Abstract
The emerging stimulant drug of abuse (3,4)-methylenedioxypyrovalerone [(R,S)-MDPV] is self-administered as a racemic mixture by intranasal, iv, oral, and smoking routes. The individual enantiomers are known to have widely different pharmacological effects, with (S)-MDPV showing much greater potency than (R)-MDPV in pharmacological testing. The goal of these studies was to develop and validate an analytical method for quantitation of (R)-MDPV, (S)-MDPV and (R,S)-MDPV in small volumes of rat serum using a chiral separation column and liquid chromatography-mass spectrometry. The method was validated for selectivity, precision, accuracy, recovery, sensitivity, and reproducibility. The method was also used to determine the enantiomeric stability of the individual enantiomers during sample cleanup and analysis. The linear dynamic range of the calibration curve was 1 – 1000 ng/ml for each enantiomer. Concentration values for the lower limit of quantitation (1 ng/ml) were within 30% of their nominal value, but all other calibration standards were <20% of their nominal value. With proper storage and handling of samples, the two MDPV enantiomers were shown to remain stable in rat serum without any apparent racemization during the time needed for analysis. Finally, the ruggedness of the method was demonstrated with diluted and undiluted serum samples collected from Sprague Dawley rats in a preliminary pharmacokinetic study at 3 mg/kg of (R,S)-MDPV. In summary, the assay used a simple sample preparation method, reversed-phase chiral chromatography, and tandem mass spectrometry to achieve accurate and selective determinations of MDPV enantiomer concentrations in small volumes of serum.
Graphical Abstract
A chiral-selective LC-MS/MS method for determination of MDPV, a drug of abuse with enantiomerically selective pharmacology, was developed and validated.
Introduction
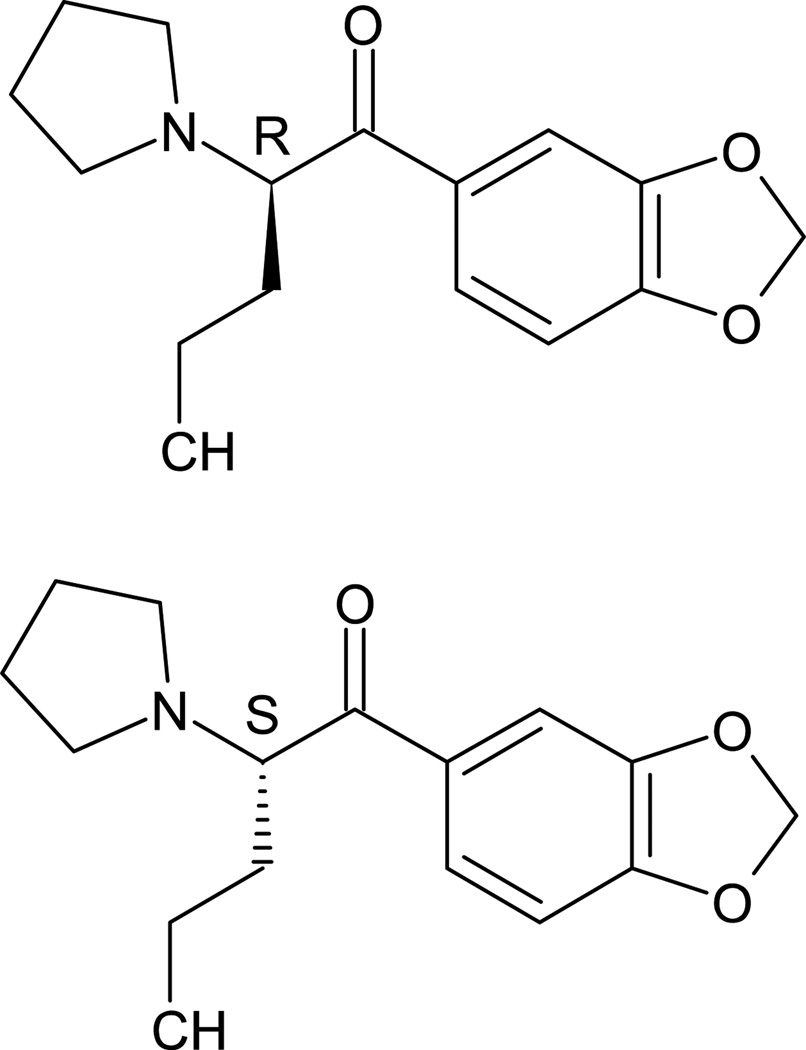
The synthetic cathinone, (3,4)-methylenedioxypyrovalerone (MDPV) is a medically dangerous stimulant drug of abuse, which can cause a variety of severe physiological and neuropsychiatric adverse effects, including tachycardia, hypertension, rhabdomyolysis, psychosis, hallucinations, agitation, and acidosis.1,2 This intoxication can lead to hospitalization or death.1,3–5 In a retrospective multicenter study of MDPV-confirmed patients, the drug was most commonly self-administered via insufflation (34%), but other patients administered the drug by the oral, intravenous (iv), or inhaled routes.1 Despite its similar structure to methamphetamine, MDPV acts by potent inhibition of dopamine reuptake rather than inducing dopamine release like methamphetamine.6,7 While the drug is so far only available to users as a racemic mixture, the (R)- and (S)-MDPV enantiomers (Figure 1) are known to produce very different pharmacological effects in animal models of (R,S)-MDPV abuse,8,9 with the (S)-MDPV enantiomer showing much greater pharmacological activity than (R)-MDPV. Given these profound enantiomeric differences in potency, it is important to understand the contribution of each enantiomer’s serum concentration and pharmacokinetic properties to the differing pharmacological effects of (R)- and (S)-MDPV. This information is essential knowledge for development of future medical treatments for (R,S)-MDPV use disorders.
