Abstract
Although hemorrhagic cystitis (HC) is a common complication of allogeneic hematopoietic cell transplantation (alloHCT), its risk factors and effects on survival are not well-known. We evaluated HC in a large cohort (n=1321, 2003 – 2012) receiving alloHCT from all graft sources, including umbilical cord blood (UCB). We compared HC patients with non-HC (control) patients and examined clinical variables at HC onset and resolution. Of these 1321 patients, 219 (16.6%) developed HC at a median of 22 days after alloHCT. BK viruria was detected in 90% of 109 tested HC patients. Median duration of HC was 27 days. At the time of HC diagnosis, acute graft-versus-host disease (GVHD), fever, severe thrombocytopenia, and steroid use were more frequent than at the time of HC resolution. In univariate analysis, male sex, age <20 years, myeloablative conditioning with cyclophosphamide and acute GVHD were associated with HC. In multivariate analysis, HC was significantly more common in males and HLA-mismatched UCB graft recipients. Severe grade HC (grade III–IV) was associated with increased treatment-related mortality (TRM) but not with overall survival at 1 year. HC remains hazardous and therefore better prophylaxis and early interventions to limit its severity are still needed.
Introduction
Hemorrhagic cystitis (HC) is a serious and common complication of hematopoietic cell transplantation (HCT) affecting both allogeneic (alloHCT) and autologous (alloHCT) recipients.1–4 Early urinary bleeding after transplant is usually attributed to toxic effects of the preparative regimens, while HC attributable to factors other than chemotherapy is usually noted more than 2 weeks after alloHCT 1. Many factors from toxic effects of the preparative regimens to viruses have been implicated in the etiology of HC 1, 5–12. Among viruses, polyoma BK virus, cytomegalovirus (CMV) and adenovirus are associated with HC, in both adults and children 1, 7, 13–18. Several predisposing factors have been reported including transplant type, age at transplantation, presence of graft-versus-host disease (GVHD), donor source, and conditioning regimen components and intensity 1, 12, 19–21. The effect of HC on survival remains controversial, but its morbidity can be substantial, even in survivors 10, 22.
We analyzed HC in a large cohort, including pediatric and adults, from one center. This large consecutive alloHCT cohort provided an opportunity to evaluate HC incidence and to define its risk factors in all age groups and among different graft sources. We also compared factors, including platelet count and coagulation tests, between HC onset and HC resolution to evaluate clinical factors contributing to the severity and duration of HC.
Patients and Methods
In this retrospective analysis, we reviewed the records of 1321 consecutive patients (787 male and 534 female) who underwent alloHCT at the University of Minnesota between July 2003 and March 2012. Patients were consented and treated according to protocols approved by the University of Minnesota Institutional Review Board and registered at clinicaltrials.gov. Of these 1321 patients, 219 (16.6%) developed HC. This subset was evaluated in detail to determine factors associated with development, and resolution, of HC.
We examined the University of Minnesota Blood and Marrow Transplant Program Database and available medical records to determine the following potential risk factors in those with HC and controls: sex, age, diagnosis and risk category, graft source and cell dose, conditioning regimen intensity, GVHD prophylaxis and ATG usage. The records of patients who developed HC were further reviewed to identify contemporaneous factors present within ± 7 days of the onset and resolution of HC including platelet count, international normalized ratio (INR), partial thromboplastin time (PTT), fibrinogen, creatinine, presence of GVHD, use of steroids, presence of fever, grade and duration of HC. Viral testing, in particular BK virus in urine and/or blood, was done at the discretion of treating providers.
Patients who underwent a reduced intensity conditioning (RIC) regimen generally received cyclophosphamide (50mg/kg IV on day −6), fludarabine (40mg/m2 IV daily from days −6 through −2) and total body irradiation (TBI, 200cGy on day −1) or fludarabine (30mg/m2 IV daily from days −6 through −2) and busulfan (3.2mg/kg IV daily on days−5 and −4). Equine anti-thymocyte globulin 15mg/kg IV every 12 hours for six doses was added for patients (n=324) who had received no multiagent chemotherapy within 3 months of alloHCT. RIC and UCB recipients received GVHD prophylaxis with cyclosporine (from day −3 to +100) and mycophenolate mofetil (from days −3 to +30). Myeloablative conditioning (MAC) most often consisted of cyclophosphamide (60 mg/kg intravenously daily for 2 days) and 1320 cGy TBI given divided in 8 fractions. The remaining group mainly received busulphan, fludrabine, and melphalan containing regimens.
Definitions
Patients were determined to have HC based on clinical presentation of cystitis, which was further classified by grade of hematuria. Thus, grade of HC was defined according to the following criteria: Grade 1 is defined as microscopic hematuria; Grade 2 as macroscopic hematuria; Grade 3 as macroscopic hematuria with small clots; and Grade 4 as gross hematuria with clots, clot retention and renal failure due to obstructive nephropathy 15, 19, 23. Patients were evaluated for HC from initiation of conditioning regimen through follow-up period.
Based on frequency of maximum HC grade, a grade variable was defined to compare Grade 1–2 versus Grade 3–4. HC resolution was determined once patients had 2 consecutive weeks without hematuria confirmed by urinalysis (UA). Clinical factors (including steroid use, GVHD) were considered present if active within ±7 days of HC onset or resolution.
Graft source and matching was defined as matched (HLA 8/8 allele matched) versus mismatched, bone marrow/peripheral blood stem cell (BM/PBSC) and UCB matched (HLA 5–6/6 locus matched) versus mismatched (HLA 4/6)24, 25.
Standard disease risk includes acute leukemia, lymphoma and other malignancies in first or second remission or chronic phase CML. All other patients were defined as high risk.
