Abstract
Objective
Sesamol, a phenolic component of lignans, has been previously shown to reduce lipopolysaccharide-induced oxidative stress and upregulate phosphatidylinositol 3-kinase/Akt/endothelial nitric oxide synthase pathways. In the present study, we synthesized a modified form of sesamol (INV-403) to enhance its properties and assessed its effects on atherosclerosis.
Methods and Results
Watanabe heritable hyperlipidemic rabbits were fed with high-cholesterol chow for 6 weeks and then randomized to receive high-cholesterol diet either alone or combined with INV-403 (20 mg/kg per day) for 12 weeks. Serial MRI analysis demonstrated that INV-403 rapidly reduced atherosclerotic plaques (within 6 weeks), with confirmatory morphological analysis at 12 weeks posttreatment revealing reduced atherosclerosis paralleled by reduction in lipid and inflammatory cell content. Consistent with its effect on atherosclerosis, INV-403 improved vascular function (decreased constriction to angiotensin II and increased relaxation to acetylcholine), reduced systemic and plaque oxidative stress, and inhibited nuclear factor–κB activation via effects on nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) phosphorylation with coordinate reduction in key endothelial adhesion molecules. In vitro experiments in cultured endothelial cells revealed effects of INV-403 in reducing IκBα phosphorylation via inhibition of IκB kinase 2 (IKK2).
Conclusion
INV-403 is a novel modified lignan derivative that potently inhibits atherosclerosis progression via its effects on IKK2 and nuclear factor–κB signaling.
Keywords: atherosclerosis, macrophages, vascular biology
Atherosclerosis is a complex, multifactorial disease that involves distinct stages that accrue over a lifetime.1,2 Oxidative stress and inflammation play a fundamental role in the genesis of all of these processes3–6 and represent targets for intervention. Current approved therapies for the prevention and treatment of atherosclerosis suffer from important drawbacks, including the inability to address multiple mechanisms involved in progression in atherosclerosis and side effects, especially those related to unrecognized off-target effects, when targeting highly specific pathways.7,8
Dietary approaches are inherently powerful as they are safe and can be initiated early and over the long term for an individual, providing for the greatest likelihood for success.9–17 A number of dietary strategies have the capability of simultaneously addressing distinct pathophysiologically relevant processes.18–25 Successful implementation, however, warrants complete understanding of the effects of the intervention on these pathways.19 In addition, no single dietary intervention combines all aspects of an ideal intervention.17,18,26–32 Thus, fortification of existing dietary regimens with simple and safe components derived from other regimens may be preferable.17,33 In many Asian cultures, ingredients used in cooking have been implicated in health benefits over thousands of years.34 Oil seeds, such as flaxseeds and soybeans, are well-known repositories of small molecule chemical components that include isoflavones, sterols, tocopherols, tocotrienols, and lignans.23,35–37 Sesamol, a phenolic component of lignan derivatives, such as sesamin and sesaminol, is generated upon roasting of sesame seeds and during the bleaching process of sesame oil, and it has previously been shown to reduce lipopolysaccharide-induced oxidative stress38 and upregulate phosphatidylinositol 3-kinase/Akt/endothelial nitric oxide synthase pathways.39 In the present study, we synthesized a modified form of sesamol (INV-403) and demonstrated that this small molecule markedly inhibited atherosclerosis in a rabbit model, paralleled by a decrease in aortic inflammation oxidative stress and improvement in endothelial function, mediated in part by its effects on inhibition of IKK2 and consequent reduction in nuclear factor-κB (NF-κB) activation without overt changes in plasma lipid profile in a Watanabe heritable hyperlipidemic (WHHL) model.
Methods
Animal Model
The Institutional Animal Care and Use Committee at the Ohio State University approved the experimental animal protocols. Ten male WHHL rabbits (2 months old) were obtained from the Brown family in Odenville, Alabama and allowed to acclimate for 2 weeks before being fed with high-cholesterol chow (fat: 2.7%, wt/wt; cholesterol: 0.5%, wt/wt; Harlan Teklad TD87251) for 6 weeks, at which time point they were randomly assigned to control or INV-403 groups (20 mg/kg per day for 12 weeks). INV-403 was dissolved in 90% ethanol and sprayed onto the high-cholesterol chow. The diet-drug mixture was then vacuum dried overnight to remove ethanol and then used to feed rabbits.
Other Methods
Other methods are described in the Supplemental Methods, available online at http://atvb.ahajournals.org.
Statistics
Results are expressed as means±SD. The unpaired Student t test was used to compare parameters in the INV-403 and control treated groups. Probability values <0.05 were reported as significant. With multiple comparisons, a Bonferroni correction was used for multiple comparisons. In vitro experiments comparing INV-403 with other antioxidants involving multiple groups were analyzed using 1-way ANOVA with a Bonferroni post hoc correction.
Results
We first assessed the effect of INV-403 on atherosclerosis in a rabbit model as illustrated in Figure 1A. To enhance the atherosclerosis progression, all rabbits were fed with high-cholesterol chow for a period of 6 weeks before being randomly assigned to the control or INV-403 group. Dietary supplementation with INV-403 started at the end of 6 weeks for a duration of 12 weeks. This dietary supplementation with INV-403 did not significantly affect rabbit weight (control versus INV-403: 3.16±0.13 versus 3.16±0.08 kg) or the level of any of the plasma lipoprotein subfractions (Supplemental Figure I).
