Abstract
Objectives. To investigate the relationship between vision and disability in the elderly.
Methods. We used a baseline visual indicator (combining near acuity with Snellen equivalent < 20/30 and self-reported distance visual loss) to explore the association between visual loss and subsequent disability (mobility, instrumental activities of daily living [IADLs], ADLs, and participation restriction) from 1999 to 2007 in 8491 elderly participants of the French Three-City Cohort (Bordeaux, Dijon, and Montpellier).
Results. In multiadjusted analyses, near visual impairment, alone or associated with distance visual function loss, was associated with greater risk of developing ADL limitations (P = .027), IADL limitations (P = .002), and participation restriction (P < .001), but not mobility (P = .848). The disabling impact of visual loss was significant for 11 of the 15 activities, when analyzed one by one.
Conclusions. Both near and distance visual loss was associated with greater functional decline over time, and the combination of the two could be even worse.
Public Health Implications. In the context of rapid aging of the population, maintaining good vision in the elderly represents a promising prevention track, visual impairment being common in the elderly, largely undermanaged, and mostly reversible. Further research, especially trials, is necessary to estimate the public health impact of such interventions.
Aging well has become a major challenge for societies around the world in which lifespans are increasing. Aging well can be defined as successful aging, active aging, and healthy aging, but also—and perhaps more pragmatically—as aging independently in daily living. According to the health data from the Centers for Disease Control and Prevention (CDC), in 2014, limitations in activities of daily living (ADLs) affected 31% of the elderly US population, representing 13.6 million people, which is considerable in terms of needs of assistance. To promote aging well, several levers for action have been identified, such as controlling cardiovascular risk factors, promoting healthy diet, and physical activity practice. Maintaining good vision has been less investigated than the others, but it could represent an interesting track. Indeed, visual impairment (VI) is highly prevalent in the elderly1,2—13.5% of those aged 65 years and older are visually impaired, according to health data from the CDC—and most often treatable.3–5 Such impairment may be attributable to functional causes, including uncorrected refractive errors (myopia, hyperopia, astigmatism, and presbyopia), or to organic causes, including cataracts, glaucoma, age-related macular degeneration, and diabetic retinopathy.
In daily living, all activities are more or less visually demanding, and several visual functions (VFs) are required for independent living.5–8 For instance, near vision is necessary for performing tasks requiring good resolution and adaptation to changing light conditions, such as reading, writing, and grasping and finely manipulating objects—for example, for preparing meals or practicing hobbies.9 Distance vision is required for driving, navigating safely and independently (particularly in unfamiliar environments), using public transportation, walking (especially down steps), and shopping.9 Finally, other VFs, such as contrast sensitivity, disability glare, visual field perception, and stereoscopic depth perception, may also affect functioning in daily life.5,10 A better knowledge of the relationships between vision and functioning in daily life may therefore have important public health implications.6,11
In the Salisbury Eye Evaluation project,12 loss of distance visual acuity was related to increased difficulty, more in instrumental activities of daily living (IADLs) than in basic ones (ADLs), as also reported elsewhere.6,12 A cross-sectional analysis of 2781 Finnish participants aged 55 years and older showed that the prevalence of ADLs, IADLs, and mobility limitations increased with decreasing distance visual acuity (P < .001). The risk of limitations in several domains of disability was 3 to 5 times greater among people with poor visual acuity than among others.13 In the Canadian Study of Health and Aging, vision was one of the major determinants of activity limitation with arthritis, heart diseases, cognitive impairment, and foot problems, in terms of population attributable risk.14 In the Medical Research Council Study, participants with VI had on average 2 fewer disability-free years than those without VI.15 Other studies also reported that elderly people with poorer VF (acuity, contrast sensitivity, and useful field of view) needed more time to complete visual IADLs (e.g., reading instructions on medicine bottles, finding a phone number in a directory).16 Finally, some studies also suggested a dose–response effect: the poorer the vision loss, the greater the risk of disability.13
However, although near vision is obviously crucial for most ADLs,1 previous studies have almost exclusively focused on distance VI, following World Health Organization (WHO) guidelines. In defining its thresholds for distance VI, the WHO specifies that vision should be measured with both eyes uncovered and the patient wearing current optical correctional lenses17; the chart used can be decimal, Snellen, or US equivalent notation. These guidelines have been restricted principally owing to a lack of population-based empirical data.1,18 However, near vision loss in the elderly population is common and largely undercorrected, in high-income as well as low-income countries.1
We cross-sectionally and prospectively explored the relationship between vision loss and specific functional changes over 7 years of follow-up in a large, prospective, population-based cohort of elderly participants. We investigated the effect of measured impairment of near vision (near VI), of self-reported distance visual function (VF) loss, and of the combined effect of the two, and explored each domain of activity limitation (mobility, IADLs, ADLs) and participation restriction.
