Abstract
The V3 loop in the HIV envelope gp120 is one of the immunogenic sites targeted by Abs. The V3 crown in particular has conserved structural elements recognized by cross-reactive neutralizing Abs, indicating its potential contribution in protection against HIV. Crystallographic analyses of anti-V3 crown mAbs in complex with the V3 peptides have revealed that these mAbs recognize the conserved sites on the V3 crown via two distinct strategies: a cradle-binding mode (V3C) and a ladle-binding (V3L) mode. However, almost all of the anti-V3 crown mAbs studied in the past were isolated from chronically HIV-infected individuals. The extents to which the two types of anti-V3 crown Abs are generated by vaccination are unknown. This study analyzed the prevalence of V3C-type and V3L-type Ab responses in HIV-infected individuals and in HIV envelope-immunized humans and animals using peptide mimotopes that distinguish the two Ab types. The results show that both V3L-type and V3C-type Abs were generated by the vast majority of chronically HIV-infected humans, although the V3L-type were more prevalent. In contrast, only one of the two V3 Ab types was elicited in vaccinated humans or animal models, irrespective of HIV-1 envelope clades, envelope constructs (oligomeric or monomeric), and protocols (DNA plus protein or protein alone) used for vaccinations. The V3C-type Abs were produced by vaccinated humans, macaques, and rabbits, whereas the V3L-type Abs were made by mice. The V3C-type and V3L-type Abs generated by the vaccinations were able to mediate virus neutralization. These data indicate the restricted repertoires and the species-specific differences in the functional V3-specific Ab responses induced by the HIV envelope vaccines. The study implies the need for improving immunogen designs and vaccination strategies to broaden the diversity of Abs in order to target the different conserved epitopes in the V3 loop and, by extension, in the entire HIV envelope.
Keywords: HIV, HIV envelope, V3, mimotopes, antibodies
1. Introduction
The HIV envelope (Env) glycoproteins are the only virus-encoded proteins on the virion surface, and the Env gp120 subunit binds to CD4 and the co-receptors CCR5 or CXCR4. The third variable (V3) loop on gp120 is the key factor determining the virus co-receptor usage [1–4]. Despite its name, the V3 loop contains conserved elements targeted by broadly reactive antibodies [5, 6], implicating the importance of V3 for HIV Env-based vaccines. Indeed, anti-V3 Abs inversely correlated with infection risk in subsets of the vaccinees in the RV144 Thai trial [7].
The crystal structures of the V3 loop in the context of the CD4-bound gp120 and as peptides in complex with V3-specific mAbs from HIV-infected individuals show that V3 can be divided into three regions: the conserved base near the disulfide bond, the flexible stem, and the distal crown [8, 9]. The conserved base of V3 and glycans at positions N301 and N332 are targeted by broadly neutralizing Abs (bNAbs) such as PGT121, PGT128, PGT135 [10–12]; however, this region is poorly immunogenic. The highly immunogenic site on V3 is the 13 amino acid-long crown; this region is targeted by the anti-V3 Abs generated during infection [13–15] and after immunization [16–20]. The V3 crown is also recognized by a large panel of V3 monoclonal Abs (mAbs), most of which neutralize many Tier 1 viruses and few Tier 2 viruses [5, 6, 21–24]. Although the V3 crown is often occluded in the membrane-bound native Env trimers of Tier 2 viruses, this region is accessible to Abs in the soluble Env gp120 monomers and gp140 oligomers, including the stabilized gp140 trimers of BG505 SOSIP.664 which are strongly bound in ELISA by anti-V3 mAbs such as 39F, 19b and 14e [25].
The crystallographic analyses of V3 peptides in complex with the different V3 crown-specific mAbs identified three antigenic regions in the V3 crown: the arch consisting of the highly conserved GPGR/Q motif at the apex of the crown, the circlet at the middle region with a conserved hydrophobic patch and a more variable hydrophilic face on the opposing faces, and the conserved band region (Fig 1A) [5]. The mAbs recognize their epitopes in the V3 crown via two distinct epitope-binding modes, designated V3 cradle (V3C) and V3 ladle (V3L). MAbs with the V3L-binding mode are exemplified by the 447-52D mAb, whose key contact residues are the GPGR/Q arch plus residues N-terminal to the arch [26]. In contrast, mAbs with the V3C-binding mode like 2557, 2219 and 1006 bind to the hydrophobic core in the circlet and the band region, without contacting the arch [5]. In a recent study, two human anti-V3 crown mAbs with V3L- and V3C-binding modes were reported to have neutralizing activities against escape variants that arose after passive transfer with three bNAbs [22]. These mAbs synergized with the passively transferred bNAbs to suppress viremia by preventing the emergence of escape viruses, demonstrating their potential contribution in protection against HIV. Nonetheless, the information currently available about the V3C- and V3L-binding Abs has come mainly from studies of mAbs isolated from a small set of HIV-infected individuals. The prevalence of these Ab types among HIV-infected individuals is unknown. More importantly, it remains unclear whether both V3C- and V3L-types Abs can be elicited by immunization and if the different animal models used in the preclinical testing also produce V3C-type and V3L-type Abs.
Fig 1. Specific recognition of V3C and V3L peptide mimotopes by V3C-type and V3L-type mAbs respectively.
