Abstract
Background. A poorer quality diet among individuals with low socioeconomic status (SES) may partly explain the higher burden of noncommunicable disease among disadvantaged populations. Because there is a link between sodium intake and noncommunicable diseases, we systematically reviewed the current evidence on the social patterning of sodium intake.
Objectives. To conduct a systematic review and a meta-analysis of the evidence on the association between SES and sodium intake in healthy adult populations of high-income countries.
Search Methods. We followed the PRISMA-Equity guidelines in conducting a literature search that ended June 3, 2016, via MEDLINE, Embase, and SciELO. We imposed no publication date limits.
Selection Criteria. We considered only peer-reviewed articles meeting the following inclusion criteria: (1) reported a measure of sodium intake disaggregated by at least 1 measure of SES (education, income, occupation, or any other socioeconomic indicator); (2) were written in English, Spanish, Portuguese, French, or Italian; and (3) were conducted in a high-income country as defined by the World Bank (i.e., per capita national gross income was higher than $12 746). We also excluded articles that exclusively sampled low-SES individuals, pregnant women, children, adolescents, elderly participants, or diseased patients or that reported results from a trial or intervention.
Data Collection and Analysis. As summary measures, we extracted (1) the direction (positive, negative, or neutral) and the magnitude of the association between each SES indicator and sodium intake, and (2) the estimated sodium intake according to SES level. When possible and if previously unreported, we calculated the magnitude of the relative difference in sodium intake between high- and low-SES groups for each article, applying this formula: ([value for high-SES group – value for low-SES group]/[value for high-SES group]) × 100. We considered an association significant if reported as such, and we set an arbitrary 10% relative difference as clinically relevant and significant. We conducted a meta-analysis of the relative difference in sodium intake between high- and low-SES groups. We included articles in the meta-analysis if they reported urine-based sodium estimates and provided the total participant numbers in the low- and high-SES groups, the estimated sodium intake means for each group (in mg/day or convertible units), and the SDs (or transformable measures). We chose a random-effects model to account for both within-study and between-study variance.
Main Results. Fifty-one articles covering 19 high-income countries met our inclusion criteria. Of these, 22 used urine-based methods to assess sodium intake, and 30 used dietary surveys. These articles assessed 171 associations between SES and sodium intake. Among urine-based estimates, 67% were negative (higher sodium intake in people of low SES), 3% positive, and 30% neutral. Among diet-based estimates, 41% were negative, 21% positive, and 38% neutral. The random-effects model indicated a 14% relative difference between low- and high-SES groups (95% confidence interval [CI] = –18, –9), corresponding to a global 503 milligrams per day (95% CI = 461, 545) of higher sodium intake among people of low SES.
Conclusions. People of low SES consume more sodium than do people of high SES, confirming the current evidence on socioeconomic disparities in diet, which may influence the disproportionate noncommunicable disease burden among disadvantaged socioeconomic groups.
Public Health Implications. It is necessary to focus on disadvantaged populations to achieve an equitable reduction in sodium intake to a population mean of 2 grams per day as part of the World Health Organization’s target to achieve a 25% relative reduction in noncommunicable disease mortality by 2025.
PLAIN-LANGUAGE SUMMARY
People with high socioeconomic status (SES) tend to have diets that are healthier than are those of people with low SES. This disparity in diet quality may partly contribute to the higher burden of noncommunicable diseases among people with low SES. Because sodium intake higher than the recommended level of 2 grams per day is associated with an increased risk of developing hypertension, cardiovascular disease, and certain cancers, we systematically reviewed the current evidence of the social patterning of sodium intake in healthy adult populations of high-income countries.
The 51 articles we reviewed, representing 19 high-income countries, indicate that people of low SES consumed 14% more sodium (approximately 503 mg/day) than did people of high SES.
High sodium intake increases the risk of several noncommunicable diseases (NCDs), including hypertension,1,2 cardiovascular disease,3,4 and certain cancers,5,6 that disproportionally affect low-SES populations7,8 In high-income countries (HICs), SES influences diet quality, whereby low-SES people tend to follow unhealthier diets—characterized by high intakes of highly processed foods and insufficient intakes of some essential nutrients—than do high-SES people.9–12 As highly processed foods—those with added sugar, salt, preservatives, and colors to enhance or preserve palatability, look, and freshness13—are generally high in sodium,14,15 it is possible that low-SES individuals consume more sodium than do their more advantaged counterparts, which could partly explain the higher NCD burden in low-SES populations.16
The World Health Organization targets a global 25% relative reduction in NCD mortality by 2025, and a reduction in sodium intake to a population mean of 2 grams per day is 1 of the identified instruments.17 A recent report found that the estimated global mean sodium intake was 3.95 grams per day, nearly twice the World Health Organization’s recommended limit. In North America, Western Europe, and Australia and New Zealand, the estimated sodium intake ranged from 3.4 to 3.8 grams per day; in East Asia, it was 5.0 grams per day.18 Concurrently, the Global Plan of Action on Social Determinants of Health aims to eliminate avoidable health inequalities.19 Thus, quantifying the impact of SES on sodium intake can provide evidence needed to guide public health policy.
A systematic review of differences in micronutrient intakes in Europe found positive associations between intake of most micronutrients and SES.10 Notably, this review failed to include sodium intake. Another review examined dietary intake in exclusively low-income European populations, finding much higher than recommended sodium intakes in 2 of 3 studies.12 Yet another systematic review, this one by Imamura et al., examining international trends in dietary quality from 1990 to 2010, found that consumption of unhealthy foods (including sodium) increased significantly worldwide, with HICs faring much worse than low- and middle-income countries (LMICs).20 However, the authors did not assess food intake trends by SES.
This gap in the literature—and the need for evidence to guide public health policy to reduce NCDs and health inequalities—prompted us to conduct a systematic review of the social patterning of sodium intake in HICs. We also conducted a meta-analysis of social differences in sodium intake for studies that assessed sodium intake through urine-based collection methods.
METHODS
Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)–Equity guidelines,21 we conducted the last literature search on June 3, 2016, via MEDLINE, Embase, and SciELO. We considered only peer-reviewed articles meeting the following inclusion criteria: (1) reported a measure of sodium intake disaggregated by at least 1 measure of SES (education, income, occupation, or any other SES indicator); (2) were written in English, Spanish, Portuguese, French, or Italian; and (3) were conducted in a HIC as defined by the World Bank (per capita national gross income above $12 746).22
We imposed no publication date limits. Details of the electronic database search strategy are available as a supplement to this article at http://www.ajph.org as Table A. Three reviewers (C. d. M., A.-L. M. and D. P.) independently screened all the titles and abstracts of articles returned in the electronic search, removed articles clearly failing to meet the inclusion criteria, and retrieved potentially eligible articles for full-text review. We also screened the reference lists of reviewed articles for potentially relevant articles that the electronic search failed to identify. As our review focused only on healthy adult populations of HICs, C. d. M., A.-L. M. and D. P. applied the following exclusion criteria during title and abstract screening and full-text reviewing: (1) sampled populations were from LMICs; (2) did not disaggregate sodium intake by a measure of SES; (3) exclusively included low-SES individuals, pregnant women, children, adolescents, elderly participants, or diseased patients; or (4) reported results from a trial or intervention.
To minimize the effect of a single study, we also excluded articles that reported previously published data, unless the analyses conducted were substantially different (e.g., one used income but another education as the predictor variable). After removing duplicates, C. d. M., A.-L. M., and D. P. agreed on a final list of articles to include in the systematic review.
