Abstract
Stroke is the second leading cause of death in the world. Stroke occurs when blood flow stops, and that stoppage results in reduced oxygen supply to neurons in the brain. The occurrence of stroke increases with age, but anyone at any age can suffer from stroke. Recent research has implicated multiple cellular changes in stroke patients, including oxidative stress and mitochondrial dysfunction, inflammatory responses, and changes in mRNA and proteins. Recent research has also revealed that stroke is associated with modifiable and non-modifiable risk factors. Stroke can be controlled by modifiable risk factors, including diet, cardiovascular, hypertension, smoking, diabetes, obesity, metabolic syndrome, depression and traumatic brain injury. Stroke is the major risk factor for vascular dementia (VaD) and Alzheimer’s disease (AD). The purpose of this article is to review the latest developments in research efforts directed at identifying 1) latest developments in identifying biomarkers in peripheral and central nervous system tissues, 2) changes in microRNAs (miRNAs) in patients with stroke, 3) miRNA profile and function in animal brain, and 4) protein biomarkers in ischemic stroke. This article also reviews research investigating circulatory miRNAs as peripheral biomarkers of stroke.
Keywords: Stroke, Vascular dementia, Alzheimer’s disease, circulatory microRNA, serum, plasma, protein biomarker
1. Introduction
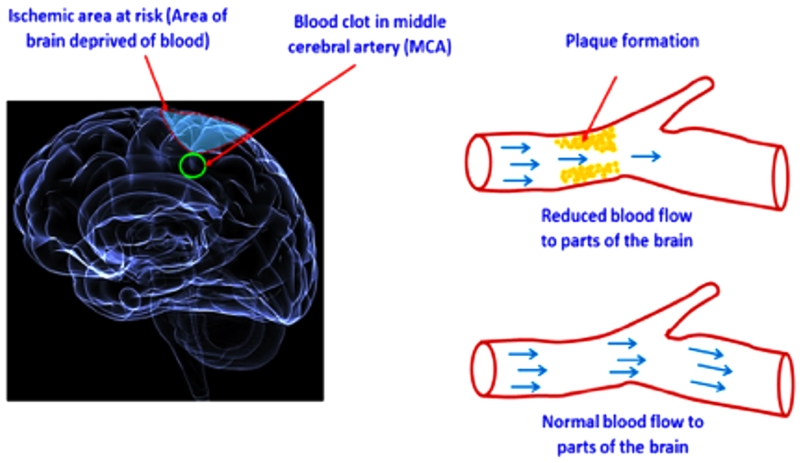
Stroke is a common neurological disease that occurs when the blood supply to the brain is interrupted, resulting in a shortage of oxygen and nutrients to brain tissue (Fig. 1). The traditional definition of stroke by the World Health Organization (WHO) is “rapidly developing clinical signs of focal or global disturbance of cerebral function, with symptoms lasting 24 hours or longer, or leading to death with no apparent cause other than vascular origin” [1]. The acute disruption of blood flow is usually caused by a clot blocking a blood vessel (ischemic stroke; IS) or by a ruptured blood vessel (hemorrhagic stroke; HS). The effects of ischemic and hemorrhagic strokes depend on the part of the brain that is injured and the severity of the injury. Patients having the same type of stroke can have differing clinical symptoms. Similarly, patients with the same clinical symptoms can have different etiopathologies. Due to its multifactorial nature, stroke may be classified as a syndrome, not as a single disease. Modern neuroimaging, typically with computed tomography (CT) or magnetic resonance imaging (MRI), is now used to accurately diagnose a stroke.
Figure 1.
Schematic representation of ischemic stroke. The shaded area (in yellow) shows the formation of plaques and reduced blood supply/flow to an area of brain leading to ischemia.
ISs, sometimes called ’cerebral infarctions,’ are caused by either cerebral thrombosis or embolism, and account for 50-85% of all strokes worldwide [2]. HSs are caused by subarachnoid hemorrhage or intracerebral hemorrhage, and account for 1-7% and 7-27% of all strokes worldwide, respectively [3].
According to Trial of Org 10172 in Acute Stroke Treatment (TOAST) diagnostic criteria [4], stroke can be classified into five subtypes: large-vessel disease, small vessel disease, cardio-embolic stroke, stroke of other determined etiology, and stroke of undetermined etiology. The subtypes are based on risk factor profiles, clinical features, and results from diagnostic tests, including CT and MRI scans, vascular imaging, electrocardiograms, echocardiography assessment, and assessment of prothrombotic syndromes.
Stroke is the third leading cause of disability-adjusted life years (DALYs) worldwide [5, 6]. According to the WHO, stroke affects 15 million people worldwide. Of these, about 5 million patients suffer from permanent disability and about 5.5 million patients succumb to their disabilities [7]. The occurrence of stroke increases with age, but anyone at any age can suffer a stroke. Globally, the prevalence rate of stroke is about 400-800/1,000,000 persons [8]. In many developed countries, the incidence of stroke is declining, largely as a result of the better control of high blood pressure and reduced levels of smoking. However, the absolute number of strokes continues to increase worldwide because people are living longer, especially in regions of rapid economic growth.
Stroke accounts for about 5.7 million (16.6%) deaths in the world, with 87% of these deaths occurring in low- and middle-income counties. Sixteen million new cases of stroke have occurred every year worldwide [9, 10] and 3 million women and 2.5 million men have died every year, worldwide from stroke [11].
Dementia syndromes established after stroke were typically considered to be vascular in origin, and post-stroke dementia might be the consequence of the effects of stroke and degenerative changes [12]. Research linking stroke and dementia has focused on shared vascular risk factors, ameliorated by lifestyle activities or medication. Vascular dementia (VaD) and Alzheimer’s disease (AD) are important because these results may be due to a variety of causes, including cerebrovascular dysfunction, but the evidence for their association with other neurodegenerative disorders is limited [13]. VaD, AD, and stroke share some common risk factors. There is no clear evidence linking stroke, VaD and AD.
In the recent years, hundreds of genes were examined and it may have an impact on stroke pathogenesis or genes that are associated with clinical risk factors such as hypertension, diabetes, hyperlipidemia and vascular disease [14-16]. But only a few were fully proven to influence the susceptibility to the disease and have generated negative or conflicting results. Stroke is both a heterogeneous and a multifactorial disease, in which heritable (genetic) and environmental factors equally contribute [17, 18]. The cellular changes and pathophysiology of stroke are complex and involve numerous processes, including oxidative stress and mitochondrial dysfunction, energy failure, increased intracellular calcium levels, cytokine mediated cytotoxicity, inflammatory responses, disruption of the blood-brain barrier (BBB), activation of glial cells, and miRNA changes [19]. The purpose of this article is to 1) latest developments in identifying biomarkers in peripheral and central nervous system tissues, 2) changes in microRNAs (miRNAs) in patients with stroke, 3) miRNA profile and function in animal brain and, 4) protein biomarkers in ischemic stroke.
2. Biomarkers and Stroke
A biomarker – such as a protein, nucleic acid, or metabolite – is a quantification of a definite biological state, typically one relevant to the risk, occurrence, severity, prognosis or projected therapeutic response of disease. Biomarkers may be useful in identifying different diseases, such as stroke, AD, cancer, and diabetes, and disease severity. Identification of biomarkers can inform researchers in their attempts to develop early detectable peripheral biomarkers. Identification of biomarkers can contribute to a better understanding of the etiologies and mechanisms underlying particular diseases, such as stroke.
