Abstract
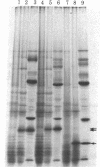
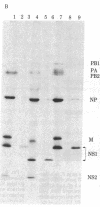
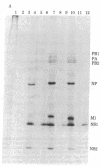
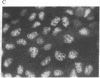
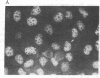
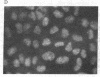
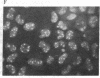
RNA segment 8 of the influenza A virus genome codes for two nonstructural proteins, NS1 and NS2, for which the functions are unknown. Cloned cDNA copies of this gene from three different influenza A virus strains were inserted into an Escherichia coli plasmid expression vector, pAS1, carrying the strong regulatable lambda phage promoter, PL. After induction, the NS1 proteins were overproduced to levels of 20-25% of total cellular protein. This was surprising in that the codon composition for these eukaryotic genes is similar to that for weakly expressed proteins in E. coli. Thus, under the appropriate conditions, it appears that high level expression of genes containing a relatively large proportion of minor codons can be obtained. The NS1 protein produced in bacteria from a cloned cDNA copy of the A/PR/8/34 virus NS gene was purified to apparent homogeneity and used to generate a high-titer monospecific rabbit antiserum. Immunoprecipitation studies showed this antibody to be crossreactive against the NS1 proteins produced by several different influenza A virus strains. Immunofluorescence experiments in Madin-Darby canine kidney cells showed the NS1 proteins to be located in the nucleoplasm early in infection for all strains examined. With some of the strains, NS1-specific immunofluorescence was observed predominantly in the nucleoli later in infection. This technology can be used to obtain other viral proteins in pure form for structural, functional, and immunological studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez M., Taussig R., Zazra J. J., Young J. F., Palese P., Reisfeld A., Skalka A. M. Complete nucleotide sequence of the influenza A/PR/8/34 virus NS gene and comparison with the NS genes of the A/Udorn/72 and A/FPV/Rostock/34 strains. Nucleic Acids Res. 1980 Dec 11;8(23):5845–5858. doi: 10.1093/nar/8.23.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez M., Zazra J. J., Elliott R. M., Young J. F., Palese P. Nucleotide sequence of the influenza A/duck/Alberta/60/76 virus NS RNA: conservation of the NS1/NS2 overlapping gene structure in a divergent influenza virus RNA segment. Virology. 1981 Aug;113(1):397–402. doi: 10.1016/0042-6822(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Bos T., Ueda M., Nayak D. P., Dowbenko D., Compans R. W. Immune response to human influenza virus hemagglutinin expressed in Escherichia coli. Gene. 1983 Mar;21(3):273–284. doi: 10.1016/0378-1119(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P., Ueda M., Hiti A. L., Dowbenko D., Kleid D. G. Expression of antigenic determinants of the hemagglutinin gene of a human influenza virus in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5376–5380. doi: 10.1073/pnas.78.9.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Tacon W. C., Catlin G. H., Jenkins B., Porter A. G., Carey N. H. Influenza antigenic determinants are expressed from haemagglutinin genes cloned in Escherichia coli. Nature. 1980 Jan 10;283(5743):171–174. doi: 10.1038/283171a0. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. N., Schulman J. L., Young J. F., Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983 Apr 15;126(1):106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Heiland I., Gething M. J. Cloned copy of the haemagglutinin gene codes for human influenza antigenic determinants in E. coli. Nature. 1981 Aug 27;292(5826):851–852. doi: 10.1038/292851a0. [DOI] [PubMed] [Google Scholar]
- Ho Y., Lewis M., Rosenberg M. Purification and properties of a transcriptional activator. The cII protein of phage lambda. J Biol Chem. 1982 Aug 10;257(15):9128–9134. [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Soeiro R. Studies on the intranuclear localization of influenza virus-specific proteins. Virology. 1975 Apr;64(2):378–387. doi: 10.1016/0042-6822(75)90114-2. [DOI] [PubMed] [Google Scholar]
- Krystal M., Buonagurio D., Young J. F., Palese P. Sequential mutations in the NS genes of influenza virus field strains. J Virol. 1983 Feb;45(2):547–554. doi: 10.1128/jvi.45.2.547-554.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Lamon E. W., Compans R. W. Surface expression of a nonstructural antigen on influenza A virus-infected cells. Infect Immun. 1981 Dec;34(3):1065–1067. doi: 10.1128/iai.34.3.1065-1067.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hampson A. W., Layton J. E., White D. O. The polypeptides of influenza virus. IV. An analysis of nuclear accumulation. Virology. 1970 Nov;42(3):744–752. doi: 10.1016/0042-6822(70)90320-x. [DOI] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]