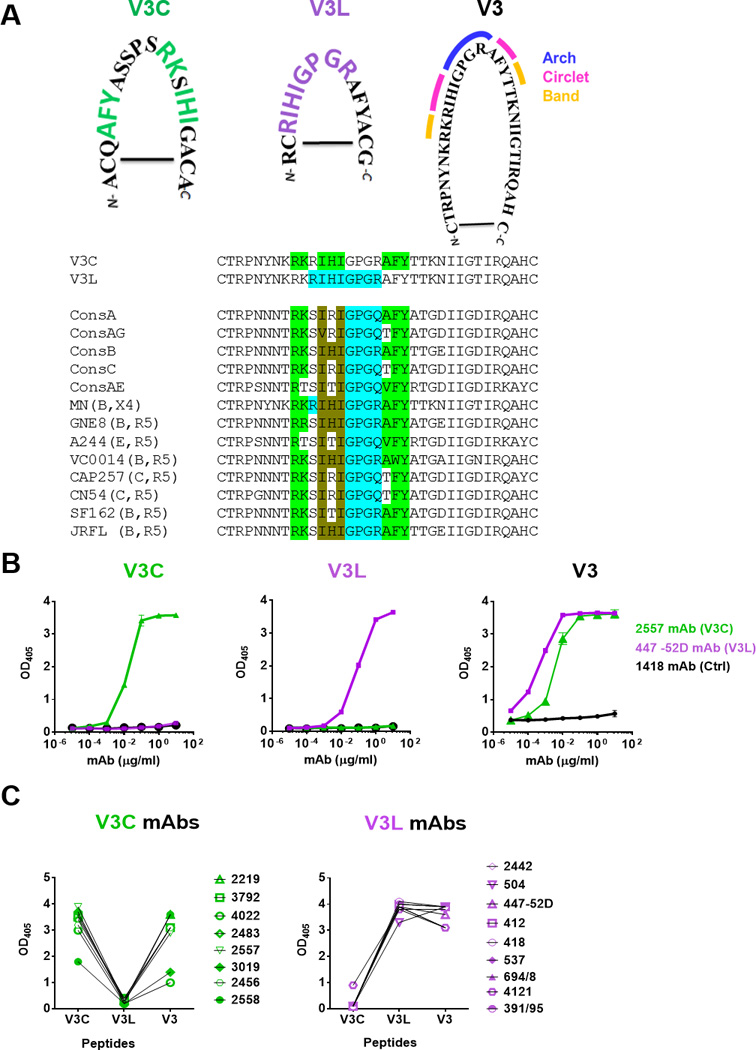
A) Amino acid sequences of cyclic V3C and V3L mimotopes as compared to the full-length cyclic V3 peptide of HIV-1 MN. The signature motifs required for recognition by V3C- and V3L-type mAbs are highlighted in green and purple respectively. The arch, circlet and band regions of the V3 crown [5] are also marked. Alignment of V3 sequences from consensus A, AG, B, C, AE and from the different Env strains used for immunogens in this study shows the presence of the V3C and V3L motifs irrespective of clades and chemokine receptor usage. The specific amino acids encompassing the V3C and V3L motifs are highlighted in green and blue, respectively. The amino acids common for both motifs are shown in brown. The V3L motif is shown to include GPGR or Q at the V3 arch, because V3L-type Abs like 44752D can recognize both sequences [26]. B) Distinct patterns of ELISA reactivity displayed by V3C mAb 2557, V3L mAb 447-52D and control 1418 Abs with the V3C, V3L or full length V3 peptides. Each of these cyclic peptides was biotinylated, coated on the Streptawell ELISA plates (1 µg/ml) and reacted with titrated concentrations of mAbs. C) Specific recognition of V3C and V3L mimotopes by eight V3C- and nine V3L-type mAbs, respectively. Each mAb was tested at a concentration of 10 µg/ml. No cross-reactivity between V3C and V3L mimotopes was observed, whereas the full-length V3 peptide was recognized by both types of the V3 mAbs. OD405, optical density at 405 nm
In this study we used cyclic peptide mimotopes designed to react exclusively with V3C-type or V3L-type mAbs [5, 19, 27, 28] to detect these Abs in sera or plasma of HIV-infected and HIV Env-vaccinated humans. Given that the human V3C-type mAb’s gene usage is restricted to VH5.51 [29] and other species have different VH genes, we also used the peptide mimotopes to evaluate the induction of V3C-type and V3L-type Abs in macaques, rabbits and mice after immunization with different HIV Env vaccines. The data show that, although HIV infection induces V3C- and V3L-type Abs to varying levels, HIV Env immunization did not recapitulate the repertoires of V3 Abs generated during the natural infection. More limited and species-specific repertoires of functional V3 Abs were elicited by immunization irrespective of the HIV Env immunogens and the vaccination protocols.
2. Materials and Methods
2.1. Sera/plasma samples
The samples tested in this study are described in Table 1.
Table 1.
Sera and plasma samples tested for the presence of V3-specific Abs with V3C- and V3L-binding modes.
| Species | Infection or vaccination | Vaccination protocol | Plasma/serum collection time |
Reference | |
|---|---|---|---|---|---|
| Humans | HIV infection | NA | Chronic infection | This paper | |
| Vaccination | VAX003 AIDSVAX® B/E (gp120 MN & gp120 A244) |
Intramuscular with alum 7x |
2 weeks after 7th immunization |
[37] | |
| VAX004 AIDSVAX® B/B (gp120 MN & gp120 GNE8) |
[35] | ||||
| Macaques |
Group 1:Co-immunization with gp160 DNA & gp140 trimeric proteins (clade B, “Early breadth” Envs from VC10014) |
DNA: intradermal by gene gun Protein: intramuscular with Adjuplex 4x |
2 weeks after 4th immunization |
[20] and this paper |
|
|
Group 2:Co-immunization with gp160 DNA (54 wk _D or 54wk_D,C,G Env clones) + gp140 trimeric protein (54wk_D Env clone) (54wk_D,C,G are clade C Env clones from subject CAP257) | |||||
| Rabbits |
Group 1: Co-immunization with gp160 DNA + gp140 trimeric protein. (clade B, “Early breadth” Envs from VC10014) |
DNA: Intradermal by gene gun Protein: intramuscular with (PEI) 4x |
2 weeks after 4th immunization |
This paper | |
| Group 2:gp140 CN54 protein (clade C) | Intramuscular with IFA 4x |
2 weeks after 4th immunization |
This paper | ||
|
Group 3:gp140 CN54 DNA + gp140 CN54 protein (clade C) |
DNA: intradermal by gene gun 3x Protein: intramuscular with IFA 2x |
2 weeks after 5th immunization |
|||
| Mice | Group 1:gp120 JRFL (clade B) | Subcutaneous with MPL/DDA 4x |
2 weeks after 4th immunization |
This paper | |
| Group 2:gp140 CN54 (clade C) | |||||
NA: Not applicable, IFA: incomplete Freund’s adjuvant, MPL/DDA: monophosphoryl lipid A dimethyldioctadecylammonium, PEI: Polyethyleneimine
2.2. Antigens
gp120 JRFL protein was purchased from Immune Technology. The full-length V3 (HIV-1 MN), V3C and V3L peptides used in ELISA were biotinylated at the N-terminus. The non-biotinylated versions were used for neutralization inhibition. All peptides were synthesized by Biopeptide.
2.3. Detection of antigen-specific IgG by ELISA
ELISA was performed as described [17] with updated modifications. Streptawell plates (Roche) were used to immobilize biotinylated peptides (1 µg/ml). After blocking, diluted plasma or sera were incubated for 1.5 hour at 37°C. The bound Abs were detected by enzyme-conjugated secondary Abs: anti-human IgG (Sigma; 1:10,000), anti-macaque IgG (NIH Non-human Primate Reagent Resource; 1:1000), anti-rabbit IgG (Southern Biotech, 1:2000) and anti-mouse IgG (Sigma, 1:5000). Alkaline phosphatase substrate (Thermoscientific) or OPD substrate (Thermoscientific) was finally added, and the optical densities (OD) were read at 405nm or 450nm, respectively. The half-maximal binding titers were calculated from titration curves by non-linear regression using GraphPad Prism 7.
2.4. Neutralization assay
Virus neutralization was measured using a beta-galactosidase assay (Promega) with TZM.bl target cells using heat-inactivated plasma or sera as described [30, 31]. The SF162 pseudovirus was produced in transfected 293T/cells using plasmids pNL4-3.Luc.R−.E− from Dr. Nathaniel Landau and SF162 from Drs. L. Stamatatos and C. Cheng-Mayer (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH). The neutralization inhibition assay was done according to published protocols [32] using 40 µg/ml of each peptide. Pooled pre-bleed or normal sera were tested for negative controls, whereas mAbs 447-52D (V3L-type) or 2557 (V3C-type) were used as positive controls.
