Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD), defined as excessive liver fat deposition and one of end-stage liver disease causes. Increased ferritin levels are associated with insulin resistance and a higher hepatic iron and fat content. Hyperferritinemia has been associated with severity of liver damage in NAFLD. The study aimed to evaluate the effects of phlebotomy on liver enzymes and histology in such patients.
Materials and Methods:
Thirty-two eligible patients who had NAFLD and after 6 months of lifestyle modification still had NAFLD, and whose ferritin serum was above 250 mg/dl, were enrolled in this clinical trial study. After written informed consent was obtained, each patient's blood serum was taken for aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALK-P), complete blood count (CBC), total iron-binding capacity (TIBC), iron, and ferritin. Then the patients underwent liver biopsy. After that patients underwent phlebotomy, giving 350 cc blood monthly. Before every phlebotomy, hemoglobin and ferritin were checked. If they were in the goal range, phlebotomy was discontinued and the patient underwent liver biopsy. A serum sample was taken for testing at the beginning of the study. The results before and after phlebotomy were compared. The maximum duration of the study was 6 months.
Results:
Thirty-two patients (26 males and 6 females) were enrolled, and the mean average age was 33.7 ± 6.74 years. Phlebotomy improved liver enzymes and histology of liver significantly (P < 0.001) and induced reduction of ferritin.
Conclusion:
Phlebotomy is effective for the improvement of liver enzymes and histology in patients with NAFLD and hyperferritinemia.
Keywords: Hyperferritinemia, nonalcoholic fatty liver disease, phlebotomy
Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses the accumulation of triglycerides in the cytoplasm of hepatocytes in the rate of 5-10% weight in the absence of liver disease, alcohol consumption, and other diseases.[1] The pathogenesis of the disease is unclear but may be the result of several factors, including insulin resistance, oxidative stress dyslipidemia, abdominal obesity, diabetes, hypertension, and some additional factors.[1,2] NAFLD is the most common liver disease and its prevalence in the general population is 20-30%.[2] It is considered a hepatic manifestation of metabolic syndrome and includes a wide range of diseases from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis.[3] In fact, 20% of those with NASH may evolve to cirrhosis during their lifetimes and it is the third leading cause of liver transplants in the world.[4] Therefore, the diagnosis and treatment of NASH and modification to address the underlying risk factors are of great importance.[2] A diagnosis of NAFLD requires confirmation of hepatic steatosis based on imaging, biochemical studies, and liver biopsy. Most patients with NAFLD are asymptomatic and it is detected during routine checkup or other imaging for other conditions such as gallbladder problems, but there are some clinical symptoms that are nonspecific and include fatigue and malaise, abdominal discomfort, pain in the right upper quadrant, nausea, and other symptoms.[3] Although the pathogenesis of this disease is still poorly understood, two hypotheses has been expressed. The first hypothesis involves triglyceride accumulation in the liver, causing steatosis, and the second hypothesis involves inflammation, oxidative stress, and inflammatory cytokines causing damage to liver cells.[2,3,4]
Currently there is no effective full treatment for NAFLD; weight loss and physical activity that increases insulin sensitivity are the most effective therapies for the treatment of this disease, but they have not been successful in curing the disease and there is no effective drug therapy for it.[5,6] The medications available are used to treat the underlying risk factors. Insulin sensitizers, hepatoprotectives, lipid-lowering agents, angiotensin receptor blockers, and antioxidants are used.[7]
Hyperferritinemia with mild hepatic iron accumulation is observed in 20-30% of patients with NAFLD and is commonly referred to as dysmetabolic iron overload syndrome.[8] Besides directly inducing liver damage, excess iron is also involved in the pathogenesis of metabolic syndrome by inducing adipose tissue, insulin resistance, and modifying the release of adipokines.[9] Furthermore, hyperferritinemia and increased iron stores have been associated with greater severity of liver disease and hepatocellular carcinoma in patients with NAFLD.[10]
Therefore, previous studies have evaluated the therapeutic value of iron depletion in NAFLD. Phlebotomy ameliorated insulin resistance more than lifestyle changes alone, whereas in a propensity score-adjusted multicenter study with the prospective evaluation of 198 patients, it increased the likelihood of alanine aminotransferase (ALT) normalization at the end of follow-up.[11] It has been associated with oxidative stress and mild hepatic iron overload, sometimes in the presence of common mutations of the C282Y and H63D polymorphisms of the HFE gene associated with hereditary hemochromatosis. Increased iron stores have been associated with an altered adipokine profile and insulin resistance in humans, and increased liver iron may induce hepatic insulin resistance.[12] Finally, in patients with NAFLD, hyperferritinemia has been associated with progressive liver damage. After the hypothesis that increased iron stores may play a role in the pathogenesis of insulin resistance and liver damage by increasing oxidative stress, a few studies have evaluated the effect of iron depletion on metabolic parameters, and it was reported to improve insulin resistance and liver enzymes in a small number of NAFLD patients without iron overload and either impaired or normal glucose tolerance.[13,14] Therefore, the aim of this study is to evaluate the role of phlebotomy in the improvement of liver enzymes and histology in NAFLD.
