Abstract
Outbreaks of porcine epidemic diarrhoea virus (PEDV) have caused great economic losses to the global pig industry. PEDV strains with variants in the spike (S) gene have been reported in several countries. To better understand the molecular epidemiology and genetic diversity of PEDV field isolates, in this study, we characterised the complete genome sequence of a novel PEDV variant JSCZ1601 from a outbreak in China in 2016. The PEDV isolate was 28,033 nucleotides (nt) in length without the polyadenylated sequences. Phylogenetic analysis based on the full-length genome sequence of JSCZ1601 grouped it with the pandemic variants determined post-2010 into group 2 (G2). However, the S gene of JSCZ1601 formed a new subgroup separated from the subgroups containing the other G2 strains. Comparative analysis of the amino acids encoded by the S genes revealed the N-terminal of the deduced JSCZ1601 S protein had a novel two-amino-acid deletion (N58 and S59) compared with all identified genogroups. Further, compared with the reference strains, a ‘G’ insertion was detected in the 5′ terminal of the 5′UTR of the JSCZ1601. The animal experiment revealed that this strain was high pathogenic to neonatal pigs. Taken together, a PEDV strain with the new molecular characterizations and phylogenies was found in mainland China. It is necessary to strengthen the monitoring of PEDV variations.
Porcine epidemic diarrhoea (PED) is characterised by severe watery diarrhoea, leading to dehydration and high mortality among piglets. The disease is caused by the PED virus (PEDV), which belongs to family Coronaviridae, genus Alphacoronavirus, and has an envelope surrounding a single-stranded positive-sense RNA genome1,2. The PEDV genome is approximately 28 kb nucleotides (nt) long and contains a 5′untranslated region (5′UTR), a 3′UTR with a polyadenylated tail, and at least seven open reading frames (ORFs) arranged in the following order: ORF1a, ORF1b, spike (S) glycoprotein gene, ORF3 hypothetical protein gene, envelope (E) gene, membrane (M) gene and nucleocapsid (N) gene3,4. The 5′UTR is about 291–296 nt long and the 3′UTR is about 334 nt long. Two long ORFs, ORF1a and ORF1b, occupy two-thirds of the genome and encode two nonstructural polyproteins (pp1a and pp1b) that direct genome replication and transcription5.
PED was first reported in feeding and fattening pigs in England in 19712. In China, PED was identified as a sporadic viral enteric disease in pig herds before 20106,7,8. However, outbreaks of PED with increased severity of diarrhoea, vomiting, and dehydration have occurred in China since 2010. The disease approached a mobility rate as high as 100% and a mortality rate of 80–100% in piglets less than 10 days old, and has been recognised as a devastating illness causing death in neonatal piglets9,10. New outbreaks associated with a novel PEDV strain that is genetically distant from the prototype PEDV strain, CV777, have been reported in China8,11,12. Since then, PED outbreaks have increased markedly and have spread rapidly across countries. In late April 2013, PED was first confirmed in the United States and subsequently spread quickly across the country13,14,15.
Among the proteins encoded by the ORFs, the S glycoprotein is located on the envelope of the virus where it makes up the large surface projections of the virion and plays an important role in the attachment of viral particles to the receptors of host cells16,17,18. Thus, the S protein is a primary target for the development of a vaccine against PED19,20. Moreover, the S protein is considered to be the most antigenic of the encoded proteins, and several investigators have reported a high degree of genetic diversity in the S glycoprotein gene12,21,22. According to the phylogenetic analyses of the spike S gene, the PEDV strains were divided into two distinct clusters designated genogroup 1 (G1; classical) and genogroup 2 (G2; field epidemic or pandemic)21,23. Concurrently, several variant PEDV strains, characterised by insertions and deletions (INDELs) in the S gene and designated S-INDEL PEDV, were found to be circulating in United States swine farms24,25. Several strains with different deletions in the S gene were also reported in previous studies22,26,27,28,29,30. Thus, the S gene may be important for understanding the genetic relatedness of PEDV field isolates, the epidemiological status of the virus, and for vaccine development.
Thereby, a comprehensive study is necessary to better understand the genetic variations and relationships between different strains, and would be helpful to find out the reason of the continuously outbreak of PEDV and develop new strategy to control and prevent PEDV infection. In this study, we found a novel PEDV field strain JSCZ1601 with amino acid deletions in the protein encoded by the S gene, and a ‘G’ insertion in the 5′ terminal of the 5′UTR. The animal experiment revealed that this strain was high pathogenic to neonatal pigs. This study showed that a PEDV strain with the new molecular characterizations and phylogenies was found in mainland China. This will be useful to take into consideration in the control and prevention of this disease.
Results
Full-length genome sequence analysis
The sequence analysis showed that the full-length genomic sequence of JSCZ1601 (accession no. KY070587) was 28,033 nt long, excluding the polyadenylated sequences, and included the following genes and UTRs: 5′UTR (293 nt), ORF1a (12,354 nt), ORF1b (8,037 nt), S (4,155 nt), ORF3 (675 nt), E (231 nt), M (681 nt), N (1,326 nt), and 3′UTR (334 nt). We compared the full-length JSCZ1601 genome with the genomes of the reference PEDV isolates shown in Table 1. The comparative analyses showed that the nucleotide identities between JSCZ1601 and the strains identified post-2010 (e.g., Chinese strains JS-HZ2012, GD-A, and AH2012, and American strains PC21A, USA/Minnesota61/2013, and USA/Michigan252/2014) ranged from 98.0–99.0%, while the nucleotide identities with genomes determined ante-2010 (CV777, JS2008, DR13, and attenuated DR13) ranged from 96.6–97.4%.
Table 1. Nucleotide and amino acid sequence identities (%) of different regions of the JSCZ1601 genome compared with those of the other viruses.
