Abstract
Introduction:
Three-dimensional (3D) planning in oral and maxillofacial surgery has become a standard in the planification of a variety of conditions such as dental implants and orthognathic surgery. By using custom-made cutting and positioning guides, the virtual surgery is exported to the operating room, increasing precision and improving results.
Materials and Methods:
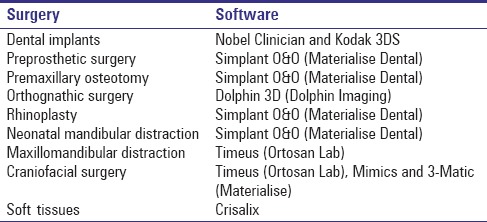
We present our experience in the treatment of craniofacial deformities with 3D planning. Software to plan the different procedures has been selected for each case, depending on the procedure (Nobel Clinician, Kodak 3DS, Simplant O&O, Dolphin 3D, Timeus, Mimics and 3-Matic). The treatment protocol is exposed step by step from virtual planning, design, and printing of the cutting and positioning guides to patients’ outcomes.
Conclusions:
3D planning reduces the surgical time and allows predicting possible difficulties and complications. On the other hand, it increases preoperative planning time and needs a learning curve. The only drawback is the cost of the procedure. At present, the additional preoperative work can be justified because of surgical time reduction and more predictable results. In the future, the cost and time investment will be reduced. 3D planning is here to stay. It is already a fact in craniofacial surgery and the investment is completely justified by the risk reduction and precise results.
Keywords: Craniofacial surgery, craniosynostosis orthognathic surgery, distraction, three-dimensional planning, virtual surgery
INTRODUCTION
Three-dimensional (3D) planning is a routine technique for some procedures such as dental implants and orthognathic surgery. Its advantages include more precision, fewer complications, and reduction of the operative time.[1,2,3]
In the 1990s, the construction of stereolithographic models begun. They were used for evaluation and treatment planning of complex facial deformities.[4]
A few years ago, surgical cutting and positioning guides were developed to treat a wider spectrum of maxillofacial deformities and conditions.
Some patients undergo surgical treatment with suboptimal results despite well-planned operations by experienced surgeons. One of the reasons for a poor outcome is the surgeon's reliance on 2D imaging for treatment planning of a 3D problem.[4] Virtual surgery, a surgical revolution, could help avoid risks. Some of its multiple advantages are:
Increased precision
Possibility to operate on the same patient for endless times
Simulate different approaches and procedure types
Compare different vectors and hardware in distraction osteogenesis
Avoid damaging neurovascular structures or teeth
Reduce operative time and improve postoperative recovery
Reduce complications and reoperations
More precise and predictable results.
Its main disadvantages are the cost of the software and 3D printing and the longer preoperative preparation and procedure planning. However, the cost tends to reduce and so does the planning time after the learning curve. On the other hand, the operative and hospitalization time is reduced.[5]
Virtual presurgical planning may be complemented with intraoperative navigation.[4] Adding intraoperative computed tomography (CT)/magnetic resonance imaging, the result can be checked, further increasing the accuracy of the procedure. Augmented reality with devices such as Google glasses could revolutionize surgery in the near future.
MATERIALS AND METHODS
Our preoperative work-up consists of craniofacial CT-scan, facial and intraoral photographs, and dental casts. To increase the resolution of the occlusal surfaces, dental casts are scanned and fused to the CT scan.[6] Different software have been used depending on the condition [Table 1]. Virtual surgery was transferred to the operating room with surgical splints or cutting and positional guides. In selected cases, 3D-models were printed to simulate the surgical procedure and the placement of the cutting guides and the hardware or distractors. Initial and follow-up photographs are taken to evaluate results. Intra- and post-operative complications have been recorded.
Table 1.