3. Results
3.1. Peptide mimotopes are recognized specifically by V3C-type or V3L-type mAbs without evidence of cross-reactivity
Peptide mimotopes recognized by V3C-type and V3L-type Abs were rationally designed [5, 19, 27, 28]. These mimotopes are cyclic peptides with the motifs specifically required for the binding of mAbs with V3C- or V3L-binding modes (Fig 1A). Fig 1A also shows that both V3C and V3L motifs are conserved among the V3 crown sequences of HIV-1 clade B and non-clade B, and are present in R5-tropic Env and X4-tropic Env. The full-length 35-mer cyclic V3 peptide of HIV-1 MN was also synthesized. To establish their specific recognition by the V3C- and V3L-type Abs, we evaluated the ELISA reactivity of these peptides with representative V3C mAb (2557) and V3L mAb (447-52D) as compared to an irrelevant control (1418). The titration data showed the exclusive recognition of these mimotopes by the corresponding mAbs (Fig 1B). In contrast, both mAbs were reactive with a full-length V3 peptide and the control mAb did not react with any of the peptides (Fig 1B). These peptide mimotopes were also tested with 17 anti-V3 mAbs (Fig 1C). The mimotopes showed distinct and non-overlapping mAb reactivity profiles. Eight mAbs were reactive with the V3C peptide but not the V3L peptide. The other nine mAbs recognized only the V3L peptide. All 17 mAbs were reactive with the full length V3. Similar reactivity profiles were observed with a larger panel of anti-V3 mAbs [19]. Importantly, no cross-reactivity was detected, indicating that these mimotopes can serve as specific probes for the two Ab types. The specificity of these mimotopes was also verified by peptide inhibition ELISA, which showed the greater blocking of anti-V3C mAb and V3C-reactive polyclonal Ab binding by the V3C peptide than the V3L peptide, and vice versa (Fig S1). Further, although the V3L peptide contains the 312GPGR315 motif common to clade B viruses, its recognition is not restricted to Abs from clade B-infected patients. The V3L-type mAbs such as 3074, 4121, and 2182 from non-clade B infected donors are reactive with the V3L peptide [19]. Conversely, some V3L-type mAbs from clade B-infected donors, as exemplified by 447-52D, bind to V3 peptides containing GPGR or GPGQ/X often present in non-clade B HIV-1, consistent with the structural data showing the ability of these mAbs to tolerate changes at position 315 [33].
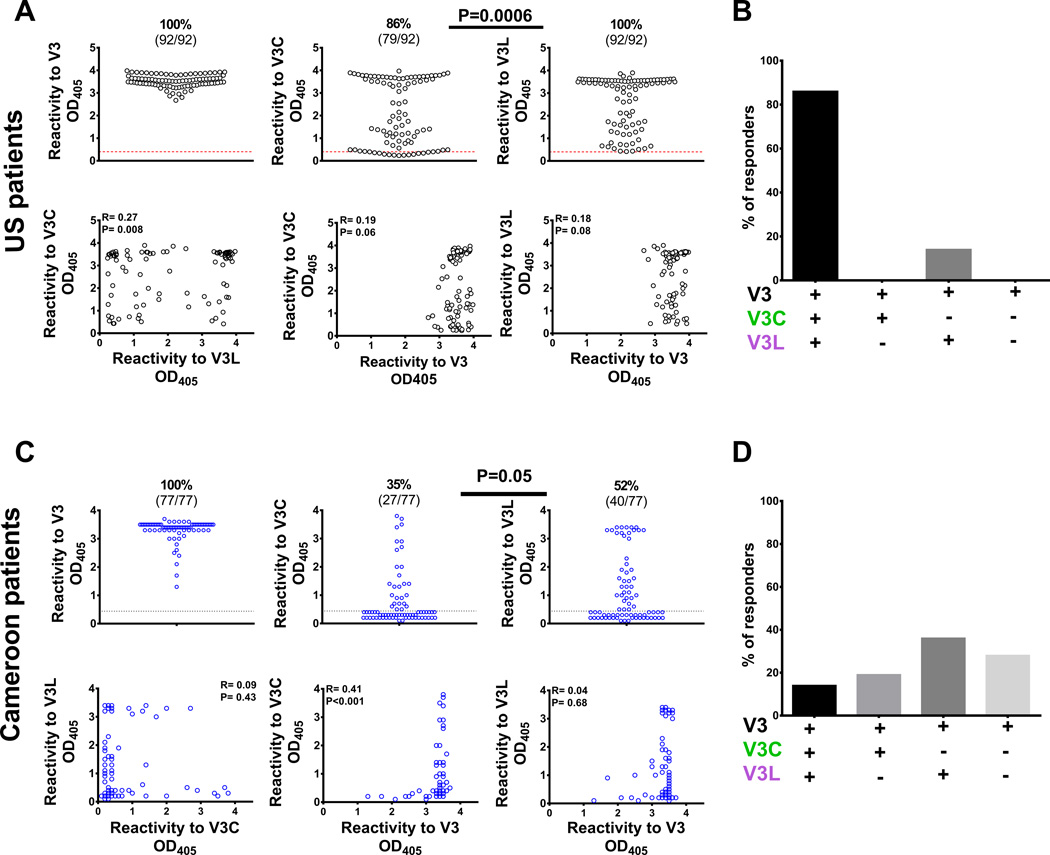
3.2. Antibodies with V3C- and V3L-binding modes are generated during HIV infection
We first evaluated the extents to which V3C-type and V3L-type Abs were present in the plasma of HIV-infected individuals. Plasma samples from 92 individuals with chronic clade B HIV infection from the US and from 77 Cameroonian individuals chronically infected with non-clade B HIV-1 were compared. All samples had reactivity against the full-length V3. Higher percentages of individuals responded to V3L than V3C among the US and Cameroonian patients (Fig 2A, 2C). In the US patients, the strengths of the V3C-type and V3L-type Ab reactivity correlated weakly to each other but not to Abs against the full-length V3. Indeed, most US patients had Ab reactivity to both V3C and V3L (Fig 2B). Among the Cameroonian patients, the strengths of V3C-type and V3L-type Abs did not correlate. The Cameroon patients also rarely produced both Ab types (Fig 2D). These patients had V3C-type Abs or V3L-type Abs or neither (Fig 2D). These results showed that V3C and V3L Abs are commonly produced during natural HIV infection, but the V3L Abs are present more frequently than the V3C Abs in both US and Cameroon patients chronically infected with diverse HIV-1 isolates of clade B and non-clade B.
Fig 2. Human subjects from the US and Cameroon infected with HIV-1 of different clades produced V3L- and V3C-type Abs, but more subjects had V3L-type Abs than V3C-type Abs.
A) ELISA reactivity of plasma IgG from chronically HIV-infected individuals (1:100 dilution) from the US was assessed against the full-length V3, V3C or V3L peptides. Comparison of the frequencies of V3C- versus V3L-responders was done by Fisher test with Yates’ continuity correction (p=0.0006). The cut-off values (red dotted lines) were based on mean plus three standard deviations of HIV-seronegative controls. OD405, optical density at 405 nm. Correlation analyses were done using the non-parametric Spearman rank-order test. The V3C- and V3L- binding strengths showed significant albeit weak correlation, but had no correlation with the full-length V3. B) The majority of HIV-infected subjects from the US responded to both V3C and V3L. A low percentage produced IgG reacting with V3L, while none responded to only V3C. C) ELISA reactivity of plasma IgG from Cameroonian individuals infected with HIV-1 of non-clade B was assessed against the full-length V3, V3C or V3L peptides. Comparison of the frequencies of V3C- versus V3L-responders was done by Fisher test with Yates’ continuity correction. The cut-off values (black dotted lines) were based on mean plus three standard deviations of HIV-seronegative controls. OD405, optical density at 405 nm. Correlation analyses were done using the non-parametric Spearman rank-order test. The binding strengths to V3C and V3L had no correlation. The full length V3-binding strengths weakly correlated with those to V3C but not V3L. D) A small fraction of HIV-infected subjects from Cameroon produced both V3C- and V3L-type Abs. The remaining subjects had V3C-type Abs, V3L-types Abs, or neither.