| Identity to JSCZ1601/ Nucleotide (Amino Acid) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| strains | Complete genome | 5′UTR | ORF1a | ORF1b | S | ORF3 | E | M | N | 3′UTR |
| PEDV-LYG | 99.0 (−) | 97.2 (−) | 99.1 (99.1) | 98.5 (99.3) | 98.3 (97.6) | 100 (100) | 100 (100) | 99.5 (100) | 99.4 (99.3) | 95.9 (−) |
| JS-HZ2012 | 99.0 (−) | 97.9 (−) | 99.0 (99.1) | 98.9 (99.7) | 98.2 (98.0) | 99.4 (100) | 99.5 (100) | 100 (100) | 98.7 (99.0) | 95.0 (−) |
| YC2014 | 99.0 (−) | 91.0 (−) | 99.2 (99.2) | 98.6 (99.4) | 98.4 (98.1) | 100 (100) | 100 (100) | 99.8 (100) | 99.5 (99.5) | 95.4 (−) |
| GD-A | 98.5 (−) | 96.9 (−) | 97.8 (98.3) | 98.6 (99.6) | 96.8 (96.8) | 95.8 (96.4) | 99.1 (100) | 98.5 (97.7) | 95.8 (96.8) | 95.6 (−) |
| AH2012 | 98.8 (−) | 98.6 (−) | 99.0 (99.2) | 93.8 (94.3) | 97.9 (97.5) | 98.6 (99.5) | 99.1 (100) | 99.7 (100) | 98.4 (98.6) | 95.0 (−) |
| AH2012/12 | 98.0 (−) | 97.2 (−) | 98.1 (98.5) | 98.6 (99.6) | 96.7 (96.5) | 95.7 (96.4) | 99.1 (100) | 98.5 (98.2) | 95.9 (96.8) | 96.5 (−) |
| CHGD-01 | 98.0 (−) | 96.2 (−) | 98.0 (98.3) | 98.5 (99.4) | 96.9 (96.8) | 96.2 (97.3) | 98.7 (98.7) | 98.3 (98.2) | 95.9 (96.8) | 98.5 (−) |
| CH/JX-1/2013 | 98.9 (−) | 98.9 (−) | 98.9 (98.9) | 98.8 (99.6) | 97.9 (97.4) | 99.7 (100) | 99.5 (100) | 99.8 (100) | 99.0 (99.0) | 99.1 (−) |
| JS2008 | 96.8 (−) | 96.9 (−) | 97.3 (97.7) | 98.0 (99.4) | 93.5 (92.5) | 38.0 (37.7) | 96.1 (94.8) | 97.7 (97.3) | 95.7 (96.8) | 95.3 (−) |
| CV777 | 96.6 (−) | 95.3 (−) | 96.7 (97.3) | 97.7 (99.3) | 93.5 (92.8) | 96.8 (96.4) | 96.9 (98.7) | 98.2 (98.6) | 95.3 (96.3) | 95.0 (−) |
| DR13 | 97.4 (−) | 97.6 (−) | 97.6 (98.1) | 98.3 (99.7) | 94.4 (93.8) | 98.5 (99.5) | 98.2 (100) | 98.3 (98.6) | 96.5 (96.8) | 94.5 (−) |
| Attenuated_DR13 | 96.7 (−) | 97.6 (−) | 97.3 (97.7) | 97.8 (99.5) | 93.3 (92.4) | 38.0 (37.7) | 87.8 (87.0) | 97.9 (97.7) | 95.7 (96.8) | 95.0 (−) |
| PC21A | 98.7 (−) | 91.1 (−) | 98.7 (99.1) | 98.5 (99.5) | 98.4 (97.9) | 100 (100) | 100 (100) | 100 (100) | 98.8 (99.0) | 93.3 (−) |
| USA/Minnesota61/2013 | 98.7 (−) | 86.6 (−) | 99.1 (99.4) | 98.7 (99.7) | 98.4 (97.9) | 100 (100) | 99.5 (100) | 100 (100) | 98.8 (99.0) | 94.5 (−) |
| USA/Michigan252/2014 | 98.7 (−) | 83.2 (−) | 98.8 (99.1) | 98.6 (99.5) | 98.3 (97.7) | 100 (100) | 100 (100) | 100 (100) | 98.8 (99.0) | 95.9 (−) |
| USA/Iowa106/2013 | 98.3 (−) | 86.6 (−) | 98.8 (99.1) | 98.9 (99.8) | 96.1 (95.3) | 100 (100) | 99.5 (100) | 99.7 (100) | 98.7 (98.8) | 94.5 (−) |
The full-length genome sequence of JSCZ1601 shared the highest levels of nucleotide identity (99.0%) with PEDV strains PEDV-LYG, JS-HZ2012, and YC2014. However, among the ORFs, the S genes had the lowest nucleotide identities 98.2–98.4% with these strains. The amino acid identity between the deduced S proteins of JSCZ1601 and PEDV-LYG was only 97.6%. These data indicate that a high level of variation may have occurred in the S protein of the JSCZ1601 strain. The deduced ORF1b protein of JSCZ1601 shared ≥99.3% amino acid identities with all the reference strains except AH2012, which shared only 94.3% identity. The 5′UTR of JSCZ1601 shared 83.2–98.9% identities with the other PEDV strains. The 3′UTR of JSCZ1601 showed higher genetic conservation than the 5′UTR and shared 94.5–99.1% homology with the other PEDV stains. Besides, the identities shared between the 5′UTR of JSCZ1601 and the three American strains (USA/Minnesota61/2013, USA/Michigan252/2014 and USA/Iowa106/2013) were only 83.2–86.6%.
Phylogenetic analyses
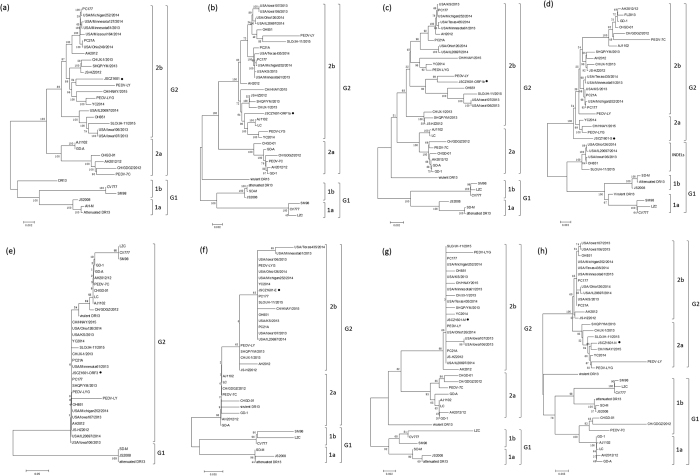
Phylogenetic trees were generated based on the nucleotide sequences of the full-length genomes and each of the seven ORFs (i.e., ORF1a, ORF1b, S, ORF3, E, M, and N) of JSCZ1601 and the other PEDV strains (Fig. 1a–h, respectively). As shown in Fig. 1a, the phylogenetic tree of the full-length genome indicated that the PEDV strains fell into two groups designated G1 and G2. G1 had two subgroups, 1a and 1b, and consisted of CV777, virulent and attenuated DR13, SM98, and an earlier Chinese strain JS2008. G2 also had two subgroups, 2a and 2b (Fig. 1a). Notably, all the strains identified from 2011 to 2015 were in G2. The phylogenetic trees of the full-length genome, ORF1a, ORF1b, E, and M showed that JSCZ1601 fell into G2b. However, the S gene of JSCZ1601 formed a sole subgroup that was separate from the other G2 strains (Fig. 1d). These data indicate that significant variation had occurred in the S gene of JSCZ1601. Moreover, the phylogenetic tree of the S genes revealed that the S-INDEL strains formed a single subgroup in G1.