Supportive care
For prevention of hemorrhagic cystitis patients receiving cyclophosphamide also received Mesna at the mg equalivalent total dose of cyclophosphamide, divided in 5 doses given 15 minutes before and 3, 6, 9 and 12 hours after each dose of cyclophosphamide. In addition, patients receive hydration at 2000 – 3000 ml/m2/day, beginning 12 hours prior and continuing 24 hours after completion of cyclophosphamide. Infectious prophylaxis, other supportive care measures, and GVHD prophylaxis have been described.24, 26, 27
Statistical Methods
Data on transplantation patient characteristics, post-transplantation complications and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized procedures. Patient and disease characteristics were summarized using descriptive statistics. Statistical comparisons of these variables between HC and control group as well as between the onset of HC and resolution of HC were completed by the nonparametric Wilcoxon test for continuous factors and Pearson chi-square test for categorical factors. Adjustments for multiple comparisons were done with Bonferroni’s method. All patients were followed longitudinally until death or last follow-up. The end points included overall survival (OS) and treatment-related-mortality (TRM) at 1 year. Kaplan-Meier was used to estimate OS 28. Cumulative incidence was used to estimate TRM 29 with relapse as a competing risk. Statistical comparison of OS between HC and the control group was completed by the log-rank test. The proportional hazards model of Fine and Gray was used to assess the independent factors associated with TRM 30. Factors considered in multivariate analysis (MVA) were: HC severity (control vs. HC grade 1–2 vs. HC grade 3–4), cell source (matched BM/PBSC, mismatched BM/PBSC, matched UCB, mismatched UCB), disease risk (standard vs. high) and graft cell dose adjusted for graft source, gender, recipient CMV serostatus, age (0–20 vs. 21–40 vs. >40 years), conditioning (MA vs. RIC), T cell depleted (yes vs. no), and ATG use (yes vs. no). All statistical analyses were performed with Statistical Analysis System software version 9.3 (SAS Institute, Inc., Cary, NC) and R Statistical Software (Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
Results
HC occurred in 219 of the 1321 alloHCT patients (16.6%) at a median of 22 days (range −7 to 786 days; 25–75 percentile 16–50 days) after alloHCT and within 100 days in 193 (88%) cases. Maximum grade of HC was 1–2 in 96 patients (44%) while grade 3–4 was seen in 123 patients (56%). Median duration of HC was 27 days (range 4–105 days; 25–75 percentile 11–45 days). CMV reactivation within 100 days of alloHCT was 31% in HC patients at a median of 41 days (range 1–99 days; 25–75 percentile 27.5–49.5 days) after transplant. In HC patients, BK viruria was detected in 90% (n=109 tested), adenovirus in 2% (n=88 tested), and HHV6 in 11% (n=36 tested).
Patient demographics for both the HC and the control group are presented in Table 1. In univariate analysis, HC was more common in males, in patients age 0–20 years, in patients with non-malignant disease or with high risk malignant disease, in patients receiving myeloablative conditioning including cyclophosphamide, in patients with acute GVHD, and in patients without CMV reactivation. When age was analyzed by decades of life, patients 10–20 years (12% of control vs. 26% of HC patients) and 30–40 years (7.8% of controls vs.13.2% of HC patients) had more HC while patients >50 years old had less HC (33.2% of controls vs.16% of HC patients). In multivariate analysis (conditioning regimen and GVHD were not included because of confounding with age and graft source). Male patients and patients receiving MM UCB transplantation (HR; 1.46; CI95%:1.06–2.00, p=0.02) had a higher frequency of HC.
Table 1.
Patient characteristics and risk factors associated with hemorrhagic cystitis
| Control (n=1102) |
HC (n=219) |
P-value (UVA) |
Multivariate Adjusted Odds Ratio (95% CI) (n=219) |
P-value (MVA) |
|
|---|---|---|---|---|---|
| Gender | <0.01 | 0.01 | |||
| Male | 640 (58.1%) | 147 (67.1%) | 1.00 | ||
| Female | 462 (41.9%) | 72 (32.9%) | 0.67 (0.49–0.92) | ||
| Median Age, in years (range) | 36.5 (0.1–75.1) | 20.8 (0.5–71.7) | <0.01 | ||
| Age ranges, in years | <0.01 | ||||
| [0–20] | 411 (37.3%) | 109 (49.8%) | |||
| [21–40] | 176 (16.0%) | 46 (21.0%) | |||
| >40 | 515 (46.7%) | 64 (29.2%) | |||
| Disease Risk | 0.04 | Not included in MVA | |||
| Non-malignant | 267 (24.2%) | 70 (32.0%) | |||
| Standard Risk | 437 (39.7%) | 73 (33.3%) | |||
| High Risk | 398 (36.1%) | 76 (34.7%) | |||
| Donor and Graft Source | 0.08 | ||||
| Matched BM/PBSC | 453 (41.1%) | 76 (34.7%) | 1.00 | ||
| MM BM/PBSC | 84 (7.6%) | 15 (6.9%) | 1.07 (0.58–1.94) | 0.84 | |
| Matched UCB | 90 (8.2%) | 13 (5.9%) | 0.87 (0.46–1.64) | 0.67 | |
| Mismatched UCB | 475 (43.1%) | 115 (52.5%) | 1.46 (1.06–2.00) | 0.02 | |
| Conditioning Regimen | <0.01 | Not included in MVA | |||
| MAC: CY | 514 (46.6%) | 149 (68%) | |||
| RIC: CY | 479 (43.5%) | 54 (24.7%) | |||
| No CY (RIC or MAC) | 109(9.9%) | 16 (7.3%) | |||
| GVHD at 100 days | Not included in MVA | ||||
| Grades II–IV | 358 (34%) | 92 (43%) | 0.01 | ||
| Grades III–IV | 142 (13%) | 47 (22%) | <0.01 | ||
| CMV Reactivation | 0.25 | Not included in MVA | |||
| No | 805 (72.8%) | 151 (69.0%) | |||
| Yes | 300 (27.2%) | 68 (31.0%) | |||
Abbreviations: GVHD, graft-versus-host disease; MAC, myeloablative conditioning; MM, mismatched; MVA, multivariate analysis; RIC, reduced-intensity conditioning; UCB, umbilical cord blood transplantation; UVA; univariate analysis.
