Abstract
A successful case of maximum voltage-directed cavo-tricuspid isthmus (CTI) ablation using a novel ablation catheter mapping technology in a myotonic dystrophy type I (DM1) patient is reported. The patient complained recurrent episodes of atrial flutter, revealed by the atrio-ventricular electrograms analysis during the routine pacemaker controls.
Key words: myotonic dystrophy, atrial flutter, atrial fibrillation, cavo-tricuspid isthmus ablation, microelectrodes
Introduction
Myotonic dystrophy type 1 (DM1) is an autosomal dominant disorder resulting from an amplified CTG trinucleotide repeat (> 50) in the 3-prime untranslated region of the dystrophia myotonica protein kinase gene (DMPK gene) on chromosome 19q13.3. DM1 is the most common muscular dystrophy of the adult life with an incidence of 1 in 8000 births and a worldwide prevalence ranging from 2.1 to 14.3/100.000 inhabitants (1). Cardiac involvement is noticed in about 80% of cases, often preceding the skeletal muscle one (2). Paroxysmal supraventricular tachy-arrhythmias (atrial fibrillation, atrial flutter, atrial tachycardia) represent a common finding on 12 lead electrocardiograms (ECG) or 24 hour ECG-Holter monitoring, with a prevalence up to 25% (3). The patients are often asymptomatic. Atrial tachycardias are observed in up to 7.3% of patients, both as un-sustained and sustained forms (4). Atrial fibrillation/flutter (AF/AFl) frequently occurs in DM1 patients up to 17% (3, 4), some times representing the first manifestation of the muscular dystrophy in young patients (5). AF/AFl seems to increase mortality in this population (6). We report a successful case of maximum voltage-directed cavo-tricuspid isthmus (CTI) ablation using a novel ablation catheter mapping technology in a DM1 patient with recurrent episodes of typical atrial flutter.
Description
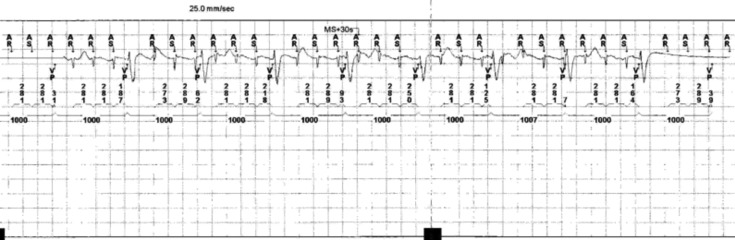
A 71-year-old woman with DM1 and arterial hypertension, with previously dual chambers pacemaker implantation for advanced atrio-ventricular block, was referred to our Division for device check and cardiologic therapy optimization. She never reported palpitations or arrhythmias-related symptoms, she didn't take antiarrhythmic medications. ECG showed a dual-chamber VDD paced rhythm at 60 bpm. Transthoracic echocardiogram showed normal left ventricular systolic function associated to grade I diastolic dysfunction. The device interrogation showed several episodes of sustained supraventricular tachycardia, with a cycle length of 289 ms (Fig. 1). AFl diagnosis was made by 24 h ECG Holter monitoring that showed the common features of typical CTI dependent atrial flutter. The patient underwent electrophysiological study (EPS) for catheter ablation of the arrhytmogenic substrate. Two decapolar diagnostic catheters were placed in the coronary sinus and in the lateral right atrium wall. An IntellaTip MiFi XP® (IntellaTip MiFi, Boston Scientific, Natick, MA) 8 mm RF catheter was used for CTI mapping and ablation (Fig. 2).
Figure 1.
Episode of sustained supraventricular tachycardia with a cycle length of 289 ms revealed by atrio-ventricular electrogram analyses during routine pacemaker control.