Data Extraction
C. d. M. extracted the data from included articles, A.-L. M. and D. P. verified this by checking the table with all extracted data against data presented in each article, and a senior reviewer (S. S.) was consulted in case of disagreement. Extracted data included the following:
country of population sample,
study period,
sample size (total and for the low- and high-SES groups),
age group,
percentage of women,
sodium intake assessment method,
SES indicators measured, and
type of adjustment for potential confounders.
As summary measures, we extracted (1) the direction (positive, negative, or neutral) and the magnitude of the association between each SES indicator and sodium intake on the basis of previous systematic reviews,23–27 and (2) the estimated sodium intake according to SES level.
When the association between sodium intake and SES indicator was reported per year, we extracted the information for each year; however, to prevent any single study from providing a disproportionately high number of associations, we included only the oldest and most recent reported data estimates from periodically repeated cross-sectional studies (e.g., the National Health and Nutrition Examination Survey). To minimize misclassification bias in intermediate categories, we selected only the lowest and highest categories per SES indicator, as recommended28 and done by previous systematic reviews.10,23–26,29–31
Quality Assessment
We performed a quality assessment of included articles’ strengths and limitations according to 3 criteria: (1) sample (2 possible points), (2) analysis (3 possible points), and (3) presentation (1 possible point), with a maximum possible score of 6, which we adapted from a previously published quality score assessment31 to better fit the objectives of our article (Table 1).
TABLE 1—
Strengths and Limitations of the Criteria Used for the Review Quality Score
| Criteria Set | Strengths | Limitations |
| Sample | Community- and population-based study design (+1) | Other study design |
| Sample | Representative sample (+1) | Nonrepresentative sample |
| Sample | More than 1 SES dimension (+1) | Only 1 SES dimension |
| Sample | Hierarchical, graded SES categories (+1) | Binary SES variables |
| Sample | SES–sodium intake association results adjusted for age, gender, BMI, energy (where applicable; +1) | No adjustment for age, gender, BMI, or energy in SES–sodium intake analysis (where applicable) |
| Presentation | SES–sodium intake association results presented in the form of mean ± SD, SE, confidence interval, and P value (+1) | Incomplete results presented (or data not shown) for SES–sodium intake analysis |
Note. BMI = body mass index; SES = socioeconomic status. We assessed articles included in the review on their strengths and limitations according to 3 sets of criteria: sample, analysis, and presentation.
We considered the article quality as higher (scores ≥ 4), intermediate (scores 2 or 3), or lower (score ≤ 1). C. d. M. and D. P. independently assigned scores, discussed differences, and agreed on a final score.
Statistical Analysis
When possible and if previously unreported, we calculated the magnitude of relative difference in sodium intake between high- and low-SES groups for each article applying the formula: ([value for high-SES group – value for low-SES group]/[value for high-SES group]) × 100.24,32 We contacted the corresponding authors to request data results whenever these were not reported in the article; 7 provided data results. (All contacted authors and the outcome are available as a supplement to this article at http://www.ajph.org as Table B.) When possible, we calculated the sodium to potassium ratio. We considered an association significant if reported as such, and we set an arbitrary 10% relative difference as clinically relevant and significant, as previously done.24,26,32,33
After assessing data comparability across articles, we conducted a meta-analysis of the relative difference in sodium intake between high- and low-SES groups, and we have reported the standardized mean difference (SMD). We included articles in the meta-analysis if they (1) reported urine-based sodium estimates (because questionnaire-based estimates were too heterogeneous or failed to provide necessary data points); and (2) provided the total participant numbers in the low- and high-SES groups, the estimated sodium intake means for each group (in mg/day or convertible units), and the SDs (or transformable measures). When both adjusted and unadjusted estimates were provided within each article, we incorporated the former into the meta-analysis. We chose a random-effects model to account for both within-study and between-study variance, as recommended.34 We conducted 6 different sensitivity analyses by doing the following:
applying a fixed-effects model assuming an equal effect size across studies,
presenting the effect size according to SES indicator,
presenting effect sizes separately for models adjusted for potential confounders and for unadjusted models,
limiting analysis to higher-quality articles,
repeating the meta-analysis with each study removed sequentially, and
meta-regressing estimated sodium intake against the level of SES (1 = lowest SES; ≥ 2 = higher SES).
We assessed the presence and effect of publication bias using the Kendall tau and the Egger test.35 We performed statistical analyses in Stata version 14 (Stata Corp., College Station, TX).
RESULTS
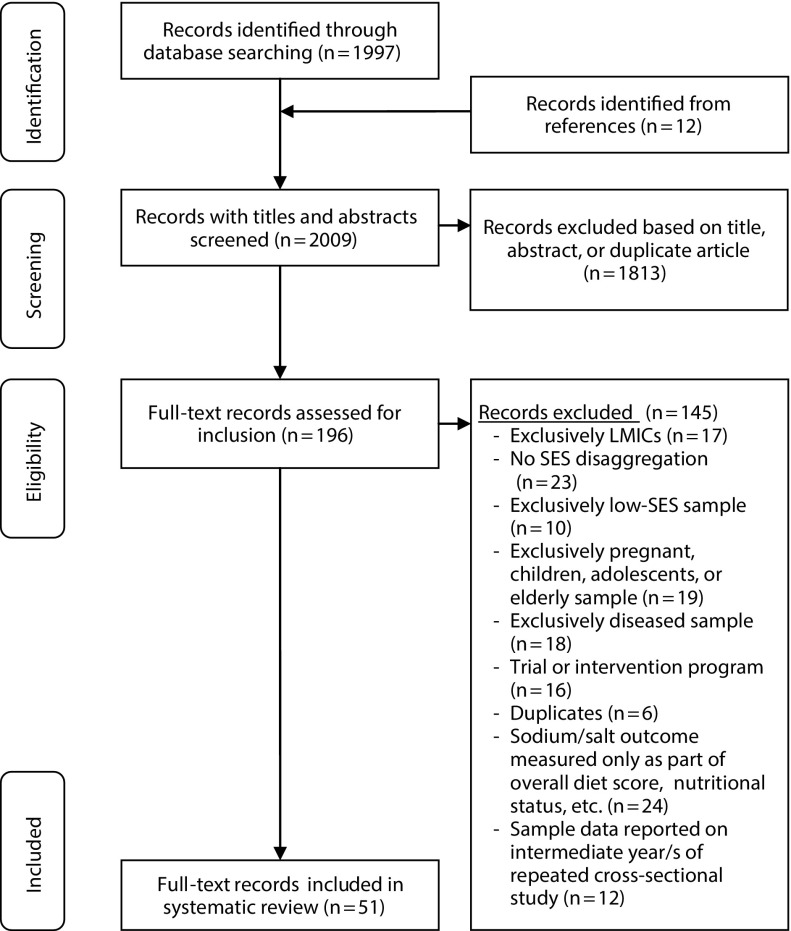
The selection procedure is summarized in Figure 1. The systematic search identified 2009 articles; after title and abstract screening, 196 remained for full-text inspection. Sixty-three articles complied with the inclusion criteria, but we excluded 12 for reporting intermediate years from repeated cross-sectional studies, leaving 51 articles for final inclusion.
FIGURE 1—
Flowchart of Articles Included in Systematic Review
Note. LMICs = low- to middle-income countries; SES = socioeconomic status.