Clinical factors within ±7 days of the onset and the resolution of HC are shown in Table 2. 58 patients died between and thus were not evaluable at resolution of HC. At the onset of HC, more patients had acute GVHD, fever, thrombocytopenia, and steroid therapy than at the time of HC resolution. Creatinine level was similar at onset and resolution of HC (0.8 mg/dL, range 0.2–5.1 mg/dL vs. 0.9 mg/dL, range, 0.2–3.0 mg/dL). There was no correlation between INR, PTT, and platelet counts and severity of HC at the time of HC onset. GVHD and CMV reactivation preceded the onset of HC in 50% and 13% of patients, respectively. Therapy of HC included IVF (45%), bladder irrigation (12%), and cidofovir (6%), and 37% of patients had no additional therapy. Eighty-nine percent (89/100) of patients receiving IVF, 75% (61/82) of patients receiving no therapy, and 63% (7/11) of patients receiving cidofovir had resolution of HC whereas HC resolved in only 26% (7/26) of patients who underwent bladder irrigation.
Table 2.
Clinical variables at onset and resolution of hemorrhagic cystitis
| N | Onset (n=219) |
N | Resolution (n=161)* |
P-value | |
|---|---|---|---|---|---|
| Platelet count (× 109 xL) | 219 | 31000 (3000–311000) | 161 | 51000 (3000–373000) | <0.01 |
| Median (min-max) | |||||
| 25th/75th percentile | 17000/51000 | 27000/110000 | |||
| INR (s) Median (Min-Max) | 174 | 1.13 (0.75–1.83) | 68 | 1.10 (0.86–1.80) | 0.06 |
| 25th/75th percentile | 1.04/1.27 | 1.025/1.19 | |||
| PTT (s) Median (Min-Max) | 81 | 35 (22–184) | 30 | 32 (21–70) | 0.25 |
| 25th/75th percentile | 31/42 | 29/38 | |||
| Steroids | 207 | 152 | <0.01 | ||
| Yes | 66 (30.1%) | 27 (16.7%) | |||
| No | 141 (64.4%) | 125 (77.6%) | |||
| Acute GVHD | 210 | 150 | 0.04 | ||
| Yes | 68 (31.1%) | 33 (20.4%) | |||
| No | 145 (66.2%) | 117 (72.6%) | |||
| Fever | 206 | 158 | <0.01 | ||
| Afebrile | 119 (54.3%) | 156 (96.8%) | |||
| Febrile | 87 (39.7%) | 2 (1.2%) | |||
| Creatinine (mg/dL) | 219 | 161 | |||
| Median (Min-Max) | 0.8 (0.2–5.1) | 0.9 (0.2–3.0) | 0.12 | ||
| 25th/75th percentile | 0.57/1.26 | 0.62/1.26 | |||
| BK Viruria | 109 | 2 | 0.64 | ||
| Positive | 98 (44.7%) | 2 (0.9%) | |||
| Negative | 11 (5.0%) | 0 | |||
| CMV | 218 | 143 | 0.24 | ||
| Positive | 12 (5.5%) | 11 (6.8%) | |||
| Negative | 206 (94.1%) | 132 (81.9%) | |||
| Adenovirus | 88 | 23 | 0.47 | ||
| Positive | 2 (0.9%) | 0 | |||
| Negative | 86 (39.3%) | 23 (14.2%) | |||
Abbreviations: CMV, cytomegalovirus; GVHD, graft-versus-host disease; MAC, myeloablative conditioning; MM, mismatched; MVA, multivariate analysis; RIC, reduced-intensity conditioning;; UCB, umbilical cord blood transplantation; UVA; univariate analysis.
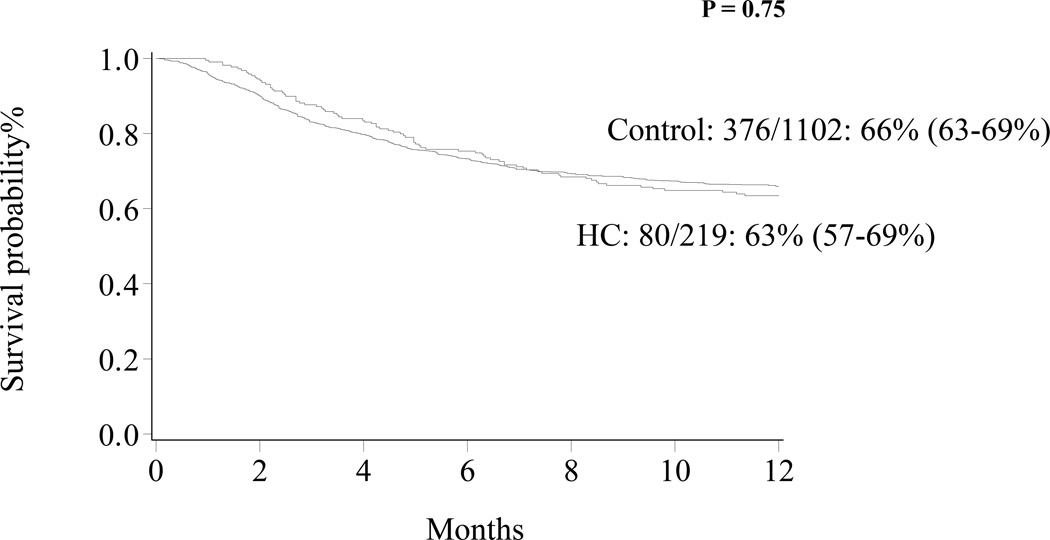
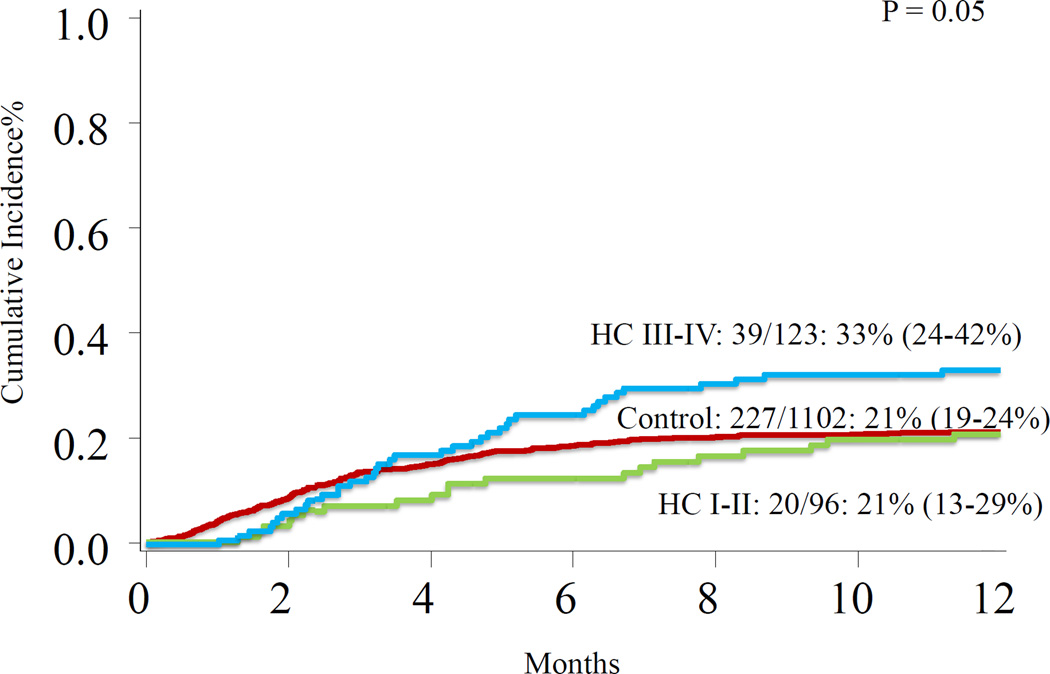
At 1 year, OS for all patients was 65% (63–68%) and was similar for HC patients [63% (57–69%)] and controls [66% (63–69%)], p= 0.75 (Figure 1). In multivariate analysis, severe grade HC (Figure 2) and HLA-mismatched status, regardless of graft source, were associated with increased TRM (Table 3). HLA-mismatched status, regardless of graft source, was associated with inferior OS (Table 3). Patients-, disease-, transplantation- characteristics were similar between severe and non-severe HC (Table 4).