3.4. Vaccinations of humans with HIV gp120 elicit low levels of V3C- or V3L-type Abs
Humans immunized with HIV gp120 generate anti-V3 Abs [34], but the V3-binding modes of the Abs elicited have not been determined. The recombinant AIDSVAX gp120 proteins of HIV-1 clades B/E and B/B used in the respective VAX003 and VAX004 trials contain signature motifs for both V3C- and V3L-type Abs (Fig 1A and [35–37]). Thereby, we sought to determine whether these Abs were induced by the VAX003 and VAX004 vaccines (Table 1). Twenty four VAX003 and 23 VAX004 plasma samples collected 2 weeks after the last boost (the 7th injection) were examined in ELISA for IgG to full-length V3, V3 crown (linear 13-mer peptide), V3C, and V3L. Abs to the full-length V3 were detected in all VAX003 vaccinees and 95% of VAX004 vaccinees. High percentages also had Abs reactive with the linear V3 crown peptide (Fig 3A, 3D), showing the immunodominance of the V3 crown. In contrast, the VAX003 and VAX004 samples lacked reactivity to N-terminal and C-terminal V3 peptides (Fig S2).
Fig 3. Higher proportions of HIV-seronegative human subjects producing V3C-type Abs than V3L-type Abs following vaccination with recombinant HIV gp120 proteins.
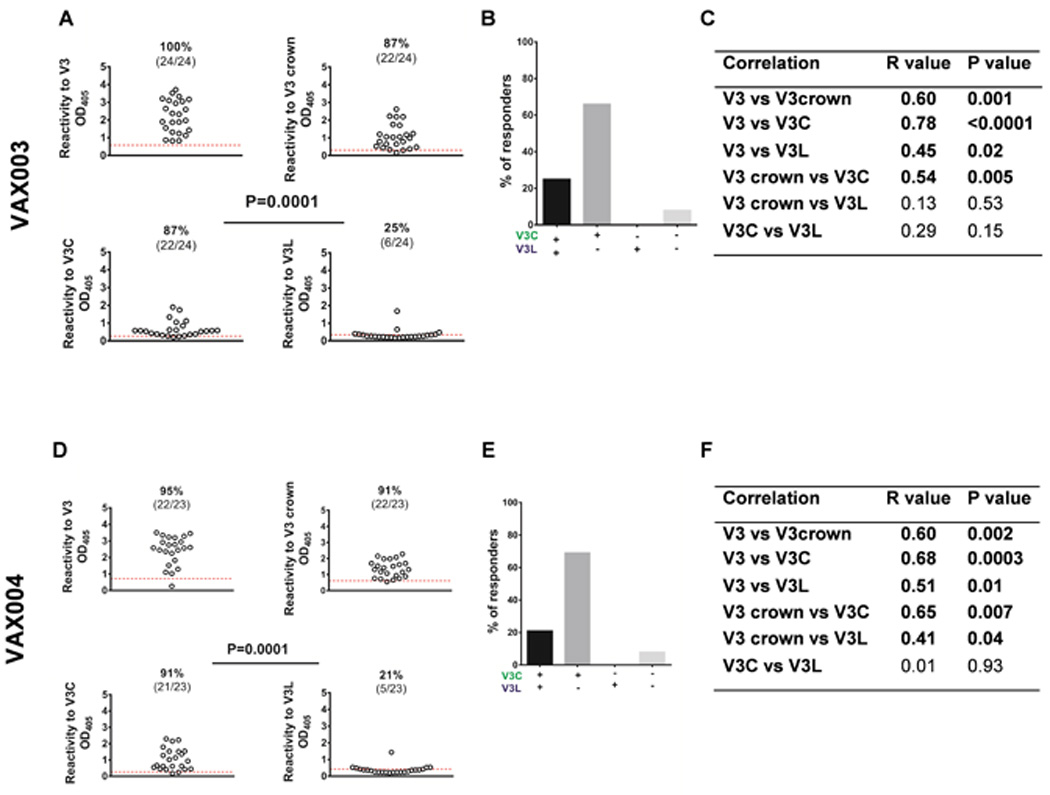
Plasma samples from participants of the VAX003 (AIDSVAX® B/E) and VAX004 (AIDSVAX® B/B) phase III vaccine trials were tested at 1:100 dilution for ELISA IgG reactivity to the full-length V3, V3C, V3L, and a linear peptide representing the V3 crown with similar sequence to V3L (RCRIHIGPGRAFY). Plasma samples collected two weeks after the final (7th) immunizations were tested. OD405, optical density at 405 nm. Red dotted lines show the cut-off values (mean + 3 standard deviations) derived from eight placebo recipients. Frequency comparisons were done by Fisher test with Yates’ continuity correction. Correlations were analyzed by the non-parametric Spearman rank-order test. A and D) The VAX003 and VAX004 participants produced IgG against the full-length V3 and the V3 crown, but the V3 crown-specific Abs raised were mainly of the V3C-type. B and E) The majority of the VAX003 and VAX004 vaccinees responded to V3C only. Few had Abs reactive to both peptides, and none reacted with V3L alone. C and F) The binding strengths of plasma Abs to V3C and V3L in the VAX003 and VAX004 vaccinees did not correlate. The binding strengths of V3C Abs correlated with those of Abs to the full-length V3 and the V3 crown.
Next we determined the presence of V3C- versus V3L-type Abs. 87% of the VAX003 vaccinees and 91% of the VAX004 vaccinees showed Abs to V3C (Fig 3A, 3D). However, lower percentages of the VAX003 (25%) and VAX004 (21%) vaccinees produced Abs to V3L. Hence, unlike the HIV-infected subjects, more vaccinees produced V3C-type Abs than V3L-type Abs. The binding strengths of Abs to V3C and V3L were low with most OD405 near the cut-off points. Moreover, only 25% of VAX003 vaccinees and 21% of VAX004 vaccinees made both V3C and V3L Abs (Fig 3B, 3E). The majority of VAX003 (66%) and VAX004 (69%) vaccinees produced V3C Abs and not V3L Abs. The binding strengths of V3C Abs were associated with those of Abs to the full-length V3 and the linear V3 crown (Fig 3C and 3F). These data show that, unlike natural HIV infection, immunization of humans with HIV gp120 elicited V3C Abs more frequently than V3L Abs, regardless of the gp120 proteins used in the vaccines. The V3C- and V3L-type Ab binding strengths were low relative to the total V3 Abs against the full-length V3 or the linear V3 crown, indicating the presence of other V3 Abs, distinct from the V3C and V3L Abs, in the VAX003 and VAX004 participants.