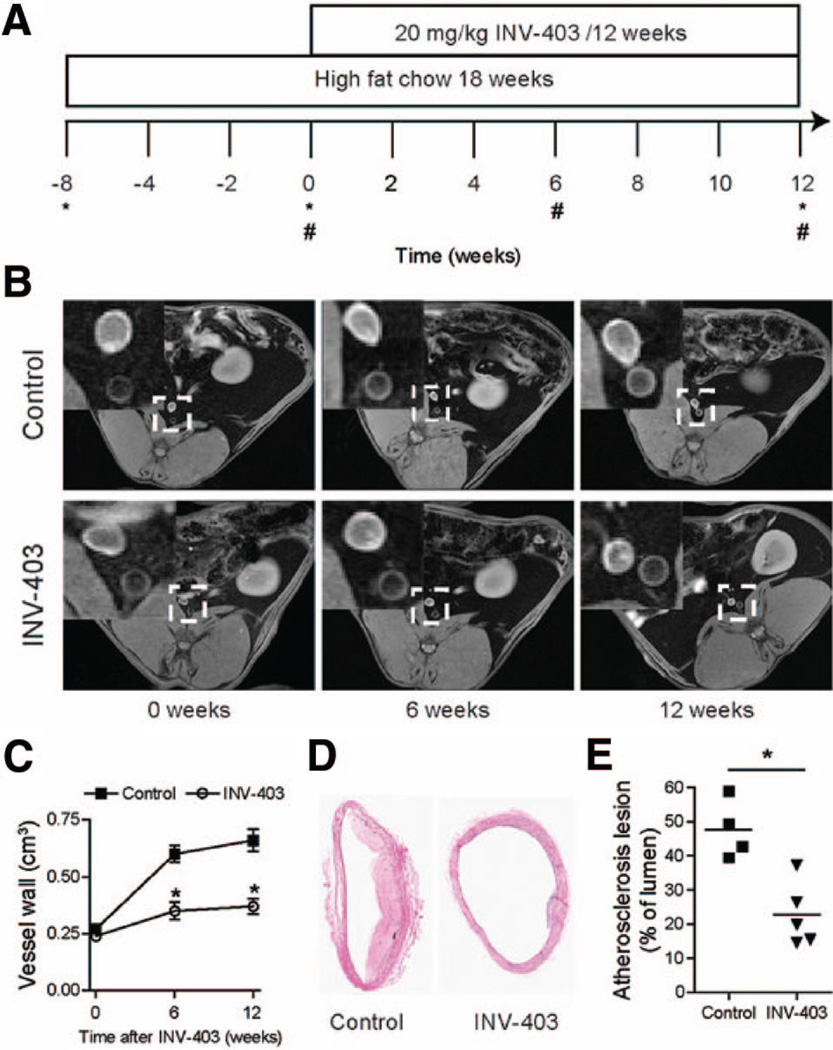
Figure 1.
INV-403 inhibits atherosclerosis. A, Outline of experimental design. *Blood sampling; #MRI scanning. B, Representative images of abdominal aorta MRI scanning. C, Quantitation of abdominal aorta MRI scanning. *P<0.05 versus control, 1-way ANOVA with Bonferroni post tests. D, Representative images (hematoxylin/eosin staining) of thoracic aorta after 12 weeks of INV-403 treatment. E, Quantitation of atherosclerotic plaque in thoracic aorta after 12 weeks of INV-403 treatment. *P<0.05 versus control, Student t test.
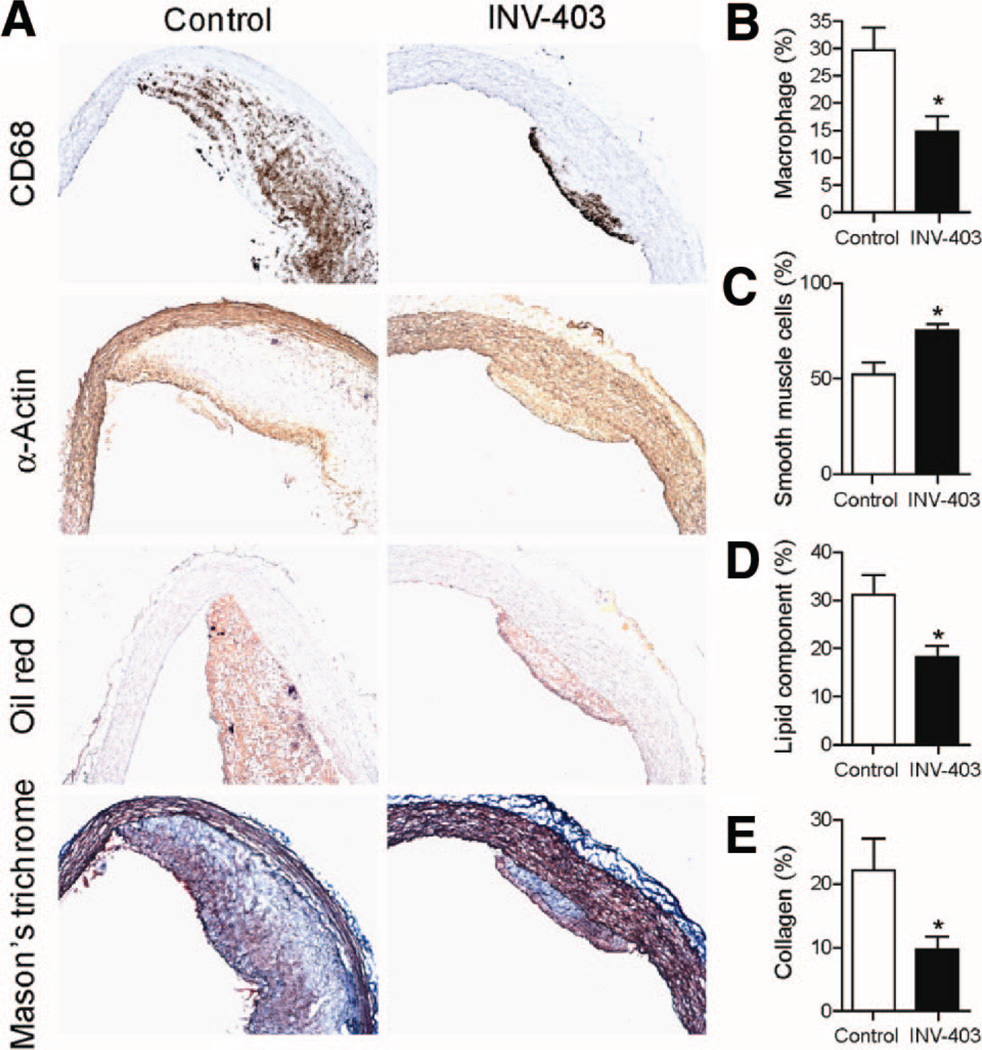
The wall volume in the rabbit abdominal aorta, an indicator of atherosclerotic plaque, was analyzed by serial in vivo MRI scanning. Figure 1B and 1C shows that after 6 weeks of dietary supplementation with INV-403, wall volume in the abdominal aorta was significantly reduced compared with that in the control arm. At the end of 12 weeks, there was a more pronounced slowing of the rate of progression of plaque in the INV-403 group (Figure 1B and 1C). Morphometric assessment of plaque burden in the thoracic aorta at the end of 12 weeks corroborated the antiatherosclerotic effects of INV-403 on the abdominal aorta (Figure 1D and 1E). Immunohistochemical analysis of thoracic aortic sections revealed a reduction in CD68+ cells in atherosclerotic plaque but increased smooth muscle proliferation (Figure 2A and 2B). INV-403 also decreased lipid accumulation and reparative fibrillar collagen (Figure 2C and 2D) in the rabbit aorta.