Software used according to the surgery
Orthognathic surgery
3D planning and the use of computer-aided design-computer-aided manufacturing (CAD-CAM) splints are a routine procedure in orthognathic surgery, thanks to its multiple advantages. The software used in our institution is Dolphin 3D Surgery (Dolphin Imaging and Management Solutions. 9200 Eton Ave. Chatsworth, CA 91311 USA) which allows simulation of the procedure, positioning of the maxilla and mandible, and print CAD-CAM splints[6] [Figures 1 and 2]. This technology is especially relevant for the correction of facial asymmetries because it analyzes the transverse plane, which is one of the limitations of 2D cephalometry. Regarding orientation, 2D allows the measurement of pitch, but not of roll or yaw.[7] It also allows checking the need of prosthesis or grafts to achieve symmetry. This is especially important in severe asymmetries, such as hemifacial microsomia.
Figure 1.
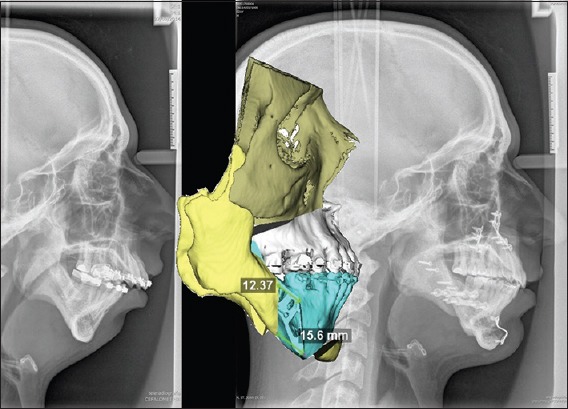
Three-dimensional planning of a bimaxillary surgery + genioplasty. Preoperative lateral teleradiograph, three-dimensional-planning, and postoperative result
Figure 2.
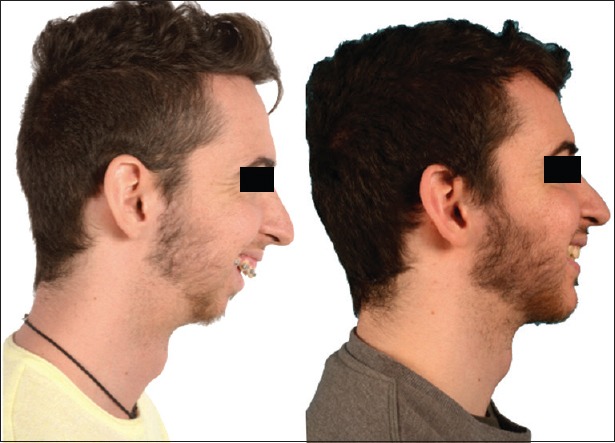
Pre- and post-operative lateral views of the patient in Figure 1
Distraction osteogenesis
Distraction is a useful procedure to lengthen facial bones. We use this technique in neonates with Pierre–Robin sequence (micrognathia, glossoptosis, and respiratory distress),[8] older children with hemifacial microsomia, and severe maxillary or mandibular hypoplasia that cannot be corrected with orthognathic surgery.
In neonates, we use two Molina unidirectional external distractors (KLS-Martin. Ludwigstaler Str. 132. D-78532 Tuttlingen, Germany) and two Kirschner needles. Osteotomies are performed through an intraoral approach using piezoelectric surgery device (VarioSurg3, NSK. Nakanishi Inc. Japan) [Figure 3]. In these cases, virtual planning is especially important to determine the osteotomy path line and the position of the needles, preserving dental germs, as well as to predict the position of the mandible that will enlarge the upper airway. No surgical splints or positioning guides are used in these patients, as there are no teeth to fix them.
Figure 3.
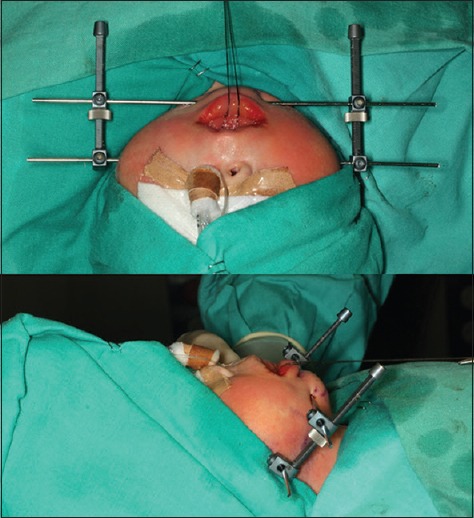
Mandibular distraction in a neonate affected with Pierre–Robin sequence
Simplant® O&O has been used for planning the osteotomies and reposition the bones. It allows studying the anatomy–distorted in most patients–and choosing the best osteotomy while avoiding damage to neurovascular structures (i.e., inferior alveolar nerve), teeth, and germs [Figures 4 and 5].