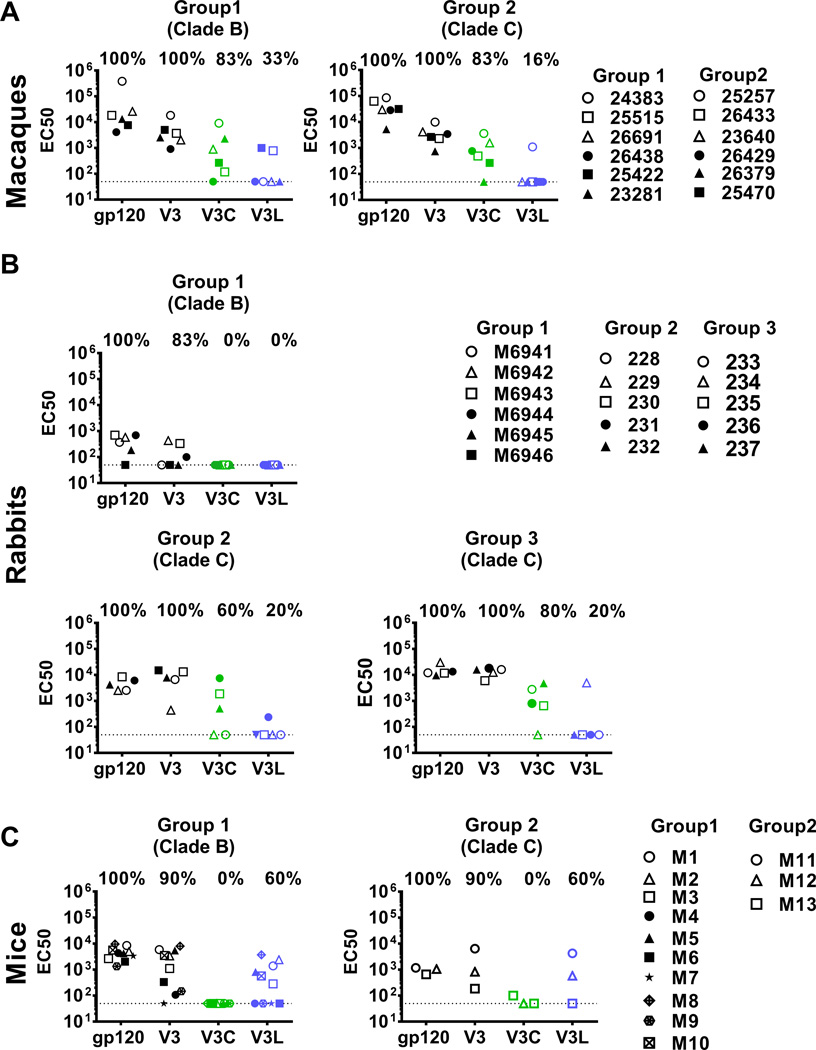
3.5. HIV Env vaccination induces mainly V3C-type Abs in macaques and rabbits, and only V3L-type Abs in mice
Although Abs against the V3 crown have been shown to be induced in different species of animals used in vaccine pre-clinical testings [16–20], their epitope-binding modes are undefined. To this end, we evaluated rhesus macaques, rabbits, and mice that were immunized with HIV-1 clade B or clade C Env immunogens, monomeric or oligomeric Env proteins, and with or without DNA priming. Plasma or serum samples were tested in ELISA for IgG against V3, V3C and V3L peptides, plus gp120 JRFL (clade B). EC50 values were calculated from the titration curves. Representative titration curves are shown in Fig S3.
A group of six macaques (Group 1) received co-immunization with HIV-1 gp160 DNA and gp140 trimeric proteins consisting of a set of HIV Env quasi-species from clade-B HIV-1-infected individuals (Table 1). Another group of six macaques (Group 2) was co-immunized with clonally-related clade C HIV-1 gp160 DNA and a clade C gp140 trimeric protein (Table 1). As expected, all clade B Env- and clade C Env-immunized macaques had similarly high IgG levels against gp120 and the full length V3 (Fig 4A). For both groups, 83% also had EC50 of >100 against V3C. In contrast, only 1–2 animals (16–33%) showed EC50 of >100 against V3L and the titers were low (p=0.014 vs. V3C for both groups). Hence, similar to human vaccinees, vaccinated macaques produced V3C-type Abs more frequently than V3L-type Abs, and this pattern was observed in macaques immunized with the HIV clade B or clade C Env vaccines.
Fig 4. HIV Env immunization elicited V3C-type Abs in macaques (A) and rabbits (B), and V3L-type Abs in mice (C).
Immunization regimens and sample collection time-points for the different animal species are shown in Table 1. Sera were diluted starting from 1:100 and tested in ELISA for IgG binding to gp120 JRFL, full length V3, V3C and V3L. The half maximal binding titers (EC50) from the individual animals are shown. Samples that did not attained half maximal binding at the lowest dilution were assigned EC50 of <100, similar to EC50 of the non-immunized controls (shown as dotted lines). The different symbols represent individual animals in each group.
We compared plasma or sera from three groups of rabbits that received a) co-immunization of HIV-1 gp160 DNA and gp140 trimeric proteins consisting of a set of HIV Env quasi-species from clade-B HIV infected individuals (Group 1), b) HIV-1 clade C trimeric gp140 CN54 protein alone (Group 2), or c) DNA gp160 CN54 prime followed by gp140 CN54 protein boosts (Group 3) (Table 1). The three groups of rabbits had IgG binding to gp120 and full length V3 (Fig 4B). However, the rabbits in Group 1 (clade B Env DNA+proteins) produced relatively low levels of Abs to gp120 and V3, and none generated V3C- or V3L-type Abs with >100 EC50. In Groups 2 and 3 (clade C Env protein without or with DNA priming), 60% and 80% of rabbits had EC50 of >100 for V3C-type Abs, while only 20% (1/5) produced V3L-type Abs. Thereby, similar to the patterns seen with immunized humans and macaques, the V3C-type Abs were made by more rabbits and to higher titers than the V3L-type Abs (p=0.03) (Fig 4B). The greater induction of the V3C-type Abs in these rabbits was attained with or without DNA priming.
Finally we tested sera from mice immunized with clade B gp120 JRFL (Group 1) or clade C gp140 CN54 (Group 2) proteins. All mice generated IgG binding to gp120 and the full-length V3, but the V3 Ab titers varied (Fig 4C). Interestingly, only V3L-type Abs were detected in both groups (Fig 4C). No V3C-type Abs were produced by these mice. This pattern was distinct from V3 Ab responses of the immunized humans, macaques, and rabbits, but was consistently observed whether the mice were immunized with monomeric clade B gp120 or oligomeric clade C gp140.
3.6. The V3C- and V3L-type Abs generated in immunized humans and animals mediate virus-neutralizing activities
Human V3C- and V3L-type mAbs showed comparable neutralizing potency against SF162, a clade B HIV-1 sensitive to V3 Abs (Table S1). To determine whether V3C- and V3L-type Abs induced by vaccination also have virus-neutralizing activities, we evaluated the VAX003 and VAX004 samples for neutralizing activity against SF162 pseudo virus. A wide range of % neutralization was observed that correlated with the binding strengths of Abs to the full-length V3 and V3C (Fig S4). Similar analyses performed with the immunized macaques and mice revealed correlation between SF162 neutralization and levels of Abs to V3C or V3L, respectively (Fig S4). For mice, correlations performed using EC50 and OD405 yielded comparable results (data not shown). Rabbits showed correlation between SF162 neutralization and Ab levels to full length V3 but not V3C (Fig S4).