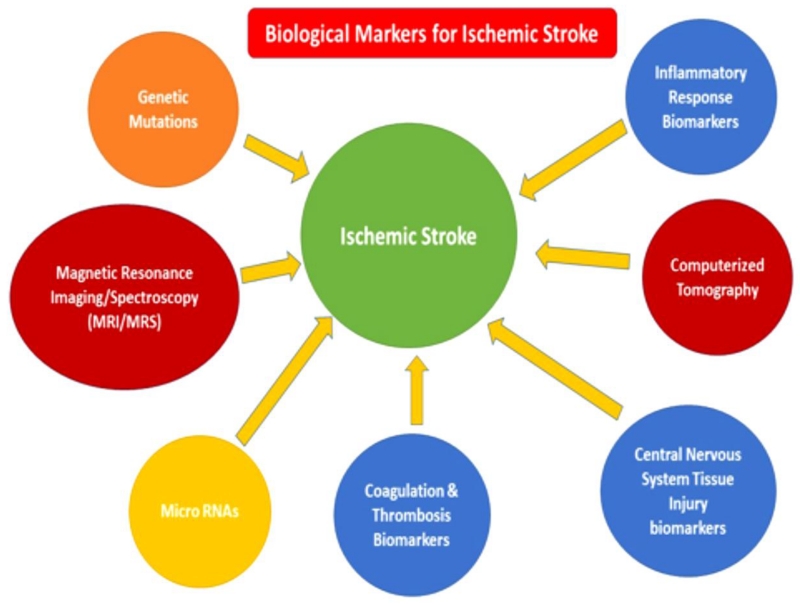
To identify peripheral biomarkers of stroke, multiple approaches have been developed, including circulatory miRNAs, blood based protein markers, coagulation and thrombosis biomarkers (Fig. 2).
Figure 2.
Clinically translatable biological markers for stroke. MRI/MRS/CT and inflammatory response biomarkers may provide the link needed to branch the basic and clinical research areas.
3. MiRNAs as Biomarkers in Stroke
MiRNAs are composed of a group of endogenous and small non-protein coding genes present in virtually all animals, plants and some viruses. MiRNAs are important regulators of several biological processes, such as cell growth, apoptosis, cell proliferation, embryonic development, and tissue differentiation [20]. According to the miRbase-21 database released in June 2014, 1881 precursor and 2588 mature miRNAs have been identified (www.mirbase.org/) in such human diseases as cancer, viral infection, diabetes, immune-related diseases, and neurodegenerative disorders. miRNAs tend to be transcribed from several dissimilar loci in the genome. Many miRNAs were found to be highly conserved [21]. These genes encode long RNAs with a hairpin structure of 17-25 nucleotides and act as an antisense regulator of other RNAs [22]. It is copied from genes that lie inside recognized exons and introns or other intergenic regions of the genome [23]. Sequence variations in miRNAs are known to alter miRNA regulations, have been associated with human disorders. MiRNAs have also been found to improve the gene-regulatory processes in cerebrovascular diseases [24]. Given the structure and localization of miRNAs, it has been suggested that miRNAs might be useful in determining peripheral biomarkers and treating human diseases [25].
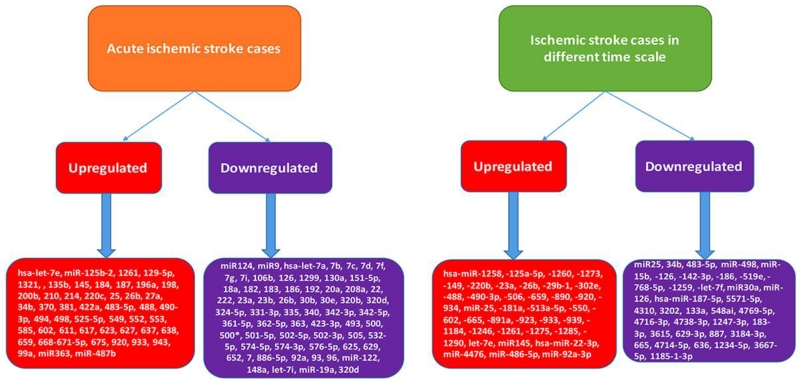
MiRNAs have also been found to be important regulators of leukocyte gene expression in acute IS cases [26]. Clinical approaches accessible for the diagnosis and prognosis of stroke were restricted to radiological imaging, which was with limited availability and higher cost. Diagnosis of early stage of stroke and its development could be improved through the finding of new biomarkers. Many studies have shown that miRNAs altered after central nervous system injury moderate processes that stimulate neuronal death with inflammation, apoptosis and oxidative stress. Furthermore, miRNAs can act as sensitive biomarkers of secondary brain damage [27]. Several studies have found circulating miRNAs change following stroke in humans (Fig. 3). Table 1 summarizes human studies investigating the role of miRNAs in the progression of IS patients.
Figure 3.
Differentially regulated miRNAs in ischemic stroke patients with different conditions i.e., acute ischemic stroke cases and ischemic stroke cases in different time scale with respect to normal healthy controls.
Table 1. Summary of miRNAs studied in patients with Stroke.
| Stroke type |
Number of Patients (M/F) |
Number of Controls (M/F) |
Age Group (yrs) |
Onset | Sampling Criteria |
Sample source |
Methodolo gy |
Total miRNAs Detected |
Upregulated | Down regulated | Reference # |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic Stroke |
8 (6/2) | 4 | 18-49 | 2-24 mont hs |
Without RF of minimal RF |
Blood | Microarray analysis |
293 | hsa-miR-1258, - 125a-5p, -1260, - 1273, -149, -220b, -23a, -26b, -29b-1, -302e, -488, -490- 3p, -506, -659, - 890, -920, -934 |
miR25, 34b, 483- 5p, miR-498 |
29 |
| Ischemic Stroke |
19(10/9) | 5 (3/2) | 18-49 | 6-18 mont hs |
With RF | Blood | Microarray analysis and selective TaqMan qPCR |
157 | miR-25, -181a, - 513a-5p, -550, - 602, -665, -891a, - 923, -933, -939, - 1184, -1246, - 1261, -1275, - 1285, -1290, let-7e |
miR-15b, -126, - 142-3p, -186, - 519e, -768-5p, - 1259, -let-7f |
28 |
| Ischemic Stroke (acute) |
31 (21/10) |
11 | 66.32 ±12.5 6 |
withi n 24 hours |
With RF | Blood | Quantitativ e real time PCR |
4 | miR124, miR9 levels were decreased | 82 | |
| Early onset post stroke depressio n |
3 | 3 | 32-93 | One week |
Without RF |
Blood | Microarray analysis |
1347 | hsa-miR-22-3p, miR-4476, miR- 486-5p, miR-92a- 3p |
hsa-miR-187-5p, 5571-5p, 4310, 3202, 133a, 548ai, 4769-5p, 4716-3p, 4738-3p, 1247-3p, 183-3p, 3615, 629-3p, 887, 3184-3p, 665, 4714-5p, 636, 1234-5p, 3667-5p, 1185-1-3p |
35 |
| Ischemic Stroke |
32 | 14 | 18-49 | -- | With RF | Blood | TaqMan miRNA assay of miR-145 |
1 | Circulating miR- 145 significantly upregulated in ischemic stroke patients |
31 | |
| Ischemic Stroke |
197 | 50 | -- | -- | Without RF of minimal RF |
Blood | Quantitativ e real time PCR |
3 | -- | miR30a, miR-126 | 34 |
| Ischemic Stroke (acute) |
136 | 116 | 50±13 | -- | Without RF of minimal RF |
Blood | Microarray analysis and selective TaqMan qPCR |
-- | 17 upregulated miRNAs and 103 downregulated miRNAs in MRI(−) acute stroke patients compared with MRI(+) acute stroke patients. 33 upregulated miRNAs and 36 downregulated miRNAs in MRI(+) acute stroke patients compared with control subjects. 105 upreupregulated miRNAs and downregulated 37 miRNAs in control subjects compared with MRI(− ) acute stroke patients |
33 | |
| Ischemic Stroke (acute) |
169 (102/67) |
24 (18/6) | -- | 1-7 days |
Without RF of minimal RF |
Blood | Microarray analysis and selective TaqMan qPCR |
105 | hsa-let-7e, miR- 125b-2, 1261, 129- 5p, 1321, , 135b, 145, 184, 187, 196a, 198, 200b, 210, 214, 220c, 25, 26b, 27a, 34b, 370, 381, 422a, 483-5p, 488, 490-3p, 494, 498, 525-5p, 549, 552, 553, 585, 602, 611, 617, 623, 627, 637, 638, 659, 668-671- 5p, 675, 920, 933, 943, 99a |
hsa-let-7a, 7b, 7c, 7d, 7f, 7g, 7i, 106b, 126, 1299, 130a, 151-5p, 18a, 182, 183, 186, 192, 20a, 208a, 22, 222, 23a, 23b, 26b, 30b, 30e, 320b, 320d, 324- 5p, 331-3p, 335, 340, 342-3p, 342- 5p, 361-5p, 362- 5p, 363, 423-3p, 493, 500, 500*, 501-5p, 502-5p, 502-3p, 505, 532- 5p, 574-5p, 574- 3p, 576-5p, 625, 629, 652, 7, 886- 5p, 92a, 93, 96 |
30 |
| Ischemic Stroke (acute) |
112 (78/34) |
60 (31/29) |
53-78 | 3-14 days |
With RF | Blood | Quantitativ e real time PCR |
1 | miR-210 level is correlated to the outcome of stroke |
32 | |
| Ischemic Stroke (acute) |
24 (12/12) |
24 (12/12) |
63.8± 8.5 |
-- | Without RF of minimal RF |
Blood | Microarray analysis and selective TaqMan qPCR |
8 | miR363, miR- 487b |
miR-122, 148a, let-7i, miR-19a, 320d |
26 |