Figure 4.
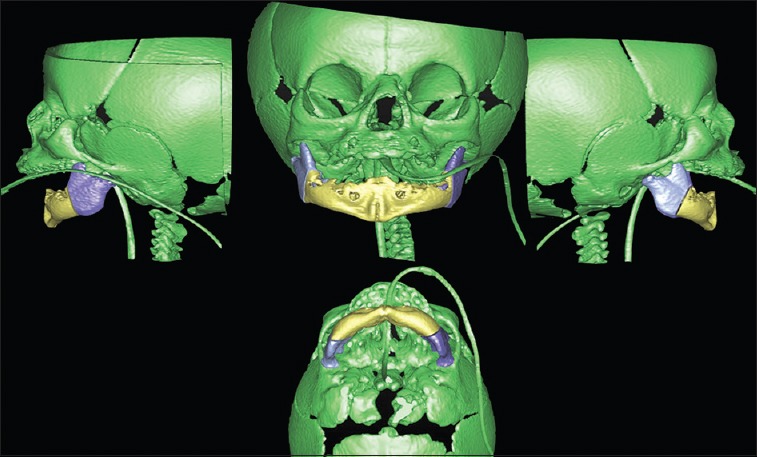
Three-dimensional planning of bilateral mandibular osteotomies for distraction
Figure 5.
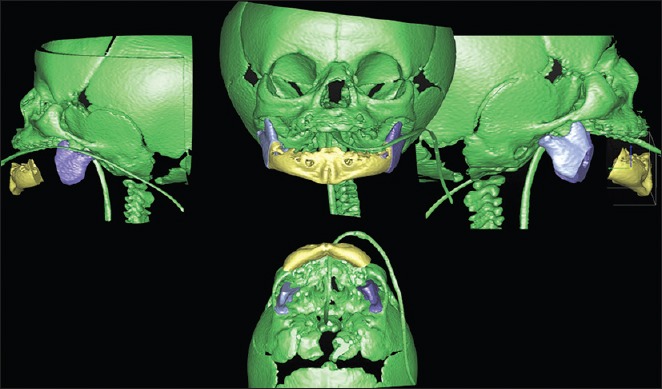
Virtual surgery for mandibular distraction
A sequel of cleft lip and palate early surgery is reduced maxillary growth, especially in bilateral cases, which can be treated with orthognathic surgery. When the sagittal discrepancy is higher than 10–12 mm, it is difficult to advance the maxilla in a single stage, due to the long gap and the limited movement of the scarred soft tissues. In case of such a severe maxillary hypoplasia, we are using the internal Zurich Pediatric Maxillary distractors (KLS-Martin. Ludwigstaler Str. 132. D-78532 Tuttlingen, Germany) which allows an advancement of the maxilla up to 20 mm.
In these cases, and in mandibular distraction for hemifacial microsomia, the software we use is Timeus (Laboratorio Ortosan, Madrid, Spain) which adds the possibility to create 3D cutting and positioning guides to transfer virtual planning to the operating room [Figures 6–8]. This is especially important to plan the desired position of the maxilla and vector of movement. It is possible to try different kinds of distractors to achieve what has been planned. This software is also useful to correct asymmetric patients by placing the distractors differently to work more on one side and center the midline.
Figure 6.
