Abstract
Sudden cardiac death in myotonic dystrophy type I (DM1) patients can be attributed to atrioventricular blocks as far as to the development of life-threatening arrhythmias which occur even in hearts with normal left ventricular systolic and diastolic function. Heterogeneity of ventricular repolarization is considered to provide an electrophysiological substrate for malignant arrhythmias. QTc dispersion (QTc-D), JTc dispersion (JTc-D) and transmural dispersion of repolarization (TDR) could reflect the physiological variability of regional and transmural ventricular repolarization. Aim of the present study was to investigate the heterogeneity of ventricular repolarization in patients with DM1 and preserved diastolic and systolic cardiac function. The study enrolled 50 DM1 patients (mean age 44 ± 5 years; M:F: 29:21) with preserved systolic and diastolic function of left ventricle among 247 DM1 patients followed at Cardiomyology and Medical Genetics of Second University of Naples, and 50 sexand age-matched healthy controls. The electrocardiographic parameters investigated were the following: Heart Rate, QRS duration, maximum and minimum QT and JT intervals, QTc- D, JTc-D and TDR.
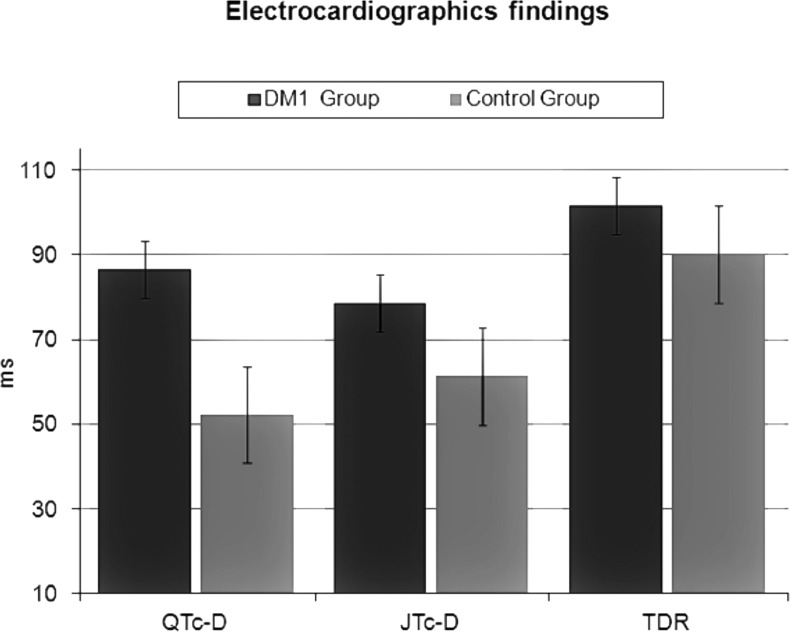
Compared to the controls, the DM1 group presented increased values of QTc-D (86.7 ± 40.1 vs 52.3 ± 11.9 ms; p = 0.03), JTc-D (78.6 ± 31.3 vs 61.3 ± 10.2 ms; p = 0.001) and TDR (101.6 ± 18.06 vs 90.1 ± 14.3 ms; p = 0.004) suggesting a significant increase in regional and transmural heterogeneity of the ventricular repolarization in these patients, despite a preserved systolic and diastolic cardiac function.
Key words: QTc dispersion, JTc dispersion, myotonic dystrophy, sudden cardiac death, repolarization, arrhythmias
Introduction
Myotonic dystrophy type 1 (DM1), or Steinert disease, is an autosomal dominant disorder with an estimated incidence of 1:8000 births, caused by an abnormal expansion of an unstable trinucleotide repeat in the 3' untranslated region of DMPK gene on chromosome 19 (1). DM1 is characterized by highly variable clinical manifestations that affect specific tissues, such as distal limb and facial muscles, smooth muscles (gastrointestinal tract, uterus), the eye (primarily the lens), the brain (especially the anterior temporal and frontal lobes), and the endocrine function (testosterone deficiency, abnormal growth hormone regulation, insulin resistance, thyroid dysfunction). Cardiac involvement is noticed in about 80% of cases, often preceding the skeletal muscle one (2), especially in men (3). Heart block is the first and most clinically significant cardiac disease in this group of patients and it is related to fibrosis of the conduction system and fatty infiltration of the His bundle (4). Heart failure occurs late in the course of the disease as the final stage of a progressive dilated cardiomyopathy. In muscular dystrophies with rapid evolution such as Duchenne muscular dystrophy, the treatment with steroids is currently considered "the gold standard" therapy able to delay the progression of the myocardial fibrosis (5); however some studies on animal models demonstrated an harmful effect of long-term deflazacort treatment on the phenotype rescued by gene therapy (6). An early onset of heart failure may occur in relation to the electromechanical delay caused by both intra- and inter-ventricular asynchrony, successfully treated with cardiac resynchronization therapy (7, 8). Ventricular arrhythmias are common findings in muscular dystrophies (9, 10). In patients with DM1, sudden cardiac death (SCD) is attributed not only to atrio-ventricular blocks, but also to the development of life-threatening arrhythmias which may occur even in the presence of normal left ventricular systolic function (11). SCD in patients with DM1 has not received sufficient recognition in the literature, and its mechanism remains of great interest. QTc dispersion (QTc-D), JTc dispersion (JTc-D) and transmural dispersion of repolarization (TDR) have been proposed as noninvasive methods to measure the regional and transmural heterogeneity of ventricular repolarization (12). Aim of the present study was to investigate the heterogeneity of regional and transmural ventricular repolarization in DM1 patients with preserved cardiac systolic function, by examining the above mentioned electrocardiographic parameters (QTc-D, JTc-D and TDR).