Figure 2.
INV-403 changes composition of thoracic aorta. After 12 weeks of INV-403 treatment, rabbit thoracic aorta was sectioned and analyzed by immunohistochemistry with indicated antibody or indicated staining. A, Representative images. B to E, Quantitation of the indicated component of thoracic aorta.
*P<0.05 versus control, Student t test.
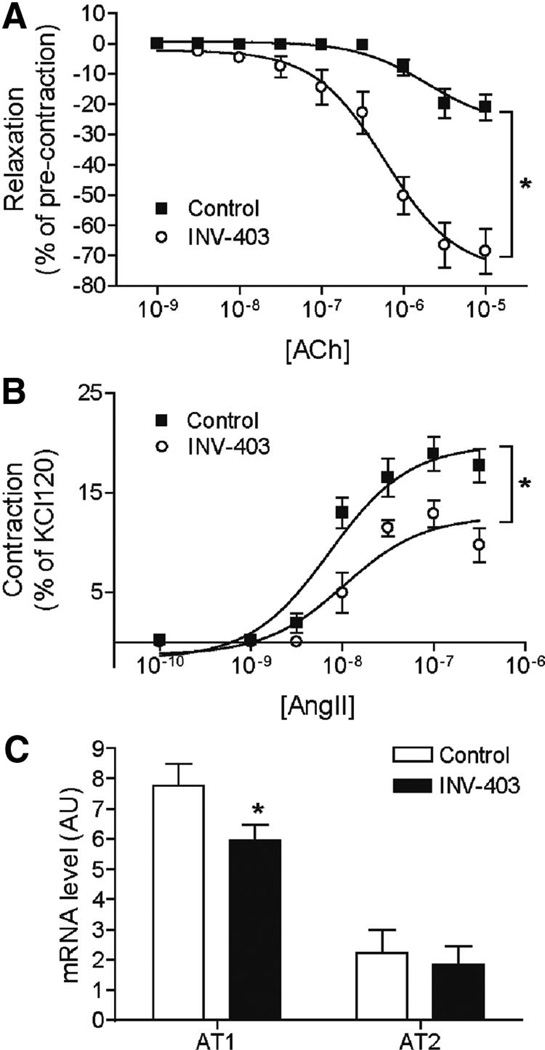
Figure 3 and Supplemental Figure II and the Table illustrate the responses to various agonists in the thoracic aorta of WHHL rabbits. Figure 3A and 3B depicts the effects of INV-403 on vascular function in response to classical agonists of endothelial and smooth muscle function. INV-403 increased relaxation to acetylcholine, whereas aortic constriction to angiotensin II was diminished. In contrast to these effects, INV-403 had no effects on responses to insulin (partial endothelium dependent agonist) or sodium nitroprusside (SNP, an endothelium independent vasodilator) (Supplemental Figure II) and had no effects on constriction in response to phycoerythrin (PE) and endothelin-1. The attenuated response to angiotensin II in INV-403 rabbits was paralleled by decreased mRNA expression of angiotensin II receptor, type 1 (AT1) receptor (Figure 3C). In contrast, treatment with INV-403 did not change the expression of AT2 receptor (Figure 3C).
Figure 3.
INV-403 alters vascular responses. After 12 weeks of INV-403 treatment, rabbit thoracic aorta segments were mounted onto organ bath chambers. A, Thoracic aorta segments were precontracted by PE (0.3 µmol/L), and then responses to indicated concentrations of acetylcholine were analyzed. Results are expressed as percentage of contraction by PE (0.3 µmol/L). *P<0.05 versus control, 2-way ANOVA with Bonferroni post tests. B, Constrictive responses of thoracic aorta segments to angiotensin II (AngII). *P<0.05 versus control, 2-way ANOVA with Bonferroni post tests. C, After 12 weeks of INV-403 treatment, the mRNA expression levels of angiotensin II receptors, AT1 and AT2, in rabbit thoracic aorta segments were measured by real-time reverse transcription–polymerase chain reaction. *P<0.05 versus control, 1-way ANOVA with Bonferroni post tests.
Table.
The Maximal Effect and Log EC50 of Aortic Responses
| PE | ET-1 | AII | Ach | SNP | Insulin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximal | Log EC50 | Maximal | Log EC50 | Maximal | Log EC50 | Maximal | Log EC50 | Maximal | Log EC50 | Maximal | Log EC50 | |
| Control | 132.2±6.5 | 6.6±0.1 | 52.3±9.5 | 7.3±0.3 | 19.8±1.2 | 8.1±0.1 | 27±4 | 5.7±0.2 | 80.8±6.7 | 6.6±0.2 | 69.3±10.7 | 3.7±1.2 |
| INV-403 | 135.4±5.1 | 6.7±0.1 | 47.3±9.1 | 7.5±0.4 | 12.6±1.1* | 8±0.2 | 73.6±2.3* | 6.3±0.1* | 72±5.9 | 6.8±0.1 | 71±9.9 | 4.2±0.7 |
Maximal effects are expressed as % of contraction by KCl (120 mM).
PE, phenylephrine; ET-1, endothelin-1; AII, angiotensin II; Ach, Acetylcholine; SNP, sodium nitroprusside.
P<0.05, two way ANOVA.