Materials and Methods
In this clinical trial study, 32 patients who according to the clinical evidence, liver enzymes, and sonographic criteria had NAFLD, and after 6 months of lifestyle modification still had fatty liver and their ferritin was above 250 mg/dl, were enrolled. Patients who were eligible to enter the study were selected and after written informed consent was obtained from them, their blood serum was taken for aspartate transaminase (AST), ALT, alkaline phosphatase (ALK-P), bilirubin, and albumin. Then the patients at baseline underwent liver biopsy by a gastroenterologist. After that the patients underwent phlebotomy, giving 350 cc blood monthly. Before phlebotomy was performed, patients had been checked for hemoglobin (Hb), so if Hb <12 mg/dl in women and Hb <13 mg/dl in men, the phlebotomy was discontinued and the patient underwent liver biopsy, and a serum sample was taken for testing at the beginning of the study. The maximum duration of the study was 6 months. The biopsy and biochemical results were compared before and after phlebotomy. To check the results, the software SPSS V.20 (SPSS Inc., Chicago, IL, USA) and statistical tests, i.e., the sample t-test and the Wilcoxon signed-rank test have been used and a P value of less than 0.05 was considered meaningful.
The inclusion criteria were: (1) Age 18-50 years, (2) nonalcoholic fatty liver on ultrasound, (3) increased serum ALT greater than 1.5 against the normal, and (4) ferritin above 250 mg/dl. The exclusion criteria were: (1) Decompensated liver failure, (2) pregnancy and lactation, (3) diabetes type 1 and 2, (4) thyroid disease, (5) alcohol consumption more than 20 g/day in women and 30 g/day in men, (6) other diseases of the liver including, hemochromatosis, chronic viral hepatitis, consumption of medications that cause nonalcoholic fatty liver such as corticosteroids, estrogen, tamoxifen, and amiodarone.
Results
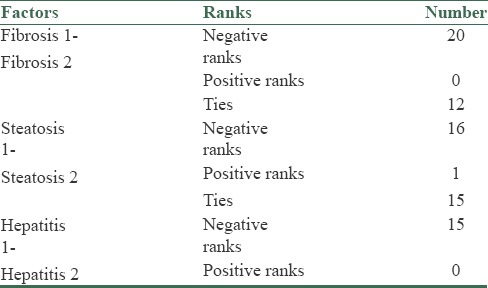
In this study, 32 patients (26 males and 6 females) were enrolled. The mean average age of the subjects was 33.7 ± 6.74 years. The mean average of ferritin at baseline was 434.2 ± 142.77 mg/dl. The mean average of liver enzymes is shown in Table 1. It was shown that phlebotomy had a significant positive effect on liver enzymes (P < 0/0001). A paired t-test showed that after 6 months of phlebotomy mean ALT, AST, and ALKP decreased significantly from baseline (P < 0/0001) [Tables 2 and 3]. The mean average of liver histology grading at baseline and after phlebotomy is described in Tables 3 and 4. The grades of histology showed significant improvement after phlebotomy (P < 0/001). The Wilcoxon test showed that the grades of fibrosis, steatosis, and hepatitis after phlebotomy were significantly reduced compared to baseline [Table 5]. Generally the results showed that in the case of fibrosis, 20 patients improved after phlebotomy, no one's condition worsened, and 12 patients remained unchanged.
Table 1.