Patients and methods
Patients selection
Among the 247 DM1 patients regularly followed at the Cardiomyology and Medical Genetics of the Second University of Naples, 50 (29M:21F) – with a mean age of 44 ± 5 years and preserved cardiac systolic function, were consecutively enrolled to participate in the study. Fifty sex- and age-matched healthy subjects were also recruited as controls. Patients with a history of hypertension (systolic/ diastolic blood pressure > 140/90 mmHg), diabetes or impaired glucose tolerance (IGT), anaemia, electrolyte imbalance, valvular heart disease, heart failure, coronary artery disease, bundle branch block or atrio-ventricular conduction abnormalities, and previous arrhythmic episodes were excluded from the study.
All patients were in sinus rhythm and not taking medications known to affect electrocardiographic intervals such as antiarrhythmic agents, tricyclic antidepressants, antihistaminics or antipsychotics.
The diagnosis of Steinert disease, firstly based on family history and clinical evaluation, was subsequently confirmed by evaluating the CTG triplet expansion. The study was conducted according to the declaration of Helsinki.
Study protocol
Medical history, physical examination, anthropometric evaluation, 12-lead surface ECG, 2D color Doppler echocardiogram and ECG Holter monitoring were performed in all patients, who were rested for at least 15 min before cardiovascular assessments, including electrocardiography and echocardiography.
Electrocardiographic measurements
All subjects underwent a standard 12-lead body surface ECG, recorded at a paper speed of 50 mm/s and a gain of 10 mm/mV in the supine position, and were invited to breath freely but not to speak during the ECG recording. To avoid diurnal variations, the ECG recordings were generally performed between 9:00 to 10:00 a.m. The analysis of the tracing was performed by the same investigator, who ignored the subjects' clinic status. ECGs were transferred to a personal computer by an optical scanner and then magnified 400 times by Adobe Photoshop software (Adobe Systems Inc., San Jose, CA). QRS duration, QT interval and JT interval were evaluated with the use of a computer software (Configurable Measurement System) using digitizer 34180 (Calcomp, Anaheim, CA, USA). The variability of the measurements was 0.36 ± 4 ms (not statistically significant). In each electrocardiogram lead, the analysis included 3 consecutive heart cycles, whenever possible. Leads were excluded from the analysis when the end of the T-wave was not clearly distinguishable, or in case of poor quality signal. The QRS interval was measured from the start of the Q wave, or, in the absence of the Q wave, from the start of R wave to the end of S wave (return to the isoelectric line). The QT interval was measured from the initial deflection of the QRS complex to the end of the T wave (return to the isoelectric line). In case of T waves not reliably determined, isoelectric or of very low amplitude, the measurements were not done and the leads excluded from the analysis. When the U wave was present, the QT was measured to the nadir of the curve between the T and U waves. QTd was determined as the difference between the maximal and minimal QT value in all leads (13). The JT interval was derived by subtracting the QRS duration from the QT interval. JTd was defined as the difference between the maximal and the minimal JT value in all leads. All measurements were corrected for heart rate using the Bazett's formula (QTc = QT/√RR; JTc = JT/√RR). TDR was defined as the interval between the peak and the end of the T-wave (14, 15). For the present study, we considered only the values of TDR measured in the precordial leads, because it has been suggested that these leads more accurately reflect transmural dispersion of repolarization (16).