Table 2 shows the characteristics of included articles. Sample sizes ranged from 8739 to 91 900,32 and most (44/51) included samples of greater than 1000. Nine articles were from East Asia (Japan, South Korea, Taiwan), 6 from Australia, 15 from North America, 20 from Europe, and 1 from Latin America (Chile), covering 19 HICs. Most articles included samples of both men and women, but 3 included samples composed of only women44,76,80 and 1 predominantly of men.78 Thirty-four articles attained higher-quality scores, with 3 scoring maximum points. Sixteen articles attained intermediate quality scores, and 1 attained a lower quality score (Table 2; detailed scoring is available as a supplement to this article at http://www.ajph.org as Table C).
TABLE 2—
Characteristics and Quality Score of Included Articles
| Reference | Country | Study Name or Sample | Age Range, Y | Sample Size, No. (% Women) | Sodium Intake Measurement Method | SES Dimension | Quality Score |
| Beard et al.36 | Australia | Hobart Salt Study | 18–70 | 194 (55) | 24-h collection | Other: Index of Relative Socioeconomic Disadvantage | 3 (intermediate) |
| Land et al.37 | Australia | Lithgow adults | 20–88 | 419 (55) | 24-h collection | Education | 2 (intermediate) |
| Nowson et al.38 | Australia | Victoria Health Monitor Survey | 18–75 | 1 040 (53) | 24-h collection | Education, income, Index of Relative Socioeconomic Disadvantage | 5 (higher) |
| Andersen et al.39 | Denmark | Copenhagen adults | 20–55 | 87 (58) | 24-h collection | Education | 1 (lower) |
| Hu et al.40 | Finland | Finnish portion of Monitoring of Trends and Determinants in Cardiovascular Disease | 25–64 | 1 935 (52) | 24-h collection | Education | 3 (intermediate) |
| Reinivuo et al.41 | Finland | Finnish Study on Risk Factors on Chronic, Non-communicable Diseases | 25–64 | 6 730 (55) | 24-h collection | Education | 3 (intermediate) |
| Cappuccio et al.42 | Italy | MINISAL-GIRCSI and Meno Sale Più Salute studies | 39–79 | 3 857 (49) | 24-h collection | Education, occupation | 5 (higher) |
| Leclercq and Ferro-Luzzi43 | Italy | Households from 3 Italian regions | ≥ 18 | 182 (50) | 24-h collection | Other: SES (education, occupation) | 2 (intermediate) |
| Murakami et al.44 | Japan | Japanese women | 18–22 | 1 105 (100) | 24-h collection | Other: neighborhood socioeconomic disadvantage | 3 (intermediate) |
| Polonia et al.45 | Portugal | Portuguese Hypertension and Salt Study | 18–90 | 3 720 (53) | 24-h collection | Education | 5 (higher) |
| Schoen et al.46 | Switzerland | Swiss Salt Survey | ≥ 15 | 1 547 (52) | 24-h collection | Education | 5 (higher) |
| Ji et al.47 | United Kingdom | National Diet and Nutrition Survey | 19–64 | 2 105 (55) | 24-h collection, 7-d diet record | Education, occupation | 4 (higher) |
| Angell and Eisenhower48 | United States | Heart Follow-up Study | ≥ 18 | 1 656 (58) | 24-h collection | Poverty status | 6 (higher) |
| Stamler et al.49 | United States | International Study of Electrolyte Excretion and Blood Pressure | 40–59 | 2 195 (50) | 24-h collection | Education | 4 (higher) |
| Yi et al.50 | United States | Heart Follow-up Study | ≥ 18 | 1 656 (58) | 24-h collection | Education, poverty or income, neighborhood poverty | 5 (higher) |
| Kho et al.51 | South Korea | Healthy Twin Study | ≥ 18 | 1 204 (54) | 12-h collection | Education, income | 5 (higher) |
| Caro52 | Chile | National Health Survey (Chile) | ≥ 15 | 5 434 (59) | Spot urine | Education, economic assistance | 5 (higher) |
| Klenow et al.53 | Germany | German Health Interview and Examination Survey for Adults | 18–79 | 6 910 (52) | Spot urine | Other: SES (education, occupation, income) | 5 (higher) |
| Hong et al.54 | South Korea | Korean National Health and Nutrition Examination Survey | ≥ 19 | 18 000 (55) | Spot urine | Education, income, occupation | 6 (higher) |
| Millett et al.55 | United Kingdom | Health Survey for England | ≥ 16 | 6 384 (56) | Spot urine | Occupation | 5 (higher) |
| Pfister et al.56 | United Kingdom | Norfolk part of the European Prospective Investigation Into Cancer and Nutrition | 39–79 | 25 639 (55) | Spot urine | Education, occupation | 4 (higher) |
| Chien et al.57 | Taiwan | Chin-Shan Community Cardiovascular Cohort Study | ≥ 35 | 1 520 (52) | First morning void | Education, occupation | 2 (intermediate) |
| McLaren et al.58 | Canada | National Canada Survey, Canadian Community Health Survey | 25–64 | 10 449 (56) | 24-h dietary recall | Education, income | 5 (higher) |
| Dubois and Girard59 | Canada, United States | Quebec Nutrition Survey, National Health and Nutrition Examination Survey | 18–74 | 2 103 (51) | 24-h dietary recall | Education, income, occupation | 4 (higher) |
| Buyck et al.60 | France | Supplementation of Antioxidant Vitamins and Minerals Study | 35–60 | 4 919 (58) | 24-h dietary recall | Education | 3 (intermediate) |
| van den Brandt et al.61 | Netherlands | Netherlands Cohort Study | 55–69 | 3 123 (51) | 24-h dietary recall | Education | 3 (intermediate) |
| Kim et al.62 | South Korea | Korean National Health and Nutrition Examination Survey | ≥ 20 | 20 777 (53) | 24-h dietary recall | Education | 4 (higher) |
| Lee et al.63 | South Korea | Korean National Health and Nutrition Examination Survey | ≥ 18 | 28 450 (50) | 24-h dietary recall | Education, income | 4 (higher) |
| Lee et al.64 | South Korea | Korean National Health and Nutrition Examination Survey | ≥ 20 | 14 539 (61) | 24-h dietary recall | Education, income, occupation | 5 (higher) |
| Beydoun and Wang65 | United States | Continuing Food Survey on Intakes by Individuals, Diet and Health Knowledge Survey | ≥ 18 | 4 356 (49) | 24-h dietary recall | Other: perceived barrier to food price | 3 (intermediate) |
| Cogswell et al.66 | United States | National Health and Nutrition Examination Survey | ≥ 20 | 12 581 (49) | 24-h dietary recall | Education, income | 5 (higher) |
| Crews et al.67 | United States | Healthy Aging in Neighborhoods of Diversity Across the Life Span Study | 30–64 | 2 058 (57) | 24-h dietary recall | Income | 3 (intermediate) |
| Greer et al.68 | United States | National Health and Nutrition Examination Survey | ≥ 20 | 8 779 (49) | 24-h dietary recall | Other: modified retail food environment index | 5 (higher) |
| Kachan et al.69 | United States | National Health and Nutrition Examination Survey | ≥ 18 | 8 987 (46) | 24-h dietary recall | Occupation | 6 (higher) |
| Meyer et al.70 | United States | Minnesota Heart Study | 25–74 | 10 863 (53) | 24-h dietary recall | Education | 4 (higher) |
| Popkin et al.71 | United States | National Food Consumption Survey | ≥ 18 | 32 406 (50) | 24-h dietary recall | Other: SES (education, income) | 4 (higher) |
| Welsh et al.72 | United States | Shawnee County Survey | ≥ 18 | 834 (52) | 24-h dietary recall | Education, income | 5 (higher) |
| Yang et al.73 | United States | National Health and Nutrition Examination Survey | ≥ 20 | 12 267 (52) | 24-h dietary recall | Education | 4 (higher) |
| Ji and Cappuccio74 | United Kingdom | National Diet and Nutrition Survey | 19–64 | 1 027 (56) | 4-d food diary | Education, occupation | 5 (higher) |
