly removed). As shown in Figure 2, the curve representing 1% of the phospholipids leaking from bilayers into the test medium (fleak = 0.01) approximated the observed experimental values that show a nonlinear curve. A modeled curve for fleak = 0.02 overestimated the observations. More plots comparing simulations for unwashed and washed beads for compounds with log DMW of 4.0, 4.5, and 5.0 can be found in the Supporting Information (Figure S4). All experimental data and modeling output suggest a phospholipid leakage of approximately 1% from the bilayers on the beads into the medium, which–if not removed from the test medium–will influence the sorption isotherms for compounds with a DMW > log 4.0.
Figure 2.
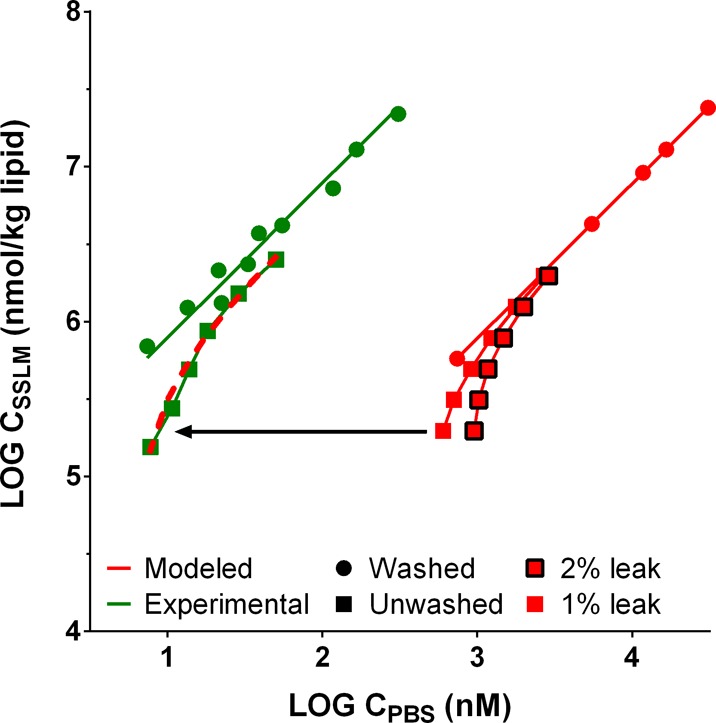
Experimental sorption data and simulated sorption data for dodecylpyridinium (PYR12) obtained with “unwashed” and “washed” sorbent dilution series, where unwashed still contains the medium from the well plates, whereas the medium was replenished with fresh PBS in “washed” samples. The simulated series show curves of a lipid leakage fraction of 0%, 1%, and 2%. The fitted linear curve for the experimental data indicates a log DMW,PBS of 4.89 (95% c.i. 4.84–4.95).
Varying the incubation time on the roller mixer (5–240 min) did not have a significant impact for fully dispersed SSLM solutions (Figure S5); 30 min was kept as standard. After centrifugation, the autosampler vials may stand for several hours in the autosampler before injection. The results of analysis after standing for 3, 12, or 21 h indicated no further leakage of phospholipids, as the measured surfactant concentrations and resulting sorption isotherm showed excellent overlap, whereas a significant fraction of leaked phospholipids would have increased the apparent concentrations in the medium (Figure S6). In test solution of pH 5.4 (washed), the DMW values of an ionizable tertiary N,N-dimethyldecylamine (“T10”) as well as of a permanently charged QAC N,N,N-trimethyldecylammonium (“Q10”) were not statistically different from the DMW in PBS (pH7.4), indicating that the <0.5% neutral fraction of T10 is not contributing to the measured DMW in PBS and that there were no confounding pH-dependent surface charge effects of the SSLM material. In contrast, pH-dependent surface charge effects in IAM-HPLC confound the DMW of organic cations in saline medium by ∼0.7 log units between pH 5 and 7.4.28 As a result, the measurements of DMW in PBS of cationic surfactants all relate to the partition coefficient of the ionic form (KMW,ion) and can thus be directly compared to COSMOmic simulations with the ionized structures and IAM-HPLC measurements made at pH 5.
