Abstract
Autophagy is an evolutionarily conserved process that plays a crucial role in maintaining a series of cellular functions. It has been found that autophagy is closely involved in the physiological process of spermatogenesis and the regulation of sperm survival and motility. However, the role of autophagy in high-fat diet (HFD)-induced impaired spermatogenesis remains unknown. This study was designed to investigate the role of autophagy in HFD-induced spermatogenesis deficiency and employed chloroquine (CQ) to inhibit autophagy and rapamycin (RAP) to induce autophagy. 3-methyladenine (3-MA) and CQ were administered via intratesticular injection in vivo. The effects of CQ and 3-MA on the parameters of spermatozoa co-cultured with palmitic acid (PA) in vitro were also investigated. Human semen samples from obese, subfertile male patients were also collected to examine the level of autophagy. The results suggested that HFD mice subjected to CQ showed improved spermatogenesis. Inhibiting autophagy with CQ improved the decreased fertility of HFD male mice. Moreover, the in vivo and in vitro results indicated that both CQ and 3-MA could suppress the pathological changes in spermatozoa caused by HFD or PA treatment. Additionally, the excessive activation of autophagy was also observed in sperm samples from obese, subfertile male patients.
Infertility is defined as the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse1,2. Approximately 25–30% of the couples experiencing infertility have male-factor infertility3. Obesity is an acknowledged risk factor for male subfertility4,5,6,7. The mechanisms underlying obesity-induced male spermatogenesis deficiency remain unclear; deciphering these molecular mechanisms could be of great therapeutic interest for spermatogenesis impairment.
The mechanisms underlying spermatogenesis impairment induced by obesity are complex. Endocrine disorders8,9,10,11, genetic components12,13,14,15 and physical or chemical factors16,17 are all involved in the development of subfertility caused by obesity. However, accumulating data indicate a potential role of autophagy in spermatogenesis18,19.
Autophagy, or programmed cell death type II, is an evolutionarily conserved process that plays a crucial role in maintaining a series of physiological functions through the formation of a double-membrane vesicle termed the autophagosome followed by subsequent fusion with lysosomes and degradation of the cytosolic components by resident hydrolases20,21. Autophagy activates apoptosis, which could lead to oligozoospermia and infertility22,23,24. Autophagy also regulates inflammation25, which is closely associated with male spermatogenesis impairment26,27,28,29. Moreover, a recent study indicated that autophagy plays a key role in the impairment of spermatogenesis after heat treatment30. Germ cell-specific knockout of autophagy-related gene 7 (Atg7) causes male infertility in mice19. Autophagy was also indicated to be involved in the regulation of sperm survival and motility18. Autophagy was found to be activated under specific circumstances such as nutrient depletion, growth factor depletion and hypoxia31,32. Previous study indicated that mice fed with high-fat diet (HFD) had reduced hepatic autophagy33, while others reported that nondiabetic HFD mice had upregulated autophagosome formation34. In addition, autophagy is associated with many HFD-related diseases such as nonalcoholic fatty liver disease35, diabetes36 and atherosclerosis37. However, the role of autophagy in obesity-induced spermatogenesis deficiency still remains unknown.
In this study, we used HFD to induce obesity. Both activator and inhibitor of autophagy were employed in this study to explore the role of autophagy in HFD-induced spermatogenesis deficiency. Rapamycin (RAP), which inhibits the mammalian target of RAP (mTOR) signalling pathway by binding directly to the mTOR complex, and acts as an inducer of autophagy by activating the initiation stage. Chloroquine (CQ), which reverses autophagy by inhibiting lysosomal acidification, results in lysosome accumulation and autophagy blockade. Then, we investigated the role of autophagy in HFD-induced spermatogenesis deficiency by employing CQ to inhibit autophagy and RAP to activate autophagy in mice. The fertility of obese mice was also investigated after CQ and RAP treatment. 3-methyladenine (3-MA), based on its inhibitory effect on class III phosphatidylinositol-3 kinase (PI3K) activity, is another widely used autophagy inhibitor. Palmitic acid (PA) was used in the in vitro study to mimic the conditions in vivo as described previously38,39. To exclude the off-target effects of CQ and the possibility that the protection of CQ was secondary to the loss of body weight, both CQ and 3-MA were administered via intratesticular injection or were co-cultured with PA in vivo and in vitro, respectively. In addition, their effects on the viability and motility of mice sperm were explored in this study. Human semen samples were also collected to examine the potential effects of autophagy on the sperm quality of obese subfertile male patients.
Results
Autophagy is over activated in the testis of mice subjected to HFD
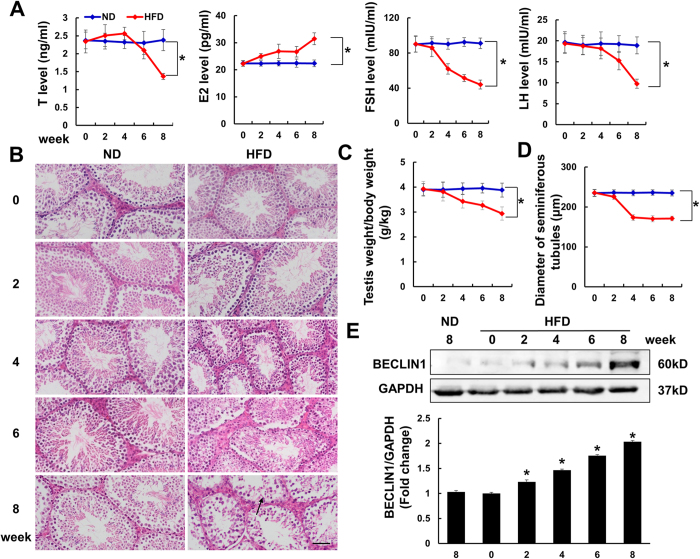
As shown in Fig. 1, the mice fed an HFD had impaired spermatogenesis, as manifested by abnormal serum sex hormone levels (Fig. 1A), decreased testis weight/body weight ratio (Fig. 1C) and pathological histological analysis (Fig. 1B), including atrophied seminiferous tubules (Fig. 1D) and an increased number of vacuoles. Meanwhile, after 8 weeks of the HFD, the mice became obese, and the serum cholesterol level was increased (see Supplementary Figure S1). The blood glucose was also detected. After 8 weeks of HFD feeding, the blood glucose was slightly increased. We also use the homeostasis model assessment of insulin resistance (HOMA-IR) to evaluate insulin resistance and found that there was no difference between the control group and HFD group (see Supplementary Figure S1). The autophagy-related protein BECLIN1 was detected to reflect the level of autophagy in the testis of mice subjected to HFD. As indicated, BECLIN1 was found to increase in the HFD group in a time-dependent manner (Fig. 1E and Supplementary Figure S4).
Figure 1. High-fat diet (HFD) induced male mice spermatogenesis impairment.
(A) Serum hormone levels of testosterone (T), oestradiol (E2), follicle stimulating hormone (FSH) and luteinizing hormone (LH) (n = 6). ND is defined as normal diet. (B) Hematoxylin and eosin (HE) staining of testis of the indicated groups. Vacuoles in the testis are marked with arrow. Magnification: x200 Scale bar: 50 μm (n = 6). (C) Statistical results of testis weight/body weight of the indicated groups (n = 6). (D) Statistical analysis of the diameter of seminiferous tubules in four groups (n = 6). (E) Protein level of autophagy marker BECLIN1 in mice testis (n = 6). Full-length gels are presented in Supplementary Figure S4. Data are expressed as mean ± standard deviation (SD). *p < 0.05, compared with control group.