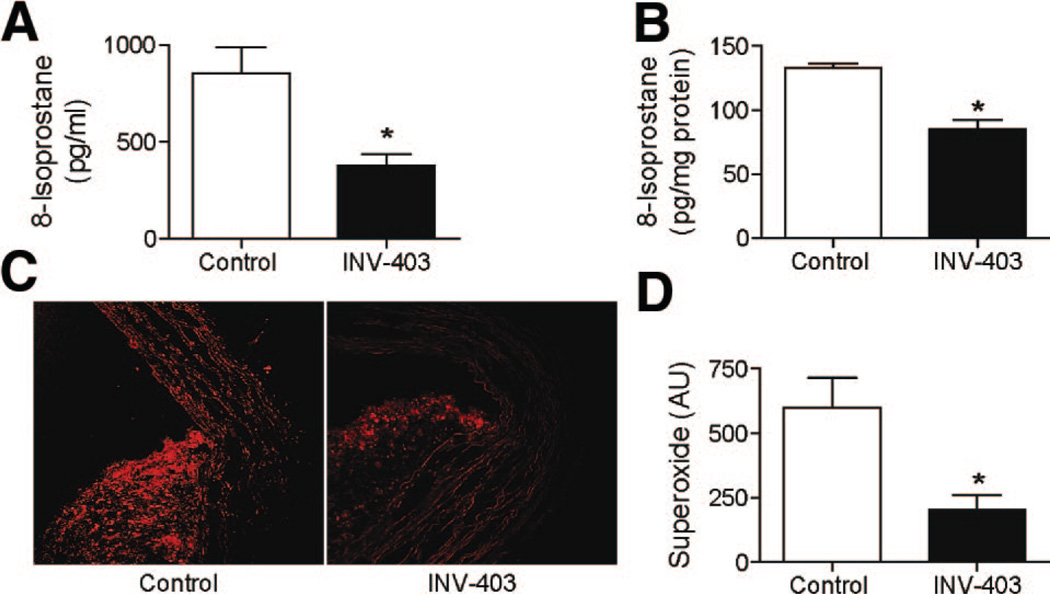
Figure 4A and 4B reveals the effect of INV-403 in reducing oxidative stress markers in plasma of animals and in aortic homogenates respectively. These results were confirmed by in situ aortic O2⨪. measurement using the dihydroethidium staining (Figure 4C and 4D). As phenolic antioxidants have been thought to exert direct antioxidant effects, we tested the effects of INV-403 on superoxide generation in response to NADPH stimulation. INV-403 (up to 1 mmol/L) did not reduce superoxide production on the addition of NADPH, indicating that INV-403 decreases oxidative stress in vivo through mechanisms other than direct scavenging of reactive oxide species (data not shown).
Figure 4.
INV-403 decreases systemic and vascular oxidative stress. A and B, 8-Isoprostane levels in plasma (A) and aortic homogenates (B) were measured by enzyme immuno assay.
*P<0.05, Student t test. C and D, Aortic superoxide generation was analyzed by dihydroethidium staining. A representative picture (C) and the summary of results (D) are shown. *P<0.01, Student t test.
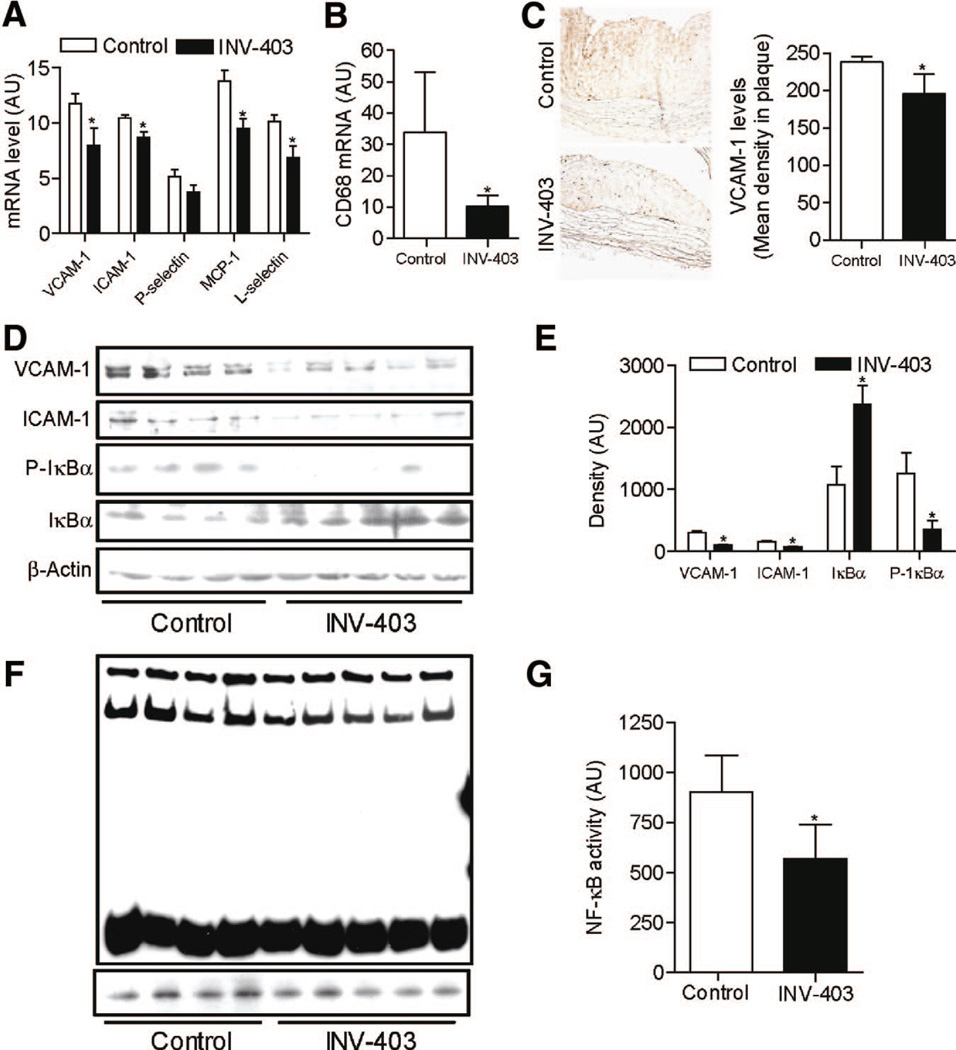
As vascular inflammation plays a pivotal role in atherosclerosis, we assessed proinflammatory gene expression and cellular correlates of inflammation. INV-403 decreased mRNA expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1, monocyte chemoattractant protein-1 (MCP-1), and L-selectin in thoracic aorta (Figure 5A). The mRNA expression of P-selectin showed a nonsignificant decrease in the INV-403 group (Figure 5A). Macrophage infiltration was decreased as evidenced by immunohistochemistry (Figure 2B) and analysis of CD68 gene expression (Figure 5B). VCAM-1 is expressed in various types of cells, including endothelial cells, smooth muscle cells, and macrophages. Figure 5C reveals that INV-403 treatment decreased VCAM-1 throughout the plaque, indicating that INV-403 may reduce VCAM-1 expression in plaque via both reduced macrophage infiltration and attenuated expression of VCAM-1 expression in other cell types. We corroborated the effect of INV-403 on VCAM-1 and intercellular adhesion molecule-1 expression by measuring expression of these adhesion molecules at the protein level (Figure 5D and 5E). Because NF-κB is a critical transcription factor regulating the expression of proinflammatory genes, such as VCAM-1, we examined the effect of INV-403 on NF-κB activity. As shown in Figure 5F and 5G, INV-403 markedly decreased the DNA binding activity of NF-κB.