METHODS
We recruited participants from the Three-City Cohort, a population-based elderly cohort on cerebral aging enlisted from the electoral rolls of 3 French cities (Bordeaux, Dijon, and Montpellier), between 1999 and 2001. At baseline, the sample comprised 9294 community-dwelling participants aged 65 years and older. The methodology has been described elsewhere.19 A standardized evaluation with a face-to-face interview and clinical examination was prospectively conducted by a specially trained neuropsychologist and by a neurologist if necessary. We performed the present analyses on the data collected at baseline and at 2, 4, and 7 years after baseline.
Activity Limitation and Participation Restriction
At each visit, interviewers (neuropsychologists) investigated the 3 domains of activity limitation (mobility, IADL, and ADL).20 They assessed mobility using 3 items from the Rosow and Breslau scale: heavy housework, walking half a mile, and using stairs.21 IADLs were telephone use, shopping, using transportation, managing drugs, and handling finances.20 They evaluated 3 additional activities for women only: housework, meal preparation, and laundry. ADLs were bathing, dressing, toileting, transferring, and eating.22 For each of these 3 domains, a participant was considered to be limited if he or she could not perform at least 1 activity on the scale without a given level of assistance. Interviewers assessed participation restriction according to the following question: “How much do you currently feel restricted in your transfers at home and your travel beyond your home?” We dichotomized this variable by distinguishing participants with no restriction from those bed bound, home bound, or neighborhood bound.
Near Visual Impairment and Distance Visual Function Loss
Interviewers measured binocular near visual acuity with the Parinaud scale (a Jaeger-like reading test commonly used by French ophthalmologists), with a standardized reading distance of 33 centimeters. They classified a Parinaud score higher than 2 (Snellen equivalent < 20/30) as near VI. They defined self-reported distance VF as inability or difficulty in recognizing a face at 4 meters. The definitions were based on current vision, using usual optical correction if any. After combining these 2 assessments, we distinguished 4 groups at baseline: no visual loss, only distance VF loss, only near VI, and both.
Controlling Factors
Sociodemographic factors included age, gender, living alone or not, and level of income. We defined educational level according to the French primary school certificate, which corresponds to about 7 years of schooling. We classified those who had not obtained this diploma as lowest level, those with only this diploma as intermediate level, and those with a greater level as high level. Lifestyle factors included diet (regular consumption of fish, fruits, and vegetables), alcohol consumption (0, 1–36, > 36 g/day), smoking (past, present, or never), and body mass index (BMI, defined as weight in kilograms divided by the square of height in meters), with less than 21.0 defined as thin, 21.0 to 24.9 as normal, 25.0 to 29.9 as overweight, and 30.0 and higher as obese. Interviewers measured blood pressure using a digital electronic blood pressure gauge; we defined hypertension as 140/90 millimeters mercury or above, or treatment for high blood pressure. Analyses also controlled for the following self-reported clinical information: ischemic heart disease (angina pectoris, cardiovascular surgery, or myocardial infarction), nonischemic heart disease (congestive heart failure or heart rhythm disorder), peripheral vascular disease (stroke or lower extremity artery surgery), diabetes (self-reported or treated), dyspnea (breathless after minor effort), hearing impairment, cognition (Mini-Mental State Examination),23 and depressive symptomatology (Center for Epidemiologic Studies Depression Scale),24 using validated French population cutoffs of 17 and 23 in men and women, respectively.25
Statistical Analyses
We conducted cross-sectional descriptive and comparative analyses using appropriate tests (t test, χ2 test, or Fisher exact test), as well as multivariate logistic regressions.
To study the occurrence of disability according to visual loss at baseline, we performed Cox models with delayed entry (with age as time scale) after exclusion of the initially disabled participants for each domain of disability (mobility, IADLs, ADLs, and confinement) or for each task when analyzed activity by activity. We excluded prevalent cases of dementia at baseline from the present study to ensure the best possible assessment of the different variables analyzed.