Autophagy is involved in spermatogenesis deficiency induced by HFD
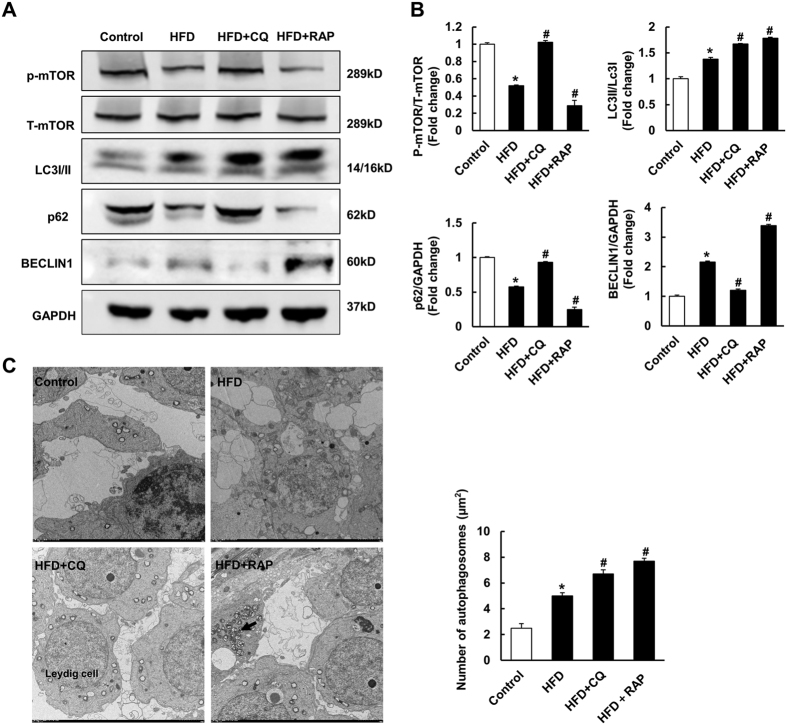
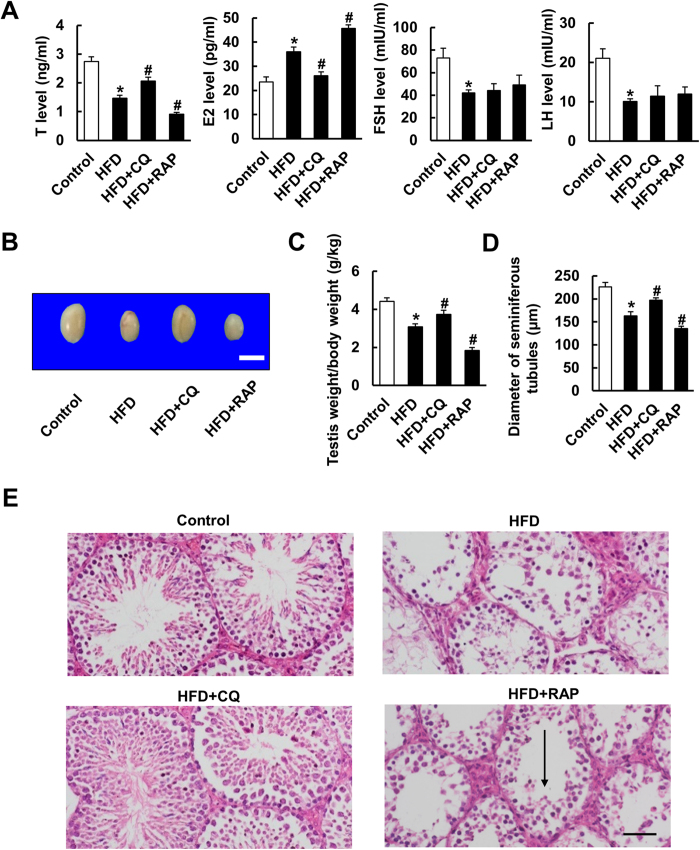
To investigate the role of autophagy in HFD-induced spermatogenesis impairment, CQ and RAP were injected intraperitoneally to inhibit autophagy and induce autophagy, respectively. As illustrated, autophagy was suppressed in mice with CQ injection, as indicated by decreased protein levels of BECLIN1 and increased LC3II/LC3I, p-mTOR and p62 levels. Conversely, the mice treated with RAP showed increased protein levels of BECLIN1 and LC3II/LC3I and decreased p-mTOR and p62 levels, which implied that autophagy was further promoted (Fig. 2A,B and Supplementary Figure S4). As CQ inhibits the degradation of autophagosomes, autophagosomes were increased in both the CQ and RAP treatment groups (Fig. 2C). Subsequently, spermatogenesis function was assessed. As shown in Fig. 3, CQ treatment attenuated HFD-induced spermatogenesis deficiency, as evidenced by increased size of the testis, an improved testis weight/body weight ratio, increased diameter of the seminiferous tubules, and improved serum hormone levels. Conversely, RAP treatment aggravated all these pathological changes. Taken together, these data demonstrate that autophagy is closely involved in HFD-induced spermatogenesis impairment and that inhibiting autophagy with CQ improved spermatogenesis deficiency induced by HFD.
Figure 2. Intraperitoneal injection of chloroquine (CQ) and rapamycin (RAP) on testis autophagy.
(A,B) The protein levels of autophagy related markers in mice testis in indicated groups (n = 6). Full-length gels are presented in Supplementary Figure S4. (C) The presence of autophagosomes (arrow) in Leydig cells of mice testis by transmission electron microscopy (TEM) examination of four groups (n = 6). Data are expressed as mean ± SD. *p < 0.05, compared with control group. #p < 0.05, compared with HFD group.
Figure 3. Suppressing autophagy by CQ ameliorated HFD-induced spermatogenesis deficiency.
(A) Serum hormone levels of T, E2, FSH and LH of the four groups (n = 6). (B) Gross morphology of testis in the indicated groups Scale bar: 2 cm. (C) Statistical results of testis weight/body weight ratio of the indicated groups (n = 10). (D) Statistical analysis of the diameter of seminiferous tubules in four groups (n = 10). (E) HE staining of testis of the indicated groups (n = 10). Vacuoles in the testis were marked with arrow. x200 Scale bar: 50 μm. Data are expressed as mean ± SD. *p < 0.05, compared with control group. #p < 0.05, compared with HFD group.