| Schröder et al.75 | Spain | Gerona adults | 25–74 | 1 577 (52) | 72-h dietary recall | Education | 4 (higher) |
| McCartney et al.76 | Ireland | Young Dublin women | 18–35 | 216 (100) | 7-d dietary recall | Other: disadvantage status | 3 (intermediate) |
| Meneton et al.77 | France | French Food Safety Agency Survey | 15–92 | 1 474 (54) | 7-d food record | Income, occupation | 5 (higher) |
| Miyaki et al.78 | Japan | Japan Hospice and Palliative Care Evaluation Study | 21–65 | 2 266 (11) | Dietary history questionnaire | Education, income | 4 (higher) |
| Fukuda and Hiyoshi79 | Japan | Comprehensive Survey of Living Conditions and the National Health and Nutrition Survey | 18–74 | 22 712 (50) | Dietary records | Income | 5 (higher) |
| Mishra et al.80 | Australia | Australia Longitudinal Study on Women’s Health | 50–55 | 10 561 (100) | FFQ | Occupation | 3 (intermediate) |
| Smith and Baghurst81 | Australia | Australian adults | ≥ 18 | 1 500 (50) | FFQ | Occupation, education, income | 3 (intermediate) |
| Zhang et al.82 | Australia | Melbourne Chinese Cohort Study | ≥ 25 | 262 (48) | FFQ | Education, income, occupation | 3 (intermediate) |
| Si Hassen et al.32 | France | Determinants of Diet and Physical Activity Study | ≥ 18 | 91 900 (78) | FFQ | Education, income, occupation | 4 (higher) |
| Ilow et al.83 | Poland | Polish–Norwegian Study | 45–65 | 3 862 (67) | FFQ | Education | 3 (intermediate) |
| Beer-Borst et al.84 | Switzerland | Bus Santé | 35–74 | 13 335 (50) | FFQ | Education, occupation | 4 (higher) |
| Gerber et al.85 | United States | Pitt County Study | 25–50 | 1 784 (58) | FFQ | Other: SES (education, occupation) | 5 (higher) |
Note. FFQ = food frequency questionnaire; SES = socioeconomic status.
Twenty-two articles used urine measurements to assess sodium intake: 15 used 24-hour urine collection, the gold standard assessment method; 1 used 12-hour urine collection51; 5 used the spot urine test; and 1 used the first morning void method.57 Thirty articles reported sodium intake measured via dietary surveys, including 24-hour dietary recalls (16 articles), 72-hour dietary recall (1 article), 7-day dietary recall (1 article), food frequency questionnaires (7 articles), dietary records (3 articles), food diary (1 article), and dietary history questionnaire (1 article; Table 2).
Overall, 45 articles included education as the SES indicator, 20 income, 20 occupation, and 15 other SES indicators (Table 2).
The 22 articles reporting urine-based sodium intake assessed 54 associations with SES: 36 were negative (67%), 2 positive, and 16 nonsignificant (the overall pattern of associations is available as a supplement to this article at http://www.ajph.org in Figure A). Figure 2 displays the relative difference in sodium intake between low- and high-SES groups for each association assessed per article for which data were available; 9 articles reported the association without showing data results.37,39,40,57,59,60,65,82,84 Associations between urine-based sodium intake and SES were predominantly negative, ranging from −19% in 4 different studies from Chile,52 England,55 Italy,43 and South Korea,54 to a nonstatistically significant −1% in a study from England.56 Only 2 studies reported positive relative differences, although none was statistically significant.36,51 Reported associations were adjusted differently per article, but most were adjusted for 1 or more potential confounders (energy, body mass index, age, gender); only 4 articles reported unadjusted associations. The corresponding absolute difference in sodium intake between low- and high-SES groups is available as a supplement to this article at http://www.ajph.org as Figure B (if data were available).
FIGURE 2—
Forest Plot of Relative Difference in Daily Sodium Intake Between Low- and High-SES Groups
Note. m = men; SES = socioeconomic status; w = women. The formula for relative difference is ([value for high-SES group – value for low-SES group]/[value for high-SES group]) × 100. We adjusted the estimated sodium intake for (a) energy, (b) body mass index, (c) age, (d) gender, and (e) other. Sample description: a specific year is indicated with its last 2 digits (e.g., 1998 is indicated as ’98); (f) Southern United States sample neighborhood poverty, (g) non-Southern United States sample neighborhood poverty, (h) Southern US sample poverty to income ratio, (i) non-Southern US sample poverty to income ratio, (j) 1977 African American sample, (k) 1989 African American sample, (l) 1989 Caucasian sample, and (m) 1977 Caucasian sample.
The 30 articles reporting diet-based sodium intake estimates assessed 117 associations with SES: 48 were negative (41%), 24 positive (21%), and 45 nonsignificant (38%; Figure A). As Figure 2 shows, there was no clear pattern in the association between diet-based sodium intake and SES, with relative differences ranging widely from −19% in 1 study in the United States72 and −17% in 1 study in Ireland76 to 14% in 1 study in South Korea64 and 13% in 1 study in the United States.66 Most associations were adjusted for potential confounders, but 6 articles reported only unadjusted associations (Figure 2; see corresponding absolute differences in Figure B).
Of the 22 articles that reported urine-based sodium association with SES, only 736,38,44,48–50,54 reported necessary and comparable data to be included in the meta-analysis. Six contacted authors provided additional data results.45,46,52,53,55,56 Therefore, we included 12 study populations from 13 articles in the meta-analysis—2 articles analyzed the same study population but each used different SES indicators48,50—representing 42 255 combined participants from 9 countries. Figure 3 shows the Forest plot from the random-effects model, presenting the overall pooled effect size in relative difference in sodium intake between low-SES and high-SES groups and separately by the sodium intake measurement method. Overall, the SMD was −0.14 (95% confidence interval [CI] = −0.18, −0.09); among articles that relied on 24-hour urine collection to estimate sodium intake, the SMD was also −0.14 (95% CI = −0.21, −0.08); among articles that relied on spot urine to estimate sodium intake, the SMD was −0.13 (95% CI = −0.19, −0.07). The I2 statistic indicated likely overall and subgroup heterogeneity (I2 > 50%; P < .01 for each).
FIGURE 3—
Forest Plot From Random-Effects Model Meta-Analysis Showing the Standardized Mean Difference in Sodium Intake Between Low- and High-SES Groups
Note. BMI = body mass index; CI = confidence interval; SES = socioeconomic status; SMD = standard mean difference. SMDs are presented separately by urine-based sodium measurement method.
The pooled relative difference translates to a global 503 milligrams per day (95% CI = 461, 545) of higher sodium intake among people of low SES, a difference ranging from 423 milligrams per day (95% CI = 352, 493) in Australia to 589 milligrams per day (95% CI = 491, 687) in the East Asian countries (South Korea and Japan).