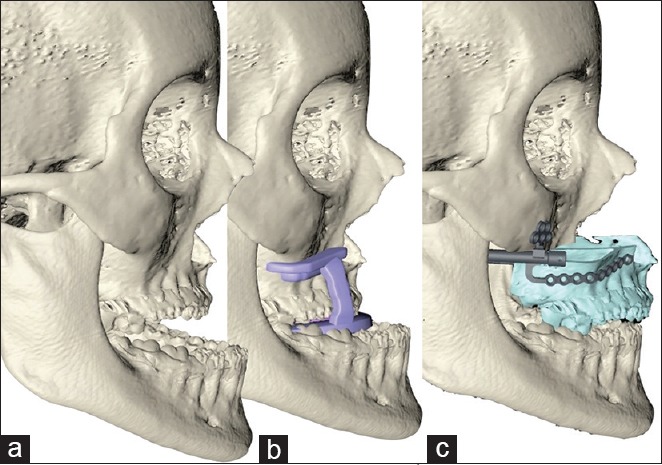
Virtual surgery for maxillary distraction. (a) Initial situation. (b) LeFort I osteotomy using cutting guides. (c) LeFort I and maxillary advancement using Zurich internal distractors and positioning guides
Figure 8.
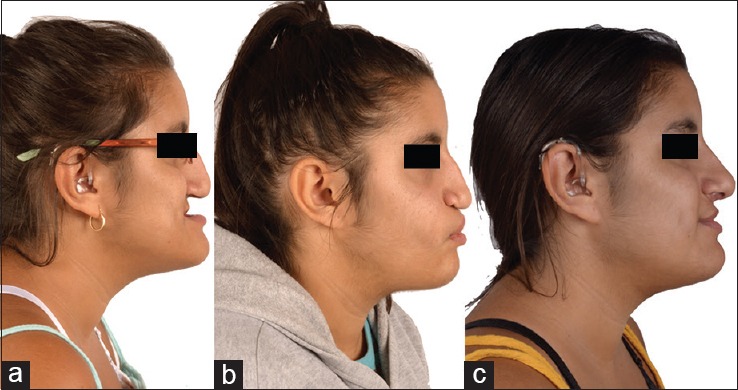
Preoperative (a), after maxillary distraction (b), and after removal of maxillary distractors, BSSO, genioplasty, and rhinoplasty (c) postoperative lateral views of the patient in Figures 6 and 7
Figure 7.
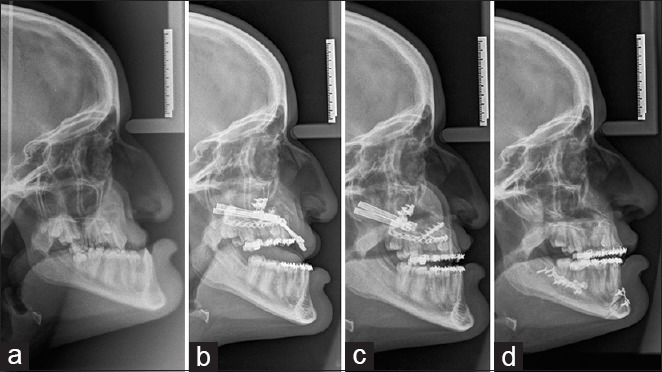
Lateral teleradiographs for maxillary distraction. (a) Initial situation. (b) Beginning of maxillary distraction. (c) End of maxillary distraction. (d) Final situation after distractors’ removal, bilateral sagittal split, genioplasty, and rhinoplasty, all in one procedure. Note the change of the nasal tip after distraction
We use 3D-printed models in these cases to simulate the LeFort I osteotomy with the cutting guide and the distractors position with the positioning guide.
Premaxillary osteotomy
Premaxillary osteotomy is done only in a selected group of complete bilateral cleft patients who have an abnormal position of the premaxilla that cannot be corrected orthopedically because it protrudes or is overerupted with hanging and retroinclined upper incisors.[9]
The work-up consists of dental casts, intraoral and facial photographies, CT-scan, and virtual positioning of the premaxilla in the correct position, which is transferred to a surgical acrylic splint [Figure 9].
Figure 9.