Intraperitoneal injection of CQ on sperm count, viability and motility
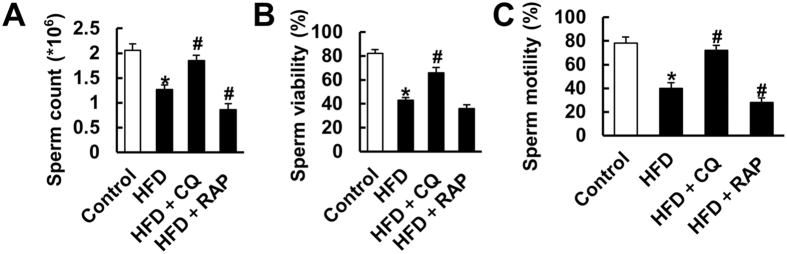
Inconsistent with a previous report showing that obese mice had no differences in total sperm count40, we found that an HFD led to a decrease in total sperm count. Moreover, HFD decreased the sperm viability and motility to 43% and 40%, respectively. Surprisingly, these pathological changes induced by HFD were attenuated after CQ treatment and, indeed, were even slightly potentiated by the addition of the activator of autophagy (Fig. 4).
Figure 4. Inhibiting autophagy by CQ intraperitoneal injection increased sperm viability and motility in mice subjected to HFD.
(A–C) Sperm parameters of mice in the indicated groups (n = 8). Data are expressed as mean ± SD. *p < 0.05, compared with control group. #p < 0.05, compared with HFD group.
Intraperitoneal injection of CQ on fertility and adverse pregnancy outcomes
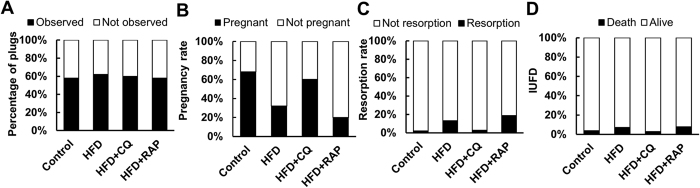
The fact that CQ could improve sperm quality in mice with HFD prompted us to explore the effect of CQ on the fertility of obese mice. Inconsistent with a previous study40, we found that the percentage of female mice with plugs did not change between the control and HFD groups (Fig. 5A). However, subsequent analysis showed that HFD decreased the pregnancy rate (68.0% vs 32.0%, p = 0.0003). As expected, the pregnancy rate increased to 60.0% (p = 0.005) after CQ treatment and decreased to 20.0% (p = 0.171) after RAP treatment (Fig. 5B). Embryo resorption was also observed in the pregnant mice (Fig. 5C). HFD led to an increase in the embryo resorption rate (2.1% vs 13.0%, p < 0.0001), and conversely, CQ treatment reduced the embryo resorption rate (2.9%, p = 0.003). Intra-uterine foetal death (IUFD) in the pregnant female mice mated with lean and obese male mice was counted, but no significant difference was found in the indicated groups (Fig. 5D).
Figure 5. Inhibiting autophagy by CQ injection improved male mice subfertility induced by HFD.
(A) Percentage of plugs of four groups. (B) Pregnancy rate of the indicated groups. (C) Embryo resorption rate of the indicated groups. (D) Intra-uterine foetal death (IUFD) of the four groups.
A single intratesticular injection of CQ and 3-MA improved the motility and viability of sperm in vivo
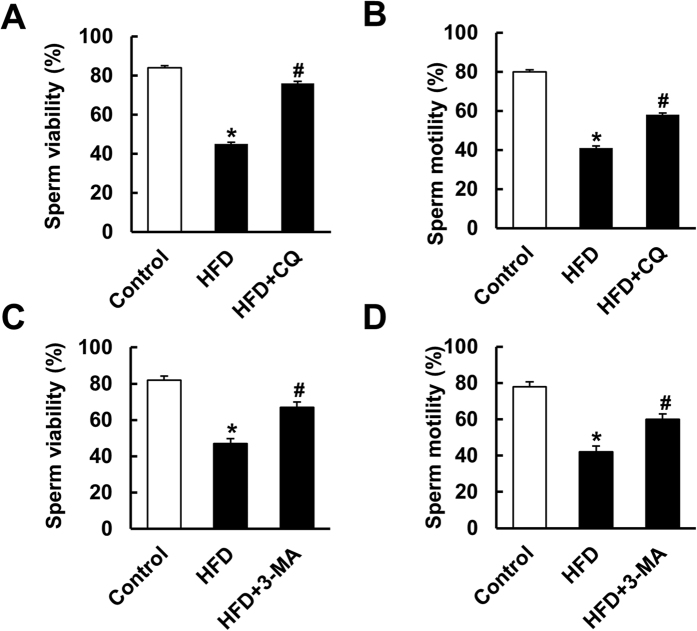
To exclude the possibility that the protection of CQ against spermatogenesis impairment was secondary to the loss of body weight, CQ was administered via a single intratesticular injection. We decided the time point of intratesticular injection for the reason that after 4 weeks HFD feeding, the serum testosterone level and the testis weight/body weight began to decrease (Fig. 1). The morphological alteration of testis was also observed at 4 weeks after HFD (Fig. 1). To exclude the nonspecific effect of chloroquine and further confirm the role of inhibiting autophagy in spermatogenesis in HFD mice, mice were subjected to another autophagy inhibitor, 3-MA. As shown in Fig. 6, both CQ and 3-MA improved the motility and viability of sperm relative to that in the HFD group.
Figure 6. The effects of single intratesticular injection of CQ and 3-methyladenine (3-MA) on sperm viability and motility.
(A,B) Sperm viability and motility after single intratesticular injection of CQ (n = 6). (C,D) Sperm viability and motility after single intratesticular injection of 3-MA (n = 6). Data are expressed as mean ± SD. *p < 0.05, compared with control group. #p < 0.05, compared with HFD group.
CQ and 3-MA attenuated the reduction in the percentages of viable and motile spermatozoa caused by PA
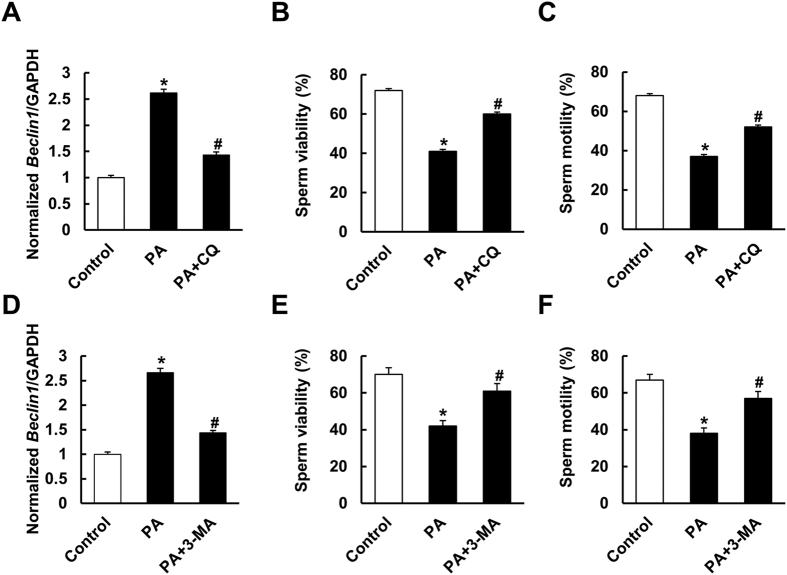
Considering the close relationship between elevated serum free fatty acid level and obesity41,42, and to further decipher the effect of autophagy inhibition, sperm were separated and co-cultured with PA in vitro to mimic the conditions in vivo as described previously38,39. Moreover, we found that the serum PA level and testis PA level were increased in the mice with HFD (see Supplementary Figure S2). Unpredictably, CQ or 3-MA impaired the viability and motility of sperm (see Supplementary Figure S3). However, both CQ and 3-MA clearly attenuated the reduction in viable and motile spermatozoa caused by PA (Fig. 7A–F).