Figure 5.
INV-403 inhibits NF-κB signaling in the vasculature. A, Total RNA were prepared from thoracic aorta of WHHL rabbits. The mRNA expression level of proinflammatory genes was then analyzed by real-time reverse transcription–polymerase chain reaction. *P<0.05, Student t test adjusted by Bonferroni correction. B, CD68 expression in rabbit aorta was assessed by real-time reverse transcription–polymerase chain reaction. *P<0.05, Student t test. C, Rabbit aortic sections were stained with anti-VCAM-1. A representative picture and the summary are presented. D and E, Total proteins were prepared from thoracic aorta of WHHL rabbits. The levels of phospho IkBα (P-IκB), total IkBα, VCAM-1, and intercellular adhesion molecule-1 (ICAM-1) were analyzed by Western blot. The Western blot (D) and densitometric quantification (E) are shown. *P<0.05; Student t test. F and G, Nuclear extracts were prepared from thoracic aorta of WHHL rabbits, and the DNA binding activity of NF-κB was measured with lightshift chemiluminescent EMSA kit. The image (F) and quantification (G) are presented.
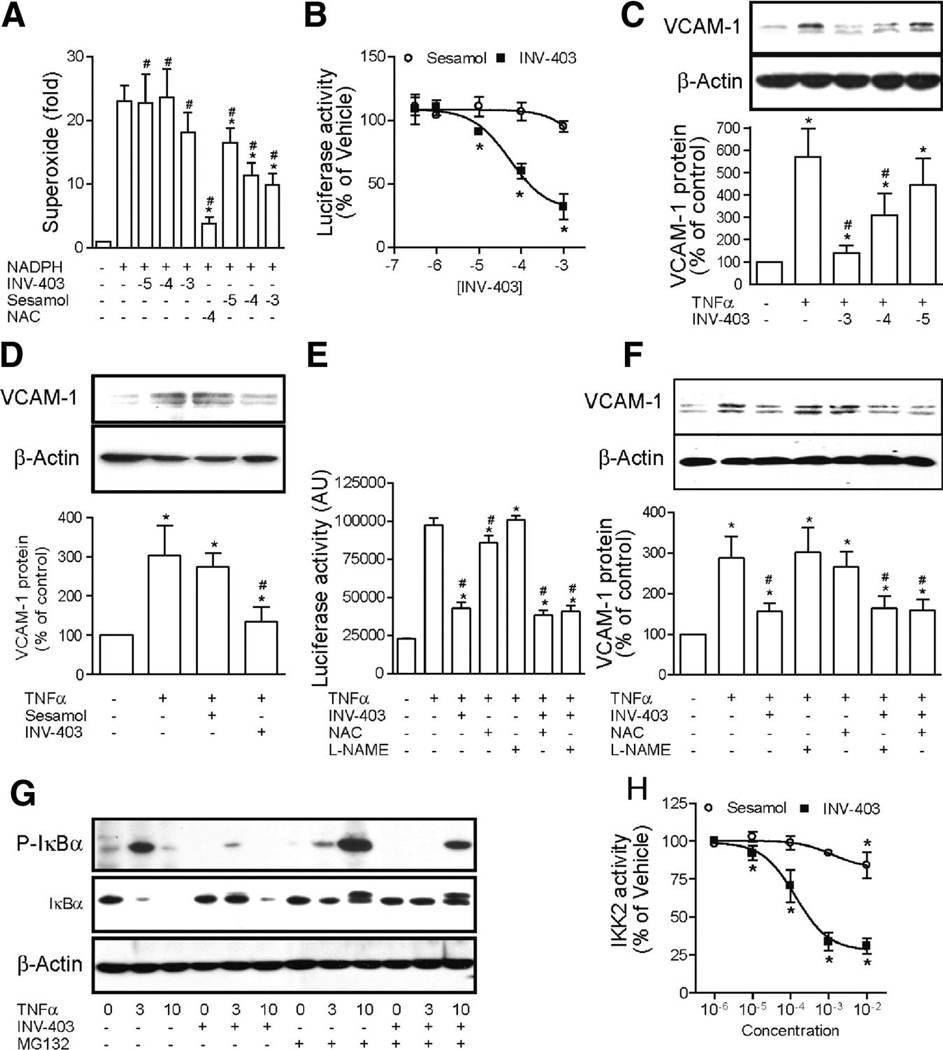
Given that NF-κB activation can be inhibited by antioxidants and INV-403 is a derivative of sesamol, which is an antioxidant in vitro, we examined whether INV-403 inhibits NF-κB dependent on its antioxidant effects. Figure 6A demonstrates that in contrast to sesamol, which scavenged superoxide induced by NADPH in liver lysates, INV-403 did not exert antioxidant effects, indicating that INV-403 inhibits NF-κB by antioxidant-independent mechanisms. To assess the mechanism by which INV-403 inhibits NF-κB, bovine aortic endothelium cells (BAECs) were transfected with NF-κB reporter construct and then treated with INV-403. Results show that INV-403 dose-dependently decreased tumor necrosis factor-α-induced NF-κB-mediated luciferase expression, whereas sesamol did not inhibit TNF-α-induced luciferase expression (Figure 6B). We corroborated the effect of INV-403 on NF-κB signaling by measuring the dose-dependent inhibition of TNF-α-induced VCAM-1 expression in BAECs (Figure 6C). In contrast, sesamol did not inhibit VCAM-1 expression (Figure 6D). NO is a potent inhibitor of NF-κB signaling and sesamol can induce NO production. We therefore tested whether the effect of INV-403 on NF-κB signaling was mediated by NO production. Figure 6E and 6F show that a NOS inhibitor, l-NAME, did not inhibit the effect of INV-403 on NF-κB mediated luciferase and VCAM-1 expression in BAECs, further supporting that the inhibition of NF-κB by INV-403 is not mediated by NO. We also tested the effects of a nonspecific antioxidant N-acetyl-L-cysteine (NAC) and compared its effects with INV-403. NAC decreased TNF-α-induced NF-κB activation, but not to the extent seen with INV-403. We did not observe significant effects of NAC on TNF-α-induced VCAM-1 expression (Figure 6E and 6F).
