Abstract
To test the hypothesis that the topographic organization of the connections of the optic tectum is determined during development by the "retinotopically" ordered input that it receives from the eye, we have mapped certain of the connections of the tectum in chicken embryos and chicks in which both eye rudiments were removed before the outgrowth of optic fibers. Because several of the connections of the tectum are normally organized retinotopically, we should expect, if this hypothesis were correct, that some or all of these connectional patterns would be significantly altered in such "eyeless" chickens. In fact, we have found that the connections formed between the optic tectum and two of the isthmic nuclei, with which it is reciprocally connected, show the same topographic organization in "eyeless" animals as in control chickens raised under the same conditions. This clearly indicates that the topographic organization of the chicken optic tectum is independently specified and is not contingent upon the input that it receives from the retina.
Full text
PDF

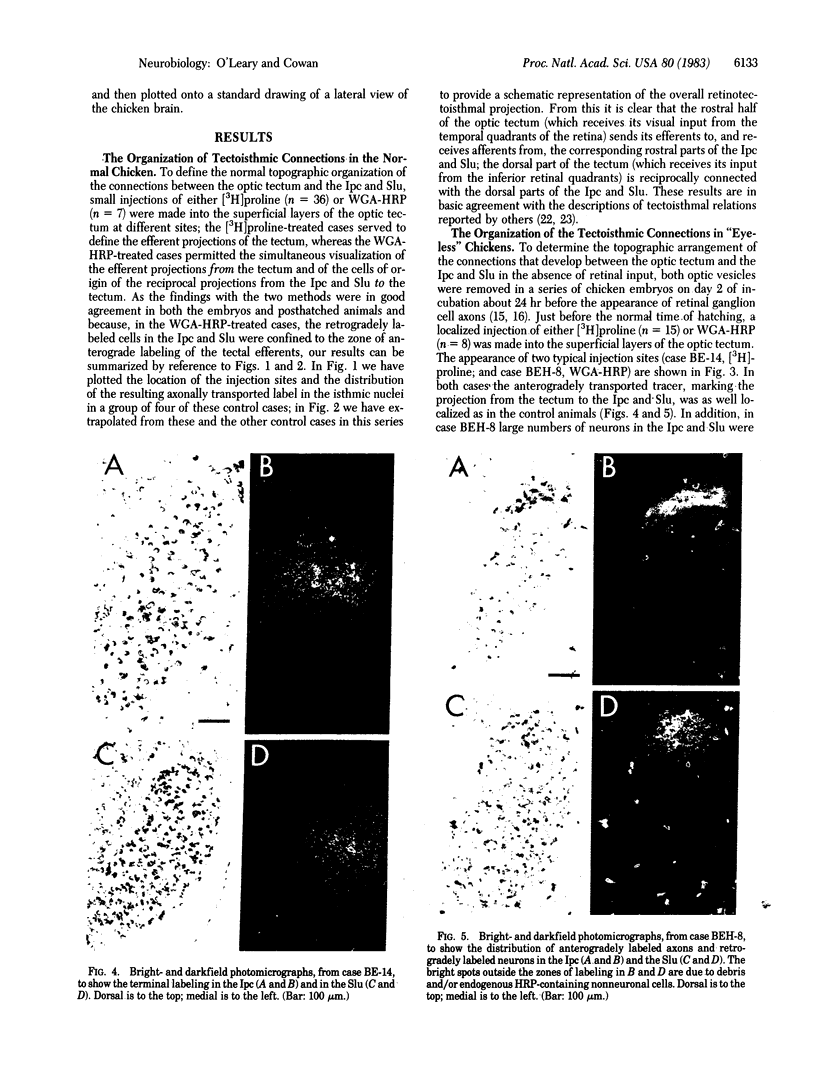


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Constantine-Paton M., Ferrari-Eastman P. Topographic and morphometric effects of bilateral embryonic eye removal on the optic tectum and nucleus isthmus of the leopard frog. J Comp Neurol. 1981 Mar 10;196(4):645–661. doi: 10.1002/cne.901960410. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Gottlieb D. I., Hendrickson A. E., Price J. L., Woolsey T. A. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972 Feb 11;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Crossland W. J., Cowan W. M., Rogers L. A., Kelly J. P. The specification of the retino-tectal projection in the chick. J Comp Neurol. 1974 May 15;155(2):127–164. doi: 10.1002/cne.901550202. [DOI] [PubMed] [Google Scholar]
- Crossland W. J., Cowan W. M., Rogers L. A. Studies on the development of the chick optic tectum. IV. An autoradiographic study of the development of retino-tectal connections. Brain Res. 1975 Jun 20;91(1):1–23. doi: 10.1016/0006-8993(75)90463-1. [DOI] [PubMed] [Google Scholar]
- Crossland W. J., Uchwat C. J. Topographic projections of the retina and optic tectum upon the ventral lateral geniculate nucleus in the chick. J Comp Neurol. 1979 May 1;185(1):87–106. doi: 10.1002/cne.901850106. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Hunt R. K. Retinotectal specificity: models and experiments in search of a mapping function. Annu Rev Neurosci. 1980;3:319–352. doi: 10.1146/annurev.ne.03.030180.001535. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Coulombre A. J. Topographical development of the ganglion cell fiber layer in the chick retina. A whole mount study. J Comp Neurol. 1972 Dec;146(4):507–518. doi: 10.1002/cne.901460406. [DOI] [PubMed] [Google Scholar]