3.1. Whole blood as sources of circulatory miRNAs
Tan et al. (2009) conducted the first study to identify the expression of miRNAs in normal healthy persons (n=5) and in patients between 18 and 49 years of age (n=19) diagnosed with IS according to WHO clinical criteria and TOAST classifications. Using microarray analysis of blood specimens and selective TaqMan qPCR of miRNAs, the researchers evaluated each patient’s functional status at the time of blood sampling. They found that among the 836 miRNAs present on the array chip, 157 miRNAs were differentially regulated in the stroke subjects. Among those miRNAs, 138 miRNAs were highly expressed, and 19 were poorly expressed. Of the highly expressed miRNAs, 17 were upregulated (miR-25, -181a, -513a-5p, -550, -602, -665, -891a, -923, -933, -939, - 1184, -1246, -1261, -1275, -1285, -1290, let-7e), and of the poorly expressed miRNAs, 8 were down regulated (miR-15b, -126, -142-3p, -186, -519e, -768-5p, -1259, -let-7f). The researchers found that analysis of miRNA profiling revealed the following key events that occur during stroke recovery: regulation of hypoxia, angiogenesis, and erythropoiesis/hematopoiesis. They concluded that these miRNAs could be used to differentiate large artery, small artery, and cardio embolic strokes from each other [28].
In 2013, the Tan research group characterized miRNA profiles in patients with low/no risk IS, who did not have pre-existing risk factors for stroke. They correlated the expressions of miRNAs to cerebrovascular lesions caused by cerebral ischemia. They found that 21 miRNAs exhibited similar expression levels in all IS patients (hsa-miR-1258, -125a-5p, -1260, -1273, -149, -220b, -23a*, -25*, -26b*, -29b-1*, -302e, -34b, -483-5p, -488, -490-3p, -498, -506, -659, -890, -920, -934). Among the 21, 17 were up-regulated and 4, down-regulated (miR25, 34b, 483-5p, miR-498). They also found that miR25 was down-regulated in all IS patients, and even the expression level of miR25 was found to be up-regulated in their previous study [28], suggesting that miR25 might be specific for stroke pathogenesis in low-risk stroke patients and might present a different molecular mechanism for its stroke pathogenesis [29].
In investigations of circulatory mRNAs in peripheral blood samples, using a customized TaqMan Low Density Array, Sepramaniam et al. (2014) examined a panel of 32 miRNAs that were hypothesized to distinguish stroke etiologies during the acute phase of stroke. Five miRNAs were consistently altered in blood specimens from acute stroke patients, irrespective of age at the time of stroke, severity of the stroke, and confounding metabolic complications: miR-125b-2*, miR-125b-27a*, miR-125b-422a, miR-125b-488, and miR-125b-627. These five miRNAs were found to be possible biomarkers for diagnosis of stroke [30].
MiRNA levels from peripheral blood cells of 48 patients with IS and 48 patients with a risk factor for IS (controls) were compared [26] using Affymetrix Gene Chip miRNA 3.0 arrays. Expression levels of six miRNAs were down-regulated (miR-122, miR-148a, let-7i, miR-19a, miR-320d, and miR-4429), and two were up-regulated (miR-363 and miR-487b) in the IS patients. These two miRNAs might be important regulators of leukocyte gene expression in acute IS.
Gan et al. (2012) also studied the role of circulatory miRNA-145 expression in IS patients (n = 32) and 14 healthy control subjects (n = 14) who had no identifiable risk factors for stroke and no history of cardiovascular and cerebrovascular diseases. Using TaqMan Real-Time PCR, they found that circulatory miRNA-145 expression was up-regulated in the IS patients but not in the controls. This finding argues for miRNA-145 being a possible biomarker for IS and useful to elucidate mechanotransduction of IS in stroke patients [31].
Zeng and colleagues. (2011) conducted the study to evaluate whether miRNA-210 could be a blood biomarker for acute cerebral ischemia because miRNA-210 is a master and pleiotropic hypoxia-miRNA, and it plays multiple roles in brain ischemia [32]. Using quantitative PCR, they measured miRNA-210 in blood samples from stroke patients (n=112) and healthy controls (n= 60). They found that, compared to healthy controls, miRNA-210 was significantly decreased in stroke patients, especially at 7 days and 14 days after stroke onset (0.56 vs. 1.36; P=0.001, respectively, and 0.50 vs. 1.36; P=0.001, respectively). They also found that the level of miR-210 in blood drawn from stroke patients was significantly higher than in blood samples from patients who never had a stroke. These findings suggest that miR-210 in blood samples from acute IS patients might be useful in diagnosing and prognosing stroke, and it might also be useful in predicting the response of stroke patients.
3.2. Plasma as sources of circulatory miRNAs
Wang et al. (2014) studied circulating miRNAs in blood plasma from subjects with acute stroke and control subjects to determine whether circulating miRNAs can serve as possible biomarkers for acute stroke in humans [33]. Using miRNA microarrays and real-time PCR analyses, they found that hsa-miR-106b-5P and hsa-miR-4306 were present in high abundance in patients of acute stroke, whereas hsa-miR- 320e and hsa-miR- 320d were present in low abundance in control subjects. The following four miRNAs were up-regulated in acute stroke patients compared to the control subjects: hsa-miR-106b-5P (3.63-fold in MRI(−) patients and 23.90-fold in MRI(+) patients), hsa-miR-4306 (3.19-fold in MRI(−) patients) and 5.30-fold in MRI(+) patients hsa-miR-320e, 0.33-fold in MRI(−) patients and 0.13-fold in MRI(+) patients and hsa-miR-320d, .23-fold in MRI(−) patients and .07-fold in MRI(+) patients. Based on the up regulation of these miRNAs, Wang et al. suggested that circulatory miRNAs in blood plasma might be promising biomarkers for the early detection of acute stroke in humans [33].
Three miRNAs were identified as possible peripheral biomarkers of IS: miR-30a, miR-126, and let-7b [34]. Expression levels of circulating miR-30a and miR-126 levels were down-regulated in IS patients until 24 weeks. The level of circulating let-7b was up-regulated in comparison to lower in patients with large-vessel atherosclerosis than healthy volunteers, whereas circulating let-7b had higher level in patients with other kinds of IS till 24 weeks [34].