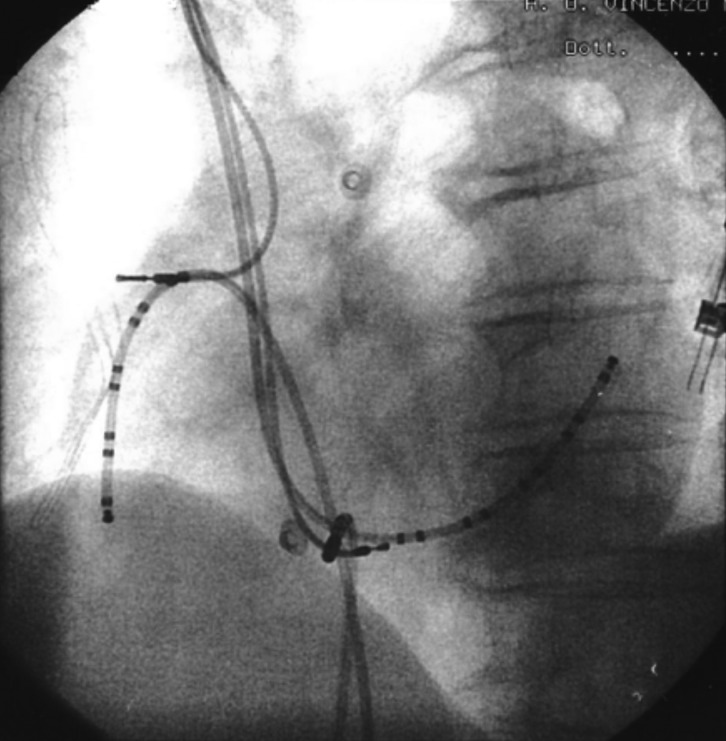
Figure 2.
Position of catheters in left anterior oblique (LAO) views during cavo-tricuspid isthmus (CTI) ablation. Two decapolar diagnostic catheters were placed in the coronary sinus and in the lateral right atrium wall. An IntellaTip MiFi XP® 8 mm RF catheter was positioned the 6 o'clock position across the CTI.
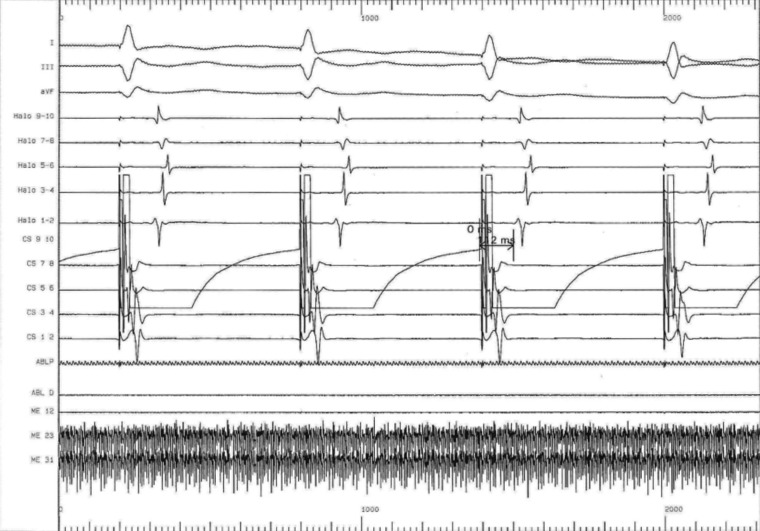
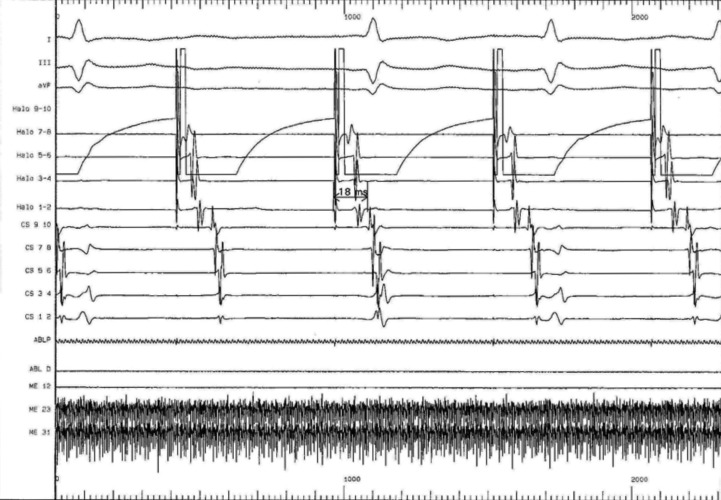
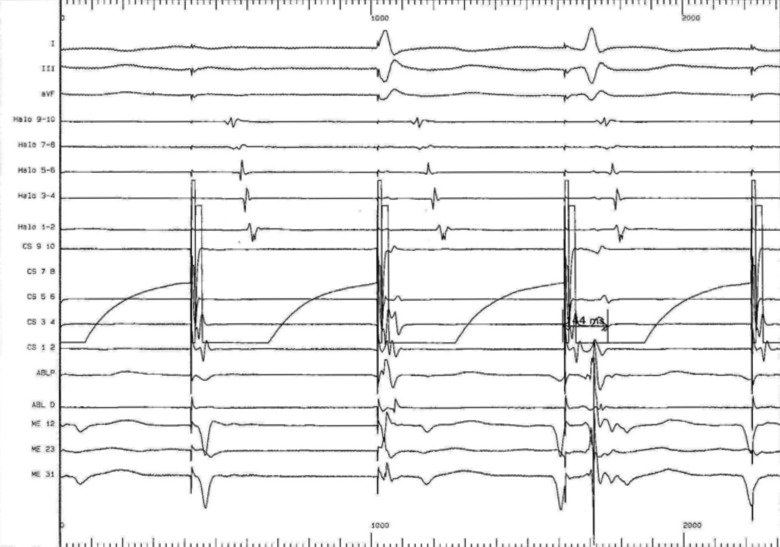
Prior to the onset of the ablation procedure, a baseline electrophysiology study was performed including the measurements of the baseline trans-isthmus interval. This was 112 ms when measured from the pacing stimulus on electrode 9/10 of the coronary sinus catheter to the atrial electrogram detected by electrode 1/2 on the Halo catheter (Fig. 3); and of 118 ms when measured from the pacing stimulus on electrode 1/2 of the HALO catheter to the atrial electrogram detected by electrode 9/10 on the coronary sinus catheter (Fig. 4). We performed a voltage-directed technique for ablating the only conducting bundles of the CTI, avoiding to ablate the intervening non-conducting fibrous tissue, according to previous experiences (7).
Figure 3.