We controlled all multivariate models for the factors described in Methods. We conducted sensitivity analyses with imputed missing data on vision. Finally, to counteract the problem of multiple comparisons, we used the Bonferroni correction. We performed the analyses using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
The final sample comprised 8491 participants (i.e., 91.4% of the initial sample), after exclusion of 612 participants because of missing data on vision and an additional 191 because of dementia at baseline. Within this sample, 251 (3.0%) had only distance VF loss, 921 (10.9%) had only near VI, and 213 (2.5%) had both. The participants with both losses had a significantly greater mean score on the Parinaud scale than those with near VI only (7.6 points [SD = 6.6] vs 4.6 points [SD = 2.2]). At baseline, 46.6% had mobility restriction, 9.3% had IADL limitation, 0.9% had ADL limitation, and 6.7% were house or neighborhood bound. As presented in Table 1, participants with vision loss were significantly older, more often women, had lower education, and were more likely to be disabled. They also had poorer health (results not shown).
TABLE 1—
Sample Characteristics in Relation to Visual Status at Baseline (n = 8491): France, 1999–2007
| Characteristic | No Visual Loss (n = 7106), No. (%) or Mean ±SD | Only Distance Function Loss (n = 251), No. (%) or Mean ±SD | Only Near Acuity Loss (n = 921), No. (%) or Mean ±SD | Both Types of Visual Loss (n = 213), No. (%) or Mean ±SD | P |
| Mean age, y | 73.7 ±5.3 | 75.4 ±6.0 | 76.3 ±5.9 | 78.7 ±6.1 | < .001 |
| Female gender | 4204 (59.2) | 186 (74.1) | 608 (66.0) | 161 (75.6) | < .001 |
| Educational level | |||||
| Low | 497 (7.0) | 24 (9.6) | 140 (15.3) | 29 (13.7) | < .001 |
| Intermediate | 1152 (16.2) | 36 (14.4) | 233 (25.4) | 38 (17.9) | |
| High | 5452 (76.8) | 190 (76.0) | 544 (59.3) | 145 (68.4) | |
| Limitation | |||||
| Mobility | 3022 (43.0) | 127 (52.5) | 598 (65.7) | 165 (79.3) | < .001 |
| IADLs | 480 (6.8) | 37 (14.8) | 173 (18.9) | 94 (44.1) | < .001 |
| ADLs | 45 (0.6) | 6 (2.4) | 16 (1.7) | 5 (2.4) | < .001 |
| Confinement | 304 (4.3) | 27 (10.8) | 161 (17.5) | 79 (37.3) | < .001 |
Note. ADL = activity of daily living; IADL = instrumental activity of daily living.
Association Between Disability and Visual Status at Baseline
The cross-sectional analyses controlling for demographic, lifestyle, and clinical factors showed that individuals with impairment of both near and distance vision were the most likely to be disabled. For IADLs, for example, the odds ratio was 6.0 (95% confidence interval [CI] = 4.1, 8.8) with both impairments and 1.6 (95% CI = 1.2, 2.0) with only near VI; there was no significant association with distance VF loss only (odds ratio [OR] = 1.1; 95% CI = 0.7, 1.9; Table 2). Of the 4 domains, ADL limitation was the only one for which the association was not confirmed in multivariate analyses (P = .244), despite odds ratios around 2 (analyses conducted on 49 prevalent cases).
