Abstract
Aims and Objectives:
The principal goal of endodontics is the prevention of periapical infection. Acute and chronic apical periodontitis occur due to the persistence of pathogenic microorganisms such as Enterococcus faecalis and Candida albicans harboring the root canal systems of the teeth. The concept of the use of probiotics in addressing endodontic disease is new and has not been studied adequately. On the basis of the success of probiotics in periodontal treatment, this preliminary work was performed (a) to evaluate the antibacterial efficacy of probiotics against common endodontic pathogens, i.e. E. faecalis and C. albicans, and (b) to evaluate the potential use of probiotic therapy as an additive in endodontic treatment procedures.
Materials and Methods:
Two commercial probiotics were selected and evaluated based upon the numbers and concentration of organisms. Pathogenic test organisms were C. albicans (ATCC 10231) and E. faecalis (ATCC 29212). Phase 1 of the study was conducted by agar cup method test to evaluate the antibacterial activity of the selected probiotics against E. faecalis and C. albicans by measuring zones of inhibition (ZOI) in mm. Microorganisms from probiotic samples were isolated following manufacturer's instructions. Pathogenic organisms were set to a 0.1 McFarland standard challenge. Circular wells of 8 mm diameter were punched in each of the poured plates. Appropriately diluted test samples were added to the above-punched wells. The volume of the solution added to each well was 100 μl. The plates were incubated in an upright position at 37°C for 24 hours under aerobic conditions. Post incubation, ZOI was measured (mm). Phase 2 was conducted by mixing 9 ml of 30% poloxamer 407 and de Man, Rogosa and Sharpe (MRS) broth in a test tube with 500 μl of either E. faecalis or C. albicans set at an optical density (OD) of 0.252, together with 500 μl of test probiotic strain, set at a respective OD. Samples were then incubated at 37°C for 48 hours, followed by serial dilutions by 1 ml till 108. This was done to calculate colony forming units (CFU)/ml counts. Controls used were endodontic pathogens in 30% poloxamer with MRS broth without any probiotic group.
Results:
Probiotic groups showed inhibitory activity against E. faecalis by the agar cup method, whereas there was no effect on C. albicans. In the biofilm stage, both the test groups had an antibacterial effect on pathogenic organisms.
Conclusion:
This study suggests that probiotic organisms of the species Lactobacillus and Bifidobacterium are effective for preventing the growth of E. faecalis and C. albicans in vitro. Because probiotics are available in varied compositions and concentrations, further evaluation for their role in treating endodontic infection is suggested and warranted. In addition, the study suggested that poloxamer 407 could be utilized as an ideal delivery vehicle for probiotics for use as a potential endodontic intracanal medicament.
KEYWORDS: Antimicrobial, bacteriotherapy, endodontics, innovative, probiotics
INTRODUCTION
Simply defined, probiotics are live bacteria that confer a health benefit to the host. The term “Probiotic” was initially coined to oppose the term “Antibiotic” by Lilley and Stillwell in 1965. The World Health Organization (WHO) recognizes probiotics to be the next, most important immune defense system in the event that current antibiotics become useless because of the development of bacterial resistance. This concept has fuelled research for using probiotics in medicine and dentistry.
The organisms that have been used in the past as probiotics are certain strains of Lactobacillus and Bifidobacteria.[1] Some of the mechanisms of action of these probiotics include the production of bacteriocin-like inhibitory substances and the alteration of local pH, competing for nutrients, forming physical barriers, and stimulating immune response.[2] Within the field of endodontics, the traditional method of treatment has been to eliminate the bacteria present in the root canal system. When root canal therapy fails, the presence of bacteria is almost always present. Enterococcus faecalis and Candida albicans are particularly found consistently in posttreatment endodontic disease.[3] Endodontic therapy aims to remove bacteria through chemomechanical methods.
To our knowledge, probiotics work is sparse in terms of their therapeutic effect in the treatment of endodontic disease. Recently, probiotics have been introduced in dentistry for the treatment or prevention of disease. Experimental studies and clinical trials have demonstrated that certain gastrointestinal bacteria may control the growth of some oral microorganisms, including those cariogenic species associated with dental decay. Oral administration of probiotics has also been explored in the control of periodontal disease by reducing plaque levels and gingival inflammation.[4,2] The purpose of this study is to evaluate the potential use of probiotic therapy as an adjunct in endodontic therapy along with its effect on the reduction or elimination of apical periodontitis.