In a recent study, Zhang et al. (2016) analyzed the circulating miRNA profile expression in patients with early onset post-stroke depression [35]. They randomly selected cases for miRNA microarray analysis and found 25 differentially expressed circulating miRNAs with fold change ≥2 and p≤0.05 between both subject groups. In the microarray analysis, four miRNAs were upregulated (hsa-miR-22-3p, miR-4476, miR-486-5p, and miR-92a-3p) and 21 miRNAs were down regulated (hsa-miR-187-5p, 5571-5p, 4310, 3202, 133a, 548ai, 4769-5p, 4716-3p, 4738-3p, 1247-3p, 183-3p, 3615, 629-3p, 887, 3184-3p, 665, 4714-5p, 636, 1234-5p, 3667-5p, and 1185-1-3p). Using microarray analysis of has-miR133, -92a-3p and 187-5p, they validated their microarray results [35].
Overall, findings from the above studies are useful and provide new information about circulating miRNAs, indicating that miRNAs are potential peripheral biomarkers for stroke.
4. MiRNA profile and function in animal brain
Recently several studies found differentially expressed miRNAs with ischemic brain damage, which were identified using miRNA profiling techniques in mice and rat MCA occlusion (MCAO) models [36, 37]. MiRNAs are a newly discovered non-coding small RNA molecules that negatively regulate target gene expression and are involved in the regulation of cell proliferation and cell apoptosis [37].
The mechanisms of neuroprotection induced by fastigial nucleus stimulation (FNS) were not entirely understood. Huang et al. (2015) investigated the association between miR- 29c expression and neuroprotective effects using a stroke animal model. MiR-29c was decreased after FNS and it attenuates ischemic neuronal apoptosis by negatively regulating apoptotic proteins Birc2 and Bak1. Therefore, miR- 29c might be involved in apoptosis processes of neuroprotection induced by FNS in stroke [38].
Liu and coworkers (2015) investigated the role of miR-424 in transient cerebral ischemia in mice with a focus on oxidative stress–induced neuronal injury. MiR-424 levels in the peri-infarct cortex increased at 1 and 4 hours then decreased 24 hours after reperfusion. Further, they have concluded that miR-424 protects against transient cerebral ischemia/reperfusion injury by inhibiting oxidative stress [39].
Chi and colleagues studied the role of miR-134 in an ischemic mouse model [36]. They found miR-134 expression levels were increased in mouse brain from 12 h to 7day reoxygenation/reperfusion after 1h MCAO treatment. MiR-134 overexpression endorsed neuronal cell death and apoptosis by decreasing HSPA12B protein levels. Conclusively, miR-134 might impact neuronal cell survival against ischemic injury in mouse brain with IS by negatively modulating HSPA12B protein expression in a posttranscriptional manner [36].
Vinciguerra et al. (2014) studied the neuroprotective effects of miRNAs in stroke and also to set up a valid therapeutical approach able to contrast the role of specific miRNAs that regulate NCX expression under experimental conditions imitating stroke. NCX1 physiological expression was dramatically reduced when cortical neurons were treated with mir-103-1. AntimiR-103-1 prevented NCX1 protein down regulation induced by the increase in miR 103-1 after brain ischemia, thereby reducing brain damage and neurological deficits. Further they concluded that blocking mir-103-1 by microRNA inhibitors was a reasonable strategy to stop neuro detrimental regulation of NCX occurring during ischemic conditions [40].
MiR-124 is the most abundant miRNA in the brain. Studies have shown that miR-124 is clearly reduced in the ischemic brain after stroke. MiR-124 was an endogenous regulator of Ku70 that improves ischemia/reperfusion (I/R)-induced brain injury and dysfunction [41]. Sun and colleagues studied the role and underlying mechanism of miR-124 in stroke. The level of miR-124 was significantly increased in ischemic penumbra as compared with that in non-ischemic area of MCAO mice. Further, they have concluded that miRNA-124 protects neurons against apoptosis in cerebral ischemic stroke [42].
Several studies reported that miRNAs can regulate amyloid precursor protein (APP) and β-site APP cleaving enzyme 1 (BACE1) expressions. Ai et al. (2013) evaluated the effect of microRNA on memory impairment in rats induced by chronic brain hypoperfusion (CBH). They found that miR-195 was down regulated in both the hippocampal and cortical tissues of rats following CBH, and in the plasma of dementia patients. APP and BACE1 proteins were down regulated by miR-195 overexpression, upregulated by miR-195 inhibition, and unchanged by binding-site mutation or miR-masks, indicating that APP and BACE1 were two potential targets for miR-195 and might be a potentially valuable anti-dementia approach [43].
Selvamani and coworkers studied the neuroprotective function of an antagomir to microRNAs which involved in the conserved IGF (insulin like growth factor) regulatory pathway [44]. They hypothesized miRNAs that target the IGF-1 signaling family for translation repression could be alternatively suppressed to promote IGF-1-like neuroprotection. An antagomir to miRNA let7f promotes neuroprotection in an IS model. Further, they have concluded that gender-specific role for miRNA, where anti-Let7f mediated neuroprotection was only seen in ovary-intact females, underscoring the importance of the endocrine environment for miRNA actions [44].
MiR-223 is also expressed in the nervous system and controls the expression and function of GluR2 and NR2B subunits of the glutamate receptor. Harraz et al. (2012) showed that miR-223 is a neuroprotective miRNA using in vivo and in vitro models of ischemic reperfusion brain injury and excitotoxic neuronal death. Further, concluded that a therapeutic role for miR-223 in stroke and other excitotoxic neuronal disorders [45].
5. Protein Biomarkers in Ischemic Stroke
A single biomarker might not be adequate to identify underlying complexities known to underlie cellular changes linked to disease and to discriminate diseased from healthy individuals. A biomarker panel that reflects diverse pathophysiological characteristics of a disease or syndrome might be needed to capture the complexities of a particular disease. For example, a biomarker panel for stroke could provide information about inflammation, atherosclerosis, cerebral ischemia, blood–brain barrier disruption, thrombus formation, oxidative stress, and endothelial injury. Biomarker panels have been sought to improve the diagnosis of stroke and its cause. A biomarker needs to identify a particular feature of disease state as accurately and specificity as possible and to be presented as clearly as possible for use by clinicians [46]. Some methods have been combined to identify protein biomarkers, such as western blot, enzyme-linked immune sorbent assay, immunohistochemical staining, and mass spectrometry.
Use of these procedures facilitates the identification of a wide variety of proteins as possible biomarkers for IS. Such proteins include: Heart-type Fatty Acid Binding Protein (H-FABP), Malondialdehyde (MDA), Low-Density Protein Oxidized (LDL-oxidized), Brain-Derived Neurotrophic Factor (BDNF), S100 Calcium Binding Protein B (S100B), Neuron-Specific Enolase (NSE), Glial Fibrillary Acidic Protein (GFAP), N-Methyl D-Aspartate Receptor Antibody (NMDA-R-Ab), Myelin Basic Protein (MBP), C-Reactive Protein (CRP), Vascular Cell Adhesion Protein 1 (VCAM-1), Matrix Metallopeptidase 2 (MMP-2), Matrix Metallopeptidase 9 (MMP-9), Apolipoprotein A2 (ApoA2), Apolipoprotein C1 (ApoC1), Apolipoprotein C3 (ApoC3), D-dimer, von Willebrand Factor (vWF), Parkinson Protein 7 (PARK7), Nucleoside Diphosphate Kinase A (NDKA), Brain-Derived Neurotrophic Factor (BDNF), Intercellular Adhesion Molecule 1 (ICAM-1), Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), Lipoprotein-Associated Phospholipase A2 (Lp-PLA2), Fibrinogen, Ubiquitin Fusion Degradation 1 (UFD-1), Inhibitor of Meristem Activity (IMA), and Insulin-Like Growth Factor Binding Protein 4 (IGFBP4). These proteins have a crucial role in Central Nervous System (CNS) tissue injury, inflammatory and coagulation/thrombosis biomarkers for IS (Table 2).