The mean average of liver enzymes and demographic characteristics
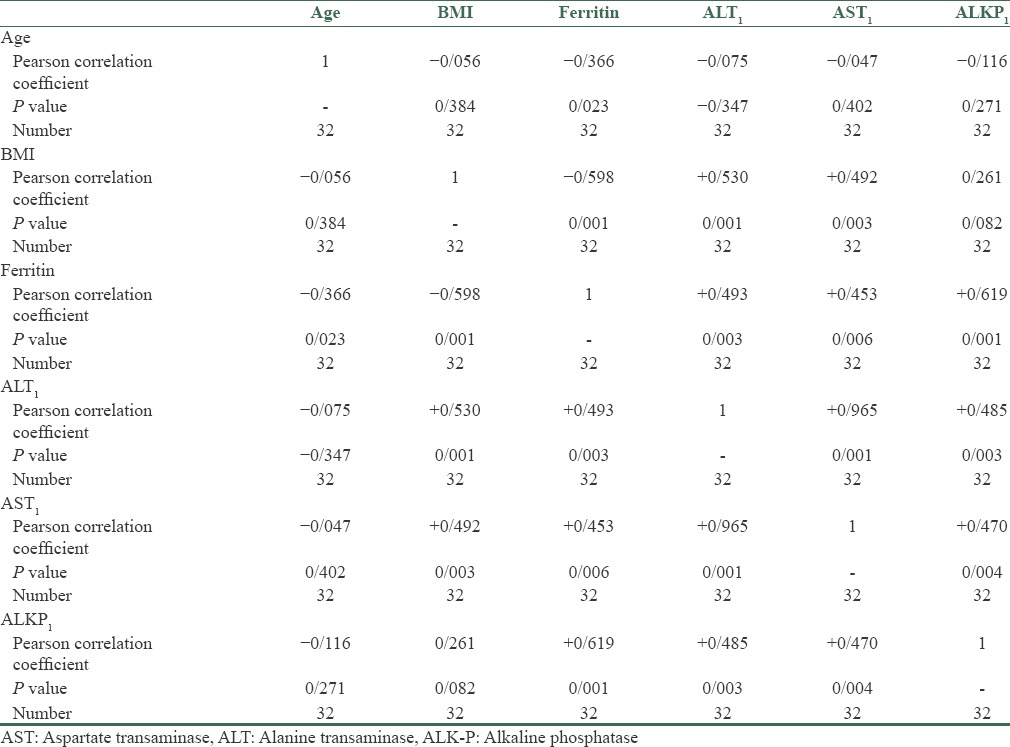
Table 2.
Mean ALT, AST, and ALKP before and after phlebotomy
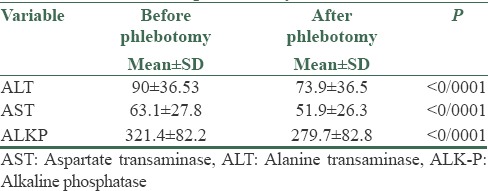
Table 3.
Differences in mean ALT, AST, and ALKP at the beginning of the study and after phlebotomy
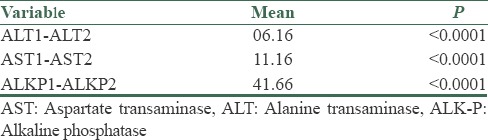
Table 4.
Grading of fibrosis, steatosis, and hepatitis before and after phlebotomy
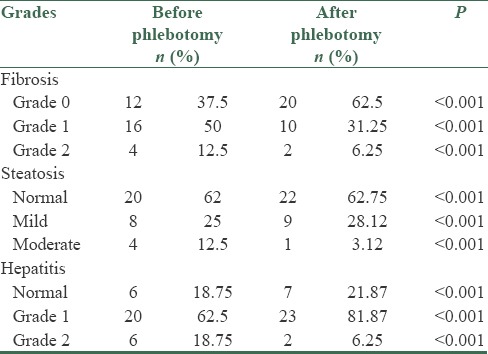
Table 5.
Grades of fibrosis, steatosis, and hepatitis after phlebotomy
Discussion
The aim of the present study was to investigate the efficacy of phlebotomy toward the improvement of liver enzymes and histology in patients with NAFLD. The results of this study showed that phlebotomy not only has a positive effect on the biochemical markers of fatty liver but is also effective on liver histology in these patients. Hyperferritenemia has been observed in patients with NAFLD and the level of it is dependent on the severity of NAFLD.[9] Hyperferritinemia is related to oxidative stress and mild hepatic iron overload.[10] An increased iron store is dependent on adipokine production and insulin resistance.[10] The results of the present study are consistent with the study of Penkova et al. (2012) where there were 60 patients: Of them 30 patients had NAFLD and 30 patients had alcoholic liver disease (ALD). The subjects underwent phlebotomy over 12 months and finally the authors concluded that phlebotomy caused a decrease in liver enzymes, and improvement in insulin resistance and also homeostasis model assessment-estimated insulin resistance (HOMA-IR). They remarked that phlebotomy is effective for NAFLD and ALD.[11] Insulin resistance plays an important role in steatosis and steatosis hepatitis, and there is a relationship between NAFLD and diabetes type 2; insulin resistance is an independent factor for diabetes 2.[5] Also, Valenti et al. (2007) noted that phlebotomy can decrease HOMA-IR and so lead to a decrease in diabetes 2. They selected 198 patients who were affected by NAFLD, and divided them into two groups: 79 people who underwent phlebotomy and 119 patients who were designated the control group. The experimental group underwent phlebotomy for 6-8 months and finally, the researchers concluded that phlebotomy can decrease liver enzymes.[12] Housechayer et al. (2012) obtained similar results in patients with metabolic syndrome,[13] and Fransescos et al. (2002) reported that phlebotomy can reduce liver enzymes and insulin resistance.[14] All in all, it seems that decreasing iron by phlebotomy can reduce hepatocellular necrosis, a mechanism ascertained in different studies by drugs sensitive to insulin, and that decrease in necrosis causes the reduction of liver enzymes.[15] Experimental studies found that a decrease in oxidative stress and necrosis reduces apoptosis and leads to an improvement in liver cells.[16] By reducing iron liver stores through phlebotomy, oxidative stress can be decreased, and an improvement in insulin resistance can also lead to the decrease in production of liver glucose, which is one mechanism of development of steatosis in NAFLD.[17] Moreover, according to in vivo and in vitro observations, they emphasized phlebotomy in the intervention of retinol-binding protein 4 (RBP4) in insulin resistance and iron overload, while RBP4 in relation to steatosis involves a different mechanism.[18] Thus, it appears that phlebotomy can improve histology and decrease liver enzymes in patients with NAFLD through the above mechanisms.