Echocardiographic measurements
All echocardiographic examinations were performed using a standard ultrasound machine with a 3.5-MHz phased-array probe (M3S). All patients were examined in the left lateral and supine positions by precordial Mmode, 2-Dimensional and Doppler echocardiography. One lead ECG was recorded continuously. Left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), interventricular septum end-diastolic thickness (IVSEDT) and left ventricular posterior wall end-diastolic thickness (LVPWEDT) were measured from M-mode in the parasternal long-axis views, according to the SOPs of the American Society of Echocardiography. Left ventricular mass (LVM) was calculated by using the Devereux's formula, and indexed for body surface area and height. Left atrium diameter (LAD) was measured during the systole along the parasternal long-axis view from the 2-dimensional guided M-mode tracing. Left Atrial (LA) length was measured from the apical 4-chamber view during systole. The maximum LA volume (LAV) was calculated from the apical 4- and 2-chamber zoomed views using the biplane method of disks. The ejection fraction (EF) was measured using a modified Simpson biplane method. Each representative value was obtained by the average of 3 consecutive measurements. The pulsed-wave Doppler examination was performed to obtain the following indices of Left Ventricle (LV) diastolic function: peak mitral inflow velocities at early (E) and late (A) diastole and E/A ratio. The average values of these indices, obtained by 5 consecutive cardiac cycles, were used for the analysis.
Statistical analysis
The continuous variables are expressed as mean ± standard deviation. The statistical analysis was performed using the Student's t-test for unpaired data. P-values < 0.05 were considered as statistically significant. The association between two variables was investigated by the Pearson's simple correlation. The analyses were performed using the SPSS 11.0 software for Windows SPSS Inc. (Chicago, IL, USA).
Results
The clinical and echocardiographic characteristics of the study population are summarized in Table 1. The DM1 group did not significantly differ from the healthy control group in body mass index (BMI), heart rate or blood pressure. No significant differences in LVPWEDT, IVSEDT, LVEDD, LVESD, LVM/height, LV shortening fraction (SF), LVEF and E wave, A wave or E/A ratio were also observed between the two groups.
Table 1.
Clinical and echocardiographic characteristics of the study population.
| DM1 patients |
Control group |
P | |
|---|---|---|---|
| Patients (n) | 50 | 50 | |
| Age (years) | 44 ± 5 | 44 ± 5 | n.s. |
| BMI (Kg/m2) | 21 ± 4 | 20 ± 5 | 0.2 |
| Sex (male/female) | 29/21 | 29/21 | |
| SBP (mmHg) | 120.5 ± 11 | 119 ± 13 | 0.5 |
| DBP (mmHg) | 71.7 ± 6 | 68.5 ± 9 | 0.3 |
| HR (bpm) | 75.8 ± 4.2 | 74.9 ± 5.5 | 0.3 |
| PR interval (ms) | 188 ± 11 | 179 ± 18 | 0.2 |
| QRS duration (ms) | 92 ± 13 | 95 ± 10 | 0.3 |
| EF (%) | 60.4 ± 7.1 | 62.6 ± 4.2 | 0.2 |
| SF (%) | 33.3 ± 5.2 | 33.8 ± 4.3 | 0.3 |
| LVEDD (mm) | 43.5 ± 8.2 | 43.3 ± 6.4 | 0.2 |
| LVESD (mm) | 25.3 ± 3.1 | 26.3 ± 2.7 | 0.4 |
| IVSEDT (mm) | 8.7 ± 1.5 | 9 ± 1.2 | 0.5 |
| LVPWEDT (mm) | 9.7 ± 1.3 | 8.9 ± 1.8 | 0.3 |
| LVM/H 2.7 (g/m 2.7) | 35.5 ± 9 | 32.4 ± 8 | 0.2 |
| E wave (cm/s) | 78.3 ± 11.3 | 80.3 ± 14.5 | 0.2 |
| A wave (cm/s) | 56.4 ± 7.3 | 57.5 ± 8.5 | 0.4 |
| E/A ratio | 1.4 ± 0.5 | 1.5 ± 0.6 | 0.3 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; EF: ejection fraction; SF: shortening fraction; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; IVSEDT: interventricular septal end diastolic thickness; LVPWEDT: left ventricular posterior wall end diastolic thickness; LVM/H: left ventricular mass/height.
The electrocardiographic characteristics of the study population are shown in Table 2. Compared to the healthy controls, patients with DM1 presented increased values of QTc-D (86.7 ± 40.1 vs 52.3 ± 11.9 ms; p = 0.03), JTc-D (78.6 ± 31.3 vs 61.3 ± 10.2 ms; p = 0.001) and TDR (101.6 ± 18.06 vs 90.1 ± 14.3 ms; p = 0.004) (Fig. 1). The intra-observer variability of QTc-D, JTc-D and TDR measurements was 7 ± 5 ms, 5 ± 2 ms, and 4 ± 2 ms, respectively. No statistically significant correlation was found between the parameters QTc-D, JTc-D, TDR, BMI (p = 0.3), LVM (p = 0.4) and EF (p = 0.2).
Table 2.