MATERIALS AND METHODS
PROBIOTIC STRAIN SELECTION
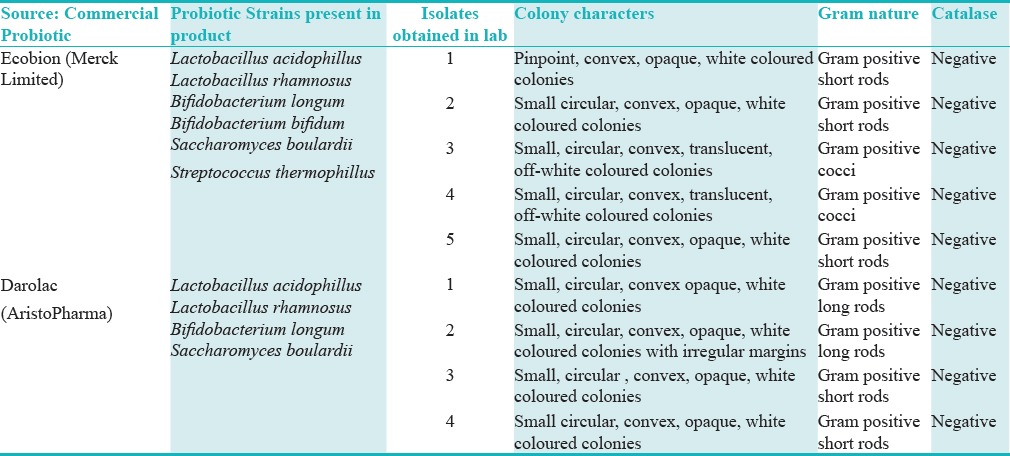
Because of financial limitations, individual probiotic strains could not be purchased from ATCC (American Type Culture Collection). After extensive research, two commercial probiotic cocktails Ecobion and Darolac were used in this study. The probiotic cocktails were delivered in wrapped ice packaging to preserve viability of the organisms. Upon arrival, the probiotics were stored in a refrigerator at 30°F. Probiotic strains were isolated as per the manufacturer's instructions [Table 1].
Table 1.
Revival of probiotics from commercial products
PATHOGENIC STRAIN SELECTION
E. faecalis ATCC 29212 was chosen for this study after extensive literature review, which revealed that this organism possesses multiple properties leading to its key role as an endodontic pathogen.[5,6]
C. albicans ATCC 10231 was chosen as another pathogenic test organism because of its biphasic nature, which allows it to be the universal co-aggregate in biofilms; it is the most frequently isolated fungus from root filled teeth with apical periodontitis.[7]
PHASE 1: TESTING FOR PROBIOTIC EFFICACY AGAINST E. FAECALIS AND C. ALBICANS
Planktonic stage evaluation
Lactobacillus strain was isolated from each of the commercially available probiotic capsules viz. Ecobion (E2) and Darolac (D4). These strains were further evaluated using different microbiological techniques.
Agar Cup Method
0.5 ml of requisite test pathogen culture of 0.1 optical density (OD) at 620 nm was inoculated into 20 ml of molten sterile agar cooled to 45°C ± 2°C. The solutions were mixed thoroughly and poured into a sterile empty petridish and allowed to solidify. Circular wells of 8 mm diameter were punched using a sterile steel cork borer in each of the poured plates. Appropriately diluted test samples were added to the above-punched wells. The volume of the solution added to each well was 100 μl. The plates were incubated in an upright position at 37°C for 24 hours under aerobic conditions. Post incubation, zone of inhibition (ZOI) was measured (mm).
Extraction of cell-free supernatant (CFS)
The pure isolate of Lactobacillus spp. was propagated in 100 ml flask containing MRS broth (pH 6.0) and incubated at 37°C for 72 hours under microaerophilic conditions. The supernatant that may contain crude bacteriocin, a cell-free solution, was obtained by centrifuging the culture at 10000 rpm for 20 minutes at 4°C. After centrifugation, the supernatant was collected in a fresh sterile tube and the pellet was discarded. The crude CFS was adjusted to pH 6.0 using 1 N NaOH prior to testing and crude CFS used as well. The crude CFS diluted to 1:2 used for testing.