Figure 1.
Overall survival patients with HC and control.
Figure 2.
Treatment-related mortality among control patients, patients with grade I–II HC, and patients with grade III–IV HC.
Table 3.
1 year overall survival and treatment-related mortality by multivariate analysis in patients with HC
| OS | TRM | ||||
|---|---|---|---|---|---|
| Parameter | Class | Hazard Ratio CI 95% |
P-value | Hazard Ratio CI 95% |
P-value |
| HC Group | Control | 1.00 | 0.14 | 1.00 | |
| Grade I-II | 0.76 (0.52-1.11) | 0.91 (0.59-1.42) | 0.69 | ||
| Grade III-IV | 1.20 (0.90-1.61) | 1.52 (1.10-2.10) | 0.01 | ||
| Graft source | M BM/PBSC | 1.00 | <0.01 | 1.00 | |
| MM BM/PBSC | 2.60 (1.90-3.55) | 2.28 (1.53-3.41) | <0.01 | ||
| M UCB | 1.14 (0.77-1.68) | 1.34 (0.85-2.12) | 0.21 | ||
| MM UCB | 1.53 (1.25-1.89) | 1.43 (1.10-1.87) | 0.01 | ||
Covariates tested in UVA were: ATG, sex, age, disease risk, conditioning regimen intensity
Abbreviations:BM, bone marrow; CMV, cytomegalovirus; HC, hemorrhagic cystitis; M, matched; MM, mismatched; PBSC, peripheral blood stem cell; UCB, umbilical cord blood.
Table 4.
Characteristic between grade I-II HC vs. III-IV HC
| HC Grade I-II | HC Grade III-IV | P-value | |
|---|---|---|---|
| Gender | 0.39 | ||
| Male | 59 (64%) | 85 (70%) | |
| Female | 33 (36%) | 37 (30%) | |
| Source | 0.57 | ||
| M BM/PBSC | 32 (35%) | 43 (35%) | |
| MM BM/PBSC | 6 (7%) | 8 (7%) | |
| M UCB | 8 (9%) | 5 (4%) | |
| MM UCB | 46 (50%) | 66 (54%) | |
| Age Group | 0.27 | ||
| 0–20 | 48 (52%) | 56 (46%) | |
| 21–40 | 15 (16%) | 31 (25%) | |
| 41- | 29 (32%) | 35 (29%) | |
| Disease Risk | 0.23 | ||
| Non Malignant | 33 (36%) | 35 (29%) | |
| Standard Risk | 33 (36%) | 39 (32%) | |
| High Risk | 26 (28%) | 48 (39%) | |
| CMV | 0.50 | ||
| Positive | 4 (5%) | 8 (7%) | |
| Negative | 87 (96%) | 114 (93%) | |
| BK Virus | 0.46 | ||
| Positive | 37 (88%) | 60 (92%) | |
| Negative | 5 (12%) | 5 (8%) | |
| Conditioning | 0.77 | ||
| MAC: CY | 60 (65%) | 85 (70%) | |
| RIC: CY | 25 (27%) | 28 (23%) | |
| NO CY | 7 (8%) | 9 (7%) | |
| DAH | 0.20 | ||
| Yes | 2 (2%) | 7 (6%) | |
| No | 90 (97%) | 115 (94%) | |
Abbreviations: BM, bone marrow; CMV, cytomegalovirus; Cy, cyclophosphamide; DAH, diffuse alveolar hemorrhage; HC, hemorrhagic cystitis; M, matched; MAC, myeloablative conditioning; MM, mismatched; PBSC, peripheral blood stem cell; RIC, reduced-intensity conditioning; UCB, umbilical cord blood.
Discussion
We examined the clinical risk factors for HC after alloHCT, with emphasis on factors contributing to the development and severity of HC. HC incidence was 16.6%, in line with other studies.8, 10, 31 Interestingly, compared to our prior study performed nearly 20 years ago, the incidence of HC has not changed.20 Male gender was the most important risk factor for developing HC as has been reported in earlier studies of relatively small number of patients 14, 31–33.