Figure 6.
INV-403 inhibits NF-κB signaling through targeting IKK2. A, In the presence of the indicated compounds, NADPH-induced superoxide production in mouse liver lysate was analyzed with lucigenin. n=3; *P<0.05 versus NADPH only; #P<0.05 versus negative control; ANOVA. B, After transfection with pGL2/NF-κB-Luc, BAECs were pretreated with the indicated concentration of INV-403 or sesamol for 30 minutes and then stimulated with TNF-α (10 ng/mL) for 4 hours, and the activity of luciferase was assessed. A summary of 3 independent experiments is presented. *P<0.05 versus TNF-α only; 1-way ANOVA. C, BAECs were pretreated with the indicated concentration of INV-403 or vehicle for 30 minutes and then stimulated with TNF-α (10 ng/mL) for 6 hours. The expression of VCAM-1 was assessed by Western blot analysis. A representative image and the summary of 4 independent experiments are presented. D, BAECs were pretreated with vehicle, INV-403 (100 µmol/L), or sesamol (100 µmol/L) for 30 minutes and then stimulated with TNF-α (10 ng/mL) for 6 hours. The expression of VCAM-1 was assessed by Western blot analysis. A representative image and the summary of 4 independent experiments are presented. E, BAECs were transfected with pGL2/NF-κB-Luc and then treated with the indicated compounds. The luciferase activity was then analyzed. n=4; *P<0.05 versus negative control; #P<0.05 versus TNF-α only; ANOVA. F, BAECs were pretreated with the indicated compounds for 30 minutes and then stimulated with TNF-α (10 ng/mL) for 6 hours. The expression of VCAM-1 was assessed by Western blot analysis. A representative image and the summary of 3 independent experiments are presented. G, BAECs were pretreated with INV-403 (100 µmol/L), MG132 (20 µmol/L), or vehicle for 30 minutes and then stimulated with TNF-α (10 ng/mL) for the indicated time. Total IκBα and phosphorylated IκBα were assessed by Western blot analysis. A representative image of 2 independent experiments with similar results is presented. H, The dose-dependent effect of INV-403 and sesamol on IKK2 activity in vitro as assessed with HTScan IKKβ Kinase Assay Kit (Cell Signaling Technology).
To determine the target of INV-403 for the inhibition of NF-κB signaling, we examined the effect of INV-403 on the TNF-α-induced IκBα phosphorylation and subsequent degradation, a mechanism central to the activation of NF-κB. Figure 6G depicts the effect of INV-403 on IκBα. INV-403 markedly reduced TNF-α-induced IκBα phosphorylation and degradation in BAECs. Because the ubiquitin-proteasome system is involved in the degradation of IκB and may serve as a mechanism by which certain drugs may modulate NF-κB activation, we tested the effects of a classic proteasomal inhibitor MG-132 alone or when used in conjunction with INV-403. As anticipated, MG-132 treatment markedly reduced IκB degradation without effects on phosphorylation of IkB. However when MG-132 was used in conjunction with INV-403, in addition to the expected reduction in IκBα degradation, there was an effect on IκBα phosphorylation. These results suggest a specific effect of INV-403 on IKK2-mediated phosphorylation rather than an effect on proteasomal degradation of IκBα.
We then tested the effects of INV-403 on IKK2 using an in vitro kinase assay. Figure 6H demonstrates that whereas sesamol almost have no effect on IKK2 activity, INV-403 dose-dependently inhibited IKK2 in vitro, supporting our in vivo data that INV-403 may inhibit NF-κB activation through IKK2-dependent mechanisms.
Discussion
The present study has multiple findings. (1) Dietary supplementation with a simple modified lignan derivative for a 12-week period significantly decreased atherosclerotic plaque burden, with changes seen as early as 6 weeks without effects on lipid profile in the WHHL model. (2) Decreased plaque burden was paralleled by marked improvements in endothelial function, vasoconstrictor responses (decreased response to angiotensin II with reduced AT1R expression) and inflammation. (3) There was inhibition of plaque NF-κB transactivation and VCAM-1 expression. (4) Reduced activation of NF-κB by INV-403 occurred via inhibition of IKK2, providing a molecular basis for its antiinflammatory effects.