PHASE 2: BIOFILM STAGE TESTING; INTRACANAL DELIVERY VEHICLE FOR PROBIOTICS
Controls testing
Probiotic isolates (E2 and D4), pathogens (C. albicans ATCC 10231and E. faecalis ATCC 29212) stocks were prepared in tryptic soy broth (TSB) broth and adjusted to a respective OD at 600 nm using UV spectrophotometer [Figure 1]. Nine millilitre of poloxamer was placed in a test tube and 500 µl of the above adjusted cultures were added and vortexed at 4°C in a refrigerated environment to allow homogenous mixing of poloxamer and microorganisms. This mixture was then incubated for 48 hours in an incubator at 37°C under aerobic conditions. After 48 hours, serial dilutions were prepared and plated on Brain Heart Infusion (BHI) agar plates to evaluate colony forming units (CFU) of the organisms. Serial dilutions were made by adding 1 ml of poloxamer mix to 9.0 ml sterile saline, followed by serially diluting the mixture by 1 ml into 9.0 ml sterile saline till 10−8 dilution followed by incubation at 37°C for 72 hours under aerobic conditions.
Figure 1.

Control testing poloxamer mixture
Testing for probiotic/pathogenic organism poloxamer mixture
9 ml of the poloxamer mixture was placed in a test tube along with 0.5ml of E. faecalis ATCC 29212 (OD, 0.252). Then, 500 µl of the test probiotic strain (E2 OD, 0.346; D4 OD, 0.328) was added. Nine millilitre of the poloxamer mixture was placed in a test tube along with 500 µl of C. albicans ATCC 10231 (OD, 0.256). Then, 500 µl of the test probiotic strain (E2 OD, 0.346; D4 OD, 0.328) was added. After 48 hours of incubation, serial dilutions of the pathogenic biofilm samples were prepared and plated on BHI agar plates to evaluate CFU of the organisms.
Serial dilutions were made by adding 1 ml of poloxamer mix to 9.0 ml sterile saline, followed by serially diluting the mixture by 1 ml into 9.0 ml sterile saline till 108 dilution. Plating was done by adding 1 ml of the dilutions onto BHI agar plates followed by incubation at 37°C for 72 hours aerobically. CFU were evaluated for all test groups and compared to controls based on the prepared dilutions to reflect the actual number of probiotics and pathogenic organisms in each group [Figure 2].
Figure 2.

Testing for probiotic (E2 and D4) against pathogenic organism (E. faecalis ATCC and C. albicans ATCC) in poloxamer mixture
RESULTS
RESULT OF AGAR CUP/WELL DIFFUSION METHOD
The inhibitory activity of the test strain was considered significantly positive if the ZOI produced by the test strain against the indicator strain (endodontic pathogen) was at least 10 mm.
The cell free suspensions (CFS) of these 2 isolates were tested against E. faecalis ATCC 29212 and C. albicans ATCC 10231 using agar cup/well diffusion method. The CFS of the two strains were used in 3 forms, i.e. (a) neat/crude; (b) CFU diluted to 1:2; and (c) CFS adjusted to pH 6.0.
(a) The crude CFS of both E2 and D4 when tested against E. faecalis ATCC 29212 showed antimicrobial activity with an average ZOI of 16.5 mm.
(b) The 1:2 diluted crude CFS of both E2 and D4 when tested against E. faecalis ATCC 29212 demonstrated antimicrobial activity with an average ZOI of 12 mm.
(c) However, when the crude CFS was adjusted to pH 6.0, it showed no activity against E. faecalis ATCC 29212 and C. albicans ATCC 10231.
(d) All the three forms of CFS did not show any antimicrobial activity against C. albicans ATCC 10231 [Table 2; Figures 3 and 4].
Table 2.
Zone of inhibition of Probiotic samples against E. faecalis and C. albicans
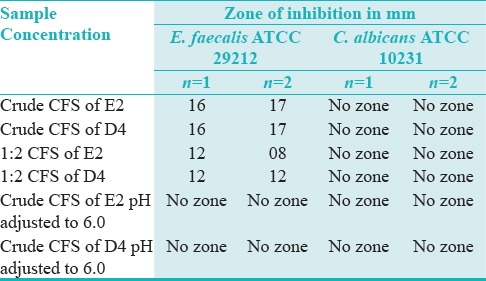
Figure 3.
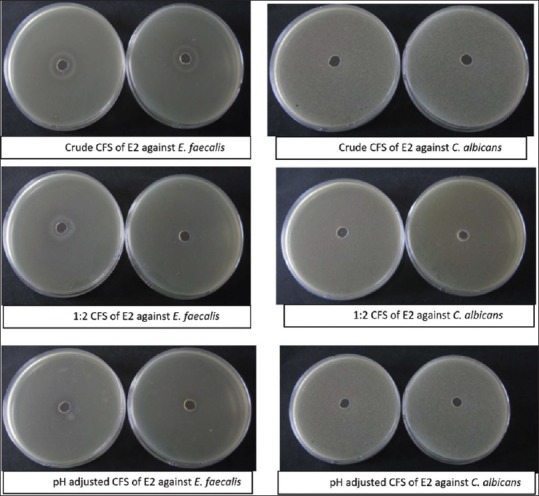
Agar cup method test of Ecobion (E2) CFS against E. faecalis and C. albicans
Figure 4.