Figure 7. Suppressing autophagy improved palmitic acid (PA)-induced reduction of sperm viability and motility.
(A) mRNA levels of Beclin1 with CQ treatment (n = 6). (B,C) The effects of CQ on sperm viability and motility in vivo (n = 6). (D) mRNA levels of Beclin1 with 3-MA treatment (n = 6). (E,F) The effects of 3-MA on sperm viability and motility in vivo (n = 6). Data are expressed as mean ± SD. *p < 0.05, compared with control group. #p < 0.05, compared with PA group.
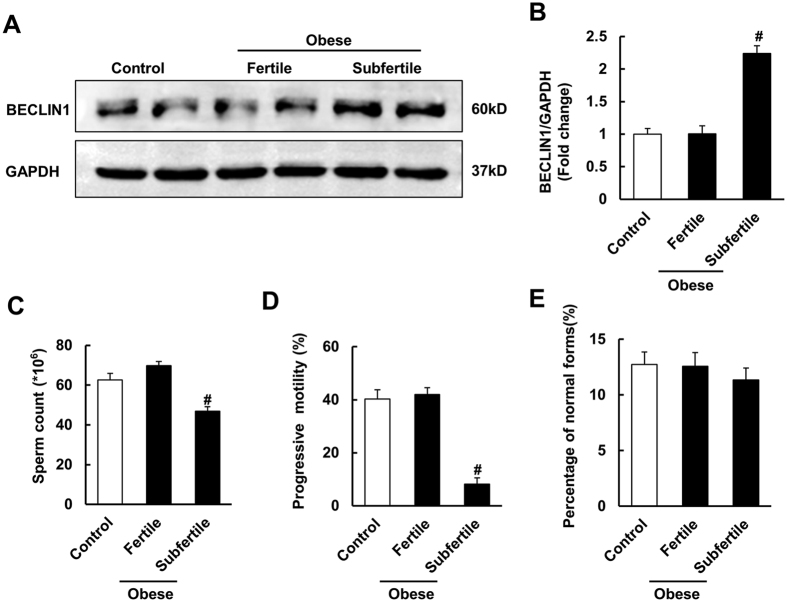
An increased level of BECLIN1 was observed in obese, subfertile male patients
According to World Health Organization guidelines (REF: WHO 2010), the lower reference limits for semen parameters are: sperm concentration ≥15 × 106 spermatozoa/ml, ≥32% progressively motile spermatozoa and ≥4% morphologically normal spermatozoa. Decreased semen quality in obese and subfertile patients reflects a condition when at least one of the parameters is under the lower reference limit. BECLINI was found to be increased in obese patients with decreased semen quality, but no significant difference was found between normal individuals and obese and fertile patients, whose semen parameters were above the lower reference limits (see Supplementary Table S1 and Fig. 8A,B), suggesting an association with impaired sperm quality.
Figure 8. Autophagy was activated in obese and subfertile patients.
(A,B) Protein level of BECLIN1 of human semen samples in the indicated groups. According to World Health Organization guidelines (REF: WHO 2010), the lower reference limits for semen parameters are: sperm concentration ≥15 × 106 spermatozoa/ml, ≥32% progressively motile spermatozoa and ≥4% morphologically normal spermatozoa. Decreased semen quality in obese and subfertile patients reflects a condition when at least one of the parameters is under the lower reference limit. Control group: lean people with normal semen quality; obese and fertile group: obese people (BMI ≥ 30) with normal semen quality; obese and subfertile group: obese people with decreased semen quality. Full-length gels are presented in Supplementary Figure S4. (C–E) Semen parameters of human subjects in the indicated groups. Data are expressed as mean ± SD. #p < 0.05, compared with control group.
Discussion
Autophagy has been implicated in the pathogenesis of different diseases such as cancer43,44, liver disease45,46,47, kidney disease48,49, neurodegenerative disease50,51 and cardiovascular disease52,53. Autophagy was also found to be associated with sperm survival54,55,56, and a recent study also showed that autophagy and apoptosis work synergistically to induce germ cell death during mouse spermatogenesis30. However, there are no related reports about the role of autophagy in HFD-induced male spermatogenesis impairment. The present study is the first to provide insight into the importance of autophagy during HFD-induced spermatogenesis deficiency and find that autophagy is over activated in HFD-induced spermatogenesis deficiency and CQ attenuates spermatogenesis impairment in HFD male mice. CQ also improves sperm count, viability and motility in mice subjected to HFD and decreases adverse pregnancy outcomes. In addition, both in vivo and in vitro studies demonstrated that CQ and 3-MA improved the reduction in sperm viability and motility induced by HFD and PA treatment, respectively. Moreover, the increased protein level of BECLIN1 in human semen samples, indicated that autophagy was activated in obese, subfertile male patients.
In this study, we showed that mice fed an HFD exhibited impaired spermatogenesis function in a time-dependent manner. Meanwhile, the serum steroid hormone levels were obviously changed, as demonstrated by an increased oestradiol (E2) level and decreased testosterone (T), follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels. In addition, these changes were consistent with those of a previous study, which found that seminiferous tubule degeneration is associated with decreased FSH and LH levels57. The fertility of HFD male mice was also found to be damaged, which was in accordance with previous studies indicating that a hypercholesterolemic diet contributed to decreased semen quality and reproductive function in male rabbits58,59. Consistent with the previous finding that autophagy was increased in the testis of mice subjected to heat stress and exposed to formaldehyde30,60, we also found that autophagy was over activated in the testis of HFD mice, implying that autophagy may play a role in HFD-induced spermatogenesis deficiency. Previous study indicated that autophagy was activated in human spermatozoa and was involved in the regulation of cell survival and motility18. Consistent with this finding, the data in our study demonstrated that BECLIN1 was increased in semen samples from obese, subfertile male patients, implying a role of autophagy in human sperm production.
To the best of our knowledge, the primary role of autophagy is as a reparative and cellular cleaning process61,62. The role of autophagy in many other HFD-related diseases has been discussed in the past few decades. Autophagy markers were increased by HFD feeding in many reports36,63, which is consistent with our results, but there is still some evidence supporting a decreased autophagy level with HFD treatment36,64, and dual effects of autophagy in HFD mice were proposed. Recent studies have demonstrated that multiple forms of metabolic stress, including HFD and diabetes, provoke an increase in autophagic flux and that suppression of excessive autophagy could reduce or even reverse the maladaptive response65,66. Moreover, Atg7 knockdown conferred protection against the heat-induced apoptosis of germ cells30. Consistent with these studies, our study demonstrated that CQ, by inhibiting autophagy, obviously attenuated HFD-induced spermatogenesis deficiency and infertility. Conversely, the mice treated with RAP displayed greater impairments in reproductive function. We also injected CQ and 3-MA directly into the testis and found that an improvement in spermatogenesis was undoubtedly produced by inhibiting autophagy. Taken together, these data demonstrated that inhibition of excessive autophagy could protect against HFD-induced spermatogenesis deficiency and subfertility.
