Abstract
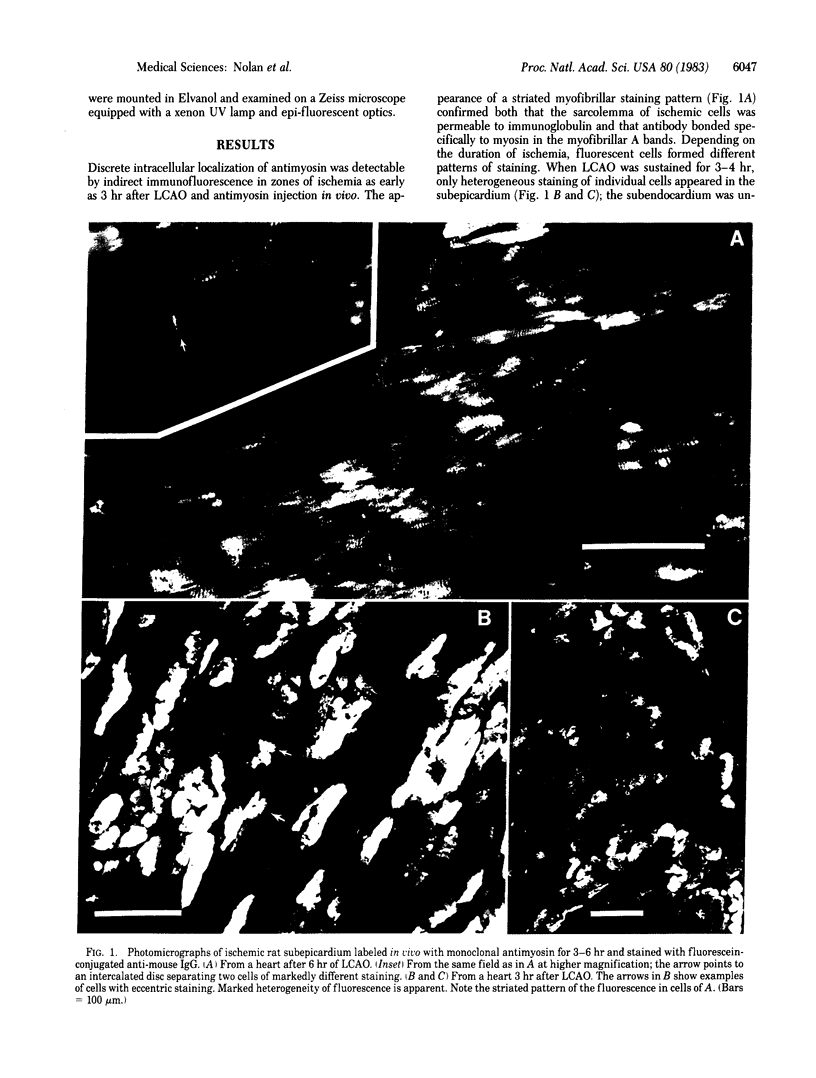
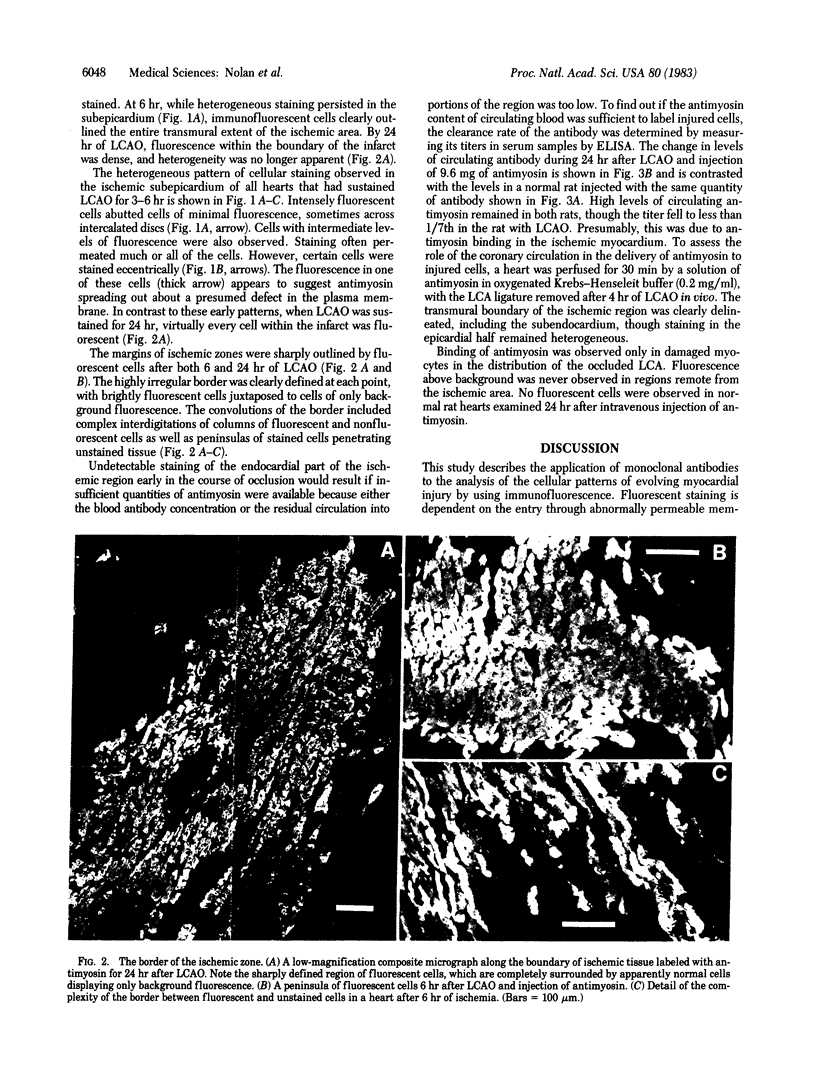
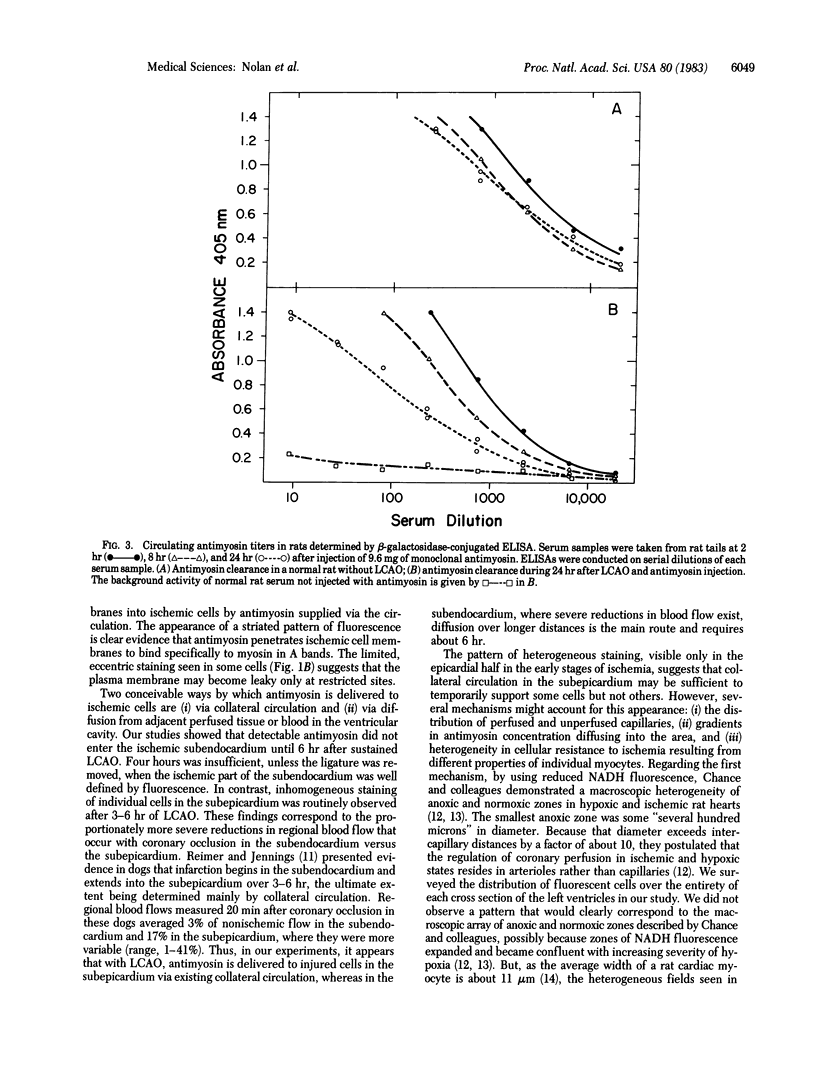
The development of cellular injury in the rat left ventricle resulting from left coronary artery occlusion was examined by immunofluorescence after intravenous injection of monoclonal antimyosin. Cardiac muscle cells that bound antimyosin during ischemia were localized by staining sections with fluorescein-conjugated anti-mouse IgG. Fluorescent staining was detectable within the ischemic region of the left ventricle 3 hr after occlusion and injection of antimyosin. After 6 hr of ischemia, the highly irregular margin of the ischemic zone was clearly outlined by fluorescent cells. At 3-6 hr after occlusion, marked heterogeneity in cellular staining was observed in the epicardial half of the ischemic area, with intensely fluorescent cells intermixed with cells of markedly lower fluorescence. By 24 hr, a homogeneous pattern of staining was observed throughout the ischemic zone. In nonischemic regions of the heart and in rats treated for 24 hr with antimyosin without occlusion, there were only background levels of staining. We conclude that: (i) visualization of ischemic cells via antimyosin provides a sensitive means for examining developing patterns of injury; (ii) the heterogeneity of staining during early ischemia may reflect variation in cellular resistance to deprivation; and (iii) the pattern of fluorescence at the margin of the occluded region indicates that the "border zone" is composed of interdigitating ischemic and nonischemic tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. M., Cohen C. J., McDonald T. F. Heterogeneity of intracellular potassium activity and membrane potential in hypoxic guinea pig ventricle. Circ Res. 1981 Nov;49(5):1181–1189. doi: 10.1161/01.res.49.5.1181. [DOI] [PubMed] [Google Scholar]
- Carey R. A., Natarajan G., Bove A. A., Coulson R. L., Spann J. F. Myosin adenosine triphosphatase activity in the volume-overloaded hypertrophied feline right ventricle. Circ Res. 1979 Jul;45(1):81–87. doi: 10.1161/01.res.45.1.81. [DOI] [PubMed] [Google Scholar]