Modeling the DMW by the Structure of Single Chain Cationic Surfactants
Isotherms were fitted with a fixed slope of 1 for all data points from tests with washed medium, where fsorbed ≥ 0.3 and the phospholipid/sorbate ratio was higher than 60 (Figure S8); an overview of all isotherm details can be found in Table S3. Standard errors were <0.05 log units, and 95% confidence intervals were <0.17 log units for all tested DMW values. The resulting DMW values (Table 1) were used to derive a simple quantitative structure activity relationship (QSAR) based on binary values for headgroup types and the length of the alkyl chain:
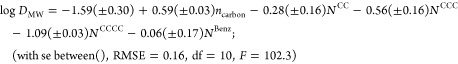 |
3 |
Where ncarbon denotes the number of carbon atoms in the alkyl chain, NBenz is a binary value indicating the presence (1) or absence (0) of a complete benzalkonium headgroup (three compounds included), and NCC (two compounds included), NCCC (three compounds included), and NCCCC (four compounds included) are binary values indicating the degree of N-alkylation. The NCCCC value for benzalkonium compounds should be 0 by default. C12-pyridinium was omitted as there was only one compound with this headgroup. Dialkylamines were omitted since they are expected to behave differently because the dual alkyl chain influences their orientation in the phospholipid membrane.24 Both types are discussed further. As shown in Figure 3, the regression model of eq 3 fits all input compounds within a factor 3 of the experimental values (root-mean-square error of 0.20). Using binary values on only 3 or 4 compounds for each parameter in the QSARs of eq 3 results in a fairly low level of redundancy in the data. However, our main aim of this excercise was to obtain insight in these simplified headgroup properties (N-methylation) and not to provide a functional well validated QSAR to predict KMW values for cationic surfactants. Considering that this data set of cationic surfactants is still structurally relatively nondiverse and relatively small, no further efforts were made to refine a QSAR based on other physicochemical or quantum-chemically derived parameters. Obviously, DMW increases with longer alkyl chains, with 0.59 ± 0.03 log units per CH2 unit, which can be used to extrapolate DMW predictions to longer chain surfactants. Comparing this fragment value with the tabulated DMW values (Table 1) it seems to be slightly higher than expected based on the experimental values obtained for the quaternary ammonium compounds, and slightly lower than expected based on the secondary and tertiary C12 compounds. Although we expect a constant CH2 unit contribution for all single linear alkyl chains, the contributions of CH2 unit in the chain near the charged amine may be slightly lower, as these may not all reside in the hydrophobic core, and this is not defined in eq 3.24 Effects of pH and possible neutral fraction for ionizable amines were ruled out based on additional tests at pH 5.4 (above and Figure S7). A consistent trend of decreasing DMW with increasing methylation of the N atom is observed for the three analogue series of C8-, C10-, and C12-amines. Primary amines have 0.28 ± 0.16 log unit higher DMW than secondary amines, which are 0.28 ± 0.16 log units higher than tertiary amines, which are 0.53 ± 16 log units higher than QACs (excluding benzalkonium compounds). Taking the average over all the C8-, C10-, and C12-amines, primary amines sorbed 1.06 log units stronger than the QAC analogues. Considering that the quaternary amine has three more CH2 units than the primary analogue, this is a remarkable feature of IOCs. The benzalkonium compounds have a DMW of 1.0 ± 0.17 log units higher than trimethylalkylammonium compounds, which reflects the effect of an additional benzyl moiety. With an experimental log DMW of 4.89, PYR12 positions in between BAC12 and Q12 compounds, corresponding to the molecular volume differences. For 16 out of 19 compounds, two or more conjoined series were tested, demonstrating both consistency of experimental results and steadiness over multiple orders of magnitude of the DMW estimates.
Table 1. Log DMW Values for All Compounds Tested with the SSLM Assay,a As Well As Uncorrected IAM-HPLC log KIAM,intr Measurements and COSMOmic Predicted log KDMPC-W Values (No Offset Correction).
| alkyl chain | method | primary amine (P) | secondary amines (S) | tertiary amines (T) | trimethyl ammonium (Q) | benzalkonium cations (BAC) | pyridinium cations (PYR) | secondary dialkyl-amines (S2) | quaternary dialkyl-ammonium (Q2) |
|---|---|---|---|---|---|---|---|---|---|
| C6 | SSLM | 2.12 | 3.15 | ||||||
| IAM | 1.32 | 2.42 | 2.88 | ||||||
| COSMO | 2.63 | 1.92 | 1.45 | 1.08 | 1.87 | 2.77 | |||
| C8 | SSLM | 3.10 | 2.76 | 2.35 | 2.18 | 3.11 | 4.65 | ||
| IAM | 2.30 | 2.33 | 2.35 | 2.18 | 3.30 | 5.04 | |||
| COSMO | 3.71 | 3.01 | 2.53 | 2.18 | 2.96 | 4.34 | |||
| C10 | SSLM | 4.30 | 3.98 | 3.65 | 3.34 | 4.01 | |||
| IAM | 3.55 | 3.59 | 3.59 | 3.44 | 4.47 | ||||
| COSMO | 4.83 | 4.14 | 3.70 | 3.30 | 4.05 | 6.26 | 7.53 | ||
| C12 | SSLM | 5.58 | 5.39 | 5.30 | 4.35 | 4.89 | |||
| IAM | 4.81 | 4.57 | |||||||
| COSMO | 6.01 | 5.28 | 4.76 | 4.47 | 5.14 | 4.77 | 8.40 | 9.79 | |
| C14 | SSLM | 5.46 | |||||||
| COSMO | 7.15 | 6.42 | 5.89 | 5.61 | 6.65 | 5.90 | 12.06 | ||
| C16 | COSMO | 8.31 | 7.56 | 7.07 | 6.72 | 7.90 | 7.03 | C16/C18 | |
| C18 | COSMO | 9.43 | 8.74 | 8.29 | 7.94 | 9.01 | 15.51 | ||
| C22 | COSMO | 10.1 |
Figure 3.