Baseline trans-isthmus interval of 112 ms as measured from the pacing stimuli on electrode 9/10 of the coronary sinus catheter to the atrial electrogram detected by electrode 1/2 on the Halo catheter. In basal conditions the electrical impulse from the coronary sinus ostium spreads with two opposite wave fronts around the tricuspid valve, counterclockwise along the interatrial septum and clockwise along the back edge of the tricuspid.
Figure 4.
Baseline pacing trans-isthmus interval of 118 ms as measured from the pacing stimuli on electrode 1/2 of the HALO catheter to the atrial electrogram detected by electrode 9/10 on the coronary sinus catheter. In basal conditions the electrical impulse from the posterolateral tricuspid spreads around the tricuspid with two wave fronts, one in a clockwise direction (along the side wall) and another counterclockwise (along the rear wall and medial).
We firstly mapped across the CTI by pulling back from the tricuspid valve annulus to the inferior vena cava at the 6 o'clock position in left anterior oblique (LAO) projection. The signal voltage was noted during this pullback, and the highest voltage was identified. We returned to this location and have ablated it with an 8 mm dry tip for 40-60 s (60-70 W, 60-70 uC); this process was then repeated and continued until all sharp atrial electrograms detected on the mini-electrodes along the CTI were eliminated and only low-voltage double potentials were visible. A total of 3 radiofrequency ablation lesions were applied.