TABLE 2—
Cross-Sectional and Longitudinal Analyses of the Relationships Between Initial Visual Status and Disability Among the Elderly (Aged ≥ 65 Years): France, 1999–2007
| Cross-Sectional Analyses |
Longitudinal Analyses |
|||
| Characteristic | Prevalent Cases, No./Total No. | OR (95% CI) | Incident Cases, No./Total No. | HR (95% CI) |
| Mobility | 3306/7361 | 2444/3841 | ||
| No impairment (Ref) | 1 | 1 | ||
| Only distance | 0.9 (0.6, 1.2) | 1.0 (0.8, 1.3) | ||
| Only near | 1.7 (1.4, 2.1) | 1.1 (0.9, 1.3) | ||
| Both | 2.3 (1.5, 3.5) | 1.1 (0.7, 1.6) | ||
| IADLs | 597/7407 | 1393/6285 | ||
| No impairment (Ref) | 1 | 1 | ||
| Only distance | 1.1 (0.7, 1.9) | 1.2 (0.9, 1.6) | ||
| Only near | 1.6 (1.2, 2.0) | 1.2 (1.0, 1.4) | ||
| Both | 6.0 (4.1, 8.8) | 1.7 (1.2, 2.4) | ||
| ADLs | 49/7413 | 207/6744 | ||
| No impairment (Ref) | 1 | 1 | ||
| Only distance | 1.9 (0.7, 5.5) | 1.5 (0.8, 2.8) | ||
| Only near | 2.0 (0.9, 4.4) | 1.6 (1.1, 2.3) | ||
| Both | 1.9 (0.6, 6.4) | 1.9 (1.0, 3.4) | ||
| Participation restriction | 424/7430 | 1034/6443 | ||
| No impairment (Ref) | 1 | 1 | ||
| Only distance | 1.3 (0.8, 2.3) | 1.1 (0.8, 1.6) | ||
| Only near | 2.7 (2.1, 3.6) | 1.3 (1.1, 1.6) | ||
| Both | 6.3 (4.2, 9.6) | 1.8 (1.3, 2.5) | ||
Note. ADL = activity of daily living; CI = confidence interval; HR = hazard ratio; IADL = instrumental activity of daily living; OR = odds ratio. Logistic regression and Cox models with delayed entry, controlled for age (as time scale for Cox models with delayed entry); gender; education; center; living alone; income; alcohol and tobacco consumption; body mass index; fish, fruit, and vegetable consumption; hypertension; cardio- and cerebrovascular diseases; diabetes; dyspnea; Mini-Mental State Examination score; hearing impairment; and depressive symptomatology.
Risk of Disability According to Initial Visual Status
Risk of developing mobility limitation.
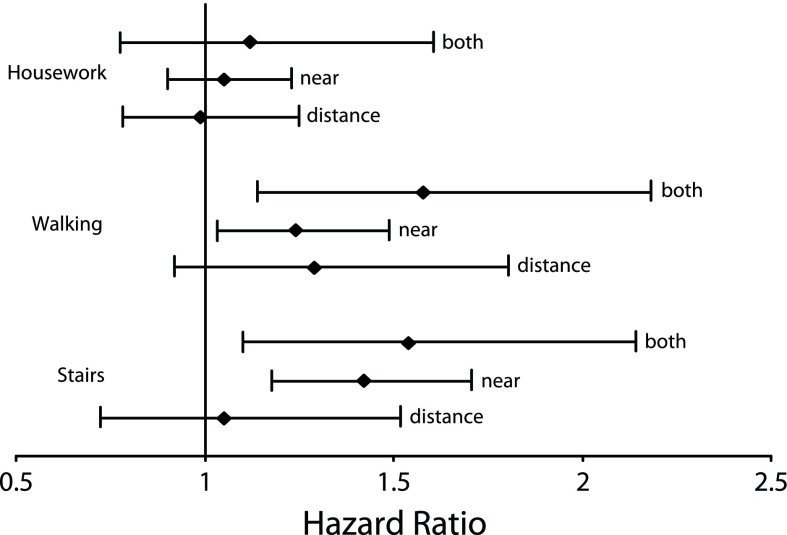
After exclusion of the numerous prevalent cases of mobility limitation at baseline (n = 3306, almost 45% of the initial sample), the significant association found in cross-sectional analysis did not persist in the fully adjusted longitudinal analysis (P = .848; Table 2). However, when we analyzed each activity one by one (still fully adjusted), the participants with near VI (with or without distance VF loss) still had an increased risk of developing limitation in using stairs (P < .001) or walking (P = .006), but not in doing heavy housework (P = .863; Figure 1). The very high incidence of limitation in doing heavy housework (63.0%, compared with 13.7% for using stairs and 14.2% for walking) probably drove the global analysis on mobility toward nonsignificance.
FIGURE 1—
Longitudinal Analyses of Relationships Between Initial Visual Status and 3 Mobility Items Among the Elderly (Aged ≥ 65 Years): France, 1999–2007
Note. The figure shows fully adjusted Cox models with delayed entry. Whiskers indicate 95% confidence intervals.
Risk of developing IADL limitation.