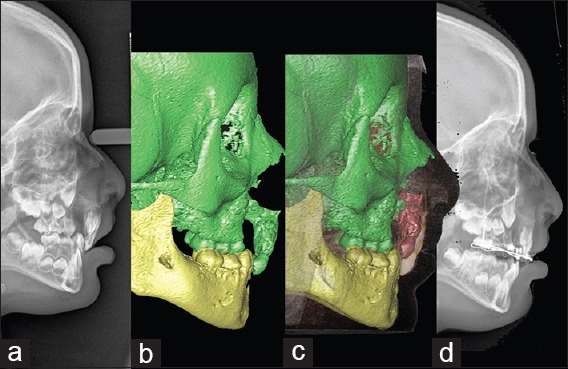
Premaxilla osteotomy. (a) Lateral teleradiograph before surgery. (b) Preoperative computed tomography scan. (c) Virtual osteotomy and reposition of the premaxilla. (d) Postoperative lateral cephalometry
3D planning is very useful in these cases to decide the best approach (intraoral or endonasal[10]) and the design of the osteotomy. It also provides information about possible interferences and the need of a second osteotomy to achieve the desired torque and position of the premaxilla.
Rhinoplasty
Severe cases of nasal bone asymmetry can be virtually planned by doing single or double osteotomies and simulate the mobilization of the bones to achieve the best possible result. The software used is Simplant® O&O (Materialise Dental/Dentsply Implants, Mölndal, Sweden). Although it is not possible to predict the soft tissue response, 3D planning shows the best way to perform the nasal osteotomies and septoplasty [Figures 10–12].
Figure 10.
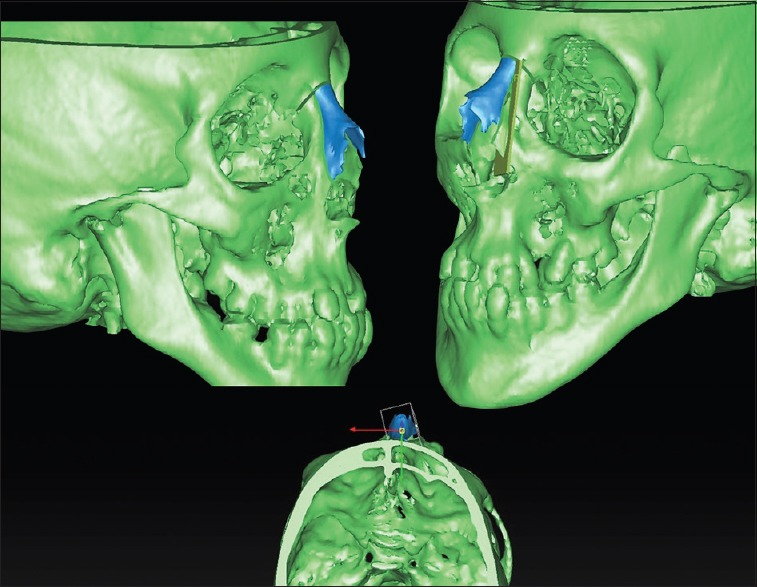
Virtual planning for rhinoplasty
Figure 12.
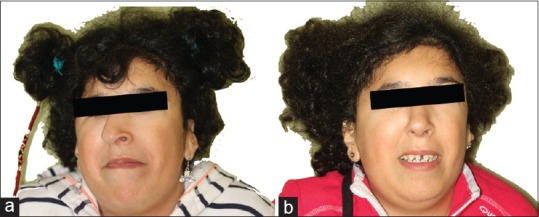
Preoperative (a) and postoperative (b) frontal images after rhinoplasty
Figure 11.