Some advantages of this study are that in patients with NAFLD confirmation has been done by liver biopsy and degrees of histological grading have been conducted individually and separately. On the other hand, in most of the other studies, liver biopsy is an invasive procedure and was not conducted to determine NAFLD, which has instead been done by biochemical criteria and imaging; obviously those tests cannot determine the grades of NAFLD. However, in this study these groups have been separated. Another factor investigated is the effect of phlebotomy on liver histology: Its effect survived during 6 months. Also, through searching different sources, the researchers evaluated the impact of phlebotomy on the biochemical criteria of NAFLD and insulin resistance, and there was no focus on liver histology. In fact, this study is the first study that seeks to determine the histology of fatty liver and its progress toward fibrosis, that is, an assessment of phlebotomy on liver histology.
The limitations in the present study are the lack of measuring chemical criteria related to insulin resistance and oxidative stress, and also the measurement of the rates of serum and liver iron.
Conclusion
All in all, it seems that phlebotomy is effective in decreasing liver cell damage and finally leads to an improvement of liver enzymes and histology in patients with NAFLD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 3.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 4.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): Treatment. Best Pract Res Clin Gastroenterol. 2004;18:1105–16. doi: 10.1016/j.bpg.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Valenti L, Dongiovanni P, Fargion S. Diagnostic and therapeutic implications of the association between ferritin level and severity of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3782–6. doi: 10.3748/wjg.v18.i29.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillygomarc’h A, Mendler MH, Moirand R, Lainé F, Quentin V, David V, et al. Venesection therapy of insulin resistance-associated hepatic iron overload. J Hepatol. 2001;35:344–9. doi: 10.1016/s0168-8278(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 11.Penkova M, Dragneva S, Marinova C, Ivanova R, Gulubova M, Ananiev J, et al. Phlebotomy in the treatment of iron overload in patients with nonalcoholic and alcoholic fatty liver diseases. Int J Bus Hum Tech. 2012;2:48–51. [Google Scholar]
- 12.Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866–7. doi: 10.1053/gast.2003.50130. [DOI] [PubMed] [Google Scholar]
- 13.Houschyar KS, Lüdtke R, Dobos GJ, Kalus U, Broecker-Preuss M, Rampp T, et al. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: Results from a randomized clinical trial. BMC Med. 2012;10:54. doi: 10.1186/1741-7015-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–9. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 15.Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K, Nakashima T, et al. Effect of iron reduction by phlebotomy in Japanese patients with nonalcoholic steatohepatitis: A pilot study. Hepatol Res. 2006;36:315–21. doi: 10.1016/j.hepres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JE, Wilson L, Brunt EM, Yeh M, Kleiner DE, Unalp-Arida A, et al. Hepatic iron deposition in reticuloendothelial cells but not hepatocytes is associated with more severe NASH: Results from the NASH Clinical Research Network. International BioIron Society Meeting 2009. Am J Hematol. 2009;84:E373–4. [Google Scholar]
- 17.Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J Hepatol. 2011;55:920–32. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Valenti L, Moscatiello S, Vanni E, Fracanzani AL, Bugianesi E, Fargion S, et al. Venesection for non-alcoholic fatty liver disease unresponsive to lifestyle counselling-a propensity score-adjusted observational study. QJM. 2011;104:141–9. doi: 10.1093/qjmed/hcq170. [DOI] [PubMed] [Google Scholar]


