Abstract
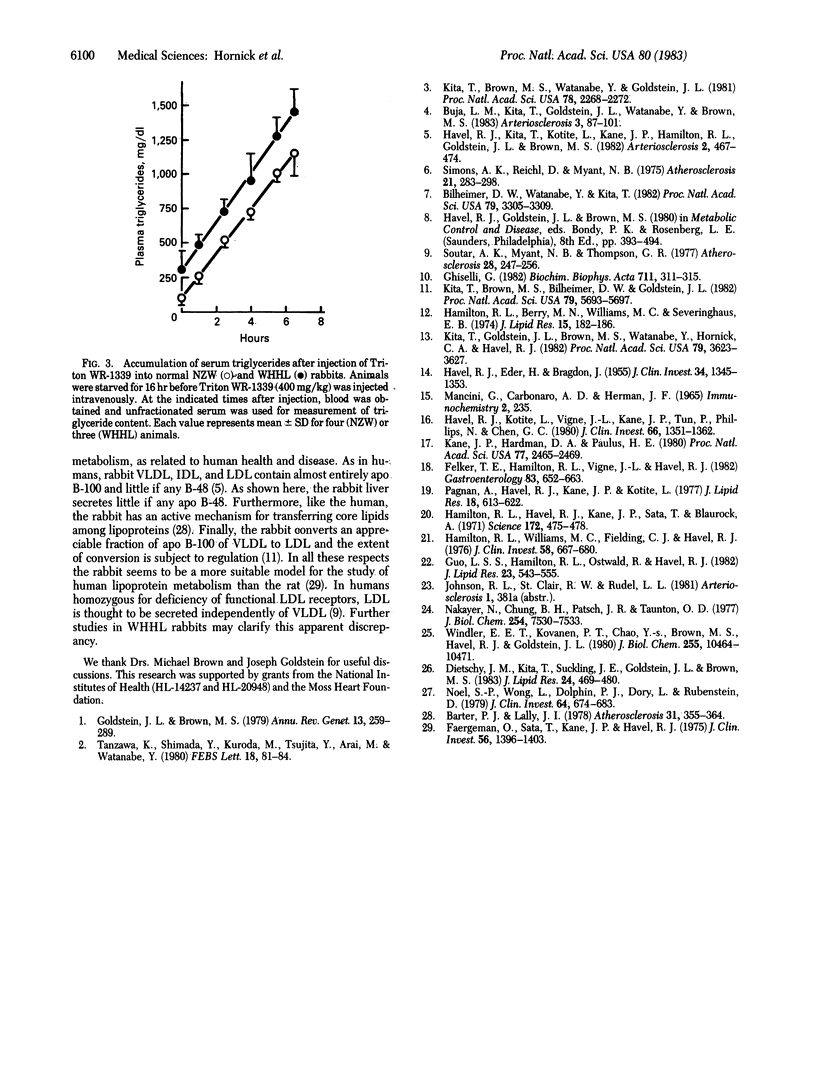
We compared the rate of accumulation of lipoproteins in perfusates of isolated livers from normal New Zealand White rabbits and Watanabe heritable hyperlipidemic (WHHL) rabbits, in which a gene mutation has produced a virtually complete deficiency of low density lipoprotein (LDL) receptors. The rate of accumulation of apolipoprotein B-100 did not differ in perfusates of livers from normal and mutant animals and little or no apolipoprotein B-48 was detected. In both groups, virtually all apolipoprotein B accumulated in very low density lipoprotein (VLDL). Experiments in which [3H]lysine was added to the perfusates showed that the apolipoprotein B that accumulated in VLDL was newly synthesized by the liver whereas the small amount of apolipoprotein B found in lipoproteins of higher density appeared to be washed out of extravascular spaces during perfusion. Perfusate VLDL from both groups contained more triglycerides and less cholesteryl esters than their counterparts from blood plasma. As compared with perfusate VLDL from normal livers, those from livers of WHHL rabbits were enriched in cholesteryl esters. Experiments in which Triton WR-1339 was injected into the blood of intact rabbits confirmed the observations with perfused livers. Previous studies have shown that the extent to which VLDL is converted to LDL is increased several-fold in WHHL rabbits. Taken together with our present results, which fail to provide evidence for increased secretion of apolipoprotein B or de novo secretion of lipoproteins other than VLDL that contain apolipoprotein B, it can be concluded that overproduction of LDL in rabbits lacking LDL receptors is solely the result of altered metabolism of VLDL.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barter P. J., Lally J. I. Metabolism of esterified cholesterol in the plasma very low density lipoproteins of the rabbit. Atherosclerosis. 1978 Nov;31(3):355–364. doi: 10.1016/0021-9150(78)90070-9. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Watanabe Y., Kita T. Impaired receptor-mediated catabolism of low density lipoprotein in the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1982 May;79(10):3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja L. M., Kita T., Goldstein J. L., Watanabe Y., Brown M. S. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 1983 Jan-Feb;3(1):87–101. doi: 10.1161/01.atv.3.1.87. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Kita T., Suckling K. E., Goldstein J. L., Brown M. S. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J Lipid Res. 1983 Apr;24(4):469–480. [PubMed] [Google Scholar]