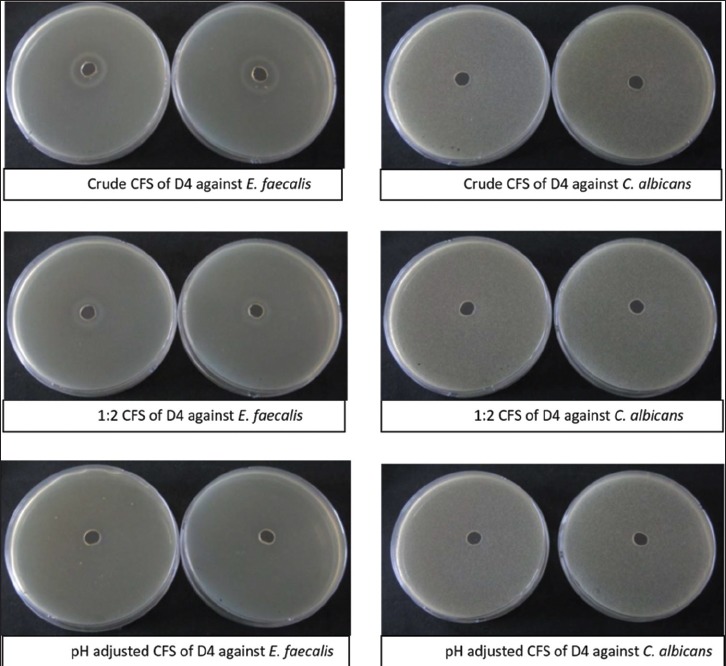
Agar cup method test of Darolac (D4) CFS against E. faecalis and C. albicans
ANTIBACTERIAL EFFICACY OF PROBIOTICS ON BIOFILM MORPHOLOGICAL STAGE OF ENDODONTIC PATHOGENS [TABLES 3 and 4]
Table 3.
Testing of probiotic Ecobion (E2) against C. albicans ATCC 10231 and E. faecalis ATCC 29212, poloxamer mixture

Table 4.
Testing of probiotic Darolac (D4) against C. albicans ATCC 10231 and E. faecalis ATCC 29212, poloxamer mixture

The two Lactobacillus strains were further evaluated for their efficacy to inhibit biofilm formation by endodontic pathogens. It was observed that
(a) Ecobion-E2 and Darolac-D4 when tested against E. faecalis ATCC 29212 were found to show a reduction of 90.60% and 77.51%, respectively.
(b) Ecobion-E2 when tested against C. albicans ATCC 10231 for its efficacy were found to show 83.79% reduction.
(c) However, Darolac-D4 when tested against C. albicans ATCC 10231 for its efficacy were found to show no reduction.
DISCUSSION
Probiotic use has been studied for the treatment of oral health problems. Specifically, the use of probiotics has been explored to aid in the treatment of periodontal problems, halitosis, and caries prevention.[2,8] The most commonly used strains belong to the Lactobacillus and Bifidobacterium genera that are commonly found in the oral cavity, including caries lesions.[9,10] These were the first probiotic species to be introduced into research.[11] Lactobacillus rhamnosus GG, ATCC 53103 has been proposed to reduce the risk for caries by producing a growth inhibitory substance against Streptococcus sobrinus.[10] Other strains of probiotics in the oral cavity include L. acidophilus, L. casei Shirota, L. paracasei, L. casei, L. johnsonii, L. reuteri, Propionibacterium, Weisella cibaria.[12]
Probiotic approach has not yet been extensively evaluated for use in endodontic therapy. Endodontics is the branch of dentistry that is concerned with the morphology, physiology, and pathology of the human dental pulp and periradicular tissues. It has been established that the primary etiology of endodontic infections is bacteria.[13] Primary infections of the necrotic pulp tissue are generally composed of a mixed bacterial community dominated by anaerobic Gram-negative bacteria.[14] Persistent infections tend to be dominated by a more specific community of bacteria. These bacteria are anaerobic and Gram positive, in particular E. faecalis.[15]
The biphasic nature of C. albicans allows it to be the universal co-aggregate in biofilms and is the most frequently isolated fungus from root filled teeth with apical periodontitis. E. faecalis appears to be highly resistant to the medicaments used in treatment and is one of the few microorganisms shown in vitro to be resistant to calcium hydroxide because of its proton pump.[16] It can also survive as a single organism without the support of other bacteria. In addition, E. faecalis has the ability to form a surface attached microbial community known as biofilm. This allows it to be protected from host defenses as well as systemic treatment. Different methods of combating E. faecalis were explored through the use of different irrigants. “Out of the box” treatments were evaluated for use against E. faecalis such as the use of passion fruit juice as an endodontic irrigant,[17] as well as the use of lasers and phytomedicine.[18,19]
Probiotics hold a potential avenue of more common and broad use for antibacterial treatment in the future. In this study, an innovative approach which might aid in increasing the success of endodontic therapy was investigated. This innovative approach involves bacteriotherapy by allowing probiotic organisms to eliminate pathogenic organisms, either by outcompeting/immune modulation or by secreting antimicrobial substances such as peroxides.[20]