Figure 1.
Phylogenetic analyses of PEDV strains based on the nucleotide sequences of the full-length genome and the ORF1a, ORF1b, S, ORF3, E, M, and N genes. Phylogenetic trees of (a) the full-length genome; (b) the ORF1a gene; (c) the ORF1b gene; (d) the S gene; (e) the ORF3 gene; (f) the E gene; (g) the M gene; (h) the N gene. The evolutionary history was inferred using the neighbour-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed using the Jukes-Cantor method and are presented as the number of base substitutions per site. The evolutionary analyses were conducted using MEGA5 software.
Comparative analysis of the deduced amino acid sequences of complete S genes
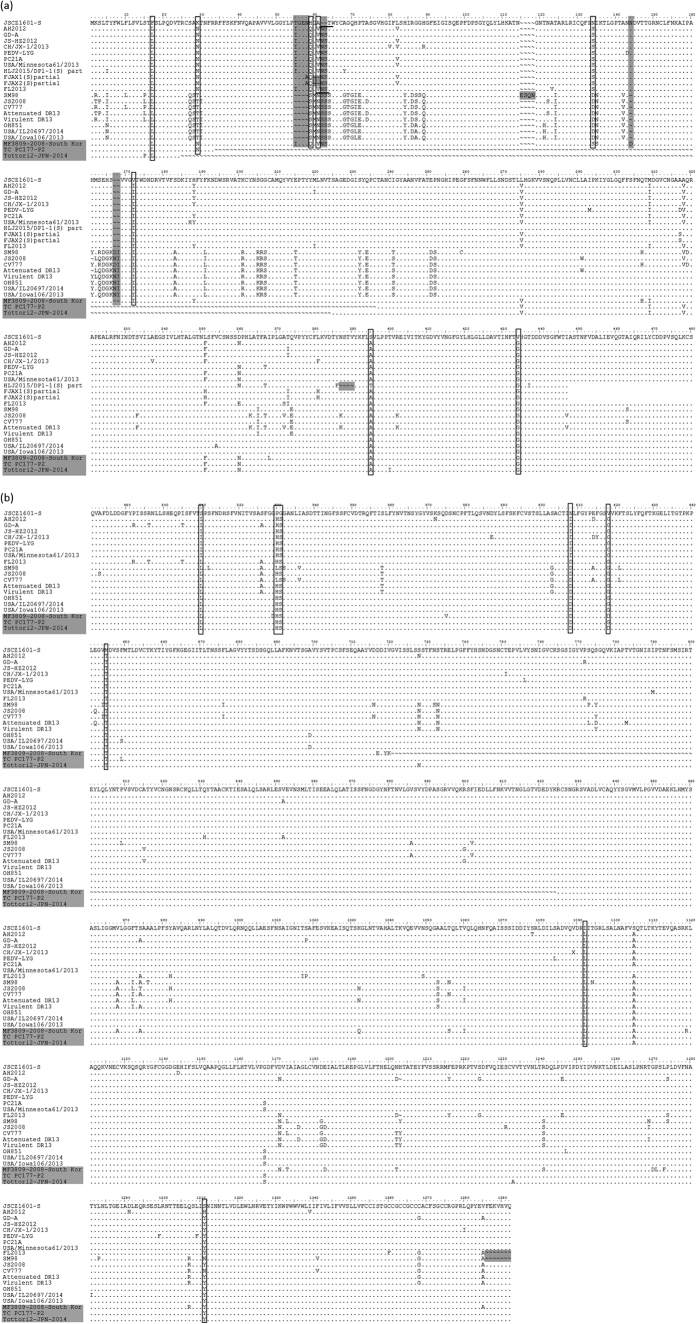
As shown in Fig. 2, compared with the S protein sequence of G1 strain CV777, the deduced S protein of JSCZ1601 had two insertions (55TGENV58 and 136 N) and one deletion (157NI158), which is the same as the S proteins of the G2 strains AH2012, GD-A, and PEDV-LYG. Notably, the N-terminal of the JSCZ1601 S protein had an additional two-amino-acid deletion (N58 and S59) compared with the S protein of the representative strain CV777. And this deletion was also existed in JSCZ1601 compared with G2 strains, such as AH2012 and GD-A. Further, the two-amino-acid deletion in the JSCZ1601 S protein eliminated a potential N-glycosylation site that was located at positions 62–64 and 57–59 in the amino acid sequences of the S proteins in AH2012 and CV777, respectively. An additional 16 unique amino acid substitutions were detected in the S protein of JSCZ1601 compared with the S proteins of all the reference strains (Fig. 2). In addition, five unique substitutions (500 L/S, 520 H/P, 521 S/G, 590 D/N, and 600 G/V) occurred in one neutralizing epitope (amino acids 499–638) of the JSCZ1601 receptor-binding subunit S1 protein compared with all the reference strains. Moreover, the deduced S proteins of three S-INDEL strains, OH851, USA/IL20697/2014, and US/Iowa106/2013, had the same amino acid deletions and insertion as the G1 strains CV777 and JS2008.
Figure 2. Alignment of the deduced amino acid sequences of the complete S proteins of JSCZ1601 and several representative PEDV strains.
Deletions and insertions are shown as grey bars. Key amino acid mutations within the different strains are shown as black boxes, and the N-glycosylation sites are indicated by short lines. (a) Alignment of the sequences of all the selected PEDV strains. (b) Alignment after removing three PEDV strains, FJAX1, FJAX2, and HLJ2015/DP1-1, in which the S genes were partial.