NF-κB signaling plays a central role in the regulation of inflammatory responses. Dietary supplementation with INV-403 markedly decreased NF-κB DNA binding activity in plaque, paralleled by decreased aortic expression levels of VCAM-1 and intercellular adhesion molecule-1, indicating that INV-403 may modulate inflammatory responses through NF-κB-dependent pathways. These effects were consistent with our in vitro studies in BAECs, which showed that INV-403 inhibited TNF-α-induced NF-κB-dependent luciferase expression and VCAM-1 expression. NF-κB activation is initiated by IκBα phosphorylation by IKK and its subsequent degradation through the ubiquitin-proteasome system. INV-403 markedly inhibited TNF-α-induced IκBα degradation in BAECs, which appeared to result from decreased IκBα phosphorylation without effects on proteasomal degradation, because its effects did not mimic those of MG-132, a classic proteasomal inhibitor. These results, in conjunction with the inhibitory effects of INV-403 on IKK2, suggest that this is the predominant pathway by which NF-κB activation is inhibited. Notably, although it has been demonstrated that NF-κB inhibitors can reduce atherosclerotic lesion and IKK2 is critical for activation of NF-κB, the effects of specific IKK2 inhibitors on atherosclerosis has not yet been assessed. INV-403 markedly decreases inhibited macrophage infiltration in plaque and was also associated with a reduction in reparative collagen. These changes were associated with potent effects in improving endothelial function. The role of NF-κB in atherosclerosis is complex with a differential cell-specific role for this pathway,40,41 which may also differ at various stages of atherosclerosis. In keeping with this concept, cell-specific conditional ablation of this pathway early in atherosclerosis has a favorable effect.41 In contrast, the NF-κB pathway may play an alternate protective role in macrophage cells, with disruption of this pathway resulting in progression of atherosclerosis.40 These contrasting effects suggest that any pharmacotherapeutic approach that modulates NF-κB may need to reconcile contrasting roles of this entity. Our data do not provide cell-specific effects of INV-403, and this is a limitation of our data.
INV-403 reduced constriction to angiotensin II but had no effects on endothelin-1 or phenylephrine-mediated constriction. This occurred through downregulation of AT-1 mRNA expression in the aorta in response to treatment with INV-403. The renin-angiotensin-aldosterone system, a crucial regulator of vascular homeostasis, has been consistently shown to play a crucial role in atherogenesis.42– 44 AT-1 is upregulated under conditions of high redox stress in atherosclerosis and in hypercholesterolemia. Conversely, blockade of AT1 receptors in atherosclerosis normalizes oxidative stress, improves endothelial function, and reduces plaque area and macrophage infiltration.45– 47 Thus, INV-403 may potentially exert additional synergistic effects with drugs that target the renin-angiotensin system (RAAS) system in atherosclerosis, a finding that may be worthy of exploration in the future. Notably, these effects occurred in the absence of any changes in plasma lipid profile in the fat-fed WHHL rabbit.
Our novel serial MRI scanning protocol allowed assessment of the time course of effects and showed that 6 weeks of dietary supplementation with INV-403 was sufficient to inhibit atherosclerotic lesion development in WHHL rabbits. The assessment of abdominal aortic atherosclerosis by MRI also served as an adjunctive measure of morphometric quantification of atherosclerosis in the thoracic aorta and confirmed effects of the drug in both these territories. Such an noninvasive approach may be helpful in assessment of time course of pharmacotherapies in rabbit models as it involved usage of a clinically relevant field strength (1.5 T) and does not require more expensive high-field MRI systems.
Notably, no significant changes in plasma lipoprotein levels were observed by the end of the 12 weeks of dietary supplementation with INV-403. In prior studies, we have demonstrated an important effect of sesame oil in reducing atherosclerosis in conjunction with reduction in plasma lipoprotein components in an low-density lipoprotein receptor−/− model.48 In this prior work, we postulated that nonsaponifiable components of sesame oil such as sesame lignans themselves or in conjunction with the fatty acid components may reduce plasma lipoprotein levels.48 In contrast to these findings, we did not see an effect of a modified lignan component alone (INV-403). These findings may suggest unique effects of individual or modified components that may be distinct from that of sesame oil. Sesame oil is rich in both polyunsaturated fatty acids and mono-unsaturated fatty acids (approximately 47% oleic acid and 39% linoleic acid) and also contains lignans, such as sesamin and sesamolin, and several antioxidant compounds, such as sesaminol. Sesamin feeding is associated with a reduction of serum lipid levels in rodents, concomitant with an increased fatty acid oxidation. This effect has been attributed to the ability of sesamin to affect peroxisome proliferator-activated receptor (PPAR)–mediated transcriptional events. Activation of PPAR has been demonstrated to modulate many aspects of lipoprotein metabolism and inflammation in vitro, as well as in animal and human studies. In our studies, we did not see in vivo effects of INV-403 consistent with PPAR-α activation (reduction in plasma triglycerides or increase in high-density lipoprotein) or increase in acyl-coenzyme A oxidase expression in the liver (data not shown). Moreover the drug did not activate PPAR-γ or PPAR-Δ in HepG2 cells using the yeast UAS-TK, Gal4 system (data not shown). In addition, it may be that the extremely high levels of non–high-density lipoprotein cholesterol in the WHHL model (related to mutations in low-density lipoprotein receptor), especially in response to high-fat chow, may render any effects on plasma lipoprotein profile indiscernible. Further studies in milder models of hyperlipidemia, preferably not involving genetic manipulation of lipoprotein clearance (eg, hamster model of diet induced hyperlipidemia) may be required to tease out the effects on plasma lipid profile.
INV-403 was synthesized from 3,4-methylenedioxyphenol, via synthetic modification to allow enhanced stability of the phenolic group and to allow NO release. Our data, however, indicate that the introduced nitro group did not directly influence acute NO release. The nitro group was nevertheless important in mediating the effects of the drug, such as the induction of low-density lipoprotein receptor in HepG2 cells. This was confirmed via structure-activity relationship analysis (data not shown).
In conclusion, INV-403 exerts important vascular protective effects in a model of severe hyperlipidemia atherosclerosis. These findings may have implications for further testing of modified forms of sesame lignans in the treatment of atherosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by intramural funds from the Ohio State University and the Wolfe Foundation. Dr Rajagopalan was also partially supported by grants from the National Institute of Health (RO1 ES015146, ES017290, and R21 DK088522).
Footnotes
Disclosures
Intellectual property in this work belongs to Ohio State University and is licensed to InVasc Therapeutics.