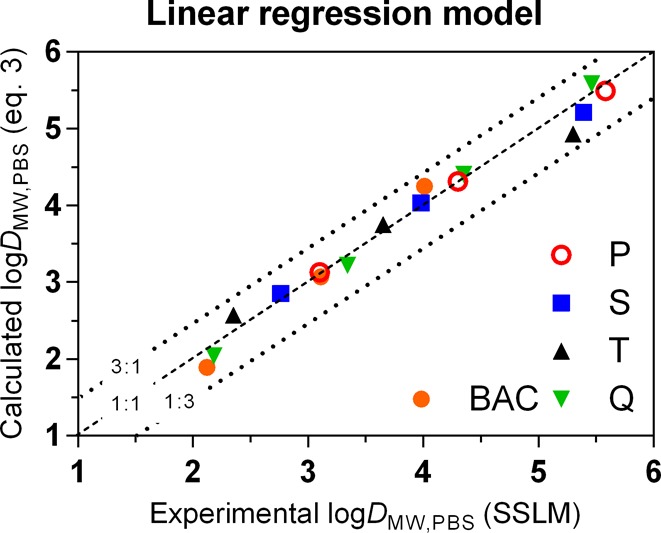
Observed and predicted log DMW,PBS values for single linear chain cationic surfactants using eq 3 (P = primary amines, S = secondary amines, T = tertiary amines, Q = trimethylalkylammonium compounds, BAC = benzalkonium chloride compounds).
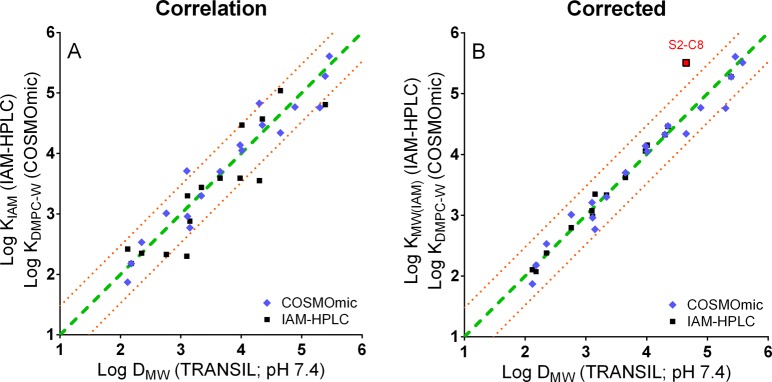
Correlation of SSLM Data with IAM-HPLC Measurements and COSMOmic Predictions
The available KIAM values obtained with IAM-HPLC (see detailed solvent series data in Figure S9, Table S3) and KDMPC-W values from COSMOmic (Table S3) are plotted against the experimental DMW results from the SSLM assay in Figure 4A. Overall, the alternative data sets and the SSLM data align reasonably well, with RMSE of 0.39 and 0.27 log units, for IAM-HPLC and COSMOmic, respectively. Instead of eq 3, now also dialkylamines and pyridinium compounds can be included. As discussed in Droge et al.,24 IAM-HPLC data and COSMOmic predictions differed in the contributions of the N-methylations to the sorption affinity. The SSLM DMW values confirm the ordering of primary > secondary > tertiary > quaternary amine analogues as predicted by COSMOmic and indicate that IAM-HPLC accounts insufficiently for effects of N-methylation. Considering that the SSLM data are obtained with relatively fluid phospholipid bilayers, and IAM-HPLC applies a covalently bound monolayer, we suggest applying corrective increments for the N-methyl headgroup contributions to KIAM values of IAM-HPLC, compared against the SSLM DMW values (δIAM-SSLM). Multiple linear regression results in δIAM-SSLM of 0.78 ± 0.07 (se) log units for primary alkylamines (i.e., KIAM values underestimate the KMW in bilayers), and 0.47 ± 0.09 log units for secondary amines. The δIAM-SSLM for tertiary amines is insignificant (−0.03 ± 0.10 log units), and very small for quaternary alkyltrimethylamines −0.11 ± 0.09. For COSMOmic δDMPC-SSLM amine type corrective increments were derived similarly. Interestingly, primary amines tended to be slightly overestimated by COSMOmic (0.17 ± 0.17 log units), while the other amine types were slightly underestimated (0.2–0.4 ± 0.16 log units). COSMOmic seems to be capable of predicting the effect of N-methylation in phospholipid bilayers. Contrary to suggested correction of COSMOmic values reported previously,24 the SSLM data support the notion that the IAM-HPLC monolayer is the most probable source of inconsistency, creating scatter between IAM-HPLC and COSMOtherm. Using these δIAM-SSLM and δDMPC-SSLM corrective increments, there is strong correspondence between SSLM DMW values, the corrected IAM-HPLC KMW(IAM) values, and corrected COSMOmic KDMPC-W values, as shown in Figure 4B. All values are well within a factor 2 of the 1:1 line and the RMSE improved from 0.39 to 0.21 (IAM-HPLC) and from 0.27 to 0.18 (COSMOmic).
Figure 4.
Experimental log DMW,PBS values with TRANSIL bilayers plotted against experimental log KIAM results from the IAM-HPLC monolayer and simulated log KDMPC-W values with DMPC bilayers using COSMOmic. In the graph on the right, IAM-HPLC values are corrected for 1° amines with +0.8 log units and 2° amines with +0.5 log units. COSMOmic values are corrected for 1° amines with −0.5 log units.