Table 2. Ischemic stroke protein biomarkers and their proposed clinical applications in humans.
| Name of Protein Biomarker |
Origin and Description of Biomarker | Association | Proposed Clinical Application |
References |
|---|---|---|---|---|
| MBP | myelin sheath of oligodendrocytes and Schwann cells in the nervous system/myelination of CNS and PNS |
CNS tissue injury |
Diagnosis, infarct volume and stroke severity |
60, 61, 83 |
| NMDA-R- Ab |
glutamate-gated ion channel protein/ efficiency of synaptic transmission and autoantibodies to the NMDA receptor |
CNS tissue injury |
Diagnosis, infarct volume and stroke severity |
58, 59, 84 |
| S100B | cytoplasm, nucleus of a wide range of cells and astrocytes/ cell cycle progression and differentiation, calcium binding protein from glial cells |
CNS tissue injury |
Diagnosis, infarct volume and stroke severity |
60, 61, 63-65 |
| Fibrinogen | blood-borne glycoprotein/ platelet activation and blood coagulation |
Coagulation /thrombosis |
Stroke risks | 49, 50, 85, 86 |
| Lp-PLA2 | inflammatory cells/ hydrolyze phospholipids into fatty acids and other lipophilic molecules |
Coagulation /thrombosis |
Stroke risks | 51-54, 87, 88 |
| ApoC-I/III | expressed in the liver, lipoproteins/ lipid metabolism |
Coagulation /thrombosis |
Diagnosis | 55, 56 |
| TNF-α | macrophages, cytokines/ cell proliferation, differentiation, apoptosis, lipid metabolism, inflammation and coagulation |
inflammatory responses |
Infarct volume, stroke severity |
67-69, 72 |
| IL-6 | T cells and macrophages/ inflammation and the maturation of B cells, acute phase response |
inflammatory responses |
Infarct volume, stroke severity |
70-74, 89 |
| CRP | liver synthesis/ acute phase response to tissue injury, infection or other inflammatory stimuli |
inflammatory responses |
Diagnosis, stroke risk | 75-81 |
5.1. Biomarkers for Coagulation and Thrombosis in Stroke
Biomarkers identifying the coagulation cascade have been linked to IS and severe thrombus in cerebral circulation; such biomarkers include the fibrogens vWF, D-Dimer, Lp-PLA2, ApoA2, ApoC1, and ApoC3. Among these fibrinogens, Lp-PLA2 and Apo CI/III have been described the most in recent years. Fibrinogen, a blood-borne glycoprotein, is a coagulation factor responsible for blood clotting. Fibrinogen has been significantly associated with stroke, coronary heart disease, and other diseases that cause vascular and nonvascular mortality [47].
The CardioVascular Disease Risk Factors Two-township Study, a community-based follow-up study, focused on identifying biomarkers for cardiovascular diseases and their risk factors. In one such study, a dose–response relationship was associated with the risk of IS and tertiles of fibrinogen (hazard ratio 3.73; 2.19-1.00). A 72% increase (hazard ratio, 1.72; 1.02-2.90) in the risk of IS was found in individuals with fibrinogen at least 8.79 μmol/L compared with those individuals with fibrinogen less than 7.03 μmol/L [48].
In one study of first IS or transient ischemic attack patients (n = 124) and healthy control subjects (n = 125) aged 18 to 75 years old, fibrinogen γ/total fibrinogen ratio was higher in patients than in control subjects during the patients’ acute phase of stroke and lower in the convalescent phase (3 months after stroke [49]. A number of studies were also documented that found elevated levels of fibrinogen in patients with risks of recurrent IS events, but the elevated levels were not as high as in previous studies of fibrinogen [49, 50].
Lp-PLA2, Apo CI/III were reported to be associated with increased risk for stroke. Lp-PLA2, 45-kDa protein of 441 amino acids catalyzes the degradation of platelet-activating factor to biologically inactive products. Lp-PLA2 is an enzyme expressed primarily by leukocytes that is active in the metabolism of low density lipoprotein to pro-inflammatory mediators. It is also a vascular specific inflammatory biomarker extremely expressed in the necrotic core of atherosclerotic plaque and is linked to plaque inflammation and variability.
The Atherosclerosis Risk in Communities Study found that the level of Lp-PLA2 might be a risk factor to identify middle-aged individuals at increased risk for their first IS event and the might be complementary beyond traditional risk factors in identifying middle-aged individuals at increased risk for IS. Further, they have concluded that future studies should regulate whether discriminating inhibition of Lp-PLA2 reduces IS and whether statins and/or fibrates were more effective for stroke prevention in patients with elevated levels of Lp-PLA2 [51].
Similarly, in a study to determine protein markers, Oei et al. (2005) conducted a prospective population based cohort study comprising 7983 men and women at least 55 years of age. They found that Lp-PLA2 is an independent predictor of IS in the general population. Further, the results suggested that the effect of Lp-PLA2 on cardiovascular disease was independent of a subject’s total cholesterol level and markers of inflammation [52].
Elkind et al. (2009) found that Lp-PLA2 (adjusted hazard ratio, 2.08; 95% confidence interval, 1.04-4.18) might be a stronger predictor of recurrent stroke risk [53]. A few years later, Elkind et al. (2009) conducted a population-based Northern Manhattan Stroke Study with first IS patients at least 40 years of age to determine protein markers and found that stroke patients with Lp-PLA2 activity levels in the highest quartile had an increased risk of another stroke after their first IS [54].
In a recent study to identify protein markers, specifically the protein Apo CI/III, Allard et al. (2004) found that apo C-I and Apo C-III were the potential markers in blood plasma to discriminate between IS and HS [55]. In a recent study, Lopez et al. (2012) used a reaction monitoring assay to a panel of nine apolipoproteins. They found that the plasma levels of specific apolipoproteins, including apo C-I and apo C-III, distinguished (with high sensitivity and specificity) IS, HS, and normal patient sample groups from each other [56].
Findings from the above studies are useful and provided new insights in understanding coagulation and thrombosis involvement in ischemic stroke.
5.2. Biomarkers for Central Nervous System Tissue Injuries in Stroke
Numerous biomarkers have been associated with central nervous system tissue injury, including S-100B, GFAP, NSE, NMDA-R-Ab and MBP. These might also be valuable in forecasting clinical consequences in patients with IS. The CNS tissue injury biomarkers were limited by some factors as markers for IS. They were not more detailed to IS, as numerous illness developments could harm the brain. BBB limits discharge of CNS biomarkers into systemic flow. As an outcome, biomarker levels might not associate with infarct volume or stroke severity given that the breakdown of the BBB is flexible between IS and the anatomic site of stroke and has dissimilar clinical impressions. NMDA is a glutamate-gated ion channel protein family. NMDA receptors are both ligand-gated and voltage-dependent and involve long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission that supposedly triggers definite classes of memory and learning [57].
In 2003, Dambinova et al. investigated the diagnostic accuracy of serum auto antibodies (aAbs) to NR2A/2B, a subtype of NMDA receptors, in evaluating transient ischemic attack (TIA) and IS and its ability to discriminate IS from ICH in 105 TIA/stroke patients and 255 age and sex matched healthy controls [58]. NR2A/2B aAbs were independent and sensitive serologic markers capable of detecting TIA with a high post-test probability and, in conjunction with neurologic observation and neuroimaging, ruling out ICH. Further, they demonstrated that some NMDA receptors were able to differentiate acute IS from ICH patients [58].
Stanca et al. (2015) sought to determine protein markers, using 49 subjects with IS, 23 subjects who had ICH, and 52 controls. Their data revealed that NMDA have significantly higher levels during an entire IS episode at all time-points, and a quantification of NMDA in IS patients might sufficiently distinguish IS patients from ICH patients. When these researchers used NMDA in combination with GFAP, also a marker, they could differentiate between ischemic and hemorrhagic, at 12 hours after stroke with a sensitivity and specificity of 94% and 91%, respectively [59].
Another possible biomarker to identify the onset of IS is myelin basic protein (MBP), a hydrophilic protein found in myelin sheaths. Higher serum levels of MBP were found in a range of acute neurological disorders. Hill et al. designed a preliminary prospective cohort study to determine whether a panel of biochemical markers could distinguish acute IS cases. They found elevated levels of MBP in only 39% patients, and peak level of MBP did not significantly correlate with discharge of modified Ranking Scale (MRS) [60].