Electrocardiographic characteristics of the study population.
| Parameters | MD1 group | Control group | P |
|---|---|---|---|
| HR (bpm) | 75.8 ± 4.2 | 74.9 ± 5.5 | 0.3 |
| QRS max (ms) | 120 ± 5.4 | 108.7 ± 5.5 | 0.5 |
| QRS min (ms) | 87.6 ± 21.3 | 69.5 ± 8.4 | 0.2 |
| QTc max (ms) | 401.4 ± 43.5 | 406.1 ± 47.1 | 0.4 |
| QTc min (ms) | 377.9 ± 70.8 | 382.5 ± 29.3 | 0.7 |
| QTc-D (ms) | 86.7 ± 40.1 | 52.3 ± 11.9 | 0.03 |
| JTc max (ms) | 345.3 ± 26.05 | 338.5 ± 25.4 | 0.2 |
| JTc min (ms) | 251.5 ± 20.6 | 259.5 ± 15.5 | 0.7 |
| JTc-D (ms) | 78.6 ± 31.3 | 61.3 ± 10.2 | 0.001 |
| TDR (ms) | 101.6 ± 18.06 | 90.1 ± 14.3 | 0.004 |
Figure 1.
Differences (mean values) in dispersion of ventricular repolarization (QTc-d; JTc-d; TDR) between the DM1 group and the healthy control group.
Discussion
Cardiac arrhythmias
Conduction defects are the most prevalent cardiac abnormalities observed in patients with DM1, and occur in 40% of them. Type 1 atrio-ventricular block has the highest prevalence (28.2%), followed by left bundle branch block (5,7%) and right bundle branch block (4.4%). The prevalence of a QRS > 120 ms and a QTc > 440 ms is 19.9% and 22%, respectively (17). Ventricular premature complex is the most prevalent arrhythmia (14.6%). Ventricular tachycardia (VT) and ventricular fibrillation (VF) may also occur. Paroxysmal supra-ventricular tachy-arrhythmias such as atrial fibrillation, atrial flutter, atrial tachycardia are a common finding on 12 lead ECG or 24 hour Holter monitoring, with a prevalence up to 25% in patients, often asymptomatic. Atrial tachycardias are observed in up to 7.3% of patients, both as unsustained and sustained forms. Atrial fibrillation/flutter (AF/AFl) frequently occurs with percentages up to 17% (18, 19).
We have previously shown that AF episodes are more frequently observed in DM1 patients who underwent pacemaker implantation, with a high percentage of right ventricular pacing and a low percentage of atrial stimulation (20). We have also observed that the right atrial septal stimulation in the Bachmann's bundle region is a safe and feasible procedure (21); in fact it allows less atrial pacing and sensing defects (22) as far as less R-wave oversensing on the atrial lead (23), compared to the right atrial appendage stimulation. However it is unable to prevent paroxysmal episodes of AF (24). Moreover, we have shown that the atrial pacing is an efficient algorithm to prevent AF episodes (25, 26) and to reduce AF burden (27) in patients with atrioventricular conduction disorders implanted with a dual-chamber pacemaker, as it is able to prevent the onset and perpetuation of atrial fibrillation and to reduce the number of atrial premature beats and P-wave dispersion (PD) (28).
Indexes of electrocardiographic arrhythmic risk
Compared to other cardiological conditions or cardiomyopathies, little is known about the mechanisms and electrocardiographic predictors of ventricular and supraventricular tachyarrhythmias in DM1 patients (29-49).
P wave dispersion (PD), a non invasive indicator of intra-atrial conduction heterogeneity producing the substrate for the re-entry known as a pathophysiological mechanism of atrial fibrillation, has been evaluated in conditions such as obesity (30), beta-thalassemia major (31, 32) and Emery-Dreifuss muscular dystrophy (33). We have recently shown (34) that DM1 patients with AF episodes have a statistically significant increase in PD and Pmax compared to those without arrhythmias, confirming that P-wave dispersion may be a simple electrocardiographic parameter to identify patients at high risk of atrial fibrillation.
QTc dispersion (QTcD), JTc dispersion (JTcD) and TDR have been proposed as noninvasive methods to evaluate the transmural and regional heterogeneity of ventricular repolarization.
An increased dispersion of ventricular repolarization is considered to provide the electrophysiological substrate for life-threatening ventricular arrhythmias in several clinical conditions such as dilated cardiomyopathy (35), obesity (36, 37), beta-thalassemia major (38), severe aortic coarctation (39, 40) and Emery-Dreifuss muscular dystrophy (10, 41, 42).
Park et al. (43) suggested that the high incidence of SCD observed in the DM1 population is associated with prolonged QTc intervals in these patients. Magrì et al. (44) showed a significant difference in the QT variability index (QTVI) between DM1 patients and healthy controls; according to their results, QTVI and age are independently associated with PR interval and CTG repeats.