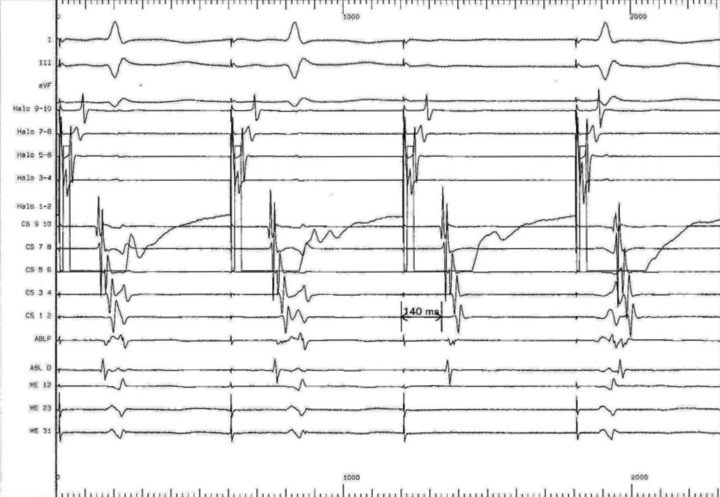
We performed a post-procedure evaluation of pacing intervals from both electrode 9/10 of coronary sinus catheter and electrode 1/2 of the HALO catheter. We showed an increased trans-isthmus interval measured by both pacing from coronary sinus electrode 9/10 to the atrial electrogram detected by electrode 1/2 on the Halo catheter and from HALO electrode 1/2 to the atrial electrogram detected by electrode 9/10 on the coronary sinus catheter, of 144 and 140 ms, respectively confirming the bidirectional block (Figs. 5, 6).
Figure 5.
Post-procedure increased trans-isthmus interval as measured from the pacing stimuli on coronary sinus electrode 9/10 (144 ms) to the atrial electrogram detected by electrode 1/2 on the Halo catheter The electrical impulse is forced to activate the atrial peritricuspidal myocardium only counterclockwise.
Figure 6.
Post-procedure increased trans-isthmus interval as measured from HALO electrode (140 ms). The electrical impulse provided by the posterolateral wall side is forced to activate the peritricuspidal myocardium only clockwise.
The procedure was completed without complications. Six months later, the ambulatory interrogation of the device did not reveal any recurrent atrial arrhythmias; so no long-term anticoagulation therapy was necessary.
Discussion
Cardiac involvement in DM1 patients occurs as a degenerative process with progressive fibrosis and fatty replacement of the myocardium, not limited to the specialized conduction system, but involving also areas – initially unaffected – of the atrial myocardium (8). This anatomopathological substrate, causing the discontinuous and inhomogeneous propagation of sinus impulses and the prolongation of atrial conduction time, may facilitate the onset and the perpetuation of atrial arrhythmias in DM1 patients (9-12), as usually it happens in other clinical conditions (13-18). The modern pacemakers, including detailed diagnostic functions and therapeutic algorithms, may facilitate the diagnosis and management of frequent paroxysmal atrial tachy-arrhythmias that may remain undetected during conventional clinical follow-up (19-29). Radiofrequency ablation of typical atrial flutter is a very successful procedure with reported acute success rates of 90-95%. The conventional ablation technique requires creation of a complete line of conduction block across CTI, from the tricuspid valve to the inferior vena cava. Such an approach has a high overall success rate but results in variable, sometimes lengthy procedure times, mainly due to anatomic variation (30-35). The "maximum voltage-guided" (MVG) ablation technique targets high-voltage isthmus electrograms to ablate the functionally important anatomic muscle bundles alone, without drawing a complete anatomic line. Previous reports suggest that this method reduces the mean ablation time and shortens the procedure duration (36, 37). The ablation tip of IntellaTip MiFi catheter may enhance the available data for such a signal dependent technique. In this catheter, bipolar signals can be recorded between the three 0.8 mm-wide electrodes that are arranged radially 1.3 mm from the end of the catheter, alongside the standard distal and proximal bipolar recordings. The use of the Intellatip MiFi radiofrequency ablation catheter has been suggested to improve mapping resolution with a more precise localization of the points with the highest amplitude, potentially allowing less but more effective RF application in less time, in a technique that relies on signal amplitude (38). These features make the maximum voltage guided cavo-tricuspid isthmus ablation using a novel catheter with three mini electrodes within the ablation tip (IntellaTip MiFi, Boston Scientific, Boston, MA) particularly suitable for efficacy and safety during the ablation procedure in DM1 patients with an higher potential risk of complications for the diffuse atrial fibrosis. This novel ablative approach is particularly useful in this category of patients for their inability to maintain for long time the supine position.