Jauch et al. (2006) sought to determine protein markers. They used an NIH stroke scale to determine stroke markers. They found that a higher 24-hour peak concentration of MBP was associated with higher National Institutes of Health Stroke Scale baseline scores (r=0.186, P<0.0001) and also that MBP became elevated within the first 24 hours after stroke, although they did not peak until some days after stroke [61].
S110B is an astroglial protein that has been studied as a serum marker for cerebral injury and disruption of the BBB. The quantity of S100B varies under normal conditions, but during an ischemic injury, S100B increases [62]. S100B has also been used as an independent predictor and diagnostic marker for stroke.
Lynch et al. [2004] sought to determine protein markers. They enrolled 65 IS patients and 157 control subjects and analyzed 26 blood-borne biochemical markers that were hypothesized to play a crucial role in the IS cascade. Out of these 26 markers, S100B correlated highly with stroke and with other inflammation and thrombosis biomarkers [63]. Similar findings were documented in other studies as well [60, 61, 64, 65].
Foerch et al. (2004) conducted a retrospective study with 275 patients with IS (mean age 69±13 years; 46% female) who had received thrombolytic therapy within 6 hours of symptom onset. They found elevated S100B serum levels before thrombolytic therapy constituted an independent risk factor for hemorrhagic transformation (HT) in patients with acute stroke [66].
Overall, these studies suggest that markers derived from brain injuries can be used as biomarkers for stroke.
5.3. Biomarkers of Inflammatory Responses in Stroke
There is extensive literature supporting the role of inflammatory responses playing a central role in IS pathogenesis. Key factors in the inflammatory responses are the transcriptional regulators, and adhesion and signaling molecules. These biomarkers are used in stroke diagnosis to differentiate clinical subtypes of stroke. TNF-α, an acute phase protein, is involved in systemic inflammation and regulation of immune cells.
According to Tuttolomondo et al. (2008) cytokines are involved in pathogenesis of IS. Furthermore, they found that TNF-α was activated in experimental ischemia. They also observed increased levels of TNF-α in patients who had been diagnosed with experimental brain ischemia [67].
Using IS patients (n = 39), HS patients (n = 38), and healthy controls (n = 47), Bokhari et al. [2014] studied the role of TNF-α in identifying protein marker. They found plasma TNF-α levels (p<0.001, r=0.503, CI: 18.197–1672.950) to be significantly elevated in stroke patients, in IS and HS subtypes, indicating that TNF-α is a promising protein marker for IS and HS patients [68].
In a recent study, Greisenegger et al. (2015) studied 15 biomarkers to determine protein markers for IS. Of the 15 biomarkers, TNF-α, IL6 were independently predictive of all-cause death. They found that IL-6 could become a pro-inflammatory cytokine and anti-inflammatory cytokine. High levels of IL-6 were observed in stroke patients, and served the vital role of a messenger molecule among leucocytes, the vascular endothelium, and parenchyma resident cells [69].
In a cross-sectional descriptive study, Shaafi et al. (2014) measured serum IL-6 levels in patients with acute IS (n=45). They found a significant positive correlation among IL-6, NIHSS, and mRS (P<0.001, r=0.6), and a significant correlation between IL-6 and infarction size, as determined by an MRI scan of the brain. They concluded that IL-6 is associated with the severity of IS as well as clinical outcomes [70].
Smith et al., (2004) sought to determine inflammatory response protein markers in stroke patients. They found that the concentration of IL-6 in the blood plasma significantly correlated (p<0.001) with the infarct volume of CT brain infarct volume (r=0.75) and MRS at 3 months post stroke (r=0.72) [71]. A series of studies from different populations have also revealed that IL-6 was associated with stroke [72-74].
Another protein that has been studied as a possible biomarker for IS is the protein CRP. CRP has already been identified as a strong biomarker for inflammation in various diseases, such as stroke, cancer, diabetes, and coronary artery disease. It is produced mainly by the liver and is regulated by inflammatory cytokines. CRP is also associated with measures of clinical stroke severity, as a major predictor of both death and functional outcomes after stroke.
To investigate protein markers, Alvarez-Perez et al. (2011) conducted a prospective controlled clinical study of 200 IS patients and 50 control subjects. They found an association between raised levels of CRP and atherothrombotic and cardio embolic strokes, suggesting that CRP might be characteristic of both the response at the acute phase of stroke and endothelial inflammatory processes [75].
Singh et al. (2014) sought to determine protein markers in stroke patients. They studied the serum CRP levels in IS patients and concluded that the level of CRP level is a good prognostic indicator of IS patients at the time of discharge and exhibits increased utility as a biomarker to identify. They also found that increased levels of CRP might predict future outcome of stroke in terms of mortality and morbidity [76]. These findings were in agreement with many other studies that sought to determine protein markers [77-81].
Overall, findings from the above studies clearly suggest that inflammatory responses can be used as biomarkers of stroke. However, further research is needed to identify precise protein markers linked to inflammatory responses, in the early stages of stroke.
6. Conclusions and Future Directions
Stroke is the second leading cause of death and the third leading cause of disability-adjusted, life-ears worldwide. The risk of having a stroke increases with age of 55 but it can occur at any age. Stroke is an interruption to the supply of blood to the brain, with developing clinical signs of disruption of cerebral function leading to death with no apparent cause other than vascular origin. Cerebral abnormalities in stroke, particularly ischemic stroke, may lead to biochemical dysfunction in the brain, ultimately leading to VaD and AD. Recent research revealed that stroke can be controlled by modifiable risk factors, including diet, cardiovascular, hypertension, smoking, diabetes, obesity, metabolic syndrome, depression and traumatic brain injury.
Multiple approaches have been developed to identify biomarkers, including circulatory miRNAs, blood based protein markers, coagulation and thrombosis biomarkers. Among these, circulatory miRNAs are reported to be promising peripheral biomarkers in stroke and stroke linked VaD and AD. Although much research has been done on ischemic stroke and its molecular and cellular links with VaD, 1) we still do not know whether stroke-associated circulatory miRNAs can be used for VaD and AD and 2) we still do not have complete understanding of the genetic basis of ischemic stroke leading to VaD and AD. Further research is needed to answer these important questions.
Highlights.
Stroke is the major risk factor for vascular dementia and Alzheimer’s disease.
Stroke occurs when blood flow stops, and that stoppage results in reduced oxygen supply to neurons in the brain.
The occurrence of stroke increases with age, but anyone at any age can suffer from stroke.
Stroke is associated with modifiable and non-modifiable risk factors.
Circulatory microRNAs can be used as peripheral biomarkers of stroke.
Acknowledgements
Work presented in this article is supported by NIH grants – AG042178 and AG47812 and Garrison Family Foundation.
Abbreviations
- CT
computed tomography
- SVD
small vessel disease
- LVD
large vessel disease
- AD
Alzheimer’s disease
- VaD
Vascular Dementia
- CT
Computerized Tomography
- MRI
Magnetic Resonance Imaging
- BBB
Blood Brain Barrier
- DALYs
Disability-Adjusted Life Years
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- IS
Ischemic Stroke
- HS
Hemorrhagic Stroke
- miRNA
microRNA
- H-FABP
Heart-type Fatty Acid Binding Protein
- MDA
Malondialdehyde
- LDL-oxidized
Low-Density Protein Oxidized
- BDNF
Brain-Derived Neurotrophic Factor
- S100B
S100 Calcium Binding Protein B
- NSE
Neuron-Specific Enolase
- GFAP
Glial Fibrillary Acidic Protein
- NMDA-R-Ab
N-Methyl D-Aspartate Receptor Antibody
- MBP
Myelin Basic Protein
- CRP
C-Reactive Protein
- VCAM-1
Vascular Cell Adhesion Protein 1
- MMP-2
Matrix Metallopeptidase 2
- MMP-9
Matrix Metallopeptidase 9
- ApoA2
Apolipoprotein A2
- ApoC1
Apolipoprotein C1
- ApoC3
Apolipoprotein C3
- vWF
D-dimer, von Willebrand Factor
- PARK7
Parkinson Protein 7
- NDKA
Nucleoside Diphosphate Kinase A
- BDNF
Brain-Derived Neurotrophic Factor
- ICAM-1
Intercellular Adhesion Molecule 1
- IL-6
Interleukin-6
- TNF-α
Tumor Necrosis Factor-α
- Lp-PLA2
Lipoprotein-Associated Phospholipase A2
- CNS
Central Nervous System
- aAbs
auto antibodies
- MRS
modified Ranking Scale
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Authors declare that they do not have conflict of interest in the content present in this manuscript.