Nucleotide sequence comparison of 5′-RNA termini
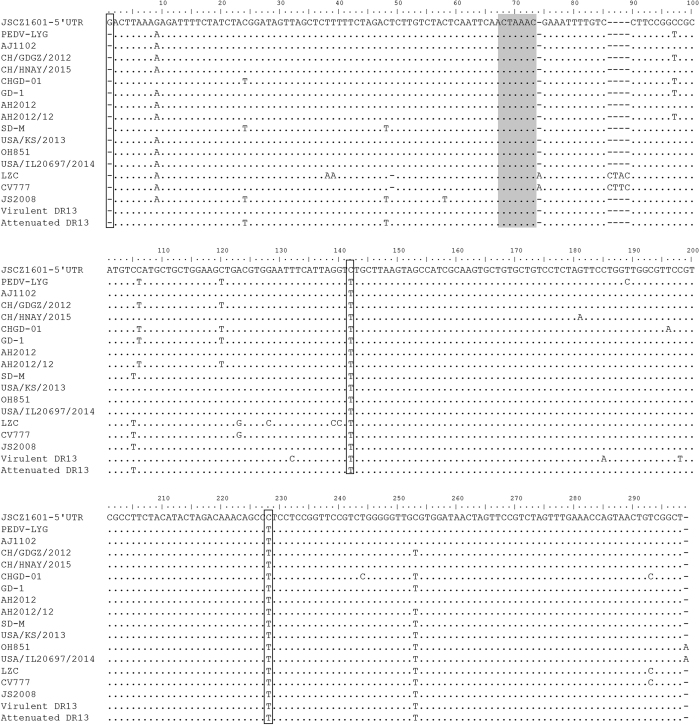
The 5′UTR of JSCZ1601 was 293 nt long, which is 3-nt shorter than the 5′UTR of CV777. As shown in Fig. 3, 1-nt deletion, 4-nt deletions, and 1-nt insertion were detected in the proximal regions of the 5′UTRs of the CV777 and LZC strains compared with the other strains. Conversely, the 5′UTRs were relatively conserved between G2 members. Interestingly, a ‘G’ insertion was observed in the 5′ terminal of the 5′UTR of JSCZ1601. And two another nucleotide substitutions, 137 C/T and 225 C/T were existed in the 5′UTR of JSCZ1601, when compared with the reference strains. Besides, the core sequence (5′-CUAAAC-3′) of the PEDV leader transcription-regulating sequence was well conserved with no nucleotide substitutions detected in any of the PEDV strains.
Figure 3. Alignment of nucleotide sequences of the 5′UTRs of JSCZ1601 and several representative PEDV strains.
Deletions and key nucleotide mutations within the different strains are shown as black boxes. The core sequences (5′-CUAAAC-3′) of the leader transcription-regulating sequences (CS-L) are shown as grey bars.
The pathogenesis of JSCZ1601 in newborn piglets
To determine the pathogenicity of JSCZ1601 in newborn piglets, suckling piglets were orally inoculated with the inocula prepared by the intestine tissue sample. While control animals were inoculated with PBS.
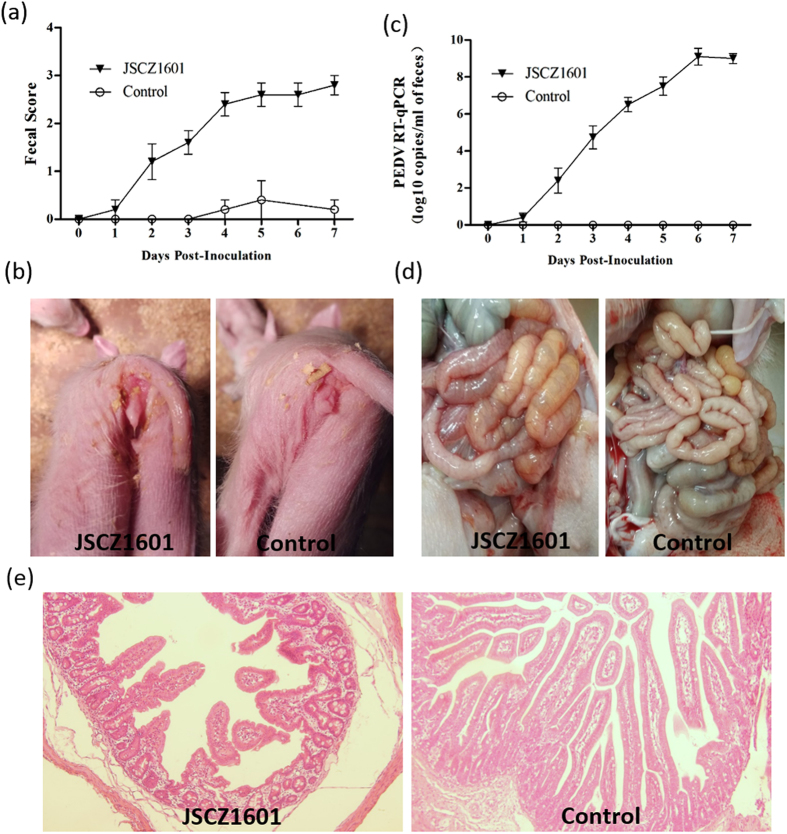
During the acclimation period, the clinical signs were monitored and piglets in the JSCZ1601 challenge group exhibited lethargy and diarrheic feces by 2 day post-inoculation (DPI) and diarrhea lasted through the study period (Fig. 4a and b). The piglets in the negative control group remained active and clinically unaffected throughout the 7-day study period. Due to severity of clinical signs, these piglets were euthanized and necropsied at 7 DPI. The quantitative genomic copies/ml of PEDV RNA in fecal samples rectal was shown in Fig. 4c. The average virus shedding in JSCZ1601 group increased about 102.5 to 109.5 genomic copies/ml through the study period. Negative control piglets remained active with normal feces and fecal shedding of PEDV remained undetected throughout the study period.
Figure 4. The pathogenesis of JSCZ1601 in newborn piglets.
(a) Mean fecal scores after viral inoculation. (b) The diarrhea symptoms of the piglet inoculated with JSCZ1601. (c) Mean RT-qPCR titers of the fecal samples. (d) Macroscopic examinations of the intestine of piglets inoculated with JSCZ1601 and control medium. (e) HE-stained small intestines of piglets inoculated with JSCZ1601 and control medium.
Necropsy examinations showed that the virus inoculated piglets displayed typical PED-like lesions. The small intestine was thin-walled and contained soft to watery contents (Fig. 4d). Small intestine contents obtained at necropsy were also tested by PEDV RT-qPCR and had 107.2 to 1010.1 genomic copies/ml. Histopathological examination showed severe necrosis and villous atrophy of the small intestinal enterocytes in JSCZ1601 inoculated pigs (Fig. 4e). Villus height and crypt depth were measured, and the mean villous height/crypt depth (VH/CD) ratio of the mock-inoculated piglets (6.5±0.8) was higher than those of JSCZ1601inoculated piglets (1.3±0.5).
Discussion
In the winter of 2010, a PED outbreak began on pig farms in southern China and immediately spread throughout the country. The death toll from the disease was over one million piglets in South China, with devastating damage to the pig industry. Our genetic analyses showed that the PEDV strains fell into two genogroups, G1 (classical) and G2 (variant)21,23. The prevalent strains that caused the outbreak in Asia in late 2010 and the recent outbreaks in North America belonged to the G2 genogroup13,31. In this study, we found a PEDV strain JSC1601 with new genetic characteristics while elucidating the molecular characteristics and phylogeny of PEDV field strains in China. The whole-genome phylogenetic analysis demonstrated that JSCZ1601 along with the strains determined post-2010 clustered into genogroup G2. However, the S gene of JSCZ1601 formed a new subgroup that was separated from the other G2 strains. Further, comparative analysis of the amino acid sequence of the S protein revealed that the N-terminal of the JSCZ1601 S protein had a two-amino-acid deletion (N58 and S59) compared with the other G2 members. Compared with the other classical and variant strains, a ‘G’ insertion was also observed in the 5′ terminal of the JSCZ1601 5′UTR. These data revealed that a PEDV strain with the new molecular characterizations and phylogenies was found in mainland China.