Heart Rate Variability (HRV) is a reliable index to assess sympathovagal balance, used to stratify the arrhythmic risk in several clinical conditions (45-53); however previous studies on autonomic modulation of heart rate in DM1 patients have obtained conflicting results (54-57).
The atrial electromechanical delay (AEMD) duration is the sum of impulse propagation from sinus node to the atria and the atrial electromechanical coupling duration (58). Previous studies evaluated the predictive role of intra-left atrial electromechanical delay in the recurrence of paroxysmal atrial fibrillation in some clinical conditions (59-62). In a recent study evaluating the AEMD in a DM1 population with normal cardiac function and its relationship with the AF onset, intra-left-AEMD and inter-AEMD were found to be independent predictors of AF-onset; in particular a cut-off value of 39.2 ms for intra-left-AEMD had a sensitivity and a specificity of 90% in identifying patients with AF-risk who need a careful cardiac monitoring (63-64). Though it is clear that the arrhythmic risk has a relevant role in the prognosis of DM1 patients, however little is still known about the electrophysiological substrate of these events and what noninvasive parameters are useful to stratify this risk (65-69).
Limitations of the study
As 12-lead surface ECG gives an incomplete picture of cardiac electric activity compared to body surface mapping or vector cardiography, QTd could not be a true manifestation of the local repolarization heterogeneity. Furthermore though QT interval and JT interval were measured on 12-lead ECGs through a computer software and digitized by an experienced cardiologist, however, in the absence of indisputable generally accepted criteria for the definition of the end of T wave, some degree of error in measurements can be occurred.
Conclusions
The present study showed a significant increase of regional and transmural heterogeneity of the ventricular repolarization in patients with DM1, despite a normal systolic and diastolic function. These results suggest that diffuse fibrosis and fat infiltration of initially unaffected myocardial areas may increase ventricular electrical instability and favor the onset of ventricular malignant tachy-arrhythmias and sudden cardiac death, even before the impairment of cardiac function. The results also confirm the needs of a continuous cardiological follow-up in these patients.
References
- 1.Walton JN. Clinical examination of the neuromuscular system. In: Walton JN, editor. Disorders of Voluntary Muscle. London: Churchill Livingstone; 1981. [Google Scholar]
- 2.Nigro G, Papa AA, Politano L. The heart and cardiac pacing in Steinert disease. Acta Myol. 2012;31:110–116. [PMC free article] [PubMed] [Google Scholar]
- 3.Cudia P, Bernasconi P, Chiodelli R, et al. Risk of arrhythmia in type I myotonic dystrophy: the role of clinical and genetic variables. J Neurol Neurosurg Psychiatry. 2009;80:790–793. doi: 10.1136/jnnp.2008.162594. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen HH, Wolfe JT, III, Holmes DR, Jr, et al. Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J Am Coll Cardiol. 1988;11:662–671. doi: 10.1016/0735-1097(88)91547-1. [DOI] [PubMed] [Google Scholar]
- 5.Nigro G, Politano L, Passamano L, et al. Cardiac treatment in neuro- muscular diseases. Acta Myol. 2006;25:119–123. [PubMed] [Google Scholar]
- 6.Rotundo IL, Faraso S, Leonibus E, et al. Worsening of cardiomyopathy using deflazacort in an animal model rescued by gene therapy. PLoS One. 2011;6:e24729–e24729. doi: 10.1371/journal.pone.0024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo V, Rago A, D'Andrea A, et al. Early onset "electrical" heart failure in myotonic dystrophy type 1 patient: the role of ICD biventricular pacing. Anadolu Kardiyol Derg. 2012;12:517–519. doi: 10.5152/akd.2012.161. [DOI] [PubMed] [Google Scholar]
- 8.Russo V, Rago A, Papa AA, et al. Cardiac resynchronization improves heart failure in one patient with myotonic dystrophy type 1. A case report. Acta Myol. 2012;31:154–155. [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro G, Russo V, Ventriglia VM, et al. Early onset of cardiomyopathy and primary prevention of sudden death in X-linked Emery- Dreifuss muscular dystrophy. Neuromuscul Disord. 2010;20:174–177. doi: 10.1016/j.nmd.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Russo V, Nigro G. ICD role in preventing sudden cardiac death in Emery-Dreifuss muscular dystrophy with preserved myocardial function: 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Europace. 2015;172:337–337. doi: 10.1093/europace/euu146. [DOI] [PubMed] [Google Scholar]