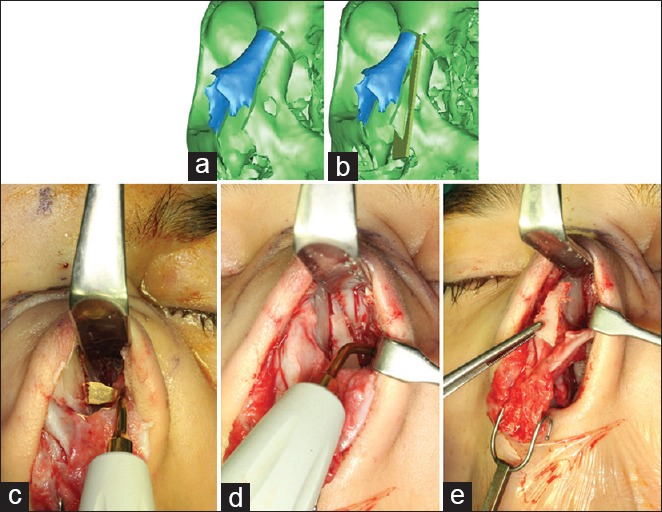
Open septorhinoplasty of a crooked nose in a Turner syndrome patient with cleft palate. (a) Three-dimensional planning of the first left osteotomy. (b) Three-dimensional planning of the second left osteotomy and segment to be removed to symmetrize the nasal bones. (c) First left osteotomy performed with piezoelectric device. (d) Second left osteotomy. (e) Removal of the left nasal bone segment
Craniofacial surgery
Complex craniofacial malformations and craniosynostosis can be treated successfully with this technique. Simulating the final result and printing the cutting and positioning guides are great aids to achieve a good, consistent result. The proposed surgery can be based on quantitative anthropometric guides, a superimposed normal side, or a mirrored normal side.[11] Operative time and bleeding is reduced, which is especially important in young patients, allowing a better recovery with a shorter hospitalization period.
The software used has been Timeus (Laboratorio Ortosan, Madrid. Spain), and 3D-models have been very useful to simulate the various steps of the surgical procedure [Figures 13–15]. In craniosynostosis, two different software have been used: 3-matic (Materialise. Technologielaan 15, 3001 Leuven, Belgium) for the design and planification, and Mimics (Materialise. Technologielaan 15, 3001 Leuven, Belgium) for the segmentation of the skulls. Cutting and positioning guides have also been used to reposition the bones to normalize the anatomy of the skull.
Figure 13.
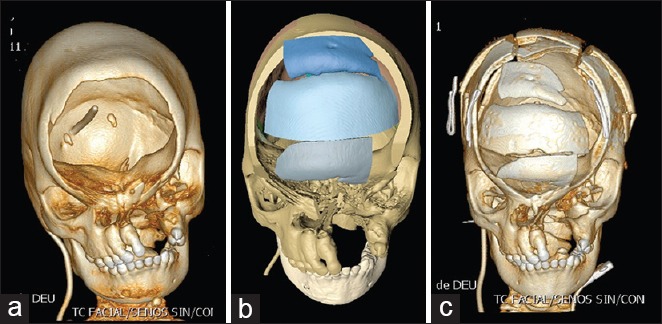
Computed tomography scan of a frontal defect causing encephalocele associated with craniofacial cleft. (a) Preoperative view. (b) Virtual simulation for the reconstruction of the frontal bone. (c) Postoperative view
Figure 15.
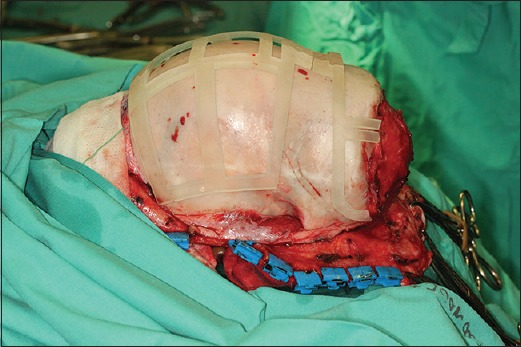
Intraoperative view of the cutting guides for cranial osteotomies in the same patient
Figure 14.