References
- 1.Stary HCCA, Dinsmore RE, Fuster V, Glagov S, Insull WJR, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Arterioscler Thromb Vasc Biol. 1995 doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 3.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D, Lewis A. Conner Memorial Lecture: oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 5.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 8.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 9.Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med. 1989;321:436–441. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- 10.Parks JS, Rudel LL. Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis. 1990;84:83–94. doi: 10.1016/0021-9150(90)90077-v. [DOI] [PubMed] [Google Scholar]
- 11.Sanders TA. Olive oil and the Mediterranean diet. Int J Vitam Nutr Res. 2001;71:179–184. doi: 10.1024/0300-9831.71.3.179. [DOI] [PubMed] [Google Scholar]
- 12.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26:995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 13.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 14.Shamir R, Feig JE, Fisher EA. Therapeutic approach to childhood hypercholesterolemia. Pediatr Endocrinol Rev. 2007;5:649–655. [PubMed] [Google Scholar]
- 15.Niinikoski H, Lagstrom H, Jokinen E, Siltala M, Ronnemaa T, Viikari J, Raitakari OT, Jula A, Marniemi J, Nanto-Salonen K, Simell O. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation. 2007;116:1032–1040. doi: 10.1161/CIRCULATIONAHA.107.699447. [DOI] [PubMed] [Google Scholar]
- 16.McCrindle BW. Hyperlipidemia in children. Thromb Res. 2006;118:49–58. doi: 10.1016/j.thromres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Getz GS, Reardon CA. Nutrition and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2499–2506. doi: 10.1161/ATVBAHA.107.155853. [DOI] [PubMed] [Google Scholar]
- 18.Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113(suppl 9B):13S–24S. doi: 10.1016/s0002-9343(01)00987-1. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 20.Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ, Sacks FM. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87:1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks FM. Major potential of diet treatment for type 2 diabetes. Curr Diab Rep. 2008;8:249–250. doi: 10.1007/s11892-008-0043-9. [DOI] [PubMed] [Google Scholar]
- 22.Salas-Salvado J, Casas-Agustench P, Murphy MM, Lopez-Uriarte P, Bullo M. The effect of nuts on inflammation. Asia Pac J Clin Nutr. 2008;17(suppl 1):333–336. [PubMed] [Google Scholar]
- 23.Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, Szapary PO. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27:65–74. doi: 10.1080/07315724.2008.10719676. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 25.Jariwalla RJ. Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res. 2001;27:17–26. [PubMed] [Google Scholar]
- 26.Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol. 2008;102:84–86. doi: 10.1016/j.amjcard.2008.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machowetz A, Gruendel S, Garcia AL, Harsch I, Covas MI, Zunft HJ, Koebnick C. Effect of olive oil consumption on serum resistin concentrations in healthy men. Horm Metab Res. 2008;40:697–701. doi: 10.1055/s-2008-1078728. [DOI] [PubMed] [Google Scholar]
- 28.El-Sabban F, Abouazra H. Effect of garlic on atherosclerosis and its factors. East Mediterr Health J. 2008;14:195–205. [PubMed] [Google Scholar]
- 29.de Lorgeril M, Salen P. The Mediterranean diet: rationale and evidence for its benefit. Curr Atheroscler Rep. 2008;10:518–522. doi: 10.1007/s11883-008-0080-5. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Jimenez F, Ruano J, Perez-Martinez P, Lopez-Segura F, Lopez-Miranda J. The influence of olive oil on human health: not a question of fat alone. Mol Nutr Food Res. 2007;51:1199–1208. doi: 10.1002/mnfr.200600273. [DOI] [PubMed] [Google Scholar]
- 31.Naruszewicz M, Kozlowska-Wojciechowska M. Plant sterols beyond low-density lipoprotein-cholesterol. Br J Nutr. 2007;98:454–455. doi: 10.1017/S0007114507781473. [DOI] [PubMed] [Google Scholar]
- 32.Malik VS, Hu FB. Dietary prevention of atherosclerosis: go with whole grains. Am J Clin Nutr. 2007;85:1444–1445. doi: 10.1093/ajcn/85.6.1444. [DOI] [PubMed] [Google Scholar]
- 33.NIH State-of-the-Science Conference Statement on Multivitamin/Mineral Supplements and Chronic Disease Prevention. NIH Consens State Sci Statements. 2006;23:1–30. [PubMed] [Google Scholar]
- 34.Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007;47:651–673. doi: 10.1080/10408390600919114. [DOI] [PubMed] [Google Scholar]
- 35.Prasad K, Mantha SV, Muir AD, Westcott ND. Reduction of hypercholesterolemic atherosclerosis by CDC-flaxseed with very low α-linolenic acid. Atherosclerosis. 1998;136:367–375. doi: 10.1016/s0021-9150(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 36.Prasad K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis. 1997;132:69–76. doi: 10.1016/s0021-9150(97)06110-8. [DOI] [PubMed] [Google Scholar]
- 37.Hirata F, Fujita K, Ishikura Y, Hosoda K, Ishikawa T, Nakamura H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122:135–136. doi: 10.1016/0021-9150(95)05769-2. [DOI] [PubMed] [Google Scholar]
- 38.Hou RC, Chen HL, Tzen JT, Jeng KC. Effect of sesame antioxidants on LPS-induced NO production by BV2 microglial cells. Neuroreport. 2003;14:1815–1819. doi: 10.1097/00001756-200310060-00011. [DOI] [PubMed] [Google Scholar]
- 39.Chen PR, Tsai CE, Chang H, Liu TL, Lee CC. Sesamol induces nitric oxide release from human umbilical vein endothelial cells. Lipids. 2005;40:955–961. doi: 10.1007/s11745-005-1456-3. [DOI] [PubMed] [Google Scholar]
- 40.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 44.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 45.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 46.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H. Comparative effect of ACE inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103:799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 47.Sorescu D, Szocs K, Griendling KK. NAD(P)H oxidases and their relevance to atherosclerosis. Trends Cardiovasc Med. 2001;11:124–131. doi: 10.1016/s1050-1738(01)00097-4. [DOI] [PubMed] [Google Scholar]
- 48.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S. Inhibition of atherosclerosis in low-density lipoprotein receptor-negative mice by sesame oil. J Med Food. 2006;9:487–490. doi: 10.1089/jmf.2006.9.487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.