- 11.Groh VJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CS, Munakata K, Reddy CP, et al. Characteristics and possible mechanisms of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1357. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 13.Bruyne MC, Hoes AW, Kors JA, et al. QT dispersion predicts cardiac mortality in the elderly: the Rotterdam Study. Circulation. 1998;97:467–472. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 14.Fuller MS, Sandor G, Punske P, et al. Estimates of repolarization dispersion from electrocardiographic measurements. Circulation. 2000;102:685–691. doi: 10.1161/01.cir.102.6.685. [DOI] [PubMed] [Google Scholar]
- 15.Antzelevitch C, Shimizu W, Yan GX, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 2001;12:1369–1378. doi: 10.1046/j.1540-8167.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 17.Petri H, Vissing J, Witting N, et al. Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol. 2012;160:82–88. doi: 10.1016/j.ijcard.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Brembilla-Perrot B, Schwartz J, Huttin O, et al. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in myotonic dystrophy. PACE. 2014;37:329–335. doi: 10.1111/pace.12260. [DOI] [PubMed] [Google Scholar]
- 19.Stabile G, Russo V, Rapacciuolo A, et al. Transesophageal echocardiograpy in patients with persistent atrial fibrillation undergoing electrical cardioversion on new oral anticoagulants: a multi center registry. Int J Cardiol. 2015;184:283–284. doi: 10.1016/j.ijcard.2015.02.075. [DOI] [PubMed] [Google Scholar]
- 20.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients? Kardiol Pol. 2013;71:1147–1153. doi: 10.5603/KP.2013.0295. [DOI] [PubMed] [Google Scholar]
- 21.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in myotonic dystrophy patients The role of Bachmann's Bundle stimulation. PACE. 2008;31:1463–1466. doi: 10.1111/j.1540-8159.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 22.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann’s bundle stimulation: a two year comparative study of electrical parameters in myotonic dystrophy type 1 patients. PACE. 2009;32:1192–1197. doi: 10.1111/j.1540-8159.2009.02464.x. [DOI] [PubMed] [Google Scholar]
- 23.Nigro G, Russo V, Politano L, et al. Does Bachmann's bundle pacing prevent atrial fibrillation in myotonic dystrophy type 1 patients? A 12 months follow-up study. Europace. 2010;12:1219–1223. doi: 10.1093/europace/euq170. [DOI] [PubMed] [Google Scholar]
- 24.Russo V, Nigro G, Papa AA, et al. Far field R-wave sensing in myotonic dystrophy type 1: right atrial appendage versus Bachmann's bundle region lead placement. Acta Myol. 2014;33:94–99. [PMC free article] [PubMed] [Google Scholar]
- 25.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: a prospective, randomized, single-bind cross over study. Europace. 2012;14:486–489. doi: 10.1093/europace/eur373. [DOI] [PubMed] [Google Scholar]
- 26.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a long term follow-up study. Acta Myol. 2012;31:139–143. [PMC free article] [PubMed] [Google Scholar]
- 27.Russo V, Nigro G, Rago A, et al. Atrial fibrillation burden in myotonic dystrophy type 1 patients implanted with dual chamber pacemaker: the efficacy of the overdrive atrial algorithm at 2 year follow-up. Acta Myol. 2013;32:142–147. [PMC free article] [PubMed] [Google Scholar]
- 28.Russo V, Nigro G, Meo F, et al. The effect of atrial preference pacing on atrial fibrillation electrophysiological substrate in myotonic dystrophy type 1 population. Acta Myol. 2014;33:127–135. [PMC free article] [PubMed] [Google Scholar]
- 29.Russo V, Puzio GF, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed. 2006;77:30–32. [PubMed] [Google Scholar]
- 30.Russo V, Ammendola E, Crescenzo I, et al. Severe obesity and P-wave dispersion: the effect of surgically induced weight loss. Obes Surg. 2008;18:90–96. doi: 10.1007/s11695-007-9340-7. [DOI] [PubMed] [Google Scholar]
- 31.Russo V, Rago A, Pannone B, et al. Early electrocardiographic evaluation of atrial fibrillation risk in beta thalassemia major patients. Int J Hematol. 2011;93:446–451. doi: 10.1007/s12185-011-0801-3. [DOI] [PubMed] [Google Scholar]