Conclusions
We showed a successful case of maximum voltageguided cavo-tricuspid isthmus ablation using a novel catheter with mini electrodes within the ablation tip in a myotonic dystrophy type 1 patient with typical atrial flutter episodes revealed by atrio-ventricular electrograms analysis during the routine pacemaker controls.
References
- 1.Nigro G, Comi LI, Politano L, Nigro Ge. Cardiomyopathies associated with muscular dystrophies. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd ed. New York: McGraw-Hill; 2004. pp. 1239–1256. Chapter 42. [Google Scholar]
- 2.Petri H, Vissing J, Witting N, et al. Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol. 2012;160:82–88. doi: 10.1016/j.ijcard.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Pelargonio G, Dello Russo A, Sanna T, et al. Myotonic dystrophy and the heart. Heart. 2002;88:665–670. doi: 10.1136/heart.88.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dello Russo A, Mangiola F, Della Bella P, et al. Risk of arrhythmias in myotonic dystrophy: trial design of the RAMYD study. J Cardiovasc Med (Hagerstown) 2009;10:51–58. doi: 10.2459/jcm.0b013e328319bd2c. [DOI] [PubMed] [Google Scholar]
- 5.Cudia P, Bernasconi P, Chiodelli R, et al. Risk of arrhythmia in type I myotonic dystrophy: the role of clinical and genetic variables. J Neurol Neurosurg Psychiatry. 2009;80:790–793. doi: 10.1136/jnnp.2008.162594. [DOI] [PubMed] [Google Scholar]
- 6.Petri H, Witting N, Ersbøll MK, et al. High prevalence of cardiac involvement in patients with myotonic dystrophy type 1: a crosssectional study. Int J Cardiol. 2014;174:31–36. doi: 10.1016/j.ijcard.2014.03.088. [DOI] [PubMed] [Google Scholar]
- 7.Stoyanov N, Winterfield J, Varma N, et al. Atrial arrhythmias in the young: early onset atrial arrhythmias preceding a diagnosis of a primary muscular dystrophy. Europace. 2014;16:1814–1820. doi: 10.1093/europace/euu141. [DOI] [PubMed] [Google Scholar]
- 8.Brembilla-Perrot B, Schwartz J, Huttin O, et al. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in myotonic dystrophy. Pacing Clin Electrophysiol. 2014;37:329–335. doi: 10.1111/pace.12260. [DOI] [PubMed] [Google Scholar]
- 9.Redfearn DP, Skanes AC, Gula LJ, et al. Cavotricuspid isthmus conduction is dependent on underlying anatomic bundle architecture: observations using a maximum voltage-guided ablation technique. J Cardiovasc Electrophysiol. 2006;17:832–838. doi: 10.1111/j.1540-8167.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips MF, Harper PS. Cardiac disease in myotonic dystrophy. Cardiovasc Res. 1997;33:13–22. doi: 10.1016/s0008-6363(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 11.Russo V, Meo F, Rago A, et al. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci. 2015;19:1241–1248. [PubMed] [Google Scholar]
- 12.Russo V, Rago A, Ciardiello C, et al. The role of the atrial electromechanical delay in predicting atrial fibrillation in myotonic dystrophy type 1 patients. J Cardiovasc Electrophysiol. 2016;27:65–72. doi: 10.1111/jce.12821. [DOI] [PubMed] [Google Scholar]
- 13.Russo V, Meo F, Rago A, et al. Atrial electromechanical delay in myotonic dystrophy type 1 patients. Eur Rev Med Pharmacol Sci. 2015;19:3991–3992. [PubMed] [Google Scholar]