In sensitivity analyses, the pooled effect size was slightly attenuated using fixed-effects models among studies that relied on urine spots to estimate sodium intake (SMD = −0.08; 95% CI = −0.10, −0.07; Figure C, available as a supplement to this article at http://www.ajph.org). Heterogeneity remained high even when fitting further random-effects models with different subgroups and exclusions (Figures D–F, available as supplements to this article at http://www.ajph.org), and the effect size remained largely unchanged after sequentially excluding each study population (Figure G, available as a supplement to this article at http://www.ajph.org). Meta-regressing the estimated sodium intake on the level of SES indicator showed a change of −126 milligrams per day per each increase in SES (P value = .03; Table D, available as a supplement to this article at http://www.ajph.org). No evidence of publication bias was found (Kendall τ = −0.11; P = .49; Egger bias = −1.56; P = .08; Figure H, available as a supplement to this article at http://www.ajph.org).
Nineteen articles provided associations between the sodium to potassium ratio and a SES indicator. We calculated the ratio from 8 articles that reported both sodium and potassium intake disaggregated by SES.36,46,66,67,70,76,80,85 Of 41 total associations, 30 were negative (73%), 2 positive (5%), and 9 nonsignificant (24%).
Among articles that reported urine-based sodium intake estimates, the pattern of association with SES was generally negative in all world regions, ranging from 50% of all associations that were negative in Australia, East Asia, and North America to 77% in Western Europe. However, no clear pattern emerged among articles that reported diet-based sodium intake estimates.
Only 7 articles reported associations between sodium intake and SES across time.55,58,62,64,70,71,74 One article found that despite a nationwide decrease in sodium intake since the year 2000 in the United Kingdom, the relative difference in sodium intake between low and high SES remained unchanged after 10 years.74 Another article from England found that the relative difference in sodium intake remained unchanged from 2004 to 2007.55 One article from South Korea found that between 1998 and 2005 the relative difference in sodium intake increased from −5% to −9% among men and from −6% to −16% among women.62 Another article found that the relative difference decreased for education but increased for income.64 Finally, 1 article found that the relative difference tended to decrease from 1985 to 2009 in the United States.70
Only 12 articles reported sodium intake by SES separately by gender. In 7 of these articles, the direction of the association was the same between men and women.48,56,62,70,73,75,85 One article found a positive association among women but none among men.32 Two articles found a negative association among women but none among men.41,58 Another article found negative associations among men for education and income but a positive association for occupation and no associations among women.82 Finally, 1 article found a negative association among women but a positive association among men.79
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis summarizing the evidence for an association of SES with sodium intake in healthy adult populations of HICs. Most observed associations and the SMD from the meta-analysis point to higher sodium intake among low-SES groups than among high-SES groups. Our results are in line with previous reports of suboptimal dietary intake among low-SES populations9–11 and confirm the existence of socioeconomic inequalities in diet in HICs.
Urine-Based Sodium Intake and Socioeconomic Status
The strongest evidence originated in reports that used urine-based sodium intake estimate. Most of the associations were negative, indicating that low-SES groups had higher sodium intake than did high-SES groups. In our meta-analysis we found that low-SES groups had 14% higher sodium intake than that of high-SES groups. Heterogeneity was present in the overall effect size, likely owing in part to different sample sizes across included studies (193–19 857), varying and disproportionate representation of SES groups (the low-SES to high-SES participants ratio ranged from 0.20 to 2.34), which contributed to widely different and nonoverlapping 95% CIs. Nevertheless, the overall effect size was similar between estimates of sodium intake from 24-hour urine collection and from urine spots. In sensitivity analyses, different subgroups by SES indicator, adjustment for confounding, and exclusions of each study sequentially failed to explain the heterogeneity observed. Importantly, the meta-regression confirmed the association between SES and sodium intake.
Because of the dose–response nature of sodium’s effect on blood pressure,2,3 our finding of 14% higher sodium intake by low-SES groups may translate into an increased risk of hypertension and cardiovascular disease. Extending previous projections of the effect of salt reduction on future cardiovascular disease to our findings, reducing the daily sodium intake of low-SES groups in the United States to the level of high-SES groups—a reduction of approximately 400 milligrams per day—could prevent annually 20 000 to 40 000 new cases of coronary heart disease, 11 000 to 23 000 new cases of stroke, and 15 000 to 32 000 deaths from any cause and save US $4.1 to US $7.0 billion in health care costs.86
Diet-Based Sodium Intake and Socioeconomic Status
Among reports of diet-based sodium intake estimates, the pattern of association with SES was less consistent. For example, 2 studies from South Korea reported opposite findings,62,64 which may be because of SES categorization and adjustment for confounding factors: the categorization of education was considerably different and the study that found a positive association failed to adjust sodium intake estimates for any potential confounding factor.64 The United States stood out as showing positive associations between SES and diet-based sodium intake, particularly in the National Health and Nutrition Examination Survey samples, regardless of adjustments for potential confounders. This may be because of strong recall and social desirability biases influencing participants’ responses to the dietary instruments—a common limitation in dietary surveys. Interestingly, the only 2 studies assessing sodium intake from 24-hour urine collections in the United States found negative associations.48,49
The predominantly negative association observed with the sodium to potassium ratios supports previous findings of significantly lower potassium intake in low- versus high-SES groups.9 Only a minority of articles reported the association between SES and sodium intake across different years, and no clear pattern could be observed; this highlights the need for improved measuring and reporting of data by SES to enable trends assessments.
Our findings also highlight the need for future research to report sodium intake in a disaggregated way using more than 1 measure of SES to better track changes in sodium intake in different population groups and to assess the impact of public health efforts to reduce socioeconomic inequalities in diet.
Public Health Implications
Although our findings indicate that low-SES populations have greater sodium intake than do high-SES populations, it is important to note that sodium intake was above the World Health Organization recommended limit for all SES groups in all included articles. Interventions aimed at reducing sodium intake should, therefore, target the overall population but need to take into account the higher salt consumption in low-SES groups. This may contribute to the increased risk of hypertension and cardiovascular disease in low-SES populations.
Thus, in line with the United Nations Global Action Plan to reduce mean population sodium intake to 2 grams per day (5 g/day of salt), as part of the global target of 25% relative reduction in NCD mortality by 2025,17 and the Global Action Plan on Social Determinants of Health to eliminate avoidable health inequalities,19 appropriate public health interventions are urgently needed. Downstream interventions that focus on individual factors (e.g., education and media programs) tend to increase socioeconomic inequalities87,88 and should be implemented with caution. In countries where cooking and table salt contributes significantly to sodium intake, inclusion of nutritional education in school curricula should be explored.89
Conversely, upstream interventions that focus on structural changes have the greatest potential to reduce socioeconomic inequalities in sodium intake.87,89,90 One option is to restrict or eliminate advertisements of unhealthy fast food, particularly those aimed at children and adolescents, as attempted in the United Kingdom.91 Another option, likely to have the most impact, is to legislatively mandate salt content reduction in processed food production, mirroring successful legislation to remove trans-fatty acids from processed foods in several HICs.90
Limitations
Our findings must be interpreted in light of several limitations. All the observed associations originated from cross-sectional analyses that used different sodium intake measurement methods. Only 15 studies used the gold standard method (24-hour urine collection), whereas the remaining studies estimated sodium intake using a variety of suboptimal methods. Estimates that derived from spot urine tests may have underestimated the relative difference in sodium intake between SES groups, because this method tends to underestimate sodium intake at high levels and to overestimate it at low levels.92 Among studies that used dietary surveys, misreporting of sodium intake was likely common, because social desirability and reporting bias may contribute to underestimations; this is unlikely to have affected urine-based estimations, however. These widely different measurement methods likely contributed to differences in reported mean sodium intake between studies.92,93 This heterogeneity is an important issue when estimating overall sodium intake or when comparing estimates between different populations. However, this is less of an issue in a study such as ours, which compared within-study differences in sodium intake between SES groups.