The three methods attribute similar effects of alkyl chain length, but also display a striking consistency in the difference between secondary dialkylamines and linear chain secondary alkylamine analogues. As discussed in Droge et al.,24 COSMOmic provides a mechanistic explanation for the relatively lower contribution of CH2 units to the DMW compared to single chain surfactants. Their design with two alkyl chains creates a steric effect, where the most favorable molecular position and orientation is mostly within the headgroup area of the phospholipid bilayer where—as a compromise—neither alkyl chain is aligned most favorably into the hydrophobic core region. The SSLM results confirm the difference in DMW between secondary linear amine N-methyldodecylamine S12 and secondary dihexylamine S2-C6 by 2.24 log units, as was observed by Droge et al.24 for IAM-HPLC (1.93 log units) and COSMOmic (2.43 log units). For all three methods the difference observed is much larger than expected with one extra CH2 fragment in S12. Between S2-C6 and dioctylamine (S2-C8), the SSLM DMW increased by 1.5 log units, while the regression model in eq 3 would predict a 2.36 log unit increase based on the addition of four CH2 units. COSMOmic also predicts a smaller value (1.73 log units) for the CH2 increment between S2-C6 and S2-C8, while IAM-HPLC showed a 2.16 log unit difference (Table S3).
DMW Values for Cationic Surfactants Compared to KOW Predictions
The most recent analysis of KMW values of neutral compounds,40 mostly obtained with liposomes, showed a strong correlation with KOW:
| 4 |
There is only a poor correlation (R2 = 0.49) between the SSLM measured log DMW at pH 7.4 and the log KOW of the neutral primary, secondary, and tertiary amines, as shown in SI Figure S10. The influence of the methyl units on the charged nitrogen are reversed for the two distribution coefficients. Each N-methyl unit increases the log KOW while it reduces the log DMW. Also, even though the sorption affinity of the protonated amines to the SSLM bilayer increases with linear alkyl chain length as the KOW predicts, the log DMW of the dialkylamines is orders of magnitude lower than the log KOW (for S2-C8 4.61 and 7.01, respectively). Typically for studies on the toxicokinetic properties of ionizable chemicals, a “log D approach” is followed, correcting the log KOW for speciation of the neutral amines at the tested pH 7.4 (99.9% ionic for primary and secondary amines, 99.7% for tertiary amines). To derive a log D, however, the affinity of the ionic species for octanol needs to be known or predicted. In the absence of measurements, often either a constant factor of ∼3 log units lower than log KOW is applied, or the affinity of the ionic species is ignored and the KOW is multiplied by the fraction of neutral species. As shown in Figure S10, both such log D approaches underestimate the sorption affinity to membranes by a up to a factor of 1000 and do not solve the poor correlation between log DMW and log KOW. Instead, log KOW could still be used to identify specific scaling factors to the difference in sorption affinity to a bilayer between neutral and ionic species, with the neutral affinity still based on eq 4. Table S3 lists the KMW values for the neutral primary, secondary and tertiary amine species calculated via eq 4 (log KOW predicted by ACD/Laboratories). Accordingly, the average difference between charged DMW,PBS and neutral KMW species (ΔMW) for primary amines is −0.05, so the affinity of charged primary amines is larger than the neutral species. For the three linear single chain N-methylalkylamines ΔMW is 0.44, for the linear N,N-dimethylalkylamines, the ΔMW is 1.25. These values closely correspond to the scaling factors suggested for the bioaccumulation model for ionogenic compounds (BIONIC) proposed by Armitage et al.,4 which were 0.3, 0.5, and 1.25 for primary, secondary and tertiary amines, respectively, based on data sets of measured KMW values for both ionic and neutral species. However, the ΔMW is 1.90 and 2.55 for the two dialkylamines S2-C6 and S2-C8, respectively, much higher than the 0.44 derived with