- 14.Rago A, Russo V, Papa AA, et al. The role of the atrial electromechanical delay in predicting atrial fibrillation in beta thalassemia major patients. JICE. 2016 Nov 22; doi: 10.1007/s10840-016-0201-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Russo V, Meo F, Rago A, et al. Impact of continuous positive airway pressure therapy on atrial electromechanical delay in obesity- hypoventilation syndrome patients. J Cardiovasc Electrophysiol. 2016;27:327–334. doi: 10.1111/jce.12879. [DOI] [PubMed] [Google Scholar]
- 16.Russo V, Rago A, Meo F, et al. Atrial septal aneurysms and supraventricular arrhythmias: the role of atrial electromechanical delay. Echocardiography. 2015;32:1504–1514. doi: 10.1111/echo.12908. [DOI] [PubMed] [Google Scholar]
- 17.Russo V, Rago A, Pannone B, et al. Atrial fibrillation and beta thalassemia major: the predictive role of the 12-lead electrocardiogram analysis. Indian Pacing Electrophysiol J. 2014;25:121–132. doi: 10.1016/s0972-6292(16)30753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo V, Rago A, Palladino A, et al. P-wave duration and dispersion in patients with Emery-Dreifuss muscular dystrophy. J Investig Med. 2011;59:1151–1154. doi: 10.2310/JIM.0b013e31822cf97a. [DOI] [PubMed] [Google Scholar]
- 19.Russo V, Rago A, Pannone B, et al. Early electrocardiographic evaluation of atrial fibrillation risk in beta-thalassemia major patients. Int J Hematol. 2011;93:446–451. doi: 10.1007/s12185-011-0801-3. [DOI] [PubMed] [Google Scholar]
- 20.Russo V, Ammendola E, Crescenzo I, et al. Severe obesity and P-wave dispersion: the effect of surgically induced weight loss. Obes Surg. 2008;18:90–96. doi: 10.1007/s11695-007-9340-7. [DOI] [PubMed] [Google Scholar]
- 21.Russo V, Nigro G, Meo F, et al. The effect of atrial preference pacing on atrial fibrillation electrophysiological substrate in myotonic dystrophy type 1 population. Acta Myol. 2014;33:127–135. [PMC free article] [PubMed] [Google Scholar]
- 22.Russo V, Nigro G, Papa AA, et al. Far field R-wave sensing in myotonic dystrophy type 1: right atrial appendage versus Bachmann's bundle region lead placement. Acta Myol. 2014;33:94–99. [PMC free article] [PubMed] [Google Scholar]
- 23.Russo V, Nigro G, Rago A, et al. Atrial fibrillation burden in myotonic dystrophy type 1 patients implanted with dual chamber pacemaker: the efficacy of the overdrive atrial algorithm at 2 year follow-up. Acta Myol. 2013;32:142–147. [PMC free article] [PubMed] [Google Scholar]
- 24.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients? Kardiol Pol. 2013;71:1147–1153. doi: 10.5603/KP.2013.0295. [DOI] [PubMed] [Google Scholar]
- 25.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a long term follow-up study. Acta Myol. 2012;31:139–143. [PMC free article] [PubMed] [Google Scholar]
- 26.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: a prospective, randomized, single-bind cross-over study. Europace. 2012;14:486–489. doi: 10.1093/europace/eur373. [DOI] [PubMed] [Google Scholar]
- 27.Nigro G, Russo V, Politano L, et al. Does Bachmann's bundle pacing prevent atrial fibrillation in myotonic dystrophy type 1 patients? A 12 months follow-up study. Europace. 2010;12:1219–1223. doi: 10.1093/europace/euq170. [DOI] [PubMed] [Google Scholar]