- Clark W. A., Jr, Chizzonite R. A., Everett A. W., Rabinowitz M., Zak R. Species correlations between cardiac isomyosins. A comparison of electrophoretic and immunological properties. J Biol Chem. 1982 May 25;257(10):5449–5454. [PubMed] [Google Scholar]
- Clark W. A., Jr, Everett A. W., Fitch F. W., Frogner K. S., Jakovcic S., Rabinowitz M., Warner A. M., Zak R. Characterization of monoclonal antibodies directed against determinants on cardiac myosin heavy chain. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1680–1686. doi: 10.1016/s0006-291x(80)80092-1. [DOI] [PubMed] [Google Scholar]
- Delcayre C., Swynghedauw B. A comparative study of heart myosin ATPase and light subunits from different species. Pflugers Arch. 1975 Mar 22;355(1):39–47. doi: 10.1007/BF00584798. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Clark W. A., Zak R. Quantitation of myosin in muscle. Anal Biochem. 1983 Apr 1;130(1):102–107. doi: 10.1016/0003-2697(83)90655-3. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Okun E. M., Kirk E. S. The histological lateral border of acute canine myocardial infarction. A function of microcirculation. Circ Res. 1981 May;48(5):640–649. doi: 10.1161/01.res.48.5.640. [DOI] [PubMed] [Google Scholar]
- Gorza L., Pauletto P., Pessina A. C., Sartore S., Schiaffino S. Isomyosin distribution in normal and pressure-overloaded rat ventricular myocardium. An immunohistochemical study. Circ Res. 1981 Oct;49(4):1003–1009. doi: 10.1161/01.res.49.4.1003. [DOI] [PubMed] [Google Scholar]
- Haber E., Katus H. A., Hurrell J. G., Matsueda G. R., Ehrlich P., Zurawski V. R., Jr, Khaw B. A. Detection and quantification of myocardial cell death: application of monoclonal antibodies specific for cardiac myosin. J Mol Cell Cardiol. 1982 Sep;14 (Suppl 3):139–146. doi: 10.1016/0022-2828(82)90142-0. [DOI] [PubMed] [Google Scholar]