the other secondary amines. The examples of the dialkylamines show that applying a single ΔMW scaling factor for all secondary amines to calculate the DMW,PBS from the KOW relationship in eq 4, can lead to erroneous values. Similarly, this exercise shows that KOW is not an adequate single descriptor to model the DMW values for ionized compounds. Measured KMW values with TRANSIL, KPLIPW values with IAM-HPLC, and even simulated KDMPC values with COSMOmic, provide much more accurate and more mechanistically sound estimates compared to KOW-based regressions.
Perspective on SSLM Assay Measurements and Associated DMW Estimates
This study showed that the adapted SSLM protocol, with SSLM beads transferred from a well plate to autosampler vials, facilitated the analysis, improved recovery in methanol reference vials, and gained experimental control over the aqueous phase. The medium renewal removed third phase liposome artifacts, and allowed for altered pH of the test medium. The problem of detached phospholipids in the original SSLM medium became significant for all compounds with a DMW higher than log 4, while using methanol controls instead of PBS controls seems mostly important for hydrophobic organic cations, and surfactants in general.41 The experimental determination of DMW becomes problematic above log 5.5, because with the required phospholipid/sorbate molar ratio >60, the aqueous phase concentrations obtained directly from the autosampler vials, are nearing LC-MS/MS detection limits. This means that to measure DMW values for cationic surfactants with alkyl chains longer than C12, and dialkylamines with alkyl chains longer than C8, either cumbersome solid phase extraction steps from larger test volumes are required—which may include uncontrolled adsorption losses—or sorption affinities need to be extrapolated with the model, or from series of smaller analogues. Alternatively, COSMOmic seems to provide a realistic and accurate predictive tool for cationic surfactants, which allows for extrapolations to longer chain cationic surfactants (Table 1) and slight alterations of the head groups. For example, the log KDMPC-W for didecyldimethylammonium chloride (DDAC), a commonly used biocide,42 and cetylpyridinium, a commonly used antiseptic, are 7.53 and 7.03 (Table 1), which are both experimentally not feasible to measure with currently applied IAM-HPLC and SSLM assays. The log KDMPC-W for behentrimonium, a trimethylalkylammonium compound with a chain length of C22 which is used in many hair cair products and which has been detected in marine sediments,43 is 10.3. The dialkylquat DODMAC (mixed chain length of C16/C18), banned for certain uses in the EU,21 has a predicted log KDMPC-W of 15.5. For these examples, the very high predicted sorption affinities to cell membrane should be considered in risk assessment models (e.g., for bioaccumulation4) alongside strong sorption affinities to environmental particles,6−9 which strongly reduces the bioavailability, and thus result in relatively low accumulation from the environment into tissues.34,44−46
Acknowledgments
This study was funded by Unilever, Safety & Environmental Assurance Center (SEAC), Colworth Science Park, Sharnbrook, United Kingdom. We thank Joop Hermens for valuable suggestions and comments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b05662.
Additional tables with information on chemicals, LC-MS/MS settings, and sorption isotherm details, as well as figures with detailed sorption data under various conditions (PDF)
Author Present Address
† S.T.J.D.: UvA-IBED, Postbus 94248, 1090 GE Amsterdam, The Netherlands.
The authors declare no competing financial interest.