References
- 1.WHO MONICA Project Principal Investigators The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J. Clin. Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Feigin V, Lawes C, Bennet D, Barker Cello S, Parag V. Worldwide stroke incidence and early case fatality in 56 population based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 7.WHO The atlas of heart disease and stroke. 2011 Retrieved 19.10.2011 from http://www.who.int/cardiovascular_diseases/resources/atlas/en/
- 8.Banarjee TK, Roy MK, Bhoi KK. Is stroke increasing in India- preventive measures that need to be implemented. J. Indian. Med. Assoc. 2005;103:162–166. [PubMed] [Google Scholar]
- 9.Sridharan SE, Unnikrishnan JP, Sukumaran S, Sylaja PN, Nayak SD, Sarma PS, Radhakrishnan K. Incidence, types, risk factors, and outcome of stroke in a developing country: the Trivandrum Stroke Registry. Stroke. 2009;40:1212–1218. doi: 10.1161/STROKEAHA.108.531293. [DOI] [PubMed] [Google Scholar]
- 10.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet. Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 11.Towfighi A, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke. 2010;41:499–503. doi: 10.1161/STROKEAHA.109.571828. [DOI] [PubMed] [Google Scholar]
- 12.Henon H, Pasquier F, Durieu I, Godefroy O, Lucas C, Lebert F, Leys D. Preexisting dementia in stroke patients. Baseline frequency, associated factors, and outcome. Stroke. 1997;28:2429–2436. doi: 10.1161/01.str.28.12.2429. [DOI] [PubMed] [Google Scholar]
- 13.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–706. doi: 10.1093/brain/awt188. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murali V, Rathika C, Ramgopal S, Padma Malini R, Arun Kumar MJ, Neethi Arasu V, Jeyaram Illiayaraja K, Balakrishnan K. Susceptible and protective associations of HLA DRB1*/DQB1* alleles and haplotypes with ischaemic stroke. Int. J. Immunogenet. 2016;43:159–165. doi: 10.1111/iji.12266. [DOI] [PubMed] [Google Scholar]
- 15.Vijayan M, Chinniah R, Ravi PM, Sivanadham R, Mosses Joseph AK, Vellaiappan NA, Krishnan JI, Karuppiah B. MTHFR (C677T) CT genotype and CT-apoE3/3 genotypic combination predisposes the risk of ischemic stroke. Gene. 2016 doi: 10.1016/j.gene.2016.06.062. pii: S0378-1119(16)30525-X. [DOI] [PubMed] [Google Scholar]
- 16.Vijayan M, Chinniah R, Ravi PM, Mosses Joseph AK, Vellaiappan NA, Krishnan JI, Karuppiah B. ACE-II genotype and I allele predicts ischemic stroke among males in south India. Meta Gene. 2014;2:661–669. doi: 10.1016/j.mgene.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123:1784–812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 18.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011;6:11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 21.Lee RV, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 22.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 23.Vemuganti R. The MicroRNAs and Stroke: No Need to be Coded to be Counted. Transl. Stroke. Res. 2010;1:158–160. doi: 10.1007/s12975-010-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Liu Y, Xin X, Kim TS, Cabeza EA, Ren J, Nielsen R, Wrana JL, Zhang Z. Evidence for positive selection on a number of MicroRNA regulatory interactions during recent human evolution. PLoS. Genet. 2012;8:e1002578. doi: 10.1371/journal.pgen.1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weidhaas J. Using microRNAs to understand cancer biology. Lancet. Oncol. 2010;11:106–107. doi: 10.1016/S1470-2045(09)70386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One. 2014;9:e99283. doi: 10.1371/journal.pone.0099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vemuganti R. All’s well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochem. Int. 2013;63:438–449. doi: 10.1016/j.neuint.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan JR, Tan KS, Koo YX, Yong FL, Wang CW, Armugam A, Jeyaseelan K. Blood microRNAs in low or no risk ischemic stroke patients. Int. J. Mol. Sci. 2013;14:2072–2084. doi: 10.3390/ijms14012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepramaniam S, Tan JR, Tan KS, DeSilva DA, Tavintharan S, Woon FP, Wang CW, Yong FL, Karolina DS, Kaur P, Liu FJ, Lim KY, Armugam A, Jeyaseelan K. Circulating microRNAs as biomarkers of acute stroke. Int. J. Mol. Sci. 2014;15:1418–1432. doi: 10.3390/ijms15011418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet. Mol. Res. 2012;11:147–152. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- 32.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front. Biosci. (Elite Ed) 2011;1:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Sun G, Zhang L, Shi L, Zeng Y. Circulating microRNAs as novel potential biomarkers for early diagnosis of acute stroke in humans. J. Stroke. Cerebrovasc. Dis. 2014;23:2607–2613. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Cheng L, Chen Y, Yang GY, Liu J, Zeng L. Clinical predictor and circulating microRNA profile expression in patients with early onset post-stroke depression. J. Affect. Disord. 2016;193:51–58. doi: 10.1016/j.jad.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 36.Chi W, Meng F, Li Y, Li P, Wang G, Cheng H, Han S, Li J. Impact of microRNA-134 on neural cell survival against ischemic injury in primary cultured neuronal cells and mouse brain with ischemic stroke by targeting HSPA12B. Brain Res. 2014;1592:22–33. doi: 10.1016/j.brainres.2014.09.072. [DOI] [PubMed] [Google Scholar]
- 37.Pang XM, Liu JL, Li JP, Huang LG, Zhang L, Xiang HY, Feng LB, Chen CY, Li SH, Su SY. Fastigial nucleus stimulation regulates neuroprotection via induction of a novel microRNA, rno-miR-676-1, in middle cerebral artery occlusion rats. J. Neurochem. 2015;133:926–934. doi: 10.1111/jnc.13094. [DOI] [PubMed] [Google Scholar]
- 38.Huang LG, Li JP, Pang XM, Chen CY, Xiang HY, Feng LB, Su SY, Li SH, Zhang L, Liu JL. MicroRNA-29c Correlates with Neuroprotection Induced by FNS by Targeting Both Birc2 and Bak1 in Rat Brain after Stroke. CNS Neurosci. Ther. 2015;21:496–503. doi: 10.1111/cns.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46:513–519. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 40.Vinciguerra A, Formisano L, Cerullo P, Guida N, Cuomo O, Esposito A, Di Renzo G, Annunziato L, Pignataro G. MicroRNA-103-1 selectively downregulates brain NCX1 and its inhibition by anti-miRNA ameliorates stroke damage and neurological deficits. Mol. Ther. 2014;22:1829–1838. doi: 10.1038/mt.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu F, Liu JL, Li JP, Xiao F, Zhang ZX, Zhang L. MicroRNA-124 (miR-124) regulates Ku70 expression and is correlated with neuronal death induced by ischemia/reperfusion. J. Mol. Neurosci. 2014;52:148–55. doi: 10.1007/s12031-013-0155-9. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci. Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ai J, Sun LH, Che H, Zhang R, Zhang TZ, Wu WC, Su XL, Chen X, Yang G, Li K, Wang N, Ban T, Bao YN, Guo F, Niu HF, Zhu YL, Zhu XY, Zhao SG, Yang BF. MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J. Neurosci. 2013;33:3989–4001. doi: 10.1523/JNEUROSCI.1997-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. U S A. 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Llamas G, de la Cuesta F, Barderas ME, Darde V, Padial LR, Vivanco F. Recent advances in atherosclerosis-based proteomics: new biomarkers and a future perspective. Expert. Rev. Proteomics. 2008;5:679–691. doi: 10.1586/14789450.5.5.679. [DOI] [PubMed] [Google Scholar]
- 47.F.S. Collaboration Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality. JAMA: the Journal of the American Medical Association. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 48.Chuang SY, Bai CH, Chen WH, Lien LM, Pan WH. Fibrinogen independently predicts the development of ischemic stroke in a Taiwanese population: CVDFACTS study. Stroke. 2009;40:1578–1584. doi: 10.1161/STROKEAHA.108.540492. [DOI] [PubMed] [Google Scholar]