The finding that CQ could improve impaired spermatogenesis does not support the notion that inhibiting autophagy is also a protective mechanism for spermatogenesis at basal condition. Conversely, mitophagy prevents paternal mitochondrial DNA transmission67. Atg7 is required for acrosome biogenesis during spermatogenesis, and germ cell-specific Atg7-knockout mice display a phenotype similar to human globozoospermia19. It was also reported that the activation of autophagy is indispensable for the process of decidualization, which was confirmed in diet-induced obesity female mice68. Consistent with these facts, we also found that CQ impaired the viability and motility of sperm in vitro and in vivo at baseline, implying that autophagy is also indispensable for the physiological process of spermatogenesis. However, with regard to the role of autophagy in the pathological process, the results differ. Zhang et al. reported that autophagy was not a protective mechanism against heat-induced apoptosis30. Our data also demonstrated that CQ could improve the quality of sperm in mice fed an HFD by inhibiting autophagy. These discrepant results possibly reflect the different roles of autophagy in physiological and pathological processes. Indeed, the dual nature of autophagy is a recurring theme in other organ systems and disease states31. Therefore, further studies are required to elucidate the dual roles of autophagy in the production of sperm.
There are several types of cells in testis, including Leydig cells, Sertoli cells and spermatogenic cells. The role of autophagy in these cells during HFD-induced impaired spermatogenesis is still unknown. The finding in our study that CQ or 3-MA could attenuate PA-induced sperm injury imply that CQ or 3-MA may directly target on the sperm. Consistent with a previous study69, we also observe the autophagosomes in the Leydig cells, and the decreased T level induced by HFD was restored after CQ treatment, indicating that Leydig cells may mediate CQ-induced protection. The results that CQ or RAP can not affect the level of LH and FSH in the mice with HFD, implying that pituitary gland was not involved in the alteration of hormones caused by the treatment of CQ or RAP.
Despite the results that autophagy was over activated in spermatogenesis when males were exposed to an HFD, we do not yet know which molecules induced such activation. Indeed, an HFD induced the decreased phosphorylation of adenosine monophosphate-activated protein kinase (AMPKα)39 and thus inhibited the activation of mTOR, which is an agonist of autophagy70,71. Previous reports also demonstrated that autophagy is activated in response to different forms of metabolic stress, including nutrient deprivation, growth factor depletion, and hypoxia31,32. Whether these metabolic stresses play roles in HFD-induced spermatogenesis impairment remains unknown. Genetic approaches to inhibit autophagy will yield more conclusive information about the biologic roles of autophagy in spermatogenesis and HFD-induced spermatogenesis deficiency. In addition, our study does not decipher whether HFD-induced autophagy occurs in response to the inciting stress, is a secondary response, promotes or antagonizes disease, or exists as an epiphenomenon.
In conclusion, we have confirmed that autophagy is over activated in HFD-induced spermatogenesis deficiency and found that inhibiting the autophagic process ameliorates HFD-induced spermatogenesis impairment in vitro and in vivo. Our study also indicate that autophagy is increased in semen samples from obese, subfertile male patients. Taken together, our finding provides a new therapeutic target for infertility induced by obesity, and further investigations are required to clarify the specific molecular mechanism and signalling pathway that are involved in the activation of autophagy, especially in human subjects.
Methods
Reagents
CQ (100421-200401) was purchased from the National Institutes for Food and Drug Control (Beijing, China), and RAP (R-5000) was purchased from LC Laboratories (Woburn, MA, USA). 3-MA (M9281) was purchased from Sigma (St. Louis, MO, USA). The rabbit anti-SQSTM1/p62 monoclonal antibody (ab109012) for western blot was purchased from Abcam (Cambridge, UK). The rabbit anti-phospho-mTOR antibody (#2974), rabbit anti-mTOR antibody (#2983), rabbit anti-BECLIN1 antibody (#3495) and rabbit anti-LC3II/I antibody (#12741) for western blot were purchased from Cell Signaling Technology (Beverly, MA, USA). The rabbit anti-GAPDH antibody (sc-25778) for western blot was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The secondary antibody was purchased from LI-COR Biosciences. Oestradiol (E2, E-EL-0065c), testosterone (T, E-EL-0072c), follicle stimulating hormone (FSH, E-EL-M0511c), and luteinizing hormone (LH, E-EL-M0057c) detection kits were purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, China). PA (P9767) was purchased from Sigma (St. Louis, MO, USA). All the other chemicals used in our study were of analytical grade.
Animal care and treatment
All the animal experiments in our study were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011) and the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee and were approved by the Animal Use Committees of Renmin Hospital of Wuhan University. Male C57BL mice (Permit number: 42000600004159, body weight: 20–22 g) were obtained from Hubei Provincial Center for Disease Control and Prevention (Wuhan, China). The mice were housed under a 12 h light: 12 h dark cycle in a temperature (20–25 °C) and humidity (50 ± 5%)-controlled environment with free access to food and water. Mice (n = 40) were randomly divided into four groups, including a control group (Normal diet + 0.1% DMSO, n = 10), an HFD group (HFD + 0.1% DMSO, n = 10), an HFD + CQ group (n = 10) and an HFD + RAP (n = 10) group. All the mice were fed ad libitum either a standard chow or a HFD. Subsequently, the mice in the HFD + CQ or HFD + RAP groups were intraperitoneally injected with CQ (10 mg/ kg)72 or RAP (1 mg/ kg)73 once per day for 8 weeks74,75, and the mice in the control group were given the same volume of vehicle. CQ was dissolved in sterile saline, and RAP was dissolved in 0.1% DMSO. After that, the mice were anaesthetized with an intraperitoneal injection of 3% sodium pentobarbital, and blood samples were obtained from the angular veins. Then, mice were euthanized with an intraperitoneal injection excessive sodium pentobarbital, the testes were collected for further experiments and the testis weight/body weight ratio was calculated. To further confirm the effect of autophagy inhibition on spermatogenesis in HFD male mice, a single intratesticular injection was performed. After mice had received the HFD for 4 weeks, 40 μl of the autophagy inhibitor CQ (300 μM) or 3-MA (10 mM, dissolved in sterile saline)19 was injected under the tunica vaginalis of the testes with a 50 μl micro-injector at a depth of 5 mm under the tunica albuginea as described previously76. After injection, the mice were fostered for another 4 weeks. Spermatozoa were taken from the epididymis to examine their viability and motility.
Determination of serum hormone levels
Blood samples were collected from angular veins. After centrifuging the samples at 1409 g and 4 °C for 15 minutes, the supernatant sera were obtained and stored at −80 °C until analysis. The levels of T, E2, FSH and LH in the serum were measured using commercially available ELISA kits according to the manufacturer’s instructions. Briefly, ninety-six-well plates were coated with the primary antibody at 37 °C for 2 h. After being blocked with 5% milk, the serum were added into the plates. The plates were incubated at 37 °C for 2 h. After that, the plates were incubated with peroxidase-conjugated second antibodies at 37 °C for 1 h. After that, the optical density values were detected by a microplate reader (Biotek, Vermont, USA).