Supplementary Material
References
- Taillardat-Bertschinger A.; Carrupt P. A.; Barbato F.; Testa B. Immobilized artificial membrane HPLC in drug research. J. Med. Chem. 2003, 46 (5), 655–65. 10.1021/jm020265j. [DOI] [PubMed] [Google Scholar]
- Pauletti G. M.; Wunderli-Allenspach H. Partition coefficients in vitro: artificial membranes as a standardized distribution model. Eur. J. Pharm. Sci. 1994, 1 (5), 273–282. 10.1016/0928-0987(94)90022-1. [DOI] [Google Scholar]
- Escher B. I.; Schwarzenbach R. P. Partitioning of substituted phenols in liposome-water, biomembrane-water, and octanol-water systems. Environ. Sci. Technol. 1996, 30, 260–270. 10.1021/es9503084. [DOI] [Google Scholar]
- Armitage J. M.; Arnot J. A.; Wania F.; Mackay D. Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environ. Toxicol. Chem. 2013, 32 (1), 115–28. 10.1002/etc.2020. [DOI] [PubMed] [Google Scholar]
- Henneberger L.; Goss K. U.; Endo S. Partitioning of organic ions to muscle protein: experimental data, modeling, and implications for in vivo distribution of organic ions. Environ. Sci. Technol. 2016, 50 (13), 7029–7036. 10.1021/acs.est.6b01417. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Goss K.-U. Effect of sodium and calcium cations on the ion-exchange affinity of organic cations for soil organic matter. Environ. Sci. Technol. 2012, 46 (11), 5894–5901. 10.1021/es204449r. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Goss K. U. Ion-exchange affinity of organic cations to natural organic matter: influence of amine type and nonionic interactions at two different pHs. Environ. Sci. Technol. 2013, 47, 798–806. 10.1021/es3033499. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Goss K. U. Sorption of organic cations to phyllosilicate clay minerals: cec-normalization, salt dependency, and the role of electrostatic and hydrophobic effects. Environ. Sci. Technol. 2013, 47 (24), 14224–14232. 10.1021/es403187w. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Goss K. U. Development and evaluation of a new sorption model for organic cations in soil: Contributions from organic matter and clay minerals. Environ. Sci. Technol. 2013, 47 (24), 14233–14241. 10.1021/es4031886. [DOI] [PubMed] [Google Scholar]
- Jolin W. C.; Sullivan J.; Vasudevan D.; MacKay A. A. Column Chromatography to Obtain Organic Cation Sorption Isotherms. Environ. Sci. Technol. 2016, 50 (15), 8196–8204. 10.1021/acs.est.6b01733. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Hermens J. L. Internal exposure: linking bioavailability to effects. Environ. Sci. Technol. 2004, 38 (23), 455A–462A. 10.1021/es0406740. [DOI] [PubMed] [Google Scholar]
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 2002, 131 (1–2), 5–17. 10.1016/S0378-4274(02)00041-3. [DOI] [PubMed] [Google Scholar]
- Ternes T. A. Occurrence of drugs in German sewage treatment plants and rivers1. Water Res. 1998, 32 (11), 3245–3260. 10.1016/S0043-1354(98)00099-2. [DOI] [Google Scholar]
- Xia W. J.; Onyuksel H. Mechanistic studies on surfactant-induced membrane permeability enhancement. Pharm. Res. 2000, 17 (5), 612–8. 10.1023/A:1007581202873. [DOI] [PubMed] [Google Scholar]
- Ying G. G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32 (3), 417–31. 10.1016/j.envint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Nunez O.; Moyano E.; Galceran M. T. Determination of quaternary ammonium biocides by liquid chromatography-mass spectrometry. J. Chromatogr. A 2004, 1058 (1–2), 89–95. 10.1016/S0021-9673(04)01442-6. [DOI] [PubMed] [Google Scholar]
- Ding W. H.; Liao Y. H. Determination of alkylbenzyldimethylammonium chlorides in river water and sewage effluent by solid phase extraction and gas chromatography mass spectrometry. Anal. Chem. 2001, 73 (1), 36–40. 10.1021/ac000655i. [DOI] [PubMed] [Google Scholar]