- 32.Russo V, Rago A, Pannone B, et al. Atrial fibrillation and Beta Thalassemia Major: the predictive role of the 12-lead electrocardiogram analysis. Indian Pacing Electrophysiol J. 2014;14:121–132. doi: 10.1016/s0972-6292(16)30753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo V, Rago A, Palladino A, et al. P wave duration and dispersion in Emery-Dreifuss muscolar distrophy. J Investig Med. 2011;59:1151–1154. doi: 10.2310/JIM.0b013e31822cf97a. [DOI] [PubMed] [Google Scholar]
- 34.Russo V, Meo F, Rago A, et al. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci. 2015;19:1241–1248. [PubMed] [Google Scholar]
- 35.Santangelo L, Ammendola E, Russo V, et al. Influence of biventricular pacing on myocardial dispersion of repolarization in dilated cardiomyopathy patients. Europace. 2006;8:502–505. doi: 10.1093/europace/eul054. [DOI] [PubMed] [Google Scholar]
- 36.Russo V, Ammendola E, Crescenzo I, et al. Effect of weight loss following bariatric surgery on myocardial dispersion of repolarization in morbidly obese patients. Obes Surg. 2007;17:857–865. doi: 10.1007/s11695-007-9160-9. [DOI] [PubMed] [Google Scholar]
- 37.Nigro G, Russo V, Salvo G, et al. Increased heterogeneity of ventricular repolarization in obese non hypertensive children. PACE. 2010;33:1533–1539. doi: 10.1111/j.1540-8159.2010.02889.x. [DOI] [PubMed] [Google Scholar]
- 38.Russo V, Rago A, Pannone B, et al. Dispersion of repolarization and beta thalassemia major: the prognostic role of QT and JT dispersion for identifying the high risk patients for sudden death. Eur J Haematol. 2011;86:324–331. doi: 10.1111/j.1600-0609.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 39.Nigro G, Russo V, Rago A, et al. The effect of aortic coarctation surgical repair on QTc and JTc dispersion in severe aortic coarctation newborns: a short-term follow-up study. Physiol Res. 2014;63:27–33. doi: 10.33549/physiolres.932491. [DOI] [PubMed] [Google Scholar]
- 40.Nigro G, Russo V, Rago A, et al. Heterogeneity of ventricular repolarization in newborns with severe aortic coarctation. Pediatr Cardiol. 2012;33:302–306. doi: 10.1007/s00246-011-0132-4. [DOI] [PubMed] [Google Scholar]
- 41.Russo V, Rago A, Politano L, et al. Increased dispersion of ventricular repolarization in Emery-Dreifuss muscular dystrophy patients. Med Sci Monit. 2012;18:CR643–CR647. doi: 10.12659/MSM.883541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nigro G, Russo V, Rago A, et al. Regional and transmural dispersion of repolarisation in patients with Emery-Dreifuss muscular dystrophy. Kardiol Pol. 2012;70:1154–1159. [PubMed] [Google Scholar]
- 43.Park KM, Shin KJ, Kim SE, et al. Prolonged corrected QT interval in patients with myotonic dystrophy type 1. J Clin Neurol. 2013;9:186–191. doi: 10.3988/jcn.2013.9.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magrì D, Piccirillo G, Bucci E, et al. Increased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1: beyond the cardiac conduction system. Int J Cardiol. 2012;156:259–264. doi: 10.1016/j.ijcard.2010.10.132. [DOI] [PubMed] [Google Scholar]
- 45.Russo V, Crescenzo I, Ammendola E, et al. Sympathovagal balance analysis in idiopathic postural orthostatic tachycardia syndrome. Acta Biomed. 2007;78:133–138. [PubMed] [Google Scholar]
- 46.Russo V, Nigro G, Chiara A, et al. The impact of selection criteria of obese patients on evaluation of heart rate variability following bariatric surgery weight loss. Letter to Editor. Obes Surg. 2009;19:397–398. doi: 10.1007/s11695-008-9784-4. [DOI] [PubMed] [Google Scholar]
- 47.Nigro G, Russo V, Chiara A, et al. Autonomic nervous system modulation before the onset of sustained atrioventricular nodal reentry tachycardia. Ann Noninvasive Electrocardiol. 2010;15:49–55. doi: 10.1111/j.1542-474X.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nigro G, Russo V, Rago A, et al. The main determinant of hypotension in nitroglycerine tilt-induced vasovagal syncope. PACE. 2012;35:739–748. doi: 10.1111/j.1540-8159.2012.03388.x. [DOI] [PubMed] [Google Scholar]
- 49.Russo V, Papa AA, Ciardiello C, et al. Which hemodynamic parameter predicts nitroglycerin-potentiated head-up tilt test response? PACE. 2015;38:507–513. doi: 10.1111/pace.12593. [DOI] [PubMed] [Google Scholar]
- 50.Russo V, Rago A, Papa AA, et al. The effect of dual-chamber closed-loop stimulation on syncope recurrence in healthy patients with tilt-induced vasovagal cardioinhibitory syncope: a prospective, randomised, single-bind, crossover study. Heart. 2013;99:1609–1613. doi: 10.1136/heartjnl-2013-303878. [DOI] [PubMed] [Google Scholar]
- 51.Nigro G, Russo V, Chiara A, et al. Which parameters describe the electrophysiological properties of successful slow pathway RF ablation in patients with common atrioventricular nodal reentrant tachycardia? Anadolu Kardiyol Derg. 2010;10:126–129. doi: 10.5152/akd.2010.036. [DOI] [PubMed] [Google Scholar]