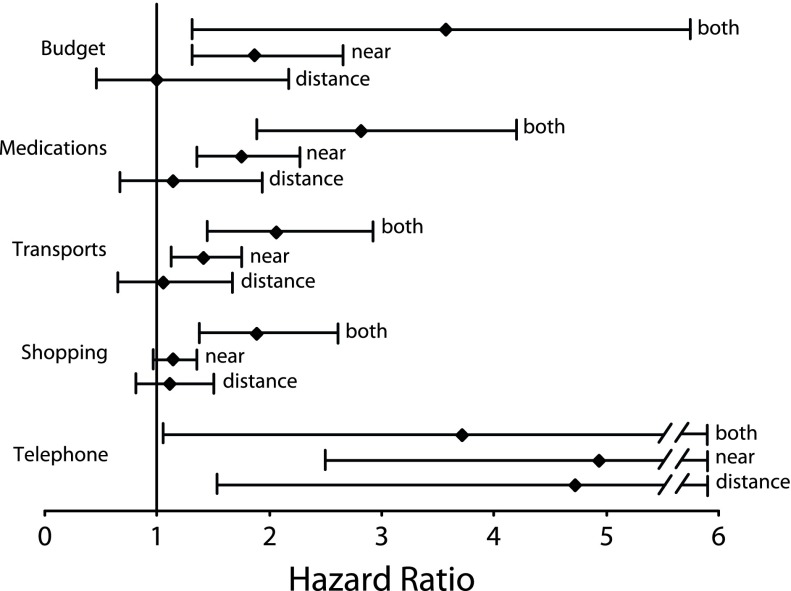
As in the cross-sectional analyses, near VI (with or without distance VF loss) was associated with greater risk of subsequent IADL limitation over time (P = .002) independently of the controlling factors (Table 2). The participants with both losses had a greater risk (hazard ratio [HR] = 1.7; 95% CI = 1.2, 2.4) than those with near VI only (HR = 1.2; 95% CI = 1.0, 1.4). As shown in Figure 2, for each single IADL, visual loss was associated with greater risk of subsequent limitation (only nearly significant for doing housework; P = .054). For 6 of the 8 IADLs (all except for using the telephone and doing shopping), the individuals with near VI were at higher risk for developing activity limitation; when combined with distance VF loss, the disabling effect was even greater, whereas distance VF loss alone was not disabling (Figure 2; results not shown for the 3 IADLs specific to women). For doing shopping, near VI alone did not significantly affect performance of the task (HR = 1.1; 95% CI = 1.0, 1.4). Finally, for using the telephone, each of the 3 visual loss statuses was associated with greater risk of subsequent limitation, with hazard ratios of around 4 (with large confidence intervals).
FIGURE 2—
Longitudinal Analyses of Relationships Between Initial Visual Status and the 5 Instrumental Activities of Daily Living Common to Both Sexes Among the Elderly (Aged ≥ 65 Years): France, 1999–2007
Note. The figure shows fully adjusted Cox models with delayed entry. Whiskers indicate 95% confidence intervals.
Risk of developing ADL limitation.
Although we found no significant relationship in cross-sectional analyses, probably from lack of statistical power, we observed a disabling impact of near VI (with or without distance VF loss) in longitudinal analyses globally (for near VI alone, HR = 1.6; 95% CI = 1.1, 2.3; Table 2), and also for bathing and dressing analyzed separately (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). Vision loss did not appear to affect the tasks of transferring and going to the toilet. Finally, because of insufficient numbers of incident cases for the feeding task (only 17 participants), we have provided no hazard ratios for this item.
Risk of developing participation restriction.
The longitudinal analyses confirmed the significant impact of visual loss in terms of participation restriction observed in cross-sectional analyses (P < .001; Table 2). The participants with both near and distance vision loss had almost twice the risk of participation restriction compared with those with no visual loss at baseline (HR = 1.8; 95% CI = 1.3, 2.5). Again, self-reported distance VF loss only was not associated with greater risk of confinement, but when combined with near VI, the disabling effect was even greater.
It is noteworthy that all the results of the analyses conducted with imputed data (comprising 6.5% of the sample) were unchanged (data not shown).