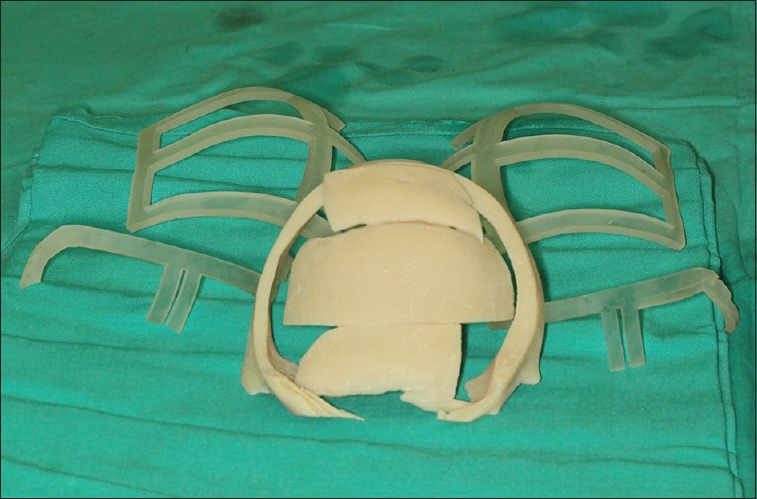
Cutting and positioning guides for frontal reconstruction of a frontal defect associated with craniofacial cleft
Soft tissues
The behavior of the soft tissues after a bone movement is very unpredictable because of its different components and status, so the simulation is not a good predictor of soft tissue outcome. Depending on the skin, fat tissue, or muscle, the response can be different in every patient; the dynamics and mimics of the patient's face have an influence as well.
We have used the software Simplant® O&O and Dolphin 3D surgery for this purpose, as well as the 3D plastic surgery simulator Crisalix (Lausanne, 1015. Switzerland). Simulation of rhinoplasty and genioplasty can be done, as well as facelift and lip surgery, among others [Figure 16]. This commercial software focuses in showing the patient's possible results, such as 3D Photoshop. It can be very helpful to increase surgeon–patient communication and to show the patient what can be expected, but there is no way to transfer the simulation to the operating theater.
Figure 16.
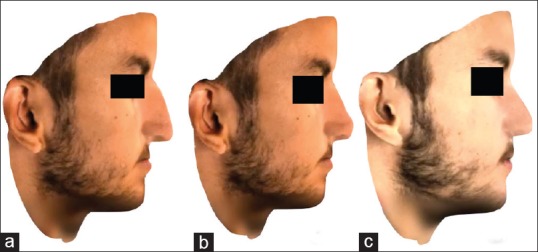
Three-dimensional simulation of orthognathic surgery and rhinoplasty using Crisalix. (a) Preoperative view. (b) Simulation of the surgical procedure. (c) Final result
DISCUSSION
Virtual surgery offers indisputable advantages. It is a big step forward in the treatment of craniomaxillofacial conditions. A step we do not want to step back from, as one feels that an incalculable amount of information is lost with 2D. Since 2010, we have been planning 3D-models in all the orthognathic surgery patients and some other conditions. Preoperative planning is time-consuming and has a learning curve. Nevertheless, these inconveniences are widely surpassed by its advantages: enhanced visualization, precise information, better tools for instruction, and accurate documentation.[5]
Little research has been done on virtual planning of midfacial distraction since the description of Gateno in 2003.[12] Virtual surgery simplifies the procedure and allows achieving good results in very complex patients. One example could be maxillary distraction in bilateral cleft patients with severe maxillary hypoplasia. In such patients, we have treated discrepancies up to 20 mm from upper incisor tip to lower incisor tip; the final position of the maxilla reaching as far as had been planned. The use of cutting and positioning guides reduces considerably the operation time and the risk of errors since the position and the vector are transferred to the guides, and from these to the patient, as planned. If an error occurs in the final position, it will happen before in the preoperative planning. Virtual surgery helps improve accurate diagnosis, optimize treatment planning, and transfer of the planning to the patient.[5,13]
Virtual planning has helped decide the approach or the osteotomy line in other surgical procedures, even when no cutting or positioning guides were made. This happens for instance in neonatal distraction with external distractors for Pierre–Robin sequence, while Steinbacher has treated these patients using cutting and positioning guides and internal distractors.[11]
Postoperative CT scans offer a good quality control of the procedure, comparing the 3D planning with the final surgical outcome.[5] This could be a useful research line to validate the accuracy of virtual surgery in other craniomaxillofacial procedures.
Regarding the soft tissues, only approximate results can be expected;[5] virtual surgery may be helpful to simulate the result after surgery, especially after rhinoplasty, and the position of the lips after orthognathic surgery, but has a lower precision when compared to the bones.