To further evaluate the contribution of V3C and V3L Abs to virus neutralization, peptide blocking experiments were performed. Fig 5A shows that pre-treatment with the V3C peptide inhibited the neutralizing activity of the V3C-type mAb 2557, whereas the V3L peptide had less inhibitory effect. The neutralization by the V3L-type mAb 447-52D, on the other hand, was suppressed more by the V3L peptide than the V3C peptide. The neutralizing activities of both mAbs were inhibited by the full-length V3 peptide. The AUCs were calculated to show the relative levels of neutralization inhibition by the different V3 peptides as compared to the control peptide.
Fig 5. Neutralization of HIV-1 SF162 by sera from vaccinated humans and animals was inhibited to varying degrees by pre-treatment with full-length V3, V3C, and V3L peptides.
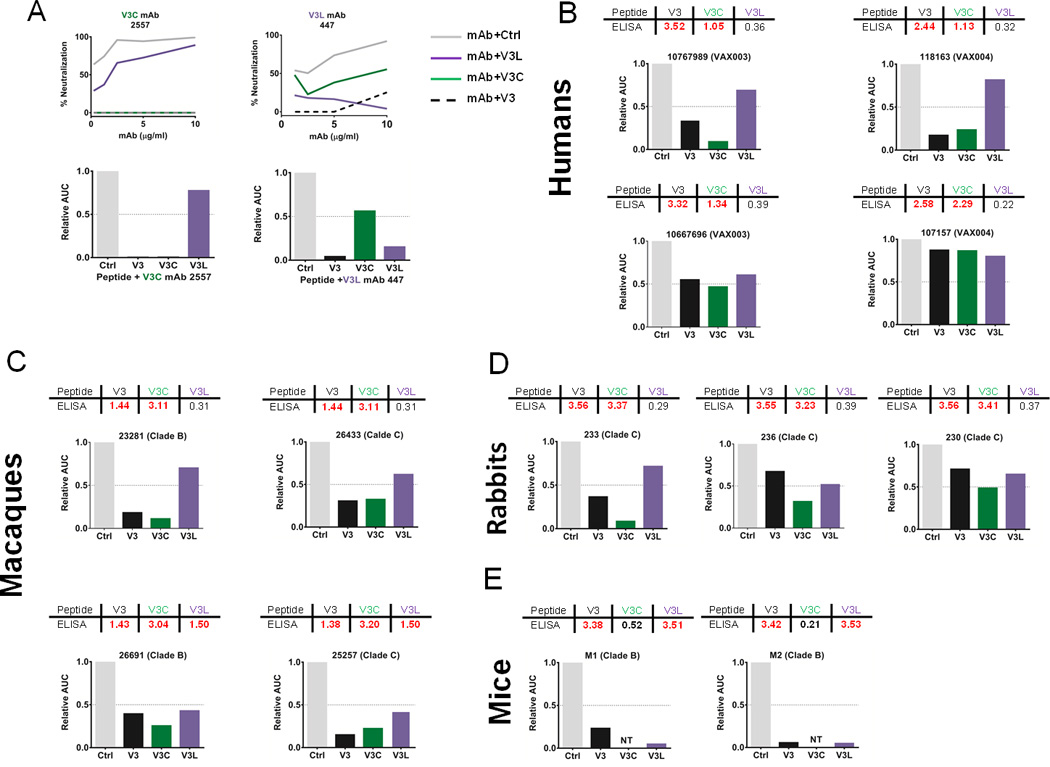
Neutralization of V3 Ab-sensitive SF162 pseudovirus was assessed with immune sera or control mAbs pre-treated with full-length V3, V3C, V3L or control scrambled peptides (40 µg/ml). The areas under the titration curves (AUC) were calculated and shown relative to the controls (normalized to 1). A) The ability of V3, V3C, and V3L peptides to block virus neutralization was verified using the V3C-type mAb 2557 or the V3L-type mAb 447-52D. The mAb titration curves are shown along with the relative AUC calculated from the respective curves. B-E) Virus neutralization by immune plasma or sera was assessed after pre-treatment with the different V3 peptides or the scrambled control. The data from selected sera from each species are shown to illustrate the varying levels of neutralization that could be attributed to the V3-specific Abs and particularly the V3C-type or V3L-type Abs elicited by vaccination. The peptide-blocking activity correlated with the presence of the respective Ab types (ELISA data are shown in the table on top of each graph) for many but not all sera.
The peptides were then used to assess the relative contribution of the V3C- and V3L-type Abs in virus neutralization. Plasma samples were selected from four human vaccinees with potent neutralizing activities and containing Abs to V3 and V3C but not to V3L (Fig 5B). Two distinct patterns were observed: a) neutralizing activities of 10767989 and 118163 were inhibited >50% by V3 and V3C peptides but not V3L peptide, b) neutralization of 10667696 and 107157 was weakly affected by the three V3 peptides including the full-length V3, indicating the contribution of non-V3 Abs to the observed neutralization.
Sera from selected macaques were similarly tested and revealed that inhibition of neutralization by V3C and V3L peptides corresponded with the presence of the respective Abs in the sera. For examples, macaques 23281 and 26433 had Abs to V3C but not V3L, and neutralization was inhibited >50% by the V3C peptide and not by the V3L peptide. Macaques 25257 and 26691 had both V3C Abs and V3L Abs, and neutralization was inhibited >50% by both V3C and V3L peptides (Fig.5C). As expected, the full-length V3 peptide inhibited neutralizing activity of all four. Hence, macaque Abs to V3C and V3L elicited by immunization contribute to virus neutralization.
Consistent with the fact that V3C-type Abs predominated over V3L-type Abs in the immune rabbit sera, neutralization by three rabbit sera tested was inhibited most by the V3C peptide. The V3L peptide did not decrease neutralization by >50%. Interestingly, the inhibition by the V3C peptide was greater than that seen with the full length V3. This pattern is different from that seen with the macaque sera and indicates a possibility that the neutralizing Abs in the rabbit sera have higher affinity for V3C than the full-length V3. Nonetheless, the neutralizing activities of rabbit sera like 236 and 230 were reduced only partially by the V3 peptides, suggesting the presence of neutralizing Abs targeting sites other than V3 (Fig 5D).
Unlike rabbits, mice immunized with HIV Env generated only V3L-type Abs. Accordingly, pre-treatment of mouse sera with the V3L peptide abolished the neutralizing activity of the sera. Neutralization was also almost completely inhibited by the full-length V3, demonstrating that neutralization is mediated solely by V3-specific Abs and particularly the V3L-type Abs (Fig 5D).
Altogether the data demonstrate that immunization of humans and animals with the HIV Env induces species-specific arrays of Abs to the V3 crown. Abs with V3C- and V3L-binding modes can be elicited by the HIV Env vaccines, and both Ab types are equally capable of mediating virus neutralization. Inducing both Ab types should enrich the Ab repertoires against HIV; however, humans and animals immunized with the HIV Env show a propensity of generating only one Ab type.