Conditioning regimen is an important factor in development of HC and its mechanism has been well described 34–41. In our study we found that conditioning regimens including cyclophosphamide increased HC risk. We also showed that cyclophosphamide in MAC was more frequently associated with HC compared to RIC containing cyclophosphamide. This might have resulted from the higher dose of cyclophosphamide 42 or higher doses of TBI in MAC 43. Some studies found no relation between dose of cyclophosphamide 19, 31, 44, 45 or interaction with TBI. Conditioning regimen intensity has also been studied and MAC has been reported to induce with higher frequency of HC 1, 8, 10, 22, 39, 46, 47. It was argued that MAC induced more HC in part due to increased risks for BK viruria 47. In our study, BK viruria was frequent in HC patients regardless of conditioning intensity.
HC incidence is higher in unrelated donor transplantation (URD) 14, 31, 48. In a study, HC patients received more URD transplantation (81%) than MRD transplantation (19%), p<0.05. 47 URD transplantation was reported as an independent risk factor for HC in children as well as adults.31 HC occurred in 16% of patients receiving MRD transplants, 30% of recipients of mismatched related (MMRD), and 40% of matched URD or UCB transplants (HR 2.9 for the comparison of MRD versus URD).21 UCB transplantation was noted as risk factor for BK virus positive HC 40, in whom the cumulative incidence of HC can be as high as 41.8% at 1 year 49. One study showed that UCB or haploidentical donor transplantation had much higher risk for HC if BK virus was positive before alloHCT.1 These findings may be attributed to greater and longer lasting immunologic and hematologic suppression leading to a higher risk of HC 8, 21, 31, 47. In our study, we found that neither HLA mismatched status nor graft source alone, but that HLA-mismatched UCB transplantation was significantly associated with higher risks of HC.
Age was found to be risk factor for HC. 19, 21, 33, 46 Older age in pediatric population and younger age in adult population seem to be associated with HC.17, 19, 21, 33, 39, 50, 51 Although age was not significant in MVA, patients in the 2nd and 4th decades of life had increased incidence of HC. Seber et al, reported a higher incidence of HC occurring among patients age 10–30; greatest in the 10–20 year age group compared to patients vs. age <10 years 19. Similarly, El-Zimaity et al found that age < 26 years was significantly associated with HC; an effect that was consistent across graft types 21.
The association between HC and GVHD has been studied, but remains unclear 1, 10, 19, 31, 32, 40, 48, 50, 52, 53. Our study identified GVHD as a significant clinical variable associated with HC and fewer patients had active GVHD at the resolution of HC compared to onset. Other studies have identified GVHD as a risk factor for the development of HC; especially severe or late-onset HC 10, 19, 22, 32, 40, 41, 54, 55. However, it remains unknown whether GVHD targeting bladder epithelium manifests as HC or whether immunosuppression from acute GVHD and/or concomitant steroid use contributes to HC and its severity 19, 56, 57. Acute GVHD preceded HC in only half of the HC patients; however, steroid use and acute GVHD were more frequent at the onset of HC than the resolution of HC. Bogdanovic et al suggested that the combination of acute GVHD and BK virus predicted development of HC better than acute GVHD alone14. BK virus was frequently found to be a risk factor for HC 1, 8, 10, 40. In this retrospective analysis, we did not aim to identify the precise role of BK virus in HC development given that BK viruria can be asymptomatic in up to 50% of alloHCT patients and that BK virus was not routinely tested at predetermined time points after alloHCT regardless of symptoms. Nearly all our tested HC patients had BK viruria. BK viruria was not associated with HC severity. We identified no significant association between adenovirus, HHV-6 or CMV in HC patients comparing the onset and resolution of HC. Some studies have associated CMV reactivation with HC 10, 22, suggesting that DNA viruses such as CMV can induce BK virus replication.58, 59. Our study also found that CMV reactivation rate was similar between control and HC group and that CMV only preceded HC in 13% of all HC patients.
We also evaluated clinical factors differing between the onset and resolution of HC. We found that the resolution of HC is associated with increased platelet counts. Brugieres et al describe thrombocytopenia at onset for all patients who developed HC (n=19) 39. It is recommended that platelet counts should be maintained above 50 × 109/L 15, 39, 60 in patients with active HC. However, in 2 small series delayed platelet engraftment was not found to be a significant variable in the development of HC 21 or in children with severe HC 31. We also analyzed serum creatinine because of its association with platelet dysfunction 61 and BK virus-induced nephropathy 62, 63, but observed no relation to risks or severity of HC. Uhm et al found worsening renal function during HC, but concluded that this resulted from concurrent CMV therapy rather than direct nephrotoxic effect of HC10. With respect to HC resolution and its therapy, bladder irrigation resulted in less resolution of HC, which most likely indicates that patients requiring invasive procedures had more severe-intractable HC, and thus poorer outcomes.
HC remains frequent and troublesome in particular when it is severe; often causing prolonged hospitalization, resource use and expense. The significance of HC grade I in BMT patients is not too clear. Attention in high risk patients to aggressive protection from both conditioning toxicity and virus associated HC is still important to limit its morbidity.