This study involved two phases: phase one (discovery phase) and phase two (application phase). Phase one was an agar cup method test utilized to determine the sensitivity or resistance of the pathogenic bacteria (E. faecalis or C. albicans)to various probiotic challenges. The pathogenic organisms were grown on blood agar in the presence of test probiotic groups placed in the wells punched in the agar plate. The absence of growth around the well was an indirect measure of the ability of the test probiotic groups to inhibit growth/out compete the pathogenic organisms E. faecalis or C. albicans. ZOI was seen for E. faecalis but not for C. albicans. Thus, in the planktonic stage, both the probioic groups exhibited antimicrobial activity against E. faecalis.
Phase two was considered to be the application portion of the study, suggesting a novel delivery vehicle for probiotics into the root canal system by utilizing 30% poloxamer 407 (also known as pluronic F-127) mixed with MRS or TSB broth containing the probiotics. Poloxamer has been utilized in the biofilm stage testing of microorganisms. Poloxamer 407 showing inverse thermosensitivity is soluble in aqueous solutions at low temperatures (mainly 4°C), but forms a gel at higher temperatures.[21] These properties make them an ideal delivery vehicle for use as an intracanal medicament between interappointment visits. Other advantages of poloxamer 407, which make it ideal for use in the root canal system, is that it has already been employed in vehicles for fluorinated dentifrices and as a dental gel product for treating patients with sensitive gums and teeth. It would be an ideal solution for apical periodontitis which is the main cause for endodontic failure.[21]
The CFU/ml count results for this study revealed significant reduction of E. faecalis colony counts for probiotic groups E2 and D4 when mixed in equal amounts with E. faecalis in the 30% poloxamer/MRS broth formula when compared with controls, however, CFU/ml counts for C. albicans in probiotic group E2 was significant whereas D4 showed no activity when mixed in equal amounts with C. albicans in the 30% poloxamer/MRS broth formula when compared with controls. Thus, in the biofilm stage, probiotic groups demonstrated a decrease in CFU/ml count of the pathogenic organisms E. faecalis but not C. albicans.
Because of the financial limitations, commercial probiotics were utilized, and probiotic organisms were extracted either in groups or individually according to manufacturer's instruction. There have been no studies involving the use of probiotics in endodontics. The effective CFU count needed to eliminate or outcompete the pathogenic organisms is unknown. However, on a positive note, the manufacturer was contacted to determine the method for the extraction of probiotic species from commercial samples. Probiotics and pathogenic organisms E. feacalis and C. albicans were tested in both planktonic and biofilm stages. Thus a standardized in-vitro study was designed.
The Human Microbiome theory is gaining acceptance in medicine but has not yet been evaluated in endodontics. Maintaining or restoring equilibrium with probiotics may show promising results in endodontic therapy. Although sterility of the endodontic system is deemed necessary for endodontic success, and achieving complete sterility is currently impossible under normal conditions.[20] Probiotics used against test organisms evaluated in vitro in both the planktonic and biofilm stages, in terms of measurement of ZOI, is an acceptable method of evaluation of efficacy of the probiotics against pathogenic organisms. This pilot study demonstrated that probiotics can be researched further as an adjunct in endodontic therapy.