DISCUSSION
The present study, conducted on a population-based cohort with a large sample of aged individuals, highlights that visual loss in the elderly significantly increases the risk of subsequent functional decline over time independently of many demographic, lifestyle, and clinical factors. Each domain and almost each activity of the full spectrum of activity limitations (mobility, IADLs, and ADLs) and activity participation were affected by visual loss. We found that near VI had a greater disabling impact than distance VF loss, and that their combined effect was even greater; both are probably a marker of specific eye diseases or of a more advanced stage of VI. Indeed, individuals with both types of loss were on average more visually impaired on the Parinaud scale than those with near VI only (7.6 vs 4.6 points). Rubin et al., who explored multiple visual measures, reported that the association with disability was consistent with an additive model in which multiple visual impairments reflect greater severity of vision loss5; our findings seem to confirm this. Finally, because of the scarcity of findings on near vision, WHO guidelines focus on distance vision; as a result, so does most of the literature on the relationship between vision and disability.1,18 However, we emphasize the disabling effect of near VI—along with that of distance VF—and highlight its importance for estimating disease burden.1,18 We thus bring important findings to the scant literature on this dimension of vision.1,18
Several studies have suggested that visual loss affects IADLs more than ADLs, with a hierarchical relationship in which IADLs are affected before ADLs.6,12,26,27 Basic ADLs (such as transferring from bed to chair or feeding) are relatively automatic and normally less visually challenging than IADLs, which are more complex, requiring greater visual input as well as other skills such as cognition.6 In the Health and Retirement Study, Berger and Porell reported that self-reported poor vision (near or distance) was not associated with greater risk of ADL limitation relative to being only IADL limited.6 In the Salisbury Eye Evaluation project, the authors reported that the direct effects of acuity loss were strongest for IADLs, at baseline (P < .001) as well as longitudinally (P < .001), whereas a significant association with ADLs was observed only in men.12 Moreover, of the 22 candidate risk factors explored by Gill et al., corrected near vision was 1 of the 5 factors found associated with ADLs, and more particularly with persistent disability (HR = 1.49; 95% CI = 1.16, 1.92).28 Mobility, less investigated in the literature, would be restricted even earlier in the disablement process, being a primary pathway leading to IADL limitation; for instance, traveling outside is required for grocery shopping.26 Finally, the studies on the impact of vision loss on participation restriction are scarce,29–31 and often limited by small sample size. However, the association between vision loss and confinement is one of our strongest findings in both cross-sectional (OR = 6.38) and longitudinal (HR = 1.83) analyses, but further research on how vision loss interferes with participation restriction is needed.32
Strengths and Limitations
Our study has several strengths. It was based on a large sample of elderly people, recruited from the general population and followed up to 3 times over 7 years, which allowed us to control for temporality between vision and incident disability and to suggest that the disabling effect of vision loss persisted over time (detailed short-, mid-, and long-term analyses not shown). Moreover, we also covered the full spectrum of activity limitation severity and participation restriction and explored 2 dimensions of vision with near VI and self-reported distance VF loss.
Nevertheless, our study also has potential limitations. First, with a participation rate of 37%, the Three-City sample was not representative of the elderly population. However, we have no reason to believe that this could affect the relationship between vision and disability. Second, distance vision was self-reported using a single question, which probably led to an underestimation of distance VF loss (only 5.5% of the sample reported such vision loss). Third, we did not cover all VFs, although some are strong predictors of disability, such as contrast sensitivity or visual field loss.5 Fourth, we combined an objective measure and a subjective perception of visual loss for 2 different visual dimensions, which makes clinical interpretation more difficult. Fifth, in the absence of ophthalmological examination, we could not study the medical causes of visual loss. Finally, even if we controlled our models for most of the well-known determinants of disability, we cannot fully ensure that the observed relationship between vision and functional decline could not be the result of an underlying nonanalyzed process (unmeasured or inadequately measured disorders, or the occurrence of disorders over time that were not analyzed).