Previous studies have demonstrated that the 5′UTR of PEDV strains were highly conserved7,13,32. In general, strict conservation of these regions is essential for the PEDV life cycle. It is known that the 5′UTRs of coronaviruses form conserved RNA structural elements that are critical for viral replication, subgenomic mRNA transcription, and translation33. In this study, we found a ‘G’ insertion in the 5′ terminal of the 5′UTR compared with the other PEDV strains. Studies on the beta-coronavirus indicated that stem-loop 4 (SL4) in the 5′UTRs was conserved among group 2 coronaviruses and may have a homolog in groups 1 and 3, and that SL4 may act as a cis-acting element for defective interfering RNA replication through interactions with cellular proteins34. In this study, the 223 T/C mutation was located in the SL4, which was identified in the 168–297nt region34. How these insertion and mutations affect the efficiency of virus replication needs to be addressed in future studies. The core sequence (5′-CUAAAC-3′) in the transcription-regulating sequence of PEDV, which was also present in JSCZ1601, was reported to be a determinant factor in transcriptional regulation in coronavirus because the synthesis of subgenomic mRNA requires the appropriate tertiary structure of the core sequence35.
The S protein of PEDV is known to play pivotal roles in viral entry and in inducing the neutralizing antibodies in natural hosts, and this makes it a primary target for the development of effective vaccines against PEDV17,22,36,37. Significant genetic variations in the S gene have been revealed between the newly determined PEDV field strains and early isolates21,38. In this study, the amino acid sequence of the JSCZ1601 S protein shared only 97.6–98.1% identities with the S proteins of the reference strains. Besides, the phylogenetic analyses showed that the S gene of JSCZ1601 formed a separated subgroup in G2. All these data implied that the S gene of JSCZ1601 had undergoing significant genetic changes. The spike ectodomain consists of a receptor-binding subunit S1 and a membrane-fusion subunit S2. S1 contains two domains, an N-terminal domain and a C-terminal domain, both of which can potentially function as receptor-binding domains39,40. Several neutralizing epitopes (499–638, 748–755, 764–771, and 1,368–1,374 amino acids) have also been identified17,36,41. In this study, the deduced JSCZ1601 S protein had a two-amino-acid deletion (N58 and S59) in the S1 N-terminal domain. And this deletion completely eliminated a potential N-glycosylation site that was present in the S proteins of the other strains. Moreover, five unique amino acid substitutions were identified in one neutralizing epitope of the S1 C-terminal domain of the JSCZ1601 S protein compared with the S proteins of all the PEDV reference strains. Whether these amino acids substitutions and the N-glycosylation site deletion influence the antigenicity and pathogenicity of PEDV remains to be investigated.
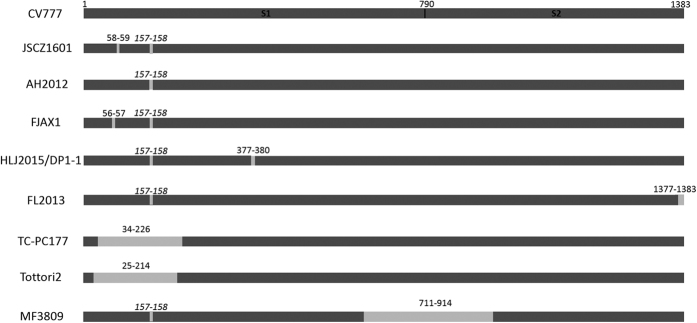
In this study, we also summarized the deletions in the S genes in the reported strains and illustrated the results in a simple schematic diagram (Fig. 5). Three strains, which has a large deletion in S gene, MF3809 with 711–914 amino acid deletion42, TC PC177 with 34–226 amino acid deletion27 and Tottori2-JPN-2014 with 25–214 amino acid deletion26 compared with the reference strain CV777 (Fig. 5). This finding suggested that positions 25–226 in subunit S1 and 711–914 in subunit S2 of the spike ectodomain may be non-essential regions in PEDV infection. The pathogenicity of the Tottori2-JPN-2014 strain has been reported to be mild26. Further, the novel PEDV strains with new amino acid deletions in their S genes have been found in fields in China. The S proteins of strains FJAX1 and FJAX2 were found to have a 66–67 amino acid deletion28 and the S protein of HLJ2015/DP1–1 had a 377–380 amino acid deletion29 compared with the CV777 S protein. However, the pathogenicity of these three strains has not been reported so far. A Chinese variant strain FL2013, which had a seven amino acid deletion (FEKVHVQ) in the C-terminus of the deduced S protein compared with the S proteins of the other G2 PEDV sequences, had reduced virulence in newborn piglets30. Together, these findings revealed that the genetic evolution of PEDV strains was variant, and the S gene of PEDV might be an important virulence gene. It is important to monitor the genetic variations in this virus, and virulent genes and regions of the PEDV genomes should be identified using the reverse genetic technologies. In addition, we also have investigated 35 samples from different pig farms in different cities and provinces (data not shown), and only one pig farm was found to have the epidemic of this strain JSCZ1601. These data indicated that the strain had a certain ability to spread, and the positive monitoring and prevention of epidemic of the strain is necessary.
Figure 5. Simple schematic diagram of the deduced S proteins of PEDV strains with the new deletions.
The reference strain was CV777. Deletions are shown as grey bars. The numbers indicate the positions of the deletion according to the CV777 sequence. Deletions that have been identified in G2 strains are labelled with italicised numbers.
The intestinal tissue samples of strain JSCZ1601 were obtained from suckling piglets less than 10 days old with severe watery diarrhoea, vomiting, and dehydration in a large-scale pig farm in Changzhou, Jiangsu Province, China. A severe diarrhoea epidemic broke out on this farm in January 2016 and caused the death of hundreds suckling piglets less than one week old. Several important pathogens, such as TGEV, RV, PRRSV, PCV, CSFV, and PRV, were confirmed to be negative in these samples (data not shown). We also checked several intestinal samples obtained from different piglets from different sows. The S gene sequences of these samples had the same characteristics as the JSCZ1601 S gene, which confirmed the severe diarrhoea epidemic was caused by the PEDV strain JSCZ1601. In order to measure the pathogenesis of JSCZ1601 in newborn piglets further, we used the virus inocula prepared from the intestine tissue sample and found that the JSCZ1601 strain could cause severe watery diarrhoea in piglets. And the low challenge dose (3 ml 1.42×104copies/ml inocula each piglet) might be one reason for the delayed time of diarrhea and the longer duration of disease. These results also revealed that the two-amino-acid deletion in the S protein did not attenuate the pathogenicity of the JSCZ1601 strain. We tried to isolate this strain in vitro using different Vero cell lines with trypsin, but failed. We will make attempts to obtain the virus isolate further.