- Martinez-Carballo E.; Gonzalez-Barreiro C.; Sitka A.; Kreuzinger N.; Scharf S.; Gans O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part II. Application to sediment and sludge samples in Austria. Environ. Pollut. 2007, 146 (2), 543–547. 10.1016/j.envpol.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Li X.; Brownawell B. J. Analysis of quaternary ammonium compounds in estuarine sediments by lc-tof-ms: very high positive mass defects of alkylamine ions as powerful diagnostic tools for identification and structural elucidation. Anal. Chem. 2009, 81 (19), 7926–7935. 10.1021/ac900900y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I.; Furlong E. T. Identification of alkyl dimethylbenzylammonium surfactants in water samples by solid-phase extraction followed by ion trap LC/MS and LC/MS/MS. Environ. Sci. Technol. 2001, 35 (12), 2583–2588. 10.1021/es001742v. [DOI] [PubMed] [Google Scholar]
- Fernandez P.; Alder A. C.; Suter M. J. F.; Giger W. Determination of the quaternary ammonium surfactant ditallowdimethylammonium in digested sludges and marine sediments by supercritical fluid extraction and liquid chromatography with postcolumn ion-pair formation. Anal. Chem. 1996, 68 (5), 921–929. 10.1021/ac9505482. [DOI] [PubMed] [Google Scholar]
- Kramer S. D.; Braun A.; Jakits-Deiser C.; Wunderli-Allenspach H. Towards the predictability of drug-lipid membrane interactions: the pH-dependent affinity of propanolol to phosphatidylinositol containing liposomes. Pharm. Res. 1998, 15 (5), 739–44. 10.1023/A:1011923103938. [DOI] [PubMed] [Google Scholar]
- Avdeef A.; Box K. J.; Comer J. E.; Hibbert C.; Tam K. Y. pH-metric logP 10. Determination of liposomal membrane-water partition coefficients of ionizable drugs. Pharm. Res. 1998, 15 (2), 209–15. 10.1023/A:1011954332221. [DOI] [PubMed] [Google Scholar]
- Droge S. T.; Hermens J. L.; Rabone J.; Gutsell S.; Hodges G. Phospholipophilicity of CxHyN+ amines: chromatographic descriptors and molecular simulations for understanding partitioning into membranes. Environ. Sci. Process Impacts 2016, 18, 1011–1023. 10.1039/C6EM00118A. [DOI] [PubMed] [Google Scholar]
- Tsopelas F.; Vallianatou T.; Tsantili-Kakoulidou A. Advances in immobilized artificial membrane (IAM) chromatography for novel drug discovery. Expert Opin. Drug Discovery 2016, 11 (5), 473–488. 10.1517/17460441.2016.1160886. [DOI] [PubMed] [Google Scholar]
- Grumetto L.; Russo G.; Barbato F. Relationships between human intestinal absorption and polar interactions drug/phospholipids estimated by IAM–HPLC. Int. J. Pharm. 2015, 489 (1–2), 186–194. 10.1016/j.ijpharm.2015.04.062. [DOI] [PubMed] [Google Scholar]
- Ledbetter M. R.; Gutsell S.; Hodges G.; Madden J. C.; O’Connor S.; Cronin M. T. D. Database of published retention factors for immobilized artificial membrane HPLC and an assessment of the effect of experimental variability. Environ. Toxicol. Chem. 2011, 30 (12), 2701–2708. 10.1002/etc.677. [DOI] [PubMed] [Google Scholar]
- Droge S. T. Dealing with confounding pH-dependent surface charges in immobilized artificial membrane HPLC columns. Anal. Chem. 2016, 88 (1), 960–7. 10.1021/acs.analchem.5b03708. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Schwarzenbach R. P.; Westall J. C. Evaluation of liposome-water partitioning of organic acids and bases. 2. Comparison of experimental determination methods. Environ. Sci. Technol. 2000, 34 (18), 3962–3968. 10.1021/es0010711. [DOI] [Google Scholar]
- Ottiger C.; Wunderli-Allenspach H. Immobilized artificial membrane (IAM)-HPLC for partition studies of neutral and ionized acids and bases in comparison with the liposomal partition system. Pharm. Res. 1999, 16 (5), 643–50. 10.1023/A:1018808104653. [DOI] [PubMed] [Google Scholar]
- Ong S.; Liu H.; Pidgeon C. Immobilized-artificial-membrane chromatography: measurements of membrane partition coefficient and predicting drug membrane permeability. J. Chromatogr. A 1996, 728 (1), 113–128. 10.1016/0021-9673(95)00837-3. [DOI] [PubMed] [Google Scholar]
- Bayerl T. M.; Bloom M. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys. J. 1990, 58 (2), 357–62. 10.1016/S0006-3495(90)82382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl-Stahlhofen A.; Hartmann T.; Schottner M.; Rohring C.; Brodowsky H.; Schmitt J.; Keldenich J. Multilamellar liposomes and solid-supported lipid membranes (TRANSIL): screening of lipid-water partitioning toward a high-throughput scale. Pharm. Res. 2001, 18 (12), 1782–8. 10.1023/A:1013343117979. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Geurts M.; Sjollema S. B.; Kramer N. I.; Hermens J. L.; Droge S. T. Acute toxicity of the cationic surfactant C12-benzalkonium in different bioassays: how test design affects bioavailability and effect concentrations. Environ. Toxicol. Chem. 2014, 33 (3), 606–15. 10.1002/etc.2465. [DOI] [PubMed] [Google Scholar]