- Hamrell B. B., Low R. B. The relationship of mechanical Vmax to myosin ATPase activity in rabbit and marmot ventricular muscle. Pflugers Arch. 1978 Nov 14;377(2):119–124. doi: 10.1007/BF00582841. [DOI] [PubMed] [Google Scholar]
- Harken A. H., Barlow C. H., Harden W. R., 3rd, Chance B. Two and three dimensional display of myocardial ischemic "border zone" in dogs. Am J Cardiol. 1978 Dec;42(6):954–959. doi: 10.1016/0002-9149(78)90681-1. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Hawkins H. K., Lowe J. E., Hill M. L., Klotman S., Reimer K. A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978 Jul;92(1):187–214. [PMC free article] [PubMed] [Google Scholar]
- Khaw B. A., Beller G. A., Haber E. Experimental myocardial infarct imaging following intravenous administration of iodine-131 labeled antibody (Fab')2 fragments specific for cardiac myosin. Circulation. 1978 Apr;57(4):743–750. doi: 10.1161/01.cir.57.4.743. [DOI] [PubMed] [Google Scholar]
- Khaw B. A., Beller G. A., Haber E., Smith T. W. Localization of cardiac myosin-specific antibody in myocardial infarction. J Clin Invest. 1976 Aug;58(2):439–446. doi: 10.1172/JCI108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun;40(6):633–644. [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Samuel J. L., Rappaport L., Mercadier J. J., Lompre A. M., Sartore S., Triban C., Schiaffino S., Schwartz K. Distribution of myosin isozymes within single cardiac cells. An immunohistochemical study. Circ Res. 1983 Feb;52(2):200–209. doi: 10.1161/01.res.52.2.200. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Deleeuw G., Barlow C., Chance B., Williamson J. R. Heterogeneity of the hypoxic state in perfused rat heart. Circ Res. 1977 Nov;41(5):606–615. doi: 10.1161/01.res.41.5.606. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Davis K. N., Medina-Ramirez G. Quantitative analysis of heterogenous NADH fluorescence in perfused rat hearts during hypoxia and ischemia. J Mol Cell Cardiol. 1982 Sep;14 (Suppl 3):29–35. doi: 10.1016/0022-2828(82)90126-2. [DOI] [PubMed] [Google Scholar]