In conclusion, here we reported the genetic characterisation of a novel PEDV strain, JSCZ1601, found on the Chinese mainland, which had significant variations in the S gene. In recent years, new S mutant strains have been constantly found and this may be related to the immune pressure of the vaccines that are used, including those from vaccine strains CV777 and DR13. Meanwhile, the virulence of the new mutants may be changed, and amino acid substitutions in the neutralizing epitopes may have conferred the capacity for immune evasion in these PEDV field strains. The monitoring of PEDV variations will be useful in the control and prevention of PED.
Methods
Clinical samples
The intestinal tissue sample was obtained from a < 10-day-old suckling piglets with severe watery diarrhea, vomiting and dehydration in a large-scale pig farm with severe diarrhea epidemic in Changzhou, Jiangsu province, China in 2016. The piglet was diagnosed as PEDV-positive using reverse transcription (RT) and polymerase chain reaction (PCR). In addition, the possible co-infections caused by porcine transmissible gastroenteritis virus (TGEV), porcine rotavirus (PRV), and other common pathogenic intestinal pathogens, had been confirmed to be negative (data not shown).
Complete Genome sequencing
Total RNA was extracted from the tissue homogenate using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and suspended in nuclease-free water immediately before use. The first-strand cDNA synthesis was performed using the SuperScript III First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols.
The entire PEDV genome was amplified by 14 pairs of primer designed with Primer 5.0 software based on the conserved regions determined by a multiple alignment analysis of the reference strains and CV777, and the 5′ and 3′ termini of the genomic sequence were synthesized by using the rapid amplification of the cDNA ends (RACE) kit (Clontech, Beijing, China) following the manufacturer’s instructions. The sequences of the primers used for the whole genome and the RACE procedure are provided in Table 2. The overlapping fragments were amplified by PCR using the Phanta Super Fidelity DNA polymerase (Vazyme, China), and the thermal cycling was performed at 95 °C for 3 min, followed by 35 cycles of 95 °C for 15 s, 52 °C to 56 °C for 15 s, and 72 °C for 2 min, with a final extension at 72 °C for 3 min. The amplified PCR products were analyzed using agarose gel electrophoresis. The PCR products were purified from the agarose gel using the AxyPrep DNA Gel Extraction Kit (Axygen, China), and cloned into the pEASY-Blunt Zero vector (Trans, China). Three clones were sequenced by a commercial service provider (Invitrogen, Shanghai, China).
Table 2. The primers of 14 overlapping fragments used for amplification of JSCZ1601 whole genome.
| Primers name | Sequence |
|---|---|
| 186-2055-F | 5′-GCGTTCCGTCGCCTTCTACATAC-3' |
| 186-2055-R | 5′-TTATAAACAGGATGTTCAATGA-3' |
| 1960-4005-F | 5′-CAGTTGTTGTTGATGGACTTGC-3' |
| 1960-4005-R | 5′-CCACTATCATTGCCTATAAAAG-3' |
| 3925-5998-F | 5′-TCGAGATACTACTGCTCTCTCC-3' |
| 3925-5998-R | 5′-TCCATCATACACCATACCAGTG-3' |
| 5922-7969-F | 5′-CATTCCTAGATAATGGTAACGG-3' |
| 5922-7969-R | 5′-ATCATAATCGCTATCACTGCTA-3' |
| 7893-9941-F | 5′-TGTTCATAGTTGCTGTTTTCTT-3' |
| 7893-9941-R | 5′-TAAGCCACCAAGTAGAACCATT-3' |
| 9869-11925-F | 5′-AGTAGTCTGTTTACGGAGAATG-3' |
| 9869-11925-R | 5′-ATGCCATCTCCTTCTGCCTTAA-3' |
| 11845-13885-F | 5′-GCGTATTGTCAAGCTCCAGAAT-3' |
| 11845-13885-R | 5′-AAGTAAGCTCAGAGCCCTCAGA-3' |
| 13808-15857-F | 5′-AACCTGGCCATTTCAATAAGGA-3' |
| 13808-15857-R | 5′-TCTTTAGGTCCTACAACCTCAT-3' |
| 15781-17829-F | 5′-ACTATCAAGGCCAAGGAGGAGA-3' |
| 15781-17829-R | 5′-CGTATGCAGCGCACTATTGTAA-3' |
| 17759-19825-F | 5′-TCAAGATTGGACCAAGTAAGAG-3' |
| 17759-19825-R | 5′-CAGCACCATAGTTATAGAGATT-3' |
| 19750-21810-F | 5′-GGTTATTCCATGCCTTCTATTT-3' |
| 19750-21810-R | 5′-GAGGTAAAACAGCCAAGAATTT-3' |
| 21729-23770-F | 5′-GCTATCCAAGTACCCTATTATTG-3' |
| 21729-23770-R | 5′-CCCTGCGAATTAACAACCTCTT-3' |
| 23707-25755-F | 5′-CTAAGGGTTTGAACACTGTGGC-3' |
| 23707-25755-R | 5′-GATATTCCATGTGAAATTCCAG-3' |
| 25688-27848-F | 5′-ATGTCTAACGGTTCTATTCCCG-3' |
| 25688-27848-R | 5′-CCACTGGCTTACCGTTGTGTGC-3' |
| 5′ RACE Primer (303-284) | 5′-TTGCTAGCCATAGCCGACAG-3' |
| 3′ RACE Primer (27724-27746) | 5′-CTATGTCCCAGGGTAGTGCCATT-3' |
The location corresponds to position within the JS-HZ2012 (KC210147) genome.
Nucleotide and amino acid sequence analyses
The overlapping sequences of the PCR products were combined to obtain the full-length genomic sequence of the JSCZ1601 strain. A nucleotide BLASTn analysis was used to compared the sequences of the JSCZ1601 genes with those of the reference strains of PEDV in the GenBank database (Table 3). The sequence alignments were generated using the Clustal W program. Phylogenetic trees of the full-length genomic, ORF1a, ORF1b, S, ORF3, E, M and N nucleotide sequences were generated using the distance-based neighbor-joining method in the MEGA, version 5.05, software. The bootstrap values were calculated based on 1000 replicates, and the evolutionary distances were computed using the Jukes-Cantor method. The deduced amino acid sequence comparisons were obtained using the BioEdit software.