4. Discussion
This study demonstrated the presence of both V3C-type and V3L-type Abs in the majority of HIV-infected persons. SHIV-infected macaques also produced Abs that recognize both V3C and V3L [22]. In contrast, immunization with the HIV Env elicited only one of the two Ab types. The V3C-type Abs were produced by immunized humans, macaques and rabbits, while the V3L-type Abs were generated in immunized mice. Very few individual humans and animals made both Abs after immunization. The reasons for the species differences and the limited V3 Ab responses induced by the HIV Env vaccines are not fully understood. One parameter we considered was the clades of HIV Env immunogens used in the vaccines. However, the V3C-type Abs was detected in humans or animals receiving HIV Env of different clades. For example, the predominance of the V3C-type Abs was seen in humans receiving the bivalent AIDVAX® B/E or AIDSVAX® B/B gp120 proteins. The two groups of macaques that received a mixture of HIV Env clones or quasi-species (clade B or clade C) also had mainly the V3C-type Abs. The different groups of rabbits receiving clade C Env immunogens also produced primarily the V3C-type Abs. A recent study reported that one of the two anti-V3 crown mAbs isolated from a rabbit immunized with the HIV Env gp120 JRFL (clade B) using a DNA prime-protein boost regimen binds to its epitope in a V3C-binding mode [23]. The frequencies of V3L responders are also unlikely determined by the GPGR motif typically present in clade B Envs [38] . Mice immunized with clade B gp120 or clade C gp140 similarly generated the V3L-type Abs, while humans, macaques, and rabbits generated very little V3L-type Abs, irrespective of the clades of the Env immunogens. Further, the addition of HIV Env DNA for priming or co-immunization yielded similar results to immunization with the HIV Env proteins alone. Hence, the various HIV Env immunogens and vaccination regimens tested in this study show a deficiency in mimicking the ability of natural HIV infection to induce an array of Abs that target both V3C and V3L epitopes in the V3 crown.
Although vaccination with the intact HIV Env immunogens induced mainly macaque Abs binding to V3C, a recent study shows that immunization with the V3C and V3L mimotopes were able to elicit Abs recognizing the respective peptides [19]. Together with the present study, the results demonstrate that, like humans, macaques have the capacity to produce both types of V3 Abs, although only one type is induced in individual animals immunized with the intact HIV Env or the mimotopes. To elicit a broader set of V3 Abs that includes both the V3C- and V3L-type Abs in the same animals, a different vaccine strategy is needed. Identifying vaccine strategies that can expand the Ab repertoires to encompass both V3C-type and V3L-type Abs would be valuable as they may be used to further broaden the Ab responses to epitopes beyond the V3 crown.
Another factor that may influence the induction of V3C- versus V3L-type Abs is their variable gene usage. The human VH5.51 gene is a signature of the V3C-type human mAbs [29]. The human V3C-type mAbs also use only Vλ light chain [27]. It is thus surprising that the AIDSVAX B/B and B/E vaccines elicited human Ab responses skewed toward the V3C-type Abs that may be more restricted in their gene usage. The V3L-type Abs that can be encoded by many different VH genes were produced only by very few individuals and at low levels. The failure of a diverse array of B cells with different VH genes to engage the V3L epitope is perplexing. One plausible reason is that the cyclic V3L mimotope presents an exceedingly constrained structure of the V3 arch that focuses the Ab binding to the conserved GPG motif. Robust Ab responses to this constrained V3L structure were generated when the cyclic V3L mimotope was used as an immunogen to vaccinate macaques [19]. By contrast, in the context of the whole soluble Env proteins, the V3 arch is a flexible segment capable of adopting many different conformations [39–41]. A large array of Abs may thus be elicited that react with the non-constrained V3 arch, but very few recognize the constrained structure presented by the V3L mimotope. Consistent with this idea, the linearized version of the V3L peptide was recognized by sera of Env-immunized humans lacking Ab reactivity to the cyclic V3L peptide (Fig 3 A,D: V3 crown vs. V3L). However, unlike the vaccinees, humans infected with either clade B or non-clade B HIV-1 generated the V3L-type Abs more frequently than the V3C-type Abs, indicating the constrained V3 arch is poorly immunogenic only in context of the Env vaccines evaluated herein and not during natural infection.
The importance of eliciting a diverse array of Abs targeting different epitopes for protection against HIV has been noted in many studies [10, 22, 42, 43]. Neutralizing Abs against V3 or other sites such as the CD4-binding site readily select for neutralization-resistant variants [44–48]. Combinations of mAbs were needed to suppress viremia after infection was established [49]. Interestingly, the endogenous induction of Abs against the V3 crown after passive transfer with broadly neutralizing Abs was found to be beneficial as the different Abs can work in synergy to virus escape [22]. We postulate that a greater diversity of Ab responses against the distinct epitopes within V3 should also be beneficial for protection against HIV. This study shows that the V3C-type and V3L-type Abs induced by vaccination are capable of mediating neutralization of SF162, a virus isolate sensitive to the V3 crown-specific Abs. However, only few immunized individuals had neutralizing activities blockable by both V3C and V3L peptides.
5. Conclusion
In conclusion, HIV infection elicits both V3C-type and V3L-type of Abs, whereas immunization with the different HIV Env vaccines induces only either type. In immunized humans, macaques and rabbits, V3C-type Abs are frequently generated, and in immunized mice, only the V3L-type Abs are produced. The V3C-type and V3L-type Abs raised by vaccination have virus-neutralizing activities. Nonetheless, induction of Ab responses dominated by only one type of V3 Abs would propel the emergence of resistant escape variants. Future vaccine regimens need to incorporate strategies that would induce broader Ab repertoires targeting simultaneously multiple distinct epitopes within V3 and also other vulnerable sites on the HIV Env glycoprotein.
Supplementary Material
Highlights.
Abs with distinct V3-binding modes (cradle and ladle) are made during HIV infection.
In contrast, HIV envelope vaccines induce either the cradle- or the ladle-type Abs.
The types of V3 Abs elicited differ depending on the vaccinated animal species.
V3 cradle- and V3 ladle- type Abs induced by vaccination can neutralize virus.
Acknowledgments
This work was supported in part by the Henry M. Jackson Foundation for the Advancement of Military Medicine (SZP, CEH), NIH grants R21 AI114520 (CEH) and HIVRAD P01 AI100151 (XPK, SZP) and by research funds from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (VA Merit Review and Research Career Scientist – CEH).
We would like to thank Michael Tuen, Chitra Upadhyay, Jing Zhang and Sara Yahyaei for general lab supports.