Acknowledgments
Qing Cao is supported by grant from the National Cancer Institute CA065493-20
Footnotes
No Conflict of Interest to disclose
References
- 1.Silva LD, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, et al. Hemorrhagic Cystitis after Allogeneic Hematopoietic Stem Cell Transplants Is the Complex Result of Bk Virus Infection, Preparative Regimen Intensity and Donor Type. Haematologica-the Hematology Journal. 2010;95(7):1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilhan O, Koc H, Akan H, Gurman G, Arslan O, Ozcan M, et al. Hemorrhagic Cystitis as a Complication of Bone Marrow Transplantation. J Chemother. 1997;9(1):56–61. doi: 10.1179/joc.1997.9.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Cesaro S, Brugiolo A, Faraci M, Uderzo C, Rondelli R, Favre C, et al. Incidence and Treatment of Hemorrhagic Cystitis in Children Given Hematopoietic Stem Cell Transplantation: A Survey from the Italian Association of Pediatric Hematology Oncology-Bone Marrow Transplantation Group. Bone Marrow Transplant. 2003;32(9):925–931. doi: 10.1038/sj.bmt.1704252. [DOI] [PubMed] [Google Scholar]
- 4.Mori Y, Miyamoto T, Kamezaki K, Kato K, Kikushige Y, Takashima S, et al. Low Incidence of Adenovirus Hemorrhagic Cystitis Following Autologous Hematopoietic Stem Cell Transplantation in the Rituximab Era. Am J Hematol. 2012;87(8):828–830. doi: 10.1002/ajh.23247. [DOI] [PubMed] [Google Scholar]
- 5.Fioriti D, Degener AM, Mischitelli M, Videtta M, Arancio A, Sica S, et al. Bkv Infection and Hemorrhagic Cystitis after Allogeneic Bone Marrow Transplant. Int J Immunopathol Pharmacol. 2005;18(2):309–316. doi: 10.1177/039463200501800213. [DOI] [PubMed] [Google Scholar]
- 6.Koskenvuo M, Dumoulin A, Lautenschlager I, Auvinen E, Mannonen L, Anttila VJ, et al. Bk Polyomavirus-Associated Hemorrhagic Cystitis among Pediatric Allogeneic Bone Marrow Transplant Recipients: Treatment Response and Evidence for Nosocomial Transmission. J Clin Virol. 2013;56(1):77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Aisa Y, Shimizu T, Ikeda Y, Okamoto S, Okada K, et al. Hemorrhagic Cystitis Caused by Adenovirus Type 34 after Allogeneic Bone Marrow Transplantation. Transplantation. 2005;79(5):624. doi: 10.1097/01.tp.0000147653.16324.ca. [DOI] [PubMed] [Google Scholar]
- 8.Giraud G, Bogdanovic G, Priftakis P, Remberger M, Svahn BM, Barkholt L, et al. The Incidence of Hemorrhagic Cystitis and Bk-Viruria in Allogeneic Hematopoietic Stem Cell Recipients According to Intensity of the Conditioning Regimen. Haematologica-the Hematology Journal. 2006;91(3):401–404. [PubMed] [Google Scholar]
- 9.Meisenberg B, Lassiter M, Hussein A, Ross M, Vredenburgh JJ, Peters WP. Prevention of Hemorrhagic Cystitis after High-Dose Alkylating Agent Chemotherapy and Autologous Bone Marrow Support. Bone Marrow Transplant. 1994;14(2):287–291. [PubMed] [Google Scholar]
- 10.Uhm J, Hamad N, Michelis FV, Shanavas M, Kuruvilla J, Gupta V, et al. The Risk of Polyomavirus Bk-Associated Hemorrhagic Cystitis after Allogeneic Hematopoietic Sct Is Associated with Myeloablative Conditioning, Cmv Viremia and Severe Acute Gvhd. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.181. [DOI] [PubMed] [Google Scholar]
- 11.Ferry C, Socie G. Busulfan-Cyclophosphamide Versus Total Body Irradiation-Cyclophosphamide as Preparative Regimen before Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia: What Have We Learned? Experimental Hematology. 2003;31(12):1182–1186. doi: 10.1016/j.exphem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Gorczynska E, Turkiewicz D, Rybka K, Toporski J, Kalwak K, Dyla A, et al. Incidence, Clinical Outcome, and Management of Virus-Induced Hemorrhagic Cystitis in Children and Adolescents after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2005;11(10):797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.van Aalderen MC, Heutinck KM, Huisman C, ten Berge IJM. Bk Virus Infection in Transplant Recipients: Clinical Manifestations, Treatment Options and the Immune Response. Netherlands Journal of Medicine. 2012;70(4):172–183. [PubMed] [Google Scholar]
- 14.Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, et al. Association between a High Bk Virus Load in Urine Samples of Patients with Graft-Versus-Host Disease and Development of Hemorrhagic Cystitis after Hematopoietic Stem Cell Transplantation. Journal of Clinical Microbiology. 2004;42(11):5394–5396. doi: 10.1128/JCM.42.11.5394-5396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dropulic LK, Jones RJ. Polyomavirus Bk Infection in Blood and Marrow Transplant Recipients. Bone Marrow Transplantation. 2008;41(1):11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megged O, Stein J, Ben-Meir D, Shulman LM, Yaniv I, Shalit I, et al. Bk-Virus-Associated Hemorrhagic Cystitis in Children after Hematopoietic Stem Cell Transplantation. Journal of Pediatric Hematology Oncology. 2011;33(3):190–193. doi: 10.1097/MPH.0b013e3181fce388. [DOI] [PubMed] [Google Scholar]
- 17.Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, et al. Blood, and Not Urine, Bk Viral Load Predicts Renal Outcome in Children with Hemorrhagic Cystitis Following Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2011;17(10):1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Miyamoto T, Kato K, Kamezaki K, Kuriyama T, Oku S, et al. Different Risk Factors Related to Adenovirus- or Bk Virus-Associated Hemorrhagic Cystitis Following Allogeneic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2012;18(3):458–465. doi: 10.1016/j.bbmt.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk Factors for Severe Hemorrhagic Cystitis Following Bmt. Bone Marrow Transplantation. 1999;23(1):35–40. doi: 10.1038/sj.bmt.1701523. [DOI] [PubMed] [Google Scholar]