- 49.Rothwell PM, Howard SC, Power DA, Gutnikov SA, Algra A, van Gijn J, et al. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke. 2004;35:2300–2305. doi: 10.1161/01.STR.0000141701.36371.d1. [DOI] [PubMed] [Google Scholar]
- 50.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–138. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 51.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch. Intern. Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 52.Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 53.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch. Intern. Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 54.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. Lipoprotein-associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc. Dis. 2009;27:42–50. doi: 10.1159/000172633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allard L, Lescuyer P, Burgess J, Leung KY, Ward M, Walter N, et al. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004;4:2242–2251. doi: 10.1002/pmic.200300809. [DOI] [PubMed] [Google Scholar]
- 56.Lopez MF, Sarracino DA, Prakash A, Athanas M, Krastins B, Rezai T, et al. Discrimination of ischemic and hemorrhagic strokes using a multiplexed, mass spectrometry-based assay for serum apolipoproteins coupled to multi-marker ROC algorithm. Proteomics. Clin. Appl. 2012;6:190–200. doi: 10.1002/prca.201100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold. Spring. Harb. Perspect. Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin. Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- 59.Stanca DM, Marginean IC, Soriț au O, Dragoș C, Marginean M, Muresanu DF, et al. GFAP and antibodies against NMDA receptor subunit NR2 as biomarkers for acute cerebrovascular diseases. J. Cell. Mol. Med. 2015;19:2253–2261. doi: 10.1111/jcmm.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill MD, Jackowski G, Bayer N, Lawrence M, Jaeschke R. Biochemical markers in acute ischemic stroke. CMAJ. 2000;162:1139–40. [PMC free article] [PubMed] [Google Scholar]
- 61.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR, NINDS rt-PA Stroke Study Group Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 62.van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC neurol. 2009;9:21. doi: 10.1186/1471-2377-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- 64.Foerch C, Otto B, Singer OC, Neumann-Haefelin T, Yan B, Berkefeld J, et al. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004;35:2160–2164. doi: 10.1161/01.STR.0000138730.03264.ac. [DOI] [PubMed] [Google Scholar]
- 65.Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch. Neurol. 2005;62:1130–1134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 66.Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- 67.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 68.Bokhari FA, Shakoori TA, Butt A, Ghafoor F. TNF-alpha: a risk factor for ischemic stroke. J. Ayub. Med. Coll. Abbottabad. 2014;26:111–114. [PubMed] [Google Scholar]
- 69.Greisenegger S, Segal HC, Burgess AI, Poole DL, Mehta Z, Rothwell PM. Biomarkers and mortality after transient ischemic attack and minor ischemic stroke: population-based study. Stroke. 2015;46:659–666. doi: 10.1161/STROKEAHA.114.007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaafi S, Sharifipour E, Rahmanifar R, Hejazi S, Andalib S, Nikanfar M, et al. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran. J. Neurol. 2014;13:70–76. M. [PMC free article] [PubMed] [Google Scholar]
- 71.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sotgiu S, Zanda B, Marchetti B, Fois ML, Arru G, Pes GM, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur. J. Neurol. 2006;13:505–513. doi: 10.1111/j.1468-1331.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 73.Montaner J, Rovira A, Molina CA, Arenillas JF, Ribó M, Chacón P, et al. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J. Cereb. Blood. Flow. Metab. 2003;23:1403–1417. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- 74.Vila N, Castillo J, Dávalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Usefulness of measurement of fibrinogen, D-dimer, D-dimer/fibrinogen ratio, C reactive protein and erythrocyte sedimentation rate to assess the pathophysiology and mechanism of ischaemic stroke. J. Neurol. Neurosurg. Psychiatry. 2011;82:986–992. doi: 10.1136/jnnp.2010.230870. [DOI] [PubMed] [Google Scholar]
- 76.Singh VK, Haria JM, Jain SK. C-Reactive Protein in Ischemic Stroke-An Experimental study. Int. J. Sci. Stud. 2014;2:25–27. [Google Scholar]
- 77.Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–2020. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 78.Arenillas JF, Alvarez-Sabín J, Molina CA, Chacón P, Montaner J, Rovira A, et al. C-reactive protein predicts further ischemic events in first-ever transient ischemic attack or stroke patients with intracranial large-artery occlusive disease. Stroke. 2003;34:2463–2468. doi: 10.1161/01.STR.0000089920.93927.A7. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhuri JR, Mridula KR, Umamahesh M, Swathi A, Balaraju B, Bandaru VC. High sensitivity C-reactive protein levels in Acute Ischemic Stroke and subtypes: A study from a tertiary care center. Iran J Neurol. 2013;12(3):92–7. [PMC free article] [PubMed] [Google Scholar]
- 80.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 81.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Zhang J, Han R, Liu H, Sun D, Liu X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J. Clin. Neurosci. 2015;22:291–295. doi: 10.1016/j.jocn.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 83.Lamers KJ, Vos P, Verbeek MM, Rosmalen F, van Geel WJ, van Engelen BG. Protein S-100B, neuron-specific enolase (NSE), myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) in cerebrospinal fluid (CSF) and blood of neurological patients. Brain. Res. Bull. 2003;61:261–264. doi: 10.1016/s0361-9230(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 84.Dambinova SA, Khounteev GA, Skoromets AA. Multiple panel of biomarkers for TIA/stroke evaluation. Stroke. 2002;33:1181–1182. doi: 10.1161/01.str.0000014922.83673.86. [DOI] [PubMed] [Google Scholar]
- 85.Cheung EY, Uitte de Willige S, Vos HL, Leebeek FW, Dippel DW, Bertina RM, et al. Fibrinogen gamma’ in ischemic stroke: a case-control study. Stroke. 2008;39:1033–1035. doi: 10.1161/STROKEAHA.107.495499. [DOI] [PubMed] [Google Scholar]
- 86.Mauriello A, Sangiorgi G, Palmieri G, Virmani R, Holmes DR, Jr, Schwartz RS, et al. Hyperfibrinogenemia is associated with specific histocytological composition and complications of atherosclerotic carotid plaques in patients affected by transient ischemic attacks. Circulation. 2000;101:744–750. doi: 10.1161/01.cir.101.7.744. [DOI] [PubMed] [Google Scholar]
- 87.Polupanov AG, Lomteva Iu.N., Khalmatov AN, Cheskidova NB, Romanova TA, Dzhumagulova AS. Lipoprotein-associated phospholipase A2: relation to development of ischemic stroke in patients with essential hypertension. Kardiologiia. 2014;54:29–34. doi: 10.18565/cardio.2014.6.29-34. [DOI] [PubMed] [Google Scholar]
- 88.Wassertheil-Smoller S, Kooperberg C, McGinn AP, Kaplan RC, Hsia J, Hendrix SL, et al. Lipoprotein-associated phospholipase A2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51:1115–1122. doi: 10.1161/HYPERTENSIONAHA.107.103721. [DOI] [PubMed] [Google Scholar]
- 89.Luna JM, Moon YP, Liu KM, Spitalnik S, Paik MC, Cheung K, et al. High-sensitivity C-reactive protein and interleukin-6-dominant inflammation and ischemic stroke risk: the northern Manhattan study. Stroke. 2014;45:979–987. doi: 10.1161/STROKEAHA.113.002289. [DOI] [PMC free article] [PubMed] [Google Scholar]