Public Health Implications
With the aging of the baby boomer generation, an explosion of the number of disabled and dependent elderly people is expected in the next decades, despite the functional improvements reported in the 2 last decades.33 In that context, prevention of functional decline has huge public health and economic implications. For France in 2011, the cost of dependency has been estimated at $23.7 billion (i.e., 1.1% of GDP).34 In the United States, a study conducted on the noninstitutionalized US civilian adult population using data from the 2002–2003 Medical Expenditure Panel Survey and state-level data from the Behavioral Risk Factor Surveillance System estimated that disability-associated health care expenditures totaled $397.8 billion (accounting for 26.7% of all health care expenditures), which represented 0.86% of GDP in 2006.35
Each prevention action and program that could soften the curve of dependency must be investigated. In the present study, we emphasize that visual loss has a significant disabling impact in each domain of disability and in almost each task of daily living. We previously showed that 20.5% of IADL limitations could be attributed to uncorrected refractive errors.4 Maintaining or recovering good vision in the elderly may represent a promising prevention track; VI is frequent among the elderly, and is largely underdiagnosed or undermanaged and mostly reversible. Further research, especially trials, must be conducted to assess the efficacy of such interventions to prevent functional decline in the elderly.
Conclusions
We highlight the strong relationship between vision and functioning in a wide range of everyday activities with potential practical public health implications. Because a substantial proportion of VI is treatable or reversible, maintaining good vision in the elderly represents a promising prevention track to explore. Improvement in ophthalmic screening and routine eye care may significantly contribute to the reduction of disability in the elderly population.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de la Recherche (ANR) under the Programme de Recherche en Santé Publique, project VISA ANR-2010 PRSP-011-01. The Three-City Study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), University of Bordeaux, and Sanofi—Aventis. The Three-City Study was also supported by the Caisse Nationale de Solidarité pour l'Autonomie; Caisse Nationale Maladie des Travailleurs Salariés; Direction Générale de la Santé; MGEN; the Institut de la Longévité; Agence Française de Sécurité Sanitaire des Produits de Santé; the Regional Governments of Aquitaine, Bourgogne and Languedoc-Roussillon; the Fondation de France; the Ministry of Research– INSERM Programme “Cohorts and Collection of Biological Material”; the ANR under the Programme National de Recherche en Alimentation et nutrition humaine, project COGINUT ANR-06-PNRA-005; the Programme Longévité et vieillissement, project 07-LVIE-004 and 07-LVIE 003 01; the Institut de Recherche en Santé Publique (IReSP), Paris, France; and Novartis. The Lille Genopole was supported by an unconditional grant from Eisai. V. Nael was supported by a CIFRE program 2015/0755, funded by Essilor.
HUMAN PARTICIPANT PROTECTION
The study protocol was approved by the Ethical Committee of the University-Hospital of Bicêtre (France) and conformed to the principles embodied in the Declaration of Helsinki. Written informed consent was obtained from each participant.
REFERENCES
- 1.He M, Abdou A, Naidoo KS et al. Prevalence and correction of near vision impairment at seven sites in China, India, Nepal, Niger, South Africa, and the United States. Am J Ophthalmol. 2012;154(1):107e1–116 e1. doi: 10.1016/j.ajo.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Stevens GA, White RA, Flaxman SR et al. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology. 2013;120(12):2377–2384. doi: 10.1016/j.ophtha.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Bourne RR, Stevens GA, White RA et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 4.Daien V, Peres K, Villain M, Colvez A, Delcourt C, Carriere I. Visual impairment, optical correction, and their impact on activity limitations in elderly persons: the POLA study. Arch Intern Med. 2011;171(13):1206–1207. doi: 10.1001/archinternmed.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin GS, Bandeen-Roche K, Huang GH et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Invest Ophthalmol Vis Sci. 2001;42(1):64–72. [PubMed] [Google Scholar]
- 6.Berger S, Porell F. The association between low vision and function. J Aging Health. 2008;20(5):504–525. doi: 10.1177/0898264308317534. [DOI] [PubMed] [Google Scholar]
- 7.Klein BE, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110(4):644–650. doi: 10.1016/S0161-6420(02)01935-8. [DOI] [PubMed] [Google Scholar]