Histological analysis
The sections of testis tissue were routinely fixed in Bouin’s solution, dehydrated, and embedded in paraffin. Testis tissue sections were stained with haematoxylin and eosin (H&E) to examine morphology. Sections (5 μm) were viewed under light microscopy (Nikon E100), and photomicrographs were captured with the Photo Imaging System (Canon 600D). The diameter of seminiferous tubules was determined using Image-Pro Plus 6.0. In each group, 100 seminiferous tubules (5 fields per mouse, 2 random seminiferous tubules per field) were measured in 10 mice, and the mean seminiferous tubule diameter was determined.
Protein extraction and western blot assay
RIPA buffer (720 μl radioimmunoprecipitation buffer, 100 mmol/l PMSF, 100 μl cocktail, 100 μl Phos-stop, 20 mmol/l NaF and 100 mmol/l Na3VO4 in 1 ml) was used to extract the proteins from testis tissue of mice and sperm samples of mice and humans77. The protein concentrations were detected with a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Proteins (20 μg) were separated on SDS-PAGE gels (LC3I/II: 15%; mTOR and p-mTOR: 6%; Others: 10%) for 2 h (75 V 30 min; 100 V 90 min) at room temperature and then transferred to a PVDF membrane (IPVH00010, Millipore, Billerica, MA, USA) at 4 °C for different times (200 mA; LC3I/II: 70 min; mTOR and p-mTOR: 120 min; Others: 90 min). After being blocked with 5% non-fat milk, the membranes were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-GAPDH antibody (1:5000), rabbit anti-BECLIN1 antibody (1:1000), rabbit anti-SQSTM/p62 monoclonal antibody (1:10000), rabbit anti-LC3II/I antibody (1:1000), rabbit anti-phospho-mTOR antibody (1:1000) and rabbit anti-mTOR antibody (1:1000). After incubation with the secondary antibody (1:5000), the membranes were scanned using a two-colour infrared imaging system (Odyssey, LI-COR, Lincoln, NE, USA). Densitometry analysis was performed by Odyssey as described previously78, and the results were normalized to GAPDH.
RNA isolation and qPCR analysis of mouse semen samples
Sperm samples of mice were homogenized in TRIzol (Invitrogen, Massachusetts, USA) on ice, and total mRNA was extracted. mRNA was reverse transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (04896866001, Roche, USA) according to the manufacturer’s instructions. All experiments were performed in triplicate, and quantitative RT-PCR analysis was carried out using the Light Cycler 480 SYBR Green 1 Master Mix (04707516001, Roche, USA). The primer sequences for Beclin1 were: forward: 5′-ATCCTGGACCGTGTCACCATCCAGG-3′; reverse: 5′-GTTGAGCTGAGTGTCCAGCTGG-3′. The primer sequences for Gapdh were: forward: 5′-GTTGTCTCCTGCGACTTCA-3′; reverse: 5′-GGTGGTCCAGGGTTTCTTA-3′. The mRNA levels were analysed using the 2−ΔΔCt method and normalized to Gapdh. The qPCR analysis was performed as described in a previous report79.
Transmission electron microscopy (TEM)
Testis tissue was fixed with 2.5% glutaraldehyde, and then these tissues were post-fixed in 1% OsO4 for 2 h. After rehydration, these tissues were embedded in epoxy resin. Ultrathin sections (80 nm) were collected on formvar-coated copper grids. Subsequently, these grids were counter-stained with uranyl acetate and lead citrate and examined on an EM2010FEF-Ω transmission electron microscope (JEOL, Tokyo, Japan). Autophagosomes were detected as described in a previous study80 and identified by the presence of a double-membrane vesicle that contained cytosol and/or organelles and looked morphologically intact.
Semen analysis in mice
The epididymis was carefully dissected away from the fat. The separated epididymides were immediately placed into Ringer’s solution and dissected to determine the count, viability and motility of the sperm as described in a previous study40. The sperm were gradually pressed out from the epididymis and collected in an Eppendorf tube. The sperm number was counted with a haemocytometer independently for three times.
Eosin-nigrosin staining solution (1% eosin and 10% nigrosin) was used to detect sperm viability. Semen samples were first incubated with the eosin-nigrosin staining solution and smeared on a microscope slide. The spermatozoa were observed under a light microscope at 100x magnification. Live spermatozoa is not stained, while dead spermatozoa with disintegrating cell membranes take up the stain81. Sperm motility was tested with computer-assisted sperm analysis (CASA). Sperm were incubated in Ringer’s solution at room temperature for 30 minutes and placed into CASA assay chambers (Hamilton Thorne Research, Beverly, MA, USA). Sperm tracks (1.5 s, 30 frames) were captured at 60 Hz and were analysed with HTM-IVOS Sperm Analyzer software (version 12.2 L; Hamilton Thorne)82. Five hundred sperm cells were randomly selected and evaluated to obtain the sperm viability and sperm motility; at least three replications were completed for each sample.
Assessment of the fertility of male mice
At the end of the 8-week HFD, the mice receiving different treatments were used in a breeding assay. Each male mouse (n = 5) was caged with two females for five days; consequently, a total of 50 females were involved in the study, and their vaginal plugs were checked every morning. The day on which the vaginal plug observed was defined as day 0.5 of gestation, and on day 14.5 of gestation, female mice were euthanized to count the number of dead foetuses and resorptions.
In vitro study
Mouse sperm samples obtained from the cauda epididymis were diluted to attain a concentration of 5 × 106 spermatozoa/ml and incubated in G-IVF (Vitrolife, Sweden) medium, fatty medium (1 mM PA)83 or fatty medium with CQ (100 μM) and 3-MA (10 mM)84. Incubations were performed under a humidified atmosphere of 5% CO2/95% O2 and 37 °C for 5 h, and sperm viability and motility were assessed as described above. Three independent experiments were carried out in the in vitro study.
Human studies
The human studies conformed to the Declaration of Helsinki and were approved by the Human Research Ethics Committees of Renmin Hospital of Wuhan University in Wuhan, China. All the participants in this study were interviewed after signing an informed consent. Semen samples were obtained from subjects who attended the infertility clinic by masturbation after 3–7 days of sexual abstinence. The interviewers collected the data of all participants, including their ethnicity, age, height, body weight, medical history, and lifestyle through a structured questionnaire. The subjects were ethnic Han Chinese from Hubei province and its nearby regions. They were men who visited the Reproductive Medical Center of Renmin Hospital of Wuhan University for infertility treatment from June 2014 to December 2014. All subjects were age-matched, and men who were unhealthy with other causes of defective spermatogenesi85, including infection, varicocele, obstruction of the vas deferens, chromosomal abnormalities or smokers, were excluded. Finally, all semen samples were divided into three groups, including the control group (n = 85), obese and fertile group (n = 65), and obese and subfertile group (n = 79). The semen samples in the control group were from individuals with normal body mass index (BMI) and normal semen parameters as described above. The semen samples in the obese and fertile group were from men whose BMIs were more than 30 and had normal semen quality, while the semen samples in the obese and subfertile group were from those whose BMIs were more than 30 and had decreased semen quality. The semen samples were liquefied for 15–30 minutes at 37 °C, and subsequently the semen parameters were tested in accordance with the World Health Organization guidelines. The residual semen samples were used for protein extraction and subsequent western blotting analysis.