- 52.Russo V, Nigro G, Calabrò R. Heart rate variability, obesity and bariatric induced weight loss: the importance of selection criteria. Letter to the Editor. Metabolism. 2008;57:622–624. doi: 10.1016/j.metabol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Ammendola E, Russo V, Politano L, et al. Is heart rate variability (HRV) a valid parameter to predict sudden death in Becker muscular dystrophy patients? Heart. 2006;92:1686–1687. doi: 10.1136/hrt.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Politano L, Palladino A, Scutifero M, et al. Usefulness of heart rate variability as a predictor of sudden cardiac death in muscular dystrophies. Acta Myol. 2008;27:114–122. [PMC free article] [PubMed] [Google Scholar]
- 55.Hardin BA, Lowe MR, Bhakta D, et al. Heart rate variability declines with increasing age and CTG repeat length in patients with myotonic dystrophy type 1. Ann Noninvasive Electrocardiol. 2003;8:227–232. doi: 10.1046/j.1542-474X.2003.08310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leo R, Rodolico C, Gregorio C, et al. Cardiovascular autonomic control in myotonic dystrophy type 1: a correlative study with clinical and genetic data. Neuromuscul Disord. 2004;14:136–141. doi: 10.1016/j.nmd.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Rakocević-Stojanović V, Milovanović B, Ivić N, et al. Cardiac autonomic nervous system in patients with myotonic dystrophy type 1. Acta Myol. 2007;26:112–114. [PMC free article] [PubMed] [Google Scholar]
- 58.Merkx KL, Vos CB, Palmans A, et al. Atrial activation time determined by transthoracic Doppler tissue imaging can be used as an estimate of thetotal duration of atrial electrical activation. J Am Soc Echocardiogr. 2005;18:940–944. doi: 10.1016/j.echo.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Calik AN, Özcan KS, Ça Da M, et al. Electromechanical delay detected by tissue Doppler echocardiography is associated with the frequency of attacks in patients with lone atrial fibrillation. Cardiol J. 2014;21:138–143. doi: 10.5603/CJ.a2013.0106. [DOI] [PubMed] [Google Scholar]
- 60.Russo V, Meo FD, Rago A, et al. Impact of continuous positive airway pressure therapy on atrial electromechanical delay in obesity- hypoventilation syndrome patients. J Cardiovasc Electrophysiol. 2016;27:327–334. doi: 10.1111/jce.12879. [DOI] [PubMed] [Google Scholar]
- 61.Russo V, Rago A, Meo F, et al. Atrial septal aneurysms and supraventricular arrhythmias: the role of atrial electromechanical delay. Echocardiography. 2015;32:1504–1514. doi: 10.1111/echo.12908. [DOI] [PubMed] [Google Scholar]
- 62.Rago A, Russo V, Papa AA, et al. The role of the atrial electromechanical delay in predicting atrial fibrillation in beta thalassemia major patients. J Interv Card Electrophysiol. 2016 Nov 16; doi: 10.1007/s10840-016-0201-y. [DOI] [PubMed] [Google Scholar]
- 63.Russo V, Rago A, Nigro G. Atrial electromechanical delay in myotonic dystrophy type 1 patients. Eur Rev Med Pharmacol Sci. 2015;19:3991–3992. [PubMed] [Google Scholar]
- 64.Russo V, Rago A, Ciardiello C, et al. The role of the atrial electromechanical delay in predicting atrial fibrillation in myotonic dystrophy type 1 patients. J Cardiovasc Electrophysiol. 2015;27:65–72. doi: 10.1111/jce.12821. [DOI] [PubMed] [Google Scholar]
- 65.Russo V, Nigro G, Papa AA, et al. Adenosine-induced sinus tachycardia in a patient with myotonic dystrophy type 1. Acta Myol. 2014;33:104–106. [PMC free article] [PubMed] [Google Scholar]
- 66.Russo V, Rago A, Meo F, et al. Ventricular fibrillation induced by coagulating mode bipolar electrocautery during pacemaker implantation in myotonic dystrophy type 1 patient. Acta Myol. 2014;33:149–151. [PMC free article] [PubMed] [Google Scholar]
- 67.Proietti R, Labos C, Davis M, et al. A systematic review and metaanalysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol. 2015;31:270–277. doi: 10.1016/j.cjca.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 68.Russo V, Rago A, Nigro G. Sudden cardiac death in neuromuscolar disorders: time to establish shared protocols for cardiac pacing. Int J Cardiol. 2016;207:284–285. doi: 10.1016/j.ijcard.2016.01.175. [DOI] [PubMed] [Google Scholar]
- 69.Dello Russo A, Mangiola F, Della Bella P, et al. Risk of arrhythmias in myotonic dystrophy: trial design of the RAMYD study. J Cardiovasc Med (Hagerstown) 2009;10:51–58. doi: 10.2459/jcm.0b013e328319bd2c. [DOI] [PubMed] [Google Scholar]