Studies also differed in the range of socioeconomic indicators included and in their categorization, making it difficult to compare relative and absolute SES differences between studies. We attempted to minimize this bias by taking only the 2 extreme groups, as previous systematic reviews have done.10,24–26,29–31 Although most articles attained higher-quality scores, more than half included only 1 measure of SES, and almost half failed to adjust the reported sodium intake by energy intake, body mass index, age, or gender. In sensitivity analysis, the meta-regression showed that the higher the SES level, the lower the sodium intake. Although the Kendall τ and the Egger test showed no indication of publication bias, it cannot be ruled out with confidence.
Finally, our review focused only on HICs, but SES has been shown to influence diet quality in LMICs as well.24 Future research should assess SES differences in sodium intake in LMICs and the impact of changes in sodium intake, particularly as these countries undergo rapid demographic, nutritional, and epidemiological transitions.
Conclusions
This systematic review and meta-analysis indicates that people of low SES likely consume more sodium than do people of high SES, which may influence the disproportionately higher burden of NCDs in socioeconomically disadvantaged groups. Our results strengthen the importance of existing global and regional targets to reduce sodium intake at the population level and should inform public health policies and interventions aimed at reducing socioeconomic disparities in diet quality and health, especially as countries work toward meeting the global target of 25% relative reduction in NCD mortality by 2025.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (PNR69 grant 406940_145187) and the European Commission and Swiss State Secretariat for Research and Innovation-SERI (Horizon 2020 grant 633666). S. Stringhini is supported by the Swiss National Science Foundation (Ambizione grant PZ00P3_147998). A.-L. Mayén is supported by a Swiss Excellence Government scholarship awarded by the Swiss Confederation. The Swiss Study on Salt Intake was supported by Swiss Federal Office of Public Health (contracts N09.004165/404.0101/-2 and 09.005791/414.0000/-74) and by logistical support of all recruitment centers.
We thank all the corresponding authors who provided additional data results for inclusion in the meta-analysis: Roman Pfister, MD, University of Cologne, Cologne, Germany; Anthony Laverty, PhD, Imperial College, London, United Kingdom; Stefanie Klenow, PhD, the Robert Koch Institute, Berlin, Germany; Juan Carlos Caro Seguel, MSc, Diego Portales University, Santiago, Chile; and Jorge Polonia, MD, PhD, Porto University, Porto, Portugal. We thank the Swiss Survey on Salt team: Isabelle Binet, Murielle Bochud, Michel Burnier, David Conen, Paul Erne, Luca Gabutti, Augusto Gallino, Idris Guessous, Daniel Hayoz, Pascal Meier, Franco Muggli, Fred Paccaud, Antoinette Péchère-Bertschi, and Paolo M. Suter.
Note. The funding sources had no involvement in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the article for publication.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not needed for this systematic review and meta-analysis because data were obtained from secondary sources.
Footnotes
See also Capewell and Kypridemos, p. 499.
REFERENCES
- 1.Sacks FM, Svetkey LP, Vollmer WM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 2.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 3.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [Erratum in Lancet. 2013;381(9867):628. Almazroa MA (added), Memish ZA (added). Lancet. 2013;381(9874):1276.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31(4):489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnson C, Raj TS, Trudeau L et al. The science of salt: a systematic review of clinical salt studies 2013 to 2014. J Clin Hypertens (Greenwich) 2015;17(5):401–411. doi: 10.1111/jch.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 8.Mackenbach JP, Bos V, Andersen O et al. Widening socioeconomic inequalities in mortality in six Western European countries. Int J Epidemiol. 2003;32(5):830–837. doi: 10.1093/ije/dyg209. [DOI] [PubMed] [Google Scholar]
- 9.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107. [Comment in: Social class and diet quality. Am J Clin Nutr. 2008.] [DOI] [PubMed] [Google Scholar]
- 10.Novaković R, Cavelaars A, Geelen A et al. Socioeconomic determinants of micronutrient intake and status in Europe: a systematic review. Public Health Nutr. 2014;17(5):1031–1045. doi: 10.1017/S1368980013001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DD, Leung CW, Li Y et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolić M, Glibetić M, Gurinović M et al. Identifying critical nutrient intake in groups at risk of poverty in Europe: the CHANCE project approach. Nutrients. 2014;6(4):1374–1393. doi: 10.3390/nu6041374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver CM, Dwyer J, Fulgoni VL, 3rd et al. Processed foods: contributions to nutrition. Am J Clin Nutr. 2014;99(6):1525–1542. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster JL, Dunford EK, Neal BC. A systematic survey of the sodium contents of processed foods. Am J Clin Nutr. 2010;91(2):413–420. doi: 10.3945/ajcn.2009.28688. [DOI] [PubMed] [Google Scholar]
- 15.Ni Mhurchu C, Capelin C, Dunford EK, Webster JL, Neal BC, Jebb SA. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households. Am J Clin Nutr. 2011;93(3):594–600. doi: 10.3945/ajcn.110.004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Fernandez R, Siopa M, Simpson SJ, Amiya RM, Breda J, Cappuccio FP. Current salt reduction policies across gradients of inequality-adjusted human development in the WHO European region: minding the gaps. Public Health Nutr. 2014;17(8):1894–1904. doi: 10.1017/S136898001300195X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaglehole R, Bonita R, Horton R et al. Measuring progress on NCDs: one goal and five targets. Lancet. 2012;380(9850):1283–1285. doi: 10.1016/S0140-6736(12)61692-4. [DOI] [PubMed] [Google Scholar]
- 18.Powles J, Fahimi S, Micha R et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3(12):e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmot M, Friel S, Bell R, Houweling TA, Taylor S Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 20.Imamura F, Micha R, Khatibzadeh S et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health. 2015;3(3):e132–e142. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch V, Petticrew M, Tugwell P et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333. doi: 10.1371/journal.pmed.1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Bank. Country and lending groups; 2014. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed February 5, 2015.
- 23.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 24.Mayén AL, Marques-Vidal P, Paccaud F, Bovet P, Stringhini S. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. Am J Clin Nutr. 2014;100(6):1520–1531. doi: 10.3945/ajcn.114.089029. [DOI] [PubMed] [Google Scholar]
- 25.Irala-Estévez JD, Groth M, Johansson L, Oltersdorf U, Prättälä R, Martínez-González MA. A systematic review of socioeconomic differences in food habits in Europe: consumption of fruit and vegetables. Eur J Clin Nutr. 2000;54(9):706–714. doi: 10.1038/sj.ejcn.1601080. [DOI] [PubMed] [Google Scholar]
- 26.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev. 2011;12(5):e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 27.Louie JC, Tapsell LC. Association between intake of total vs added sugar on diet quality: a systematic review. Nutr Rev. 2015;73(12):837–857. doi: 10.1093/nutrit/nuv044. [DOI] [PubMed] [Google Scholar]
- 28.Kunst AE, Bos V, Mackenbach JP. Monitoring socio-economic inequalities in health in the European Union: guidelines and illustrations. 2001. Available at: http://ec.europa.eu/health/ph_projects/1998/monitoring/fp_monitoring_1998_frep_06_a_en.pdf. Accessed March 8, 2015.