Data analysis
Group data are reported as the mean ± standard deviation (SD). All statistical tests were performed with SPSS 19.0. All the data in the present study were normally distributed (p < 0.05), as determined by the Kolmogorov-Smirnov’s test. Two-group comparisons were analysed with an unpaired Student’s t-test after confirming equality of variance with the F test. Multiple-group comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test70 when ANOVA analysis indicated a significant value of F and no variance in homogeneity; otherwise, Tamhane’s T2 post hoc test was used. The percentage of plugs, the pregnancy rate, the resorption rate and the IUFD between different groups were compared using a chi-square test. P-values less than 0.05 were considered significant.
Additional Information
How to cite this article: Mu, Y. et al. Diet-induced obesity impairs spermatogenesis: a potential role for autophagy. Sci. Rep. 7, 43475; doi: 10.1038/srep43475 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81370767, grant recipient J.Y.), the National Natural Science Foundation of China (81401255, grant recipient J.L.) and the Natural Science Foundation of Hubei Province (2015CFB722, grant recipient Y.Z.).
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.M., W.J.Y., T.L.Y., Y.Z. and J.Y. were involved in the study design and preparation of the manuscript. Y.M. carried out the experiments in the study with W.J.Y., T.L.Y. and J.L. analyzed the data, drafted the manuscript and had critical discussion with Y.Z., J.Y. and W.J.Y.
References
- Chandra A., Martinez G. M., Mosher W. D., Abma J. C. & Jones J. Fertility, family planning, and reproductive health of US women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23, 1–160 (2005). [PubMed] [Google Scholar]
- Chandra A. & Stephen E. H. Infertility service use among US women: 1995 and 2002. FERTIL STERIL 93, 725–736 (2010). [DOI] [PubMed] [Google Scholar]
- Hammoud A. O., Gibson M., Peterson C. M., Hamilton B. D. & Carrell D. T. Obesity and male reproductive potential. J Androl 27, 619–626 (2006). [DOI] [PubMed] [Google Scholar]
- Jensen T. K. et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. FERTIL STERIL 82, 863–870 (2004). [DOI] [PubMed] [Google Scholar]
- Kort H. I. et al. Impact of body mass index values on sperm quantity and quality. J Androl 27, 450–452 (2006). [DOI] [PubMed] [Google Scholar]
- Bieniek J. M. et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. FERTIL STERIL 106, 1070–1075 (2016). [DOI] [PubMed] [Google Scholar]
- Andersen J. M. et al. Body Mass Index Is Associated with Impaired Semen Characteristics and Reduced Levels of Anti-Mullerian Hormone across a Wide Weight Range. PLOS ONE 10, e130210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fui M. N., Dupuis P. & Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. ASIAN J ANDROL 16, 223–231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho E. M. et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. EUR J ENDOCRINOL 168, 445–455 (2013). [DOI] [PubMed] [Google Scholar]
- Tajar A. et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 95, 1810–1818 (2010). [DOI] [PubMed] [Google Scholar]
- Ayanian S. & Irwig M. S. Hypogonadism in a male-to-female transsexual with super obesity. ANDROLOGIA 45, 285–288 (2013). [DOI] [PubMed] [Google Scholar]
- Aksglaede L. et al. Serum concentrations of Anti-Mullerian Hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism. ACTA PAEDIATR 100, 839–845 (2011). [DOI] [PubMed] [Google Scholar]
- Dupont C. et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. ASIAN J ANDROL 15, 622–625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud A. et al. An aromatase polymorphism modulates the relationship between weight and estradiol levels in obese men. FERTIL STERIL 94, 1734–1738 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro J. E., Toth T. L., Wright D. L., Meeker J. D. & Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. FERTIL STERIL 93, 2222–2231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud A. O. et al. Obesity and male infertility: a practical approach. SEMIN REPROD MED 30, 486–495 (2012). [DOI] [PubMed] [Google Scholar]
- Hammoud A. O., Carrell D. T., Gibson M., Peterson C. M. & Meikle A. W. Updates on the relation of weight excess and reproductive function in men: sleep apnea as a new area of interest. ASIAN J ANDROL 14, 77–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio I. M. et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep 6, 33647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. CELL RES 24, 852–869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. & Klionsky D. J. Eaten alive: a history of macroautophagy. NAT CELL BIOL 12, 814–822 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide T. & Huber T. B. Implications of autophagy for glomerular aging and disease. CELL TISSUE RES 343, 467–473 (2011). [DOI] [PubMed] [Google Scholar]
- Rotter V. et al. Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc Natl Acad Sci USA 90, 9075–9079 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. M., Tung K. S., Tourtellotte W. G., Brown G. A. & Korsmeyer S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. SCIENCE 270, 96–99 (1995). [DOI] [PubMed] [Google Scholar]
- Lee H. W. et al. Essential role of mouse telomerase in highly proliferative organs. NATURE 392, 569–574 (1998). [DOI] [PubMed] [Google Scholar]
- Levine B., Mizushima N. & Virgin H. W. Autophagy in immunity and inflammation. NATURE 469, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumber J., Sabeur K., Vo A. & Ball B. A. Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. THERIOGENOLOGY 60, 1239–1247 (2003). [DOI] [PubMed] [Google Scholar]
- Baccetti B., Collodel G. & Piomboni P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9). J Submicrosc Cytol Pathol 28, 587–596 (1996). [PubMed] [Google Scholar]
- Oosterhuis G. J. et al. Measuring apoptosis in human spermatozoa: a biological assay for semen quality? FERTIL STERIL 74, 245–250 (2000). [DOI] [PubMed] [Google Scholar]
- Haidl G., Allam J. P. & Schuppe H. C. Chronic epididymitis: impact on semen parameters and therapeutic options. ANDROLOGIA 40, 92–96 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang M. et al. Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLOS ONE 7, e41412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J., Hara H. & Kuwano K. Autophagy in the pathogenesis of pulmonary disease. Intern Med 52, 2295–2303 (2013). [DOI] [PubMed] [Google Scholar]
- Malicdan M. C., Noguchi S. & Nishino I. Autophagy in a mouse model of distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. AUTOPHAGY 3, 396–398 (2007). [DOI] [PubMed] [Google Scholar]
- Liu H. Y. et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J BIOL CHEM 284, 31484–31492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato C. et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. CELL METAB 8, 325–332 (2008). [DOI] [PubMed] [Google Scholar]
- Mao Y. et al. Ghrelin Attenuated Lipotoxicity via Autophagy Induction and Nuclear Factor-kappaB Inhibition. CELL PHYSIOL BIOCHEM 37, 563–576 (2015). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. DIABETES 64, 36–48 (2015). [DOI] [PubMed] [Google Scholar]
- Wang B., Zhong Y., Huang D. & Li J. Macrophage autophagy regulated by miR-384-5p-mediated control of Beclin-1 plays a role in the development of atherosclerosis. AM J TRANSL RES 8, 606–614 (2016). [PMC free article] [PubMed] [Google Scholar]
- Zeng W. et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep 5, 16013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara Y. et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. CIRC RES 116, 279–288 (2015). [DOI] [PubMed] [Google Scholar]
- Ghanayem B. I., Bai R., Kissling G. E., Travlos G. & Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. BIOL REPROD 82, 96–104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C. et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J CLIN INVEST 84, 205–213 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco M. et al. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. METABOLISM 49, 220–224 (2000). [DOI] [PubMed] [Google Scholar]