This paper is dedicated to the memory of our colleague and friend, Dr. Phillipe Nyambi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest
References
- 1.Ivanoff LA, Dubay JW, Morris JF, Roberts SJ, Gutshall L, Sternberg EJ, et al. V3 loop region of the HIV-1 gp120 envelope protein is essential for virus infectivity. Virology. 1992;187:423–432. doi: 10.1016/0042-6822(92)90444-t. [DOI] [PubMed] [Google Scholar]
- 2.Nabatov AA, Pollakis G, Linnemann T, Kliphius A, Chalaby MI, Paxton WA. Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies. Journal of Virology. 2004;78:524–530. doi: 10.1128/JVI.78.1.524-530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang Y, Liu W, Chen Y, Zhang C, Su W, Zhang Y, et al. The variable loop 3 in the envelope glycoprotein is critical for the atypical coreceptor usage of an HIV-1 strain. PloS one. 2014;9:e98058. doi: 10.1371/journal.pone.0098058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung CS, Vander Heyden N, Ratner L. Analysis of the critical domain in the V3 loop of human immunodeficiency virus type 1 gp120 involved in CCR5 utilization. Journal of Virology. 1999;73:8216–8226. doi: 10.1128/jvi.73.10.8216-8226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, et al. Conserved structural elements in the V3 crown of HIV-1 gp120. Nature structural & molecular biology. 2010;17:955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 6.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, et al. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PloS one. 2010;5:e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PloS one. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CC, Lam SN, Acharya P, Tang M, Xiang SH, Hussan SS, et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolla-Pazner S. Improving on nature: focusing the immune response on the V3 loop. Human antibodies. 2005;14:69–72. [PubMed] [Google Scholar]
- 14.Davis KL, Bibollet-Ruche F, Li H, Decker JM, Kutsch O, Morris L, et al. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1 V3-specific antibodies in human plasma. Journal of Virology. 2009;83:1240–1259. doi: 10.1128/JVI.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, et al. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Tuen M, Liu J, Nadas A, Pan R, Kong X, et al. Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines. Vaccine. 2013;31:5413–5421. doi: 10.1016/j.vaccine.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visciano ML, Tuen M, Gorny MK, Hioe CE. In vivo alteration of humoral responses to HIV-1 envelope glycoprotein gp120 by antibodies to the CD4-binding site of gp120. Virology. 2008;372:409–420. doi: 10.1016/j.virol.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zolla-Pazner S, Cohen S, Pinter A, Krachmarov C, Wrin T, Wang S, et al. Cross-clade neutralizing antibodies against HIV-1 induced in rabbits by focusing the immune response on a neutralizing epitope. Virology. 2009;392:82–93. doi: 10.1016/j.virol.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessell AJ, McBurney S, Pandey S, Sutton W, Liu L, Li L, et al. Induction of neutralizing antibodies in rhesus macaques using V3 mimotope peptides. Vaccine. 2016;34:2713–2721. doi: 10.1016/j.vaccine.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, et al. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. Journal of immunology. 2016;196:3064–3078. doi: 10.4049/jimmunol.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sholukh AM, Mukhtar MM, Humbert M, Essono SS, Watkins JD, Vyas HK, et al. Isolation of monoclonal antibodies with predetermined conformational epitope specificity. PloS one. 2012;7:e38943. doi: 10.1371/journal.pone.0038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein F, Nogueira L, Nishimura Y, Phad G, West AP, Jr, Halper-Stromberg A, et al. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. The Journal of experimental medicine. 2014;211:2361–2372. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan R, Sampson JM, Chen Y, Vaine M, Wang S, Lu S, et al. Rabbit anti-HIV-1 monoclonal antibodies raised by immunization can mimic the antigen-binding modes of antibodies derived from HIV-1-infected humans. Journal of Virology. 2013;87:10221–10231. doi: 10.1128/JVI.00843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar R, Pan R, Upadhyay C, Mayr L, Cohen S, Wang XH, et al. Functional and Structural Characterization of Human V3-Specific Monoclonal Antibody 2424 with Neutralizing Activity against HIV-1 JRFL. Journal of Virology. 2015;89:9090–9102. doi: 10.1128/JVI.01280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS pathogens. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanfield RL, Gorny MK, Williams C, Zolla-Pazner S, Wilson IA. Structural rationale for the broad neutralization of HIV-1 by human monoclonal antibody 447-52D. Structure. 2004;12:193–204. doi: 10.1016/j.str.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang XH, et al. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure. PloS one. 2011;6:e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killikelly A, Zhang HT, Spurrier B, Williams C, Gorny MK, Zolla-Pazner S, et al. Thermodynamic signatures of the antigen binding site of mAb 447-52D targeting the third variable region of HIV-1 gp120. Biochemistry. 2013;52:6249–6257. doi: 10.1021/bi400645e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorny MK, Wang XH, Williams C, Volsky B, Revesz K, Witover B, et al. Preferential use of the VH5-51 gene segment by the human immune response to code for antibodies against the V3 domain of HIV-1. Molecular immunology. 2009;46:917–926. doi: 10.1016/j.molimm.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhyay C, Mayr LM, Zhang J, Kumar R, Gorny MK, Nadas A, et al. Distinct mechanisms regulate exposure of neutralizing epitopes in the V2 and V3 loops of HIV-1 envelope. Journal of Virology. 2014;88:12853–12865. doi: 10.1128/JVI.02125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. In: Coligan John E, et al., editors. Current protocols in immunology. 2005. Chapter 12:Unit 12 1. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Tuen M, Li H, Tse DB, Hioe CE. Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation. Vaccine. 2011;29:9064–9074. doi: 10.1016/j.vaccine.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke V, Williams C, Sukumaran M, Kim SS, Li H, Wang XH, et al. Structural basis of the cross-reactivity of genetically related human anti-HIV-1 mAbs: implications for design of V3-based immunogens. Structure. 2009;17:1538–1546. doi: 10.1016/j.str.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. The Journal of infectious diseases. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 35.Shmelkov E, Nadas A, Swetnam J, Zolla-Pazner S, Cardozo T. Indirect detection of an epitope-specific response to HIV-1 gp120 immunization in human subjects. PloS one. 2011;6:e27279. doi: 10.1371/journal.pone.0027279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 37.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 38.Felsovalyi K, Nadas A, Zolla-Pazner S, Cardozo T. Distinct sequence patterns characterize the V3 region of HIV type 1 gp120 from subtypes A and C. AIDS research and human retroviruses. 2006;22:703–708. doi: 10.1089/aid.2006.22.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanfield R, Cabezas E, Satterthwait A, Stura E, Profy A, Wilson I. Dual conformations for the HIV-1 gp120 V3 loop in complexes with different neutralizing fabs. Structure. 1999;7:131–142. doi: 10.1016/s0969-2126(99)80020-3. [DOI] [PubMed] [Google Scholar]
- 40.Sharon M, Kessler N, Levy R, Zolla-Pazner S, Gorlach M, Anglister J. Alternative conformations of HIV-1 V3 loops mimic beta hairpins in chemokines, suggesting a mechanism for coreceptor selectivity. Structure. 2003;11:225–236. doi: 10.1016/s0969-2126(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 41.Almond DKT, Kong X, Zolla-Pazner S&, Cardozo T. Dynamic characterization of the V3 loop crown. Antiviral Therapy. 2007;12:13–31. [Google Scholar]
- 42.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS pathogens. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong R, Louder MK, Wagh K, Bailer RT, deCamp A, Greene K, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. Journal of Virology. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders KO, Wang L, Joyce MG, Yang ZY, Balazs AB, Cheng C, et al. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection. Journal of Virology. 2015;89:8334–8345. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoddart CA, Galkina SA, Joshi P, Kosikova G, Long BR, Maidji E, et al. Efficacy of broadly neutralizing monoclonal antibody PG16 in HIV-infected humanized mice. Virology. 2014;462–463:115–125. doi: 10.1016/j.virol.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund NT, Horwitz JA, Nogueira L, Sievers SA, Scharf L, Scheid JF, et al. A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1 In Vivo. PLoS pathogens. 2015;11:e1005238. doi: 10.1371/journal.ppat.1005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.