Table 3. Representative PEDV strains used in this study.
| Strain | Country | Year | Accession No. | Strain | Country | Year | Accession No. |
|---|---|---|---|---|---|---|---|
| PEDV-LYG | China | 2014 | KM609212 | CV777 | Europe | JN599150 | |
| JS-HZ2012 | China | 2012 | KC210147 | Virulent DR13 | South Korea | 2009 | DQ862099 |
| YC2014 | China | 2014 | KU252649 | Attenuated_DR13 | South Korea | JQ023162 | |
| GD-A | China | 2012 | JX112709 | SM98 | South Korea | 2010 | GU937797 |
| LZC | China | 2006 | EF185992 | MF3809 | South Korea | 2008 | KF779469 |
| JS2008 | China | 2008 | KC109141 | Tottori2-JPN-2014 | Japan | 2014 | LC022792 |
| CHGD-01 | China | 2011 | JX261936 | SLO/JH-11/2015 | Slovenia | 2015 | KU297956 |
| PEDV-7C | China | 2011 | KM609204 | PC21A | USA | 2013 | KR078299 |
| GD-1 | China | 2011 | JX647847 | USA/Minnesota61/2013 | USA | 2013 | KJ645705 |
| AJ1102 | China | 2011 | JX188454 | USA/KS/2013 | USA | 2013 | KJ184549 |
| LC | China | 2011 | JX489155 | USA/Minnesota52/2013 | USA | 2013 | KJ645704 |
| CH/CG/11 | China | 2011 | JQ627654 | PC177 | USA | 2013 | KR078300 |
| CH8 | China | 2011 | JQ239436 | USA/Iowa106/2013 | USA | 2013 | KJ645695 |
| AH2012 | China | 2012 | KC210145 | USA/Iowa107/2013 | USA | 2013 | KJ645696 |
| AH2012/12 | China | 2012 | KU646831 | TC PC177 | USA | 2013 | KM392229 |
| CH/GDGZ/2012 | China | 2012 | KF384500 | USA/IL20697/2014 | USA | 2014 | KT860508 |
| AH2012/12 | China | 2012 | KU646831 | USA/Michigan252/2014 | USA | 2014 | KR265822 |
| CH/AHHF-2/2012 | China | 2012 | JX018182 | USA/Texas435/2014 | USA | 2014 | KR265834 |
| FL2013 | China | 2013 | KP765609 | USA/Ohio126/2014 | USA | 2014 | KJ645702 |
| CH/JX-1/2013 | China | 2013 | KF760557 | OH851 | USA | 2014 | KJ399978 |
| SHQP/YM/2013 | China | 2013 | KJ196348 | USA/Iowa303/2014 | USA | 2014 | KR265827 |
| CH/HNAY/2015 | China | 2015 | KR809885 | JSCZ1601 | China | 2016 | KY070587 |
Experimental design of infection
For the preparation of inocula for piglets, the intestine tissue sample was homogenized, diluted 1:4 in phosphate-buffered saline (PBS, pH 7.4; Sigma-Aldrich, St. Louis, MO), and centrifuged. The supernatant was filtered through a 0.45-μm-pore-size syringe filter (Millipore, Billerica, MA, USA) and collected as the inocula. The RNA titers of the inocula was 1.42 log10 genomic copies/mL by using quantitative real-time reverse transcription-PCR (RT-qPCR) as reported43.
Eight 3-day-old piglets, selected from one sow that was PEDV RNA and antibody negative, were divided into two groups (four piglets each) and housed in separate rooms. Pigs were fed a mixture of liquid milk replacer and yogurt and had free access to water. Groups A was challenged orally with 3 ml inocula. The four control piglets (Group B) were inoculated with PBS. All animals were monitored daily for clinical signs of disease, including diarrhea and vomiting. Rectal swabs were collected for scoring fecal denseness (scores: 0 normal; 1 pasty stool; 2 semiliquid diarrhea; and 3 liquid diarrhea) and for enumerating fecal viral RNA shedding by RT-qPCR. At necropsy, intestinal tissues and contents were grossly evaluated. Additionally, a portion of the jejunum and ileum were fixed in 10% neutral buffered formalin for histopathology. The villous atrophy is a typical histopathological change of the infection of a highly virulent PEDV44,45,46,47. And the villous height and crypt depth (VH:CD) ratios would be calculated using a computerized image system with as previous described44. All of the animal experiments were performed with the approval of the Jiangsu Academy of Agricultural Sciences Experimental Animal Ethics Committee (NKYVET 2015–0127) and were performed in accordance with relevant guidelines and regulations. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Statistical analysis
All data were analyzed using GraphPad Prism (Version 5.03, San Diego, CA, USA) software. Differences among groups were examined using one-way analysis of variance (ANOVA), followed by Tukey’s tests.
Additional Information
How to cite this article: Fan, B. et al. Characterization of Chinese Porcine Epidemic Diarrhea Virus with Novel Insertions and Deletions in Genome. Sci. Rep. 7, 44209; doi: 10.1038/srep44209 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by the National Key Research and Development Program (2016YFD0500101), the Special Fund for Independent innovation of Agricultural Science and Technology in Jiangsu province (CX(15)1056), the National Natural Sciences Foundation of China (31472204).
Footnotes
The authors declare no competing financial interests.
Author Contributions B.F., B.L. and K.H. designed the experiment. B.F. and B.L. wrote the manuscript. Clinical samples were collected by Q.X., Z.Y. and W.Y., D.J., F.P., P.Z. and A.M. detected the samples by RT-PCR. X.Z. analyzed the histopathology. B.F., B.L. and R.G. analyzed the data. All authors took part in discussion and interpretation of results. All authors read, advised and approved the final manuscript.