- 8.West SK, Rubin GS, Broman A, Muñoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002;120(6):774–780. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- 9.Mangione CM, Phillips RS, Seddon JM et al. Development of the “Activities of Daily Vision Scale.” A measure of visual functional status. Med Care. 1992;30(12):1111–1126. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663–2668. [PubMed] [Google Scholar]
- 11.Jin YP, Wong DT. Self-reported visual impairment in elderly Canadians and its impact on healthy living. Can J Ophthalmol. 2008;43(4):407–413. doi: 10.3129/i08-077. [DOI] [PubMed] [Google Scholar]
- 12.Lam BL, Christ SL, Zheng DD et al. Longitudinal relationships among visual acuity and tasks of everyday life: the Salisbury Eye Evaluation study. Invest Ophthalmol Vis Sci. 2013;54(1):193–200. doi: 10.1167/iovs.12-10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitinen A, Sainio P, Koskinen S, Rudanko SL, Laatikainen L, Aromaa A. The association between visual acuity and functional limitations: findings from a nationally representative population survey. Ophthalmic Epidemiol. 2007;14(6):333–342. doi: 10.1080/01658100701473713. [DOI] [PubMed] [Google Scholar]
- 14.Griffith L, Raina P, Wu H, Zhu B, Stathokostas L. Population attributable risk for functional disability associated with chronic conditions in Canadian older adults. Age Ageing. 2010;39(6):738–745. doi: 10.1093/ageing/afq105. [DOI] [PubMed] [Google Scholar]
- 15.Jagger C, Matthews R, Matthews F, Robinson T, Robine JM, Brayne C. The burden of diseases on disability-free life expectancy in later life. J Gerontol A Biol Sci Med Sci. 2007;62(4):408–414. doi: 10.1093/gerona/62.4.408. [DOI] [PubMed] [Google Scholar]
- 16.Owsley C, McGwin G, Jr, Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optom Vis Sci. 2001;78(5):350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 17.International Statistical Classification of Diseases and Health Related Problems, 10th Revision. Vol 1. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 18.Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008;86(1):63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 21.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 23.Folstein M, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Radloff S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25.Fuhrer R, Rouillon F. La version française de l’échelle CES-D (Center for Epidemiologic Studies–Depression Scale) Psychiatr Psychobiol. 1989;4:163–166. [Google Scholar]
- 26.Hochberg C, Maul E, Chan ES et al. Association of vision loss in glaucoma and age-related macular degeneration with IADL disability. Invest Ophthalmol Vis Sci. 2012;53(6):3201–3206. doi: 10.1167/iovs.12-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edjolo A, Proust-Lima C, Delva F, Dartigues JF, Peres K. Natural history of dependency in the elderly: a 24-year population-based study using a longitudinal item response theory model. Am J Epidemiol. 2016;183(4):277–285. doi: 10.1093/aje/kwv223. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Murphy TE, Barry LC, Allore HG. Risk factors for disability subtypes in older persons. J Am Geriatr Soc. 2009;57(10):1850–1855. doi: 10.1111/j.1532-5415.2009.02443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desrosiers J, Wanet-Defalque MC, Temisjian K et al. Participation in daily activities and social roles of older adults with visual impairment. Disabil Rehabil. 2009;31(15):1227–1234. doi: 10.1080/09638280802532456. [DOI] [PubMed] [Google Scholar]
- 30.Lamoureux EL, Pallant JF, Pesudovs K et al. Assessing participation in daily living and the effectiveness of rehabilitation in age related macular degeneration patients using the impact of vision impairment scale. Ophthalmic Epidemiol. 2008;15(2):105–113. doi: 10.1080/09286580701840354. [DOI] [PubMed] [Google Scholar]
- 31.Lamoureux EL, Hassell JB, Keeffe JE. The determinants of participation in activities of daily living in people with impaired vision. Am J Ophthalmol. 2004;137(2):265–270. doi: 10.1016/j.ajo.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Rees G, Tee HW, Marella M, Fenwick E, Dirani M, Lamoureux EL. Vision-specific distress and depressive symptoms in people with vision impairment. Invest Ophthalmol Vis Sci. 2010;51(6):2891–2896. doi: 10.1167/iovs.09-5080. [DOI] [PubMed] [Google Scholar]
- 33.Pérès K, Edjolo A, Dartigues JF, Barberger-Gateau P. Recent trends in disability-free life expectancy in the French elderly: twenty years follow-up of the Paquid Cohort. Annu Rev Gerontol Geriatr. 2013;33(1):293–311. [Google Scholar]
- 34.Renoux A, Roussel R, Zaidman C. Le compte de la dépendance en 2011 et à l’horizon 2060. Solidarité et Santé. 2014;50(Fév):1–41. [Google Scholar]
- 35.Anderson WL, Armour BS, Finkelstein EA, Wiener JM. Estimates of state-level health-care expenditures associated with disability. Public Health Rep. 2010;125(1):44–51. doi: 10.1177/003335491012500107. [DOI] [PMC free article] [PubMed] [Google Scholar]