- Ong S.; Pidgeon D. Thermodynamics of solute partitioning into immobilized artificial membranes. Anal. Chem. 1995, 67 (13), 2119–2128. 10.1021/ac00109a034. [DOI] [PubMed] [Google Scholar]
- Bittermann K.; Spycher S.; Endo S.; Pohler L.; Huniar U.; Goss K. U.; Klamt A. Prediction of phospholipid-water partition coefficients of ionic organic chemicals using the mechanistic model COSMOmic. J. Phys. Chem. B 2014, 118 (51), 14833–42. 10.1021/jp509348a. [DOI] [PubMed] [Google Scholar]
- Austin R. P.; Barton P.; Davis A. M.; Fessey R. E.; Wenlock M. C. The thermodynamics of the partitioning of ionizing molecules between aqueous buffers and phospholipid membranes. Pharm. Res. 2005, 22 (10), 1649–1657. 10.1007/s11095-005-6336-7. [DOI] [PubMed] [Google Scholar]
- Seelig A.; Allegrini P. R.; Seelig J. Partitioning of local anesthetics into membranes: surface charge effects monitored by the phospholipid head-group. Biochim. Biophys. Acta, Biomembr. 1988, 939 (2), 267–76. 10.1016/0005-2736(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Schrap S. M.; Opperhuizen A. On the contradictions between experimental sorption data and the sorption partitioning model. Chemosphere 1992, 24 (9), 1259–1282. 10.1016/0045-6535(92)90052-S. [DOI] [Google Scholar]
- Endo S.; Escher B. I.; Goss K. U. Capacities of membrane lipids to accumulate neutral organic chemicals. Environ. Sci. Technol. 2011, 45 (14), 5912–21. 10.1021/es200855w. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Sinnige T. L.; Hermens J. L. M. Analysis of freely dissolved alcohol ethoxylate homologues in various seawater matrixes using solid-phase microextraction. Anal. Chem. 2007, 79 (7), 2885–2891. 10.1021/ac0620260. [DOI] [PubMed] [Google Scholar]
- Juergensen L.; Busnarda J.; Caux P.; Kent R. A. Fate, behavior, and aquatic toxicity of the fungicide DDAC in the Canadian environment. Environ. Toxicol. 2000, 15, 174–200. . [DOI] [Google Scholar]
- Lara-Martin P. A.; Li X.; Bopp R. F.; Brownawell B. J. Occurrence of alkyltrimethylammonium compounds in urban estuarine sediments: behentrimonium as a new emerging contaminant. Environ. Sci. Technol. 2010, 44 (19), 7569–7575. 10.1021/es101169a. [DOI] [PubMed] [Google Scholar]
- Comber S. D. W.; Rule K. L.; Conrad A. U.; Höss S.; Webb S. F.; Marshall S. Bioaccumulation and toxicity of a cationic surfactant (DODMAC) in sediment dwelling freshwater invertebrates. Environ. Pollut. 2008, 153 (1), 184–191. 10.1016/j.envpol.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Van Wijk D.; Gyimesi-Van Den Bos M.; Garttener-Arends I.; Geurts M.; Kamstra J.; Thomas P. Bioavailability and detoxification of cationics: I. Algal toxicity of alkyltrimethyl ammonium salts in the presence of suspended sediment and humic acid. Chemosphere 2009, 75 (3), 303–309. 10.1016/j.chemosphere.2008.12.047. [DOI] [PubMed] [Google Scholar]
- Thomas P. C.; Velthoven K.; Geurts M.; Van Wijk D. Bioavailability and detoxification of cationics: II. Relationship between toxicity and CEC of cationic surfactants on Caenorhabditis elegans (Nematoda) in artificial and natural substrates. Chemosphere 2009, 75 (3), 310–318. 10.1016/j.chemosphere.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hou W. C.; Moghadam B. Y.; Corredor C.; Westerhoff P.; Posner J. D. Distribution of functionalized gold nanoparticles between water and lipid bilayers as model cell membranes. Environ. Sci. Technol. 2012, 46 (3), 1869–1875. 10.1021/es203661k. [DOI] [PubMed] [Google Scholar]
- Loidl-Stahlhofen A.; Ulrich A. S.; Kaufmann S.; Bayerl T. M. Protein binding to supported lecithin bilayers controlled by the lipid phase state: A new concept for highly selective protein purification. Eur. Biophys. J. 1996, 25 (2), 151–153. 10.1007/s002490050026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.