- 20.Sencer SF, Haake RJ, Weisdorf DJ. Hemorrhagic Cystitis after Bone-Marrow Transplantation - Risk-Factors and Complications. Transplantation. 1993;56(4):875–879. doi: 10.1097/00007890-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 21.El-Zimaity M, Saliba R, Chan K, Shahjahan M, Carrasco A, Khorshid O, et al. Hemorrhagic Cystitis after Allogeneic Hematopoietic Stem Cell Transplantation: Donor Type Matters. Blood. 2004;103(12):4674–4680. doi: 10.1182/blood-2003-08-2815. [DOI] [PubMed] [Google Scholar]
- 22.Arai Y, Maeda T, Sugiura H, Matsui H, Jo T, Ueda T, et al. Risk Factors for and Prognosis of Hemorrhagic Cystitis after Allogeneic Stem Cell Transplantation: Retrospective Analysis in a Single Institution. Hematology. 2012;17(4):207–214. doi: 10.1179/1607845412Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 23.Droller MJ, Saral R, Santos G. Prevention of Cyclophosphamide-Induced Hemorrhagic Cystitis. Urology. 1982;20(3):256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- 24.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 Partially Hla-Matched Umbilical Cord Blood Units to Enhance Engraftment in Adults with Hematologic Malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 25.Ustun C, Bachanova V, Shanley R, MacMillan ML, Majhail NS, Arora M, et al. Importance of Donor Ethnicity/Race Matching in Unrelated Adult and Cord Blood Allogeneic Hematopoietic Cell Transplant. Leuk Lymphoma. 2014;55(2):358–364. doi: 10.3109/10428194.2013.800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ustun C, Wiseman AC, DeFor TE, Yohe S, Linden MA, Oran B, et al. Achieving Stringent Cr Is Essential before Reduced-Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation in Aml. Bone Marrow Transplantation. 2013;48(11):1415–1420. doi: 10.1038/bmt.2013.124. [DOI] [PubMed] [Google Scholar]
- 27.Hagen P, Wagner JE, DeFor TE, Dolan M, Arora M, Warlick E, et al. The Effect of Equine Antithymocyte Globulin on the Outcomes of Reduced Intensity Conditioning for Aml. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 29.Lin DY. Non-Parametric Inference for Cumulative Incidence Functions in Competing Risks Studies. Statistics in Medicine. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 31.Hale GA, Rochester RJ, Heslop HE, Krance RA, Gingrich JR, Benaim E, et al. Hemorrhagic Cystitis after Allogeneic Bone Marrow Transplantation in Children: Clinical Characteristics and Outcome. Biology of Blood and Marrow Transplantation. 2003;9(11):698–705. doi: 10.1016/s1083-8791(03)00269-6. [DOI] [PubMed] [Google Scholar]
- 32.Asano Y, Kanda Y, Ogawa N, Sakata-Yanagimoto M, Nakagawa M, Kawazu M, et al. Male Predominance among Japanese Adult Patients with Late-Onset Hemorrhagic Cystitis after Hematopoietic Stem Cell Transplantation. Bone Marrow Transplantation. 2003;32(12):1175–1179. doi: 10.1038/sj.bmt.1704274. [DOI] [PubMed] [Google Scholar]
- 33.Kondo M, Kojima S, Kato K, Matsuyama T. Late-Onset Hemorrhagic Cystitis after Hematopoietic Stem Cell Transplantation in Children. Bone Marrow Transplantation. 1998;22(10):995–998. doi: 10.1038/sj.bmt.1701482. [DOI] [PubMed] [Google Scholar]
- 34.Kopterides P, Theodorakopoulou M, Mentzelopoulos S, Armaganidis A. Cyclophosphamide-Induced Hemorrhagic Cystitis Successfully Treated with Conjugated Estrogens. Am J Hematol. 2005;80(2):166–167. doi: 10.1002/ajh.20403. [DOI] [PubMed] [Google Scholar]
- 35.Walker RD. Cyclophosphamide Induced Hemorrhagic Cystitis. J Urol. 1999;161(6):1747. [PubMed] [Google Scholar]
- 36.Haselberger MB, Schwinghammer TL. Efficacy of Mesna for Prevention of Hemorrhagic Cystitis after High-Dose Cyclophosphamide Therapy. Ann Pharmacother. 1995;29(9):918–921. doi: 10.1177/106002809502900914. [DOI] [PubMed] [Google Scholar]
- 37.Wang CC, Weng TI, Wu ET, Wu MH, Yang RS, Liu SH. Involvement of Interleukin-6-Regulated Nitric Oxide Synthase in Hemorrhagic Cystitis and Impaired Bladder Contractions in Young Rats Induced by Acrolein, a Urinary Metabolite of Cyclophosphamide. Toxicol Sci. 2013;131(1):302–310. doi: 10.1093/toxsci/kfs270. [DOI] [PubMed] [Google Scholar]
- 38.Souza-Fiho MV, Lima MV, Pompeu MM, Ballejo G, Cunha FQ, Ribeiro Rde A. Involvement of Nitric Oxide in the Pathogenesis of Cyclophosphamide-Induced Hemorrhagic Cystitis. Am J Pathol. 1997;150(1):247–256. [PMC free article] [PubMed] [Google Scholar]
- 39.Brugieres L, Hartmann O, Travagli JP, Benhamou E, Pico JL, Valteau D, et al. Hemorrhagic Cystitis Following High-Dose Chemotherapy and Bone-Marrow Transplantation in Children with Malignancies - Incidence, Clinical Course, and Outcome. Journal of Clinical Oncology. 1989;7(2):194–199. doi: 10.1200/JCO.1989.7.2.194. [DOI] [PubMed] [Google Scholar]
- 40.Gilis L, Morisset S, Billaud G, Ducastelle-Lepretre S, Labussiere-Wallet H, Nicolini FE, et al. High Burden of Bk Virus-Associated Hemorrhagic Cystitis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2014;49(5):664–670. doi: 10.1038/bmt.2013.235. [DOI] [PubMed] [Google Scholar]
- 41.Russell SJ, Vowels MR, Vale T. Haemorrhagic Cystitis in Paediatric Bone Marrow Transplant Patients: An Association with Infective Agents, Gvhd and Prior Cyclophosphamide. Bone Marrow Transplant. 1994;13(5):533–539. [PubMed] [Google Scholar]