References
- Pensaert M. B. & de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Archives of virology 58, 243–247 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. N. An apparently new syndrome of porcine epidemic diarrhoea. The Veterinary record 100, 243–244 (1977). [DOI] [PubMed] [Google Scholar]
- Bridgen A., Kocherhans R., Tobler K., Carvajal A. & Ackermann M. Further analysis of the genome of porcine epidemic diarrhoea virus. Advances in experimental medicine and biology 440, 781–786 (1998). [DOI] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M. & Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus genes 23, 137–144 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D. & Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus genes 44, 167–175, doi: 10.1007/s11262-012-0713-1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. Molecular epidemiology of porcine epidemic diarrhea virus in China. Archives of virology 155, 1471–1476, doi: 10.1007/s00705-010-0720-2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J., Zeng S., Xiao S., Chen H. & Fang L. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 isolated from a suckling piglet with acute diarrhea in China. Journal of virology 86, 10910–10911, doi: 10.1128/JVI.01919-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. New variants of porcine epidemic diarrhea virus, China, 2011. Emerging infectious diseases 18, 1350–1353, doi: 10.3201/eid1808.120002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R. Q. et al. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerging infectious diseases 18, 161–163, doi: 10.3201/eid1801.111259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F. F. et al. Epidemiological survey of porcine epidemic diarrhea virus in swine farms in Shanghai, China. Archives of virology 158, 2227–2231, doi: 10.1007/s00705-013-1722-7 (2013). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) samples from field cases in Fujian, China. Virus genes 45, 499–507, doi: 10.1007/s11262-012-0794-x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. L. et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus genes 45, 181–185, doi: 10.1007/s11262-012-0735-8 (2012). [DOI] [PubMed] [Google Scholar]
- Huang Y. W. et al. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio 4, e00737–00713, doi: 10.1128/mBio.00737-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D. et al. Complete Genome Sequence of Porcine Epidemic Diarrhea Virus Strain USA/Colorado/2013 from the United States. Genome announcements 1, doi: 10.1128/genomeA.00555-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima G. Fighting a deadly pig disease. Industry, veterinarians trying to contain PED virus, new to the US. Journal of the American Veterinary Medical Association 243, 469–470 (2013). [PubMed] [Google Scholar]
- Bosch B. J., van der Zee R., de Haan C. A. & Rottier P. J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. Journal of virology 77, 8801–8811 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H. et al. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Molecules and cells 14, 295–299 (2002). [PubMed] [Google Scholar]
- Godet M., Grosclaude J., Delmas B. & Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. Journal of virology 68, 8008–8016 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T. J., Han S. C., Yang M. S. & Jang Y. S. Expression of synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat-labile enterotoxin in tobacco plants. Protein expression and purification 46, 16–22, doi: 10.1016/j.pep.2005.07.026 (2006). [DOI] [PubMed] [Google Scholar]
- Oszvald M. et al. Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Molecular biotechnology 35, 215–223 (2007). [DOI] [PubMed] [Google Scholar]
- Lee D. K., Park C. K., Kim S. H. & Lee C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus research 149, 175–182, doi: 10.1016/j.virusres.2010.01.015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J. et al. Sequence analysis of the partial spike glycoprotein gene of porcine epidemic diarrhea viruses isolated in Korea. Virus genes 35, 321–332, doi: 10.1007/s11262-007-0096-x (2007). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Detection and molecular diversity of spike gene of porcine epidemic diarrhea virus in China. Viruses 5, 2601–2613, doi: 10.3390/v5102601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A. N. et al. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerging infectious diseases 20, 1620–1628, doi: 10.3201/eid2010.140491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B. & Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerging infectious diseases 20, 917–919, doi: 10.3201/eid2005.140195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T. et al. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Archives of virology 160, 2565–2568, doi: 10.1007/s00705-015-2522-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T. et al. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Veterinary microbiology 173, 258–269, doi: 10.1016/j.vetmic.2014.08.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. et al. Genetic characteristics of porcine epidemic diarrhea virus in Chinese mainland, revealing genetic markers of classical and variant virulent parental/attenuated strains. Gene 588, 95–102, doi: 10.1016/j.gene.2016.05.011 (2016). [DOI] [PubMed] [Google Scholar]
- Wang E. et al. Molecular Characterization of the ORF3 and S1 Genes of Porcine Epidemic Diarrhea Virus Non S-INDEL Strains in Seven Regions of China, 2015. PloS one 11, e0160561, doi: 10.1371/journal.pone.0160561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. Identification and pathogenicity of a variant porcine epidemic diarrhea virus field strain with reduced virulence. Virology journal 12, 88, doi: 10.1186/s12985-015-0314-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virology journal 12, 193, doi: 10.1186/s12985-015-0421-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D. et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea viruses associated with outbreaks of severe diarrhea in piglets in Jiangxi, China 2013. PloS one 10, e0120310, doi: 10.1371/journal.pone.0120310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S., Bouma P., Williams G. D. & Brian D. A. Stem-loop III in the 5′ untranslated region is a cis-acting element in bovine coronavirus defective interfering RNA replication. Journal of virology 77, 6720–6730 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S. & Brian D. A. Stem-loop IV in the 5′ untranslated region is a cis-acting element in bovine coronavirus defective interfering RNA replication. Journal of virology 79, 12434–12446, doi: 10.1128/JVI.79.19.12434-12446.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S., Izeta A., Sola I. & Enjuanes L. Transcription regulatory sequences and mRNA expression levels in the coronavirus transmissible gastroenteritis virus. Journal of virology 76, 1293–1308 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. et al. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Veterinary microbiology 131, 73–81, doi: 10.1016/j.vetmic.2008.02.022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D. J., Kim C. J. & Shin H. J. Phage-displayed peptides having antigenic similarities with porcine epidemic diarrhea virus (PEDV) neutralizing epitopes. Virology 354, 28–34, doi: 10.1016/j.virol.2006.04.027 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. et al. Phylogenetic analysis of porcine epidemic diarrhea virus field strains prevailing recently in China. Archives of virology 158, 711–715, doi: 10.1007/s00705-012-1541-2 (2013). [DOI] [PubMed] [Google Scholar]
- Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. Journal of virology 89, 1954–1964, doi: 10.1128/JVI.02615-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. Journal of virology 86, 2856–2858, doi: 10.1128/JVI.06882-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D. J., Kim C. J. & Shin H. J. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize Porcine epidemic diarrhea virus. Virus research 132, 192–196, doi: 10.1016/j.virusres.2007.10.015 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim S., Song D. & Park B. Novel porcine epidemic diarrhea virus variant with large genomic deletion, South Korea. Emerging infectious diseases 20, 2089–2092, doi: 10.3201/eid2012.131642 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B. et al. Characterization of a pathogenic full-length cDNA clone of a virulent porcine epidemic diarrhea virus strain AH2012/12 in China. Virology 500, 50–61, doi: 10.1016/j.virol.2016.10.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. et al. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerging infectious diseases 20, 662–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Veterinary research 46, 109, doi: 10.1186/s13567-015-0249-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson D. M. et al. Characterization of Porcine Epidemic Diarrhea Virus Isolate US/Iowa/18984/2013 Infection in 1-Day-Old Cesarean-Derived Colostrum-Deprived Piglets. Veterinary pathology 53, 44–52, doi: 10.1177/0300985815591080 (2016). [DOI] [PubMed] [Google Scholar]
- Stevenson G. W. et al. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc 25, 649–654, doi: 10.1177/1040638713501675 (2013). [DOI] [PubMed] [Google Scholar]