- 29.Sánchez-Villegas A, Martínez JA, Prättälä R, Toledo E, Roos G, Martínez-González MA. A systematic review of socioeconomic differences in food habits in Europe: consumption of cheese and milk. Eur J Clin Nutr. 2003;57(8):917–929. doi: 10.1038/sj.ejcn.1601626. [DOI] [PubMed] [Google Scholar]
- 30.López-Azpiazu I, Sánchez-Villegas A, Johansson L, Petkeviciene J, Prättälä R, Martínez-González MA. Disparities in food habits in Europe: systematic review of educational and occupational differences in the intake of fat. J Hum Nutr Diet. 2003;16(5):349–364. doi: 10.1046/j.1365-277x.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 31.Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev. 2013;35:98–111. doi: 10.1093/epirev/mxs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si Hassen W, Castetbon K, Cardon P et al. Socioeconomic indicators are independently associated with nutrient intake in French adults: a DEDIPAC study. Nutrients. 2016;8(3):158. doi: 10.3390/nu8030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giskes K, Avendaňo M, Brug J, Kunst A. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev. 2010;11(6):413–429. doi: 10.1111/j.1467-789X.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, UK: Wiley; 2006. [Google Scholar]
- 36.Beard TC, Woodward DR, Ball PJ, Hornsby H, von Witt RJ, Dwyer T. The Hobart Salt Study 1995: few meet national sodium intake target. Med J Aust. 1997;166(8):404–407. doi: 10.5694/j.1326-5377.1997.tb123189.x. [DOI] [PubMed] [Google Scholar]
- 37.Land MA, Webster J, Christoforou A et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open. 2014;4(1):e003720. doi: 10.1136/bmjopen-2013-003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowson C, Lim K, Grimes C et al. Dietary salt intake and discretionary salt use in two general population samples in Australia: 2011 and 2014. Nutrients. 2015;7(12):10501–10512. doi: 10.3390/nu7125545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen L, Rasmussen LB, Larsen EH, Jakobsen J. Intake of household salt in a Danish population. Eur J Clin Nutr. 2009;63(5):598–604. doi: 10.1038/ejcn.2008.18. [DOI] [PubMed] [Google Scholar]
- 40.Hu G, Jousilahti P, Peltonen M, Lindström J, Tuomilehto J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia. 2005;48(8):1477–1483. doi: 10.1007/s00125-005-1824-1. [DOI] [PubMed] [Google Scholar]
- 41.Reinivuo H, Valsta LM, Laatikainen T, Tuomilehto J, Pietinen P. Sodium in the Finnish diet. II: Trends in dietary sodium intake and comparison between intake and 24-h excretion of sodium. Eur J Clin Nutr. 2006;60(10):1160–1167. doi: 10.1038/sj.ejcn.1602431. [DOI] [PubMed] [Google Scholar]
- 42.Cappuccio FP, Ji C, Donfrancesco C et al. Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL–GIRCSI programme. BMJ Open. 2015;5(9):e007467. doi: 10.1136/bmjopen-2014-007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leclercq C, Ferro-Luzzi A. Total and domestic consumption of salt and their determinants in three regions of Italy. Eur J Clin Nutr. 1991;45(3):151–159. [PubMed] [Google Scholar]
- 44.Murakami K, Sasaki S, Takahashi Y, Uenishi K Japan Dietetic Students’ Study for Nutrition and Biomarkers Group. Neighborhood socioeconomic disadvantage is associated with higher ratio of 24-hour urinary sodium to potassium in young Japanese women. J Am Diet Assoc. 2009;109(9):1606–1611. doi: 10.1016/j.jada.2009.06.391. [DOI] [PubMed] [Google Scholar]
- 45.Polonia J, Martins L, Pinto F, Nazare J. Prevalence, awareness, treatment and control of hypertension and salt intake in Portugal: changes over a decade. The Portuguese Hypertension and Salt study. J Hypertens. 2014;32(6):1211–1221. doi: 10.1097/HJH.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 46.Schoen T, Blum J, Paccaud F, Burnier M, Bochud M, Conen D. Factors associated with 24-hour urinary volume: the Swiss Salt Survey. BMC Nephrol. 2013;14:246. doi: 10.1186/1471-2369-14-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji C, Kandala NB, Cappuccio FP. Spatial variation of salt intake in Britain and association with socioeconomic status. BMJ Open. 2013;3(1):e002246. doi: 10.1136/bmjopen-2012-002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angell SY, Yi S, Eisenhower D et al. Sodium intake in a cross-sectional, representative sample of New York City adults. Am J Public Health. 2014;104(12):2409–2416. doi: 10.2105/AJPH.2013.301542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamler J, Elliott P, Appel L et al. Higher blood pressure in middle-aged American adults with less education-role of multiple dietary factors: the INTERMAP study. J Hum Hypertens. 2003;17(9):655–775. doi: 10.1038/sj.jhh.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi SS, Ruff RR, Jung M, Waddell EN. Racial/ethnic residential segregation, neighborhood poverty and urinary biomarkers of diet in New York City adults. Soc Sci Med. 2014;122:122–129. doi: 10.1016/j.socscimed.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 51.Kho M, Lee JE, Song YM et al. Genetic and environmental influences on sodium intake determined by using half-day urine samples: the Healthy Twin Study. Am J Clin Nutr. 2013;98(6):1410–1416. doi: 10.3945/ajcn.113.067967. [DOI] [PubMed] [Google Scholar]
- 52.Caro S JC. Determinantes sociales y conductuales en salud nutricional: evidencia para Chile. Rev Chil Nutr. 2015;42(1):23–29. [Google Scholar]
- 53.Klenow S, Thamm M, Mensink GB. Sodium intake in Germany estimated from sodium excretion measured in spot urine samples. BMC Nutrition. 2016;2(1):1–11. [Google Scholar]
- 54.Hong JW, Noh JH, Kim DJ. Factors associated with high sodium based on estimated 24-hour urinary sodium excretion: the 2009–2011 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2016;95(9):e2864. doi: 10.1097/MD.0000000000002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millett C, Laverty AA, Stylianou N, Bibbins-Domingo K, Pape UJ. Impacts of a national strategy to reduce population salt intake in England: serial cross sectional study. PLoS One. 2012;7(1):e29836. doi: 10.1371/journal.pone.0029836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2014;16(4):394–402. doi: 10.1002/ejhf.56. [DOI] [PubMed] [Google Scholar]
- 57.Chien KL, Hsu HC, Chen PC et al. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community-based cohort study in Taiwan. J Hypertens. 2008;26(9):1750–1756. doi: 10.1097/HJH.0b013e328306a0a7. [DOI] [PubMed] [Google Scholar]
- 58.McLaren L, Heidinger S, Dutton DJ, Tarasuk V, Campbell NR. A repeated cross-sectional study of socio-economic inequities in dietary sodium consumption among Canadian adults: implications for national sodium reduction strategies. Int J Equity Health. 2014;13:44. doi: 10.1186/1475-9276-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubois L, Girard M. Social position and nutrition: a gradient relationship in Canada and the USA. Eur J Clin Nutr. 2001;55(5):366–373. doi: 10.1038/sj.ejcn.1601165. [DOI] [PubMed] [Google Scholar]
- 60.Buyck JF, Blacher J, Kesse-Guyot E et al. Differential associations of dietary sodium and potassium intake with blood pressure: a focus on pulse pressure. J Hypertens. 2009;27(6):1158–1164. doi: 10.1097/hjh.0b013e328329bc08. [DOI] [PubMed] [Google Scholar]
- 61.van den Brandt PA, Botterweck AA, Goldbohm RA. Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands) Cancer Causes Control. 2003;14(5):427–438. doi: 10.1023/a:1024979314124. [DOI] [PubMed] [Google Scholar]