- 42.Ayas M, Siddiqui K, Al-Jefri A, El-Solh H, Al-Ahmari A, Khairy A, et al. Factors Affecting the Outcome of Related Allogeneic Hematopoietic Cell Transplantation in Patients with Fanconi Anemia. Biol Blood Marrow Transplant. 2014;20(10):1599–1603. doi: 10.1016/j.bbmt.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Alesawi AM, El-Hakim A, Zorn KC, Saad F. Radiation-Induced Hemorrhagic Cystitis. Curr Opin Support Palliat Care. 2014;8(3):235–240. doi: 10.1097/SPC.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 44.Cox PJ. Cyclophosphamide Cystitis--Identification of Acrolein as the Causative Agent. Biochem Pharmacol. 1979;28(13):2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- 45.Al-Rawithi S, El-Yazigi A, Ernst P, Al-Fiar F, Nicholls PJ. Urinary Excretion and Pharmacokinetics of Acrolein and Its Parent Drug Cyclophosphamide in Bone Marrow Transplant Patients. Bone Marrow Transplant. 1998;22(5):485–490. doi: 10.1038/sj.bmt.1701355. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto R, Kami M, Kanda Y, Kusumi E, Nakai K, Hamaki T, et al. Reduced Incidence of Late Hemorrhagic Cystitis after Reduced Intensity Hematopoietic Stem-Cell Transplantation. Blood. 2001;98(11):187a–187a. doi: 10.1038/sj.bmt.1704261. [DOI] [PubMed] [Google Scholar]
- 47.Giraud G, Priftakis P, Bogdanovic G, Remberger M, Dubrulle M, Hau A, et al. Bk-Viruria and Haemorrhagic Cystitis Are More Frequent in Allogeneic Haematopoietic Stem Cell Transplant Patients Receiving Full Conditioning and Unrelated-Hla-Mismatched Grafts. Bone Marrow Transplantation. 2008;41(8):737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 48.Dalianis T, Ljungman P. Full Myeloablative Conditioning and an Unrelated Hla Mismatched Donor Increase the Risk for Bk Virus-Positive Hemorrhagic Cystitis in Allogeneic Hematopoetic Stem Cell Transplanted Patients. Anticancer Research. 2011;31(3):939–944. [PubMed] [Google Scholar]
- 49.Tomonari A, Takahashi S, Ooi J, Fukuno K, Takasugi K, Tsukada N, et al. Hemorrhagic Cystitis in Adults after Unrelated Cord Blood Transplantation: A Single-Institution Experience in Japan. Int J Hematol. 2006;84(3):268–271. doi: 10.1532/IJH97.05169. [DOI] [PubMed] [Google Scholar]
- 50.Kloos RQ, Boelens JJ, de Jong TP, Versluys B, Bierings M. Hemorrhagic Cystitis in a Cohort of Pediatric Transplantations: Incidence, Treatment, Outcome, and Risk Factors. Biol Blood Marrow Transplant. 2013;19(8):1263–1266. doi: 10.1016/j.bbmt.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Laskin BL, Denburg M, Furth S, Diorio D, Goebel J, Davies SM, et al. Bk Viremia Precedes Hemorrhagic Cystitis in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2013;19(8):1175–1182. doi: 10.1016/j.bbmt.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bedi A, Miller CB, Hanson JL, Goodman S, Ambinder RF, Charache P, et al. Association of Bk Virus with Failure of Prophylaxis against Hemorrhagic Cystitis Following Bone Marrow Transplantation. J Clin Oncol. 1995;13(5):1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 53.Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of Polyoma Bk Viruria in Hemorrhagic Cystitis Complicating Bone Marrow Transplantation. Blood. 2001;98(6):1971–1978. doi: 10.1182/blood.v98.6.1971. [DOI] [PubMed] [Google Scholar]
- 54.Leung AY, Mak R, Lie AK, Yuen KY, Cheng VC, Liang R, et al. Clinicopathological Features and Risk Factors of Clinically Overt Haemorrhagic Cystitis Complicating Bone Marrow Transplantation. Bone Marrow Transplant. 2002;29(6):509–513. doi: 10.1038/sj.bmt.1703415. [DOI] [PubMed] [Google Scholar]
- 55.Trotman J, Nivison-Smith I, Dodds A. Haemorrhagic Cystitis: Incidence and Risk Factors in a Transplant Population Using Hyperhydration. Bone Marrow Transplantation. 1999;23(8):797–801. doi: 10.1038/sj.bmt.1701644. [DOI] [PubMed] [Google Scholar]
- 56.Ost L, Lonnqvist B, Eriksson L, Ljungman P, Ringden O. Hemorrhagic Cystitis - a Manifestation of Graft Versus Host-Disease. Bone Marrow Transplantation. 1987;2(1):19–25. [PubMed] [Google Scholar]
- 57.Lee GW, Lee JH, Choi SJ, Kim S, Seol M, Kim WK, et al. Hemorrhagic Cystitis Following Allogeneic Hematopoietic Cell Transplantation. Journal of Korean Medical Science. 2003;18(2):191–195. doi: 10.3346/jkms.2003.18.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bielorai B, Shulman LM, Rechavi G, Toren A. Cmv Reactivation Induced Bk Virus-Associated Late Onset Hemorrhagic Cystitis after Peripheral Blood Stem Cell Transplantation. Bone Marrow Transplant. 2001;28(6):613–614. doi: 10.1038/sj.bmt.1703187. [DOI] [PubMed] [Google Scholar]
- 59.Pari GS, Stjeor SC. Human Cytomegalovirus Major Immediate Early Gene-Product Can Induce Sv40 DNA-Replication in Human Embryonic Lung-Cells. Virology. 1990;179(2):785–794. doi: 10.1016/0042-6822(90)90146-i. [DOI] [PubMed] [Google Scholar]
- 60.Cheuk DKL, Lee TL, Chiang AKS, Ha SY, Lau YL, Chan GCF. Risk Factors and Treatment of Hemorrhagic Cystitis in Children Who Underwent Hematopoietic Stem Cell Transplantation. Transplant International. 2007;20(1):73–81. doi: 10.1111/j.1432-2277.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 61.Boccardo P, Remuzzi G, Galbusera M. Platelet Dysfunction in Renal Failure. Semin Thromb Hemost. 2004;30(5):579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 62.Costa C, Cavallo R. Polyomavirus-Associated Nephropathy. World J Transplant. 2012;2(6):84–94. doi: 10.5500/wjt.v2.i6.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. Pathology of Resolving Polyomavirus-Associated Nephropathy. Am J Transplant. 2013 doi: 10.1111/ajt.12218. [DOI] [PubMed] [Google Scholar]