- Faergeman O., Sata T., Kane J. P., Havel R. J. Metabolism of apoprotein B of plasma very low density lipoproteins in the rat. J Clin Invest. 1975 Dec;56(6):1396–1403. doi: 10.1172/JCI108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker T. E., Hamilton R. L., Vigne J. L., Havel R. J. Properties of lipoproteins in blood plasma and liver perfusates of rats with cholestasis. Gastroenterology. 1982 Sep;83(3):652–663. [PubMed] [Google Scholar]
- Ghiselli G. Evidence that two synthetic pathways contribute to the apolipoprotein B pool of the low density lipoprotein fraction of rabbit plasma. Biochim Biophys Acta. 1982 May 13;711(2):311–315. doi: 10.1016/0005-2760(82)90040-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 1979;13:259–289. doi: 10.1146/annurev.ge.13.120179.001355. [DOI] [PubMed] [Google Scholar]
- Guo L. S., Hamilton R. L., Ostwald R., Havel R. J. Secretion of nascent lipoproteins and apolipoproteins by perfused livers of normal and cholesterol-fed guinea pigs. J Lipid Res. 1982 May;23(4):543–555. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Hamilton R. L., Havel R. J., Kane J. P., Blaurock A. E., Sata T. Cholestasis: lamellar structure of the abnormal human serum lipoprotein. Science. 1971 Apr 30;172(3982):475–478. doi: 10.1126/science.172.3982.475. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kita T., Kotite L., Kane J. P., Hamilton R. L., Goldstein J. L., Brown M. S. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 1982 Nov-Dec;2(6):467–474. doi: 10.1161/01.atv.2.6.467. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980 Dec;66(6):1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Bilheimer D. W., Goldstein J. L. Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5693–5697. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Watanabe Y., Goldstein J. L. Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2268–2272. doi: 10.1073/pnas.78.4.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Goldstein J. L., Brown M. S., Watanabe Y., Hornick C. A., Havel R. J. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nakaya N., Chung B. H., Patsch J. R., Taunton O. D. Synthesis and release of low density lipoproteins by the isolated perfused pig liver. J Biol Chem. 1977 Nov 10;252(21):7530–7533. [PubMed] [Google Scholar]
- Noel S. P., Wong L., Dolphin P. J., Dory L., Rubenstein D. Secretion of cholesterol-rich lipoproteins by perfused livers of hypercholesterolemic rats. J Clin Invest. 1979 Aug;64(2):674–683. doi: 10.1172/JCI109508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Myant N. B., Thompson G. R. Simultaneous measurement of apolipoprotein B turnover in very-low-and low-density lipoproteins in familial hypercholesterolaemia. Atherosclerosis. 1977 Nov;28(3):247–256. doi: 10.1016/0021-9150(77)90174-5. [DOI] [PubMed] [Google Scholar]
- Tanzawa K., Shimada Y., Kuroda M., Tsujita Y., Arai M., Watanabe H. WHHL-rabbit: a low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Lett. 1980 Aug 25;118(1):81–84. doi: 10.1016/0014-5793(80)81223-3. [DOI] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]