- 62.Kim K, Hong SA, Kim MK. Trends in nutritional inequality by educational level: a case of South Korea. Nutrition. 2010;26(7–8):791–798. doi: 10.1016/j.nut.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Lee WJ, Kim HC, Oh SM, Choi DP, Cho J, Suh I. Factors associated with a low-sodium diet: the Fourth Korean National Health and Nutrition Examination Survey. Epidemiol Health. 2013;35:e2013005. doi: 10.4178/epih/e2013005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HS, Duffey KJ, Popkin BM. Sodium and potassium intake patterns and trends in South Korea. J Hum Hypertens. 2013;27(5):298–303. doi: 10.1038/jhh.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beydoun MA, Wang Y. How do socioeconomic status, perceived economic barriers and nutritional benefits affect quality of dietary intake among US adults? Eur J Clin Nutr. 2008;62(3):303–313. doi: 10.1038/sj.ejcn.1602700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cogswell ME, Zhang Z, Carriquiry AL et al. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96(3):647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crews DC, Kuczmarski MF, Miller ER, 3rd, Zonderman AB, Evans MK, Powe NR. Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr. 2015;25(2):103–110. doi: 10.1053/j.jrn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greer S, Schieb L, Schwartz G, Onufrak S, Park S. Association of the neighborhood retail food environment with sodium and potassium intake among US adults. Prev Chronic Dis. 2014;11:E70. doi: 10.5888/pcd11.130340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kachan D, Lewis JE, Davila EP et al. Nutrient intake and adherence to dietary recommendations among US workers. J Occup Environ Med. 2012;54(1):101–105. doi: 10.1097/JOM.0b013e31823ccafa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer KA, Harnack LJ, Luepker RV, Zhou X, Jacobs DR, Steffen LM. Twenty-two-year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J Am Heart Assoc. 2013;2(5):e000478. doi: 10.1161/JAHA.113.000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Popkin BM, Siega-Riz AM, Haines PS. A comparison of dietary trends among racial and socioeconomic groups in the United States. N Engl J Med. 1996;335(10):716–720. doi: 10.1056/NEJM199609053351006. [DOI] [PubMed] [Google Scholar]
- 72.Welsh EM, Perveen G, Clayton P, Hedberg R. Sodium reduction in Communities Shawnee County Survey 2011: methods and baseline key findings. J Public Health Manag Pract. 2014;20(1 suppl 1):S9–S15. doi: 10.1097/PHH.0b013e31829d48df. [DOI] [PubMed] [Google Scholar]
- 73.Yang Q, Liu T, Kuklina EV et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171(13):1183–1191. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 74.Ji C, Cappuccio FP. Socioeconomic inequality in salt intake in Britain 10 years after a national salt reduction programme. BMJ Open. 2014;4(8):e005683. doi: 10.1136/bmjopen-2014-005683. [Erratum in BMJ Open. 2014;4(12):e005683corr1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schröder H, Rohlfs I, Schmelz EM, Marrugat J REGICOR investigators. Relationship of socioeconomic status with cardiovascular risk factors and lifestyle in a Mediterranean population. Eur J Nutr. 2004;43(2):77–85. doi: 10.1007/s00394-004-0443-9. [DOI] [PubMed] [Google Scholar]
- 76.McCartney DM, Younger KM, Walsh J, O’Neill M, Sheridan C, Kearney JM. Socioeconomic differences in food group and nutrient intakes among young women in Ireland. Br J Nutr. 2013;110(11):2084–2097. doi: 10.1017/S0007114513001463. [DOI] [PubMed] [Google Scholar]
- 77.Meneton P, Lafay L, Tard A et al. Dietary sources and correlates of sodium and potassium intakes in the French general population. Eur J Clin Nutr. 2009;63(10):1169–1175. doi: 10.1038/ejcn.2009.57. [Erratum in Eur J Clin Nutr. 2010;64(2):230.] [DOI] [PubMed] [Google Scholar]
- 78.Miyaki K, Song Y, Taneichi S et al. Socioeconomic status is significantly associated with dietary salt intakes and blood pressure in Japanese workers (J-HOPE Study) Int J Environ Res Public Health. 2013;10(3):980–993. doi: 10.3390/ijerph10030980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda Y, Hiyoshi A. High quality nutrient intake is associated with higher household expenditures by Japanese adults. Biosci Trends. 2012;6(4):176–182. doi: 10.5582/bst.2012.v6.4.176. [DOI] [PubMed] [Google Scholar]
- 80.Mishra G, Ball K, Patterson A, Brown W, Hodge A, Dobson A. Socio-demographic inequalities in the diets of mid-aged Australian women. Eur J Clin Nutr. 2005;59(2):185–195. doi: 10.1038/sj.ejcn.1602057. [DOI] [PubMed] [Google Scholar]
- 81.Smith AM, Baghurst KI. Public health implications of dietary differences between social status and occupational category groups. J Epidemiol Community Health. 1992;46(4):409–416. doi: 10.1136/jech.46.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Hsu-Hage BH, Wahlqvist ML. Longitudinal changes in nutrient intakes in the Melbourne Chinese Cohort Study. Public Health Nutr. 2002;5(3):433–439. doi: 10.1079/phn2001259. [DOI] [PubMed] [Google Scholar]
- 83.Ilow R, Regulska-Ilow B, Różańska D et al. Evaluation of mineral and vitamin intake in the diet of a sample of Polish population—baseline assessment from the prospective cohort ‘PONS’ study. Ann Agric Environ Med. 2011;18(2):235–240. [PubMed] [Google Scholar]
- 84.Beer-Borst S, Costanza MC, Péchère-Bertschi A, Morabia A. Twelve-year trends and correlates of dietary salt intakes for the general adult population of Geneva, Switzerland. Eur J Clin Nutr. 2009;63(2):155–164. doi: 10.1038/sj.ejcn.1602922. [DOI] [PubMed] [Google Scholar]
- 85.Gerber AM, James SA, Ammerman AS et al. Socioeconomic status and electrolyte intake in Black adults: the Pitt County Study. Am J Public Health. 1991;81(12):1608–1612. doi: 10.2105/ajph.81.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bibbins-Domingo K, Chertow GM, Coxson PG et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGill R, Anwar E, Orton L et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health. 2015;15:457. doi: 10.1186/s12889-015-1781-7. [Erratum in: Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health. 2015.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health. 2013;67(2):190–193. doi: 10.1136/jech-2012-201257. [DOI] [PubMed] [Google Scholar]
- 89.Silveira JA, Taddei JA, Guerra PH, Nobre MR. The effect of participation in school-based nutrition education interventions on body mass index: a meta-analysis of randomized controlled community trials. Prev Med. 2013;56(3–4):237–243. doi: 10.1016/j.ypmed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Jørgensen T, Capewell S, Prescott E et al. [Population-level changes to promote cardiovascular health] Vnitr Lek. 2012;58(12):943–954. [PubMed] [Google Scholar]
- 91.Boyland EJ, Halford JC. Television advertising and branding. Effects on eating behaviour and food preferences in children. Appetite. 2013;62:236–241. doi: 10.1016/j.appet.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 92.Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk—measurement matters. N Engl J Med. 2016;375(6):580–586. doi: 10.1056/NEJMsb1607161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cobb LK, Anderson CA, Elliott P et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129(10):1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]