- Guo J. Y., Xia B. & White E. Autophagy-mediated tumor promotion. CELL 155, 1216–1219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Cell biology: autophagy and cancer. NATURE 446, 745–747 (2007). [DOI] [PubMed] [Google Scholar]
- Alavian S. M. et al. Virus-triggered autophagy in viral hepatitis - possible novel strategies for drug development. J Viral Hepat 18, 821–830 (2011). [DOI] [PubMed] [Google Scholar]
- Wang K., Damjanov I. & Wan Y. J. The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury. LAB INVEST 90, 257–265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osna N. A., Thomes P. G. & Donohue T. M. Jr. Involvement of autophagy in alcoholic liver injury and hepatitis C pathogenesis. World J Gastroenterol 17, 2507–2514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K. et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J 17, 1165–1167 (2003). [DOI] [PubMed] [Google Scholar]
- Chien C. T., Shyue S. K. & Lai M. K. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. TRANSPLANTATION 84, 1183–1190 (2007). [DOI] [PubMed] [Google Scholar]
- Banerjee R., Beal M. F. & Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. TRENDS NEUROSCI 33, 541–549 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. NATURE 441, 885–889 (2006). [DOI] [PubMed] [Google Scholar]
- Marzetti E. et al. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol 305, H459–H476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemchenko A., Chiong M., Turer A., Lavandero S. & Hill J. A. Autophagy as a therapeutic target in cardiovascular disease. J MOL CELL CARDIOL 51, 584–593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo B. J. et al. Autophagy and apoptosis have a role in the survival or death of stallion spermatozoa during conservation in refrigeration. PLOS ONE 7, e30688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos J. M. et al. During cooled storage the extender influences processed autophagy marker light chain 3 (LC3B) of stallion spermatozoa. ANIM REPROD SCI 145, 40–46 (2014). [DOI] [PubMed] [Google Scholar]
- Aparicio I. M., Martin M. P., Salido G. M., Pena F. J. & Tapia J. A. The autophagy-related protein LC3 is processed in stallion spermatozoa during short-and long-term storage and the related stressful conditions. ANIMAL 10, 1182–1191 (2016). [DOI] [PubMed] [Google Scholar]
- Sharma S. et al. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LbetaT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. ENDOCRINOLOGY 154, 2188–2199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L. T. et al. Semen quality and sperm function loss by hypercholesterolemic diet was recovered by addition of olive oil to diet in rabbit. PLOS ONE 8, e52386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L. T. et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLOS ONE 5, e13457 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. P. et al. Formaldehyde exposure induces autophagy in testicular tissues of adult male rats. ENVIRON TOXICOL 30, 323–331 (2015). [DOI] [PubMed] [Google Scholar]
- Levine B. & Klionsky D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. DEV CELL 6, 463–477 (2004). [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Limback D., McGinnis L. K., Plancha C. E. & Albertini D. F. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. REPRODUCTION 137, 709–720 (2009). [DOI] [PubMed] [Google Scholar]
- Sun Q. et al. Factors that Affect Pancreatic Islet Cell Autophagy in Adult Rats: Evaluation of a Calorie-Restricted Diet and a High-Fat Diet. PLOS ONE 11, e151104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codogno P. & Meijer A. J. Autophagy: a potential link between obesity and insulin resistance. CELL METAB 11, 449–451 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang T. Z. et al. Suppressing autophagy protects photoreceptor cells from light-induced injury. Biochem Biophys Res Commun 450, 966–972 (2014). [DOI] [PubMed] [Google Scholar]
- Ojha R., Jha V., Singh S. K. & Bhattacharyya S. Autophagy inhibition suppresses the tumorigenic potential of cancer stem cell enriched side population in bladder cancer. Biochim Biophys Acta 1842, 2073–2086 (2014). [DOI] [PubMed] [Google Scholar]
- Al R. S. et al. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. SCIENCE 334, 1144–1147 (2011). [DOI] [PubMed] [Google Scholar]
- Rhee J. S. et al. Diet-induced obesity impairs endometrial stromal cell decidualization: a potential role for impaired autophagy. HUM REPROD 31, 1315–1326 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. M., Clermont Y. & Hermo L. Origin and fate of autophagosomes in Leydig cells of normal adult rats. J Androl 9, 284–293 (1988). [DOI] [PubMed] [Google Scholar]
- Ma Z. G. et al. Asiatic Acid Protects against Cardiac Hypertrophy through Activating AMPKalpha Signalling Pathway. INT J BIOL SCI 12, 861–871 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z. G. et al. Protection against cardiac hypertrophy by geniposide involves the GLP-1 receptor/AMPKalpha signalling pathway. Br J Pharmacol 173, 1502–1516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanlawon A. O., Noronha C. C. & Ashiru O. A. An investigation into the effects of chloroquine on fertility of male rats. West Afr J Med 12, 118–121 (1993). [PubMed] [Google Scholar]
- Scarpace P. J. et al. Rapamycin Normalizes Serum Leptin by Alleviating Obesity and Reducing Leptin Synthesis in Aged Rats. J Gerontol A Biol Sci Med Sci 71, 891–899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. et al. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe (−/−) Mice Fed with High-Fat Diet. J DIABETES RES 2016, 1748065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D. Y. et al. Reduced cell proliferation and neuroblast differentiation in the dentate gyrus of high fat diet-fed mice are ameliorated by metformin and glimepiride treatment. NEUROCHEM RES 36, 2401–2408 (2011). [DOI] [PubMed] [Google Scholar]
- Sato M., Ishikawa A. & Kimura M. Direct injection of foreign DNA into mouse testis as a possible in vivo gene transfer system via epididymal spermatozoa. MOL REPROD DEV 61, 49–56 (2002). [DOI] [PubMed] [Google Scholar]
- Mu Y., Yan W. J., Yin T. L. & Yang J. Curcumin ameliorates highfat dietinduced spermatogenesis dysfunction. MOL MED REP 14, 3588–3594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Toll-interacting protein (Tollip) negatively regulates pressure overload-induced ventricular hypertrophy in mice. CARDIOVASC RES 101, 87–96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. CLIN CHEM 55, 611–622 (2009). [DOI] [PubMed] [Google Scholar]
- Klionsky D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. AUTOPHAGY 8, 445–544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokilavani P. et al. Antioxidant mediated ameliorative steroidogenesis by Commelina benghalensis L. and Cissus quadrangularis L. against quinalphos induced male reproductive toxicity. Pestic Biochem Physiol 109, 18–33 (2014). [DOI] [PubMed] [Google Scholar]
- Wei Z. T. et al. The long-term effects of superovulation on fertility and sexual behavior of male offspring in mice. J Assist Reprod Genet 31, 555–560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. et al. Rapamycin improves palmitate-induced ER stress/NF kappa B pathways associated with stimulating autophagy in adipocytes. Mediators Inflamm 2015, 272313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng A. C. et al. Metformin increases degradation of phospholamban via autophagy in cardiomyocytes. Proc Natl Acad Sci USA 112, 7165–7170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B. et al. Cigarette smoking is associated with abnormal histone-to-protamine transition in human sperm. FERTIL STERIL 101, 51–57 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.