A useful advantage of 3D planning is that it increases the knowledge of the anatomy of each particular patient; this is because the real surgical intervention is not the first time the surgeon operates on this particular patient - the procedure has been performed several times before on “the same” virtual patient and with 3D printed models. Virtual surgery is also a very powerful tutorial and communication tool to present, train, and document cases and procedures for colleagues, trainees, and patients.
CONCLUSION
The purpose of this article is to show new applications of 3D planning in craniomaxillofacial surgery (rhinoplasty, premaxillary osteotomy, distraction, and craniofacial surgery). Preoperative planning time is increased and needs a learning curve. The cost of the software and 3D printing will be reduced over time. This technology allows more precise results and reduces surgical time and complications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to thank the orthodontic team in our institution for their close collaboration with craniomaxillofacial patients.
Many thanks also to the anesthesiologists and pediatric ICU for the perioperative management of these complex patients.
REFERENCES
- 1.Gateno J, Xia JJ, Teichgraeber JF, Christensen AM, Lemoine JJ, Liebschner MA, et al. Clinical feasibility of computer-aided surgical simulation (CASS) in the treatment of complex cranio-maxillofacial deformities. J Oral Maxillofac Surg. 2007;65:728–34. doi: 10.1016/j.joms.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Swennen GR, Mollemans W, Schutyser F. Three-dimensional treatment planning of orthognathic surgery in the era of virtual imaging. J Oral Maxillofac Surg. 2009;67:2080–92. doi: 10.1016/j.joms.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Aboul-Hosn Centenero S, Hernández-Alfaro F. 3D planning in orthognathic surgery: CAD/CAM surgical splints and prediction of the soft and hard tissues results – Our experience in 16 cases. J Craniomaxillofac Surg. 2012;40:162–8. doi: 10.1016/j.jcms.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Bell RB. Computer planning and intraoperative navigation in cranio-maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:135–56. doi: 10.1016/j.coms.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Adolphs N, Haberl EJ, Liu W, Keeve E, Menneking H, Hoffmeister B. Virtual planning for craniomaxillofacial surgery-7 years of experience. J Craniomaxillofac Surg. 2014;42:e289–95. doi: 10.1016/j.jcms.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Rubio Palau J, Hueto Madrid JA, González Lagunas J. 3D planning in orthognathic surgery. Rev Esp Ortod. 2012;42:17–21. [Google Scholar]
- 7.Gateno J, Xia JJ, Teichgraeber JF. New 3-dimensional cephalometric analysis for orthognathic surgery. J Oral Maxillofac Surg. 2011;69:606–22. doi: 10.1016/j.joms.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sesenna E, Magri AS, Magnani C, Brevi BC, Anghinoni ML. Mandibular distraction in neonates: Indications, technique, results. Ital J Pediatr. 2012;38:7. doi: 10.1186/1824-7288-38-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen J, Kuseler A, Klit Pedersen T, Norholt S. Premaxillary osteotomy and bone grafting for secondary BCLP repair – Long-term evaluation of growth. J Oral Maxillofac Surg. 2011;69:e16–7. [Google Scholar]
- 10.Ferrer Fuertes A, García Díez E, Rivera Baró A, Sieira Gil R, Cho-Lee GY, Martí Pagés C, et al. Endonasal approach in premaxilla osteotomy of complete bilateral cleft lip palate. Int J Oral Maxillofac Surg. 2013;42:1197. [Google Scholar]
- 11.Steinbacher DM. Three-dimensional analysis and surgical planning in craniomaxillofacial surgery. J Oral Maxillofac Surg. 2015;73(12 Suppl):S40–56. doi: 10.1016/j.joms.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Gateno J, Teichgraeber JF, Xia JJ. Three-dimensional surgical planning for maxillary and midface distraction osteogenesis. J Craniofac Surg. 2003;14:833–9. doi: 10.1097/00001665-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Markiewicz MR, Bell RB. Modern concepts in computer-assisted craniomaxillofacial reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011;19:295–301. doi: 10.1097/MOO.0b013e328348a924. [DOI] [PubMed] [Google Scholar]