- 28.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann’s bundle stimulation: a two-year comparative study of electrical parameters in myotonic dystrophy type-1 patients. Pacing Clin Electrophysiol. 2009;32:1191–1196. doi: 10.1111/j.1540-8159.2009.02464.x. [DOI] [PubMed] [Google Scholar]
- 29.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in myotonic dystrophy patients: the role of Bachmann's Bundle stimulation. Pacing Clin Electrophysiol. 2008;31:1463–1466. doi: 10.1111/j.1540-8159.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 30.Russo V, Rago A, Papa A, Nigro G. Cardiac resynchronization improves heart failure in one patient with myotonic dystrophy type 1. A case report. Acta Myol. 2012;31:154–155. [PMC free article] [PubMed] [Google Scholar]
- 31.Russo V, Rago A, D'Andrea A, et al. Early onset "electrical" heart failure in myotonic dystrophy type 1 patient: the role of ICD biventricular pacing. Anadolu Kardiyol Derg. 2012;12:517–519. doi: 10.5152/akd.2012.161. [DOI] [PubMed] [Google Scholar]
- 32.Heidbuchel H, Willems R, Rensburg H, et al. Right atrial angiographic evaluation of the posterior isthmus: Relevance for ablation of typical atrial flutter. Circulation. 2000;101:2178–2184. doi: 10.1161/01.cir.101.18.2178. [DOI] [PubMed] [Google Scholar]
- 33.Costa A, Cucherat M, Pichon N, et al. Comparison of the efficacy of cooled-tip and 8-mm-tip catheters for radiofrequency catheter ablation of the cavotricuspid isthmus: a meta-analysis. Pacing Clin Electrophysiol. 2005;28:1081–1087. doi: 10.1111/j.1540-8159.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 34.Morales GX, Macle L, Khairy P, et al. Adenosine testing in atrial flutter ablation: unmasking of dormant conduction across the cavotricuspid isthmus and risk of recurrence. J Cardiovasc Electrophysiol. 2013;24:995–1001. doi: 10.1111/jce.12174. [DOI] [PubMed] [Google Scholar]
- 35.Tai CT, Liu TY, Lee PC, et al. Non-contact mapping to guide radiofrequency ablation of atypical right atrial flutter. J Am Coll Cardiol. 2004;44:1080–1086. doi: 10.1016/j.jacc.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 36.Russo V, Rago A, Meo F, et al. Ventricular fibrillation induced by coagulating mode bipolar electrocautery during pacemaker implantation in myotonic dystrophy type 1 patient. Acta Myol. 2014;33:149–151. [PMC free article] [PubMed] [Google Scholar]
- 37.Russo V, Nigro G, Papa AA, et al. Adenosine-induced sinus tachycardia in a patient with Myotonic Dystrophy type 1. Acta Myol. 2014;33:104–106. [PMC free article] [PubMed] [Google Scholar]
- 38.Bauernfeind T, Kardos A, Foldesi C, et al. Assessment of the maximum voltage-guided technique for cavotricuspid isthmus ablation during ongoing atrial flutter. J Interv Card Electrophysiol. 2007;19:195–199. doi: 10.1007/s10840-007-9158-1. [DOI] [PubMed] [Google Scholar]
- 39.Gula LJ, Redfearn DP, Veenhuyzen GD, et al. Reduction in atrial flutter ablation time by targeting maximum voltage: Results of a prospective randomized clinical trial. J Cardiovasc Electrophysiol. 2009;20:1108–1111. doi: 10.1111/j.1540-8167.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Taylor M. Rapid ablation of recurrent atrial flutter using a novel ablation catheter. J Innov Cardiac Rhythm Manage. 2014;5:1808–1812. [Google Scholar]