- Goldberg S. Studies on the mechanics of development of the visual pathways in the chick embryo. Dev Biol. 1974 Jan;36(1):24–43. doi: 10.1016/0012-1606(74)90188-2. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. An abnormal retinogeniculate projection in Siamese cats. Brain Res. 1969 Aug;14(3):739–741. doi: 10.1016/0006-8993(69)90213-3. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Aberrant visual projections in the Siamese cat. J Physiol. 1971 Oct;218(1):33–62. doi: 10.1113/jphysiol.1971.sp009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. P., Künzle H. Selective uptake and transport of label within three identified neuronal systems after injection of 3H-GABA into the pigeon optic tectum: an autoradiographic and Golgi study. J Comp Neurol. 1976 Nov 15;170(2):173–189. doi: 10.1002/cne.901700204. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Levine R. L. Stability of implanted duplicate tectal positional markers serving as targets for optic axons in adult frogs. Brain Res. 1975 Jul 18;92(3):468–471. doi: 10.1016/0006-8993(75)90332-7. [DOI] [PubMed] [Google Scholar]
- Kaiserman-Abramof I. R., Graybiel A. M., Nauta W. J. The thalamic projection to cortical area 17 in a congenitally anophthalmic mouse strain. Neuroscience. 1980;5(1):41–52. doi: 10.1016/0306-4522(80)90069-x. [DOI] [PubMed] [Google Scholar]
- Keating M. J., Feldman J. D. Visual deprivation and intertectal neuronal connexions in Xenopus laevis. Proc R Soc Lond B Biol Sci. 1975 Dec 16;191(1105):467–474. doi: 10.1098/rspb.1975.0139. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Cowan W. M. Studies on the development of the chick optic tectum. 3. Effects of early eye removal. Brain Res. 1972 Jul 20;42(2):263–288. doi: 10.1016/0006-8993(72)90530-6. [DOI] [PubMed] [Google Scholar]
- Krayanek S., Goldberg S. Oriented extracellular channels and axonal guidance in the embryonic chick retina. Dev Biol. 1981 May;84(1):41–50. doi: 10.1016/0012-1606(81)90368-7. [DOI] [PubMed] [Google Scholar]
- Levine R., Jacobson M. Deployment of optic nerve fibers is determined by positional markers in the frog's tectum. Exp Neurol. 1974 Jun;43(3):527–538. doi: 10.1016/0014-4886(74)90192-7. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Romeskie M., Sharma S. C. Retinal projection to a rotated tectal reimplant following long-term tectal denervation in adult goldfish. Brain Res. 1980 Nov 10;201(1):202–205. doi: 10.1016/0006-8993(80)90786-6. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. T. Retinal fibers alter tectal positional markers during the expansion of the retinal projection in goldfish. J Comp Neurol. 1978 Jan 15;177(2):279–295. doi: 10.1002/cne.901770207. [DOI] [PubMed] [Google Scholar]
- Sharma S. C., Gaze R. M. The retinotopic organization of visual responses from tectal reimplants in adult goldfish. Arch Ital Biol. 1971 Dec;109(4):357–366. [PubMed] [Google Scholar]
- Shatz C. A comparison of visual pathways in Boston and Midwestern Siamese cats. J Comp Neurol. 1977 Jan 15;171(2):205–228. doi: 10.1002/cne.901710206. [DOI] [PubMed] [Google Scholar]
- Udin S. B., Keating M. J. Plasticity in a central nervous pathway in xenopus: anatomical changes in the isthmotectal projection after larval eye rotation. J Comp Neurol. 1981 Dec 20;203(4):575–594. doi: 10.1002/cne.902030403. [DOI] [PubMed] [Google Scholar]
- Yoon M. G. Readjustment of retinotectal projection following reimplantation of a rotated or inverted tectal tissue in adult goldfish. J Physiol. 1975 Oct;252(1):137–158. doi: 10.1113/jphysiol.1975.sp011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg C., Willshaw D. J. How to label nerve cells so that they can interconnect in an ordered fashion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5176–5178. doi: 10.1073/pnas.74.11.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]