The protocol for the probiotic medicament would be, instead of a microbial “elimination therapy” by the use of antimicrobial agents, substituted by a microbial “replacement therapy.” Instead of attempting to eliminate pathogenic organisms by antimicrobial agents, probiotics would eliminate the endodontic pathogenic flora, allowing a more favorable/biocompatible environment using it as an intracanal medicament. This is in light of current and emerging findings in microbiology, which states that a reasonable and better approach to addressing and dealing with microbial infection should be to maintain a state of equilibrium within the Human Microbiome. In addition to eliminating and outcompeting the pathogens that originally entered from the carious process, probiotic organisms could not only eliminate disease causing bacteria but might also prevent their re-establishment after treatment has been completed, thus decreasing the incidence of endodontic failure.[20]
CONCLUSION
This pilot study demonstrated that probiotics show a potential in root canal therapy. Despite significant promises, endodontic works are sparse and need validation by further in-vitro, in-vivo research and large randomized trials. This will determine the full potential of bacteriotherapy and its application in endodontics.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Gupta G. Probiotics and Periodontal Health. J Med Life. 2011;4:387–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic lactobacillus reuteri. Swedish Dent J. 2006;30:55–60. [PubMed] [Google Scholar]
- 3.Johal S, Baumgartner JC, Marshall JG. Comparison of the antimicrobial efficacy of 1.3% NaOCl/BioPure MTAD to 5.25% NaOCl/15% EDTA for root canal irrigation. J Endod. 2007;33:48–51. doi: 10.1016/j.joen.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Evans M, Davies J K, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis; Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 7.Nair PNR. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 8.Malathi L, Jayasrikrupaa, Balachander N, Vidya Rani, Anbazhagan V, Masthan KMK. Probiotics in Dentistry – A Review. Biosciences Biotechnology Research Asia. 2014;11:193–7. [Google Scholar]
- 9.Haukioja A, Knuuttila YH, Loimaranta V, Kari K, Ouwehand AC, Meurman JH, et al. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral Microbiol Immunol. 2006;21:326–32. doi: 10.1111/j.1399-302X.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 10.Menon AM. Implications of probiotics on oral health: Past-to-present. J Dent Res Rev. 2016;3:36–41. [Google Scholar]
- 11.Patel R, DuPont HL. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60(Suppl 2):S108–21. doi: 10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gungor OE, Kirzioglu Z, Kivanc M. Probiotics: Can they be used to improve oral health? Benef Microbes. 2015;6:647–56. doi: 10.3920/BM2014.0167. [DOI] [PubMed] [Google Scholar]
- 13.Sundqvist G. Bacteriological studies of necrotic dental pulps. Umea, Sweden: University of Umea. Dissertation; 1976. [Google Scholar]
- 14.Łysakowska ME, Ciebiada-Adamiec A, Sienkiewicz M, Sokołowski J, Banaszek K. The cultivable microbiota of primary and secondary infected root canals, their susceptibility to antibiotics and association with the signs and symptoms of infection. Int Endod J. 2016;49:422–30. doi: 10.1111/iej.12469. [DOI] [PubMed] [Google Scholar]
- 15.Carbajal Mejía JB, Aguilar Arrieta A. Reduction of viable Enterococcus faecalis in human radicular dentin treated with 1% cetrimide and conventional intracanal medicaments. Dent Traumatol. 2016;32:321–7. doi: 10.1111/edt.12250. [DOI] [PubMed] [Google Scholar]
- 16.Siqueira JF, Rocas IN. The Oral Microbiota in Health and Disease: An Overview of Molecular Findings. Methods Mol Biol. 2016;1537:127–38. doi: 10.1007/978-1-4939-6685-1_7. [DOI] [PubMed] [Google Scholar]
- 17.Jayahari NK, Niranjan NT, Kanaparthy A. The efficacy of passion fruit juice as an endodontic irrigant compared with sodium hypochlorite solution: An in vitro study. J Investig Clin Dent. 2013;5:154–60. doi: 10.1111/jicd.12023. [DOI] [PubMed] [Google Scholar]
- 18.Bohora A, Hegde V, Kokate S. Comparison of antibacterial efficacy of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. albicans and mixed culture – an in vitro study. Endodontology. 2010;22:8–12. [Google Scholar]
- 19.Sinha DJ, Sinha AA. Natural medicaments in dentistry. Ayu. 2014;35:113–8. doi: 10.4103/0974-8520.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohora A, Kokate S. Concept of Probiotics in Endodontics. Int J Adv Res. 2016;4:1137–42. [Google Scholar]
- 21.Patel HR, Patel RP, Patel MM. Poloxamers: A pharmaceutical excipients with therapeutic behaviors. Int J PharmTech Res. 2009;1 [Google Scholar]