Fig. 1.
Chemical structures of (R)- and (S)-MDPV.
Quantitative analytical methods for measuring blood and tissue concentrations of MDPV have been reported,10,11 but these methods lack chiral selectivity.12 Chiral separation via diastereomeric salt formation of MDPV enantiomers has been reported.13 Another non-quantitative method uses a proprietary chiral column to determine ratios of (R)- and (S)-MDPV in illicit products and to investigate the chiral selectivity in a rat hepatotoxicity cell culture model.14 This method shows excellent chromatographic resolution and selectivity for each enantiomer, which allowed the investigators to separate a total of 100 mg of (R,S)-MDPV in multiple runs of 10 mg/ml solutions into over 40 mg of each enantiomer. Baciu et al. report a chiral quantitative capillary electrophoresis method for the determination of MDPV enantiomers in hair, with a lower limit of quantitation (LLOQ) of 0.4 ng/mg which equates to about 400 ng/ml.15 Considered together, none of these chiral selective methods were designed for quantitation of the low ng/ml concentrations of MDPV enantiomers expected in rodent pharmacokinetic studies with small serum or plasma sample volumes (20 – 50 µl).
The studies reported here demonstrate the development of a novel liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the quantitation of (R)-, (S)- and (R,S)-MDPV in serum samples using a chiral analytical column operated under reversed-phase conditions to achieve accurate and selective enantiomer determinations in small volumes of serum. The analytical method is suitable for studies of pharmacokinetic properties of (R,S)-MDPV and its two enantiomers in serum samples, and for determination of the stability of (R)- and (S)-MDPV in solution for in vivo and in vitro experiments.
Experimental
Materials
Reference standards
Calibration standards of (R,S)-MDPV HCl (1 mg/ml free base in methanol; Cerilliant, Round Rock, TX) were prepared in non-hemolyzed normal rat serum (NRS; Pel-Freeze Biologicals, Rogers, AR). Standards at concentrations of 1, 2, 6, 20, 60, 200, 600, 2000, and 6000 ng/ml of (R,S)-MDPV were prepared, which produced (R)- and (S)-enantiomer standards of 0.5, 1, 3, 10, 30, 100, 300, 1000, and 3000 ng/ml. Quality control (QC) standards were prepared from a separate stock of (R,S)-MDPV in Sprague Dawley (SD) rat serum from Innovative Research (Novi, MI) at concentrations of 3, 100, and 1600 ng/ml to allow for analysis of (R)- and (S)-enantiomer concentrations of 1.5, 50, and 800 ng/ml. 3,4-Methylenedioxypyrovalerone-D8 HCl (MDPV-D8, 0.1 mg/ml free base in methanol; Cerilliant) was diluted to 30 ng/ml in acetonitrile for use as an internal standard. (R,S)-, (R)-, and (S)-MDPV (≥95% pure) for in vivo and single enantiomer analysis was acquired from the National Institute on Drug Abuse (Bethesda, MD) Drug Supply Program. All drug concentrations are reported as the free base.
Methods
Sample preparation
Samples and standards were thawed under refrigeration for between 15 min and 1 hr (unless otherwise stated). Extraction of MDPV enantiomers was similar to the method of Anizan et al.11 If a standard concentration was ≥3 ng/ml or greater of each enantiomer, 20 µl of each sample was added to a 1.5 ml centrifuge tube. The 3000 ng/ml sample was diluted 4-fold with NRS prior to extraction. If the concentration was <3 ng/ml, 50 µl of serum was added to a 1.5 ml centrifuge tube. A volume of 100 or 250 µl of ice-cold MDPV-D8 30 ng/ml internal standard in acetonitrile was then added to 20 or 50 µl of serum samples, respectively. This was followed immediately by 2 sec of full speed vortex mixing and an additional 10 sec after the internal standard solution was added to every sample in the extraction run. Precipitated samples were then held at 4°C for 10 min prior to centrifugation for 5 min at 20,817 rcf (at 4°C). About 90% of the supernatant was carefully removed from each tube and taken to dryness with a Zymark TurboVap LV evaporator (SOTAX Corporation, Westborough, MA) under nitrogen in a 40°C water bath.
Each sample was reconstituted in 75 µl of mobile phase, which consisted of 50:50 mixture of 5 mM ammonium acetate buffer (pH 8.9) and acetonitrile, followed by vortexing for 20 sec. The reconstituted sample was then centrifuged at 20,817 rcf (at 4°C) for 5 min to remove any potential precipitates. Next, 65 µl was transferred to an autoinjector vial. Only 60 µl of reconstituted brain homogenates was transferred to avoid contamination from the residual precipitate.
Instrumentation
The LC-MS/MS analytical instrumentation included an Acquity UPLC Chromatographic system interfaced to a Quattro Premier XE mass spectrometer (Waters Corp, Milford, MA). The extracted sample (7.5 µl) was injected in Partial Loop Needle Overfill mode (10 µl loop) onto a Lux 5 µm Amylose-2 100 × 4.6 mm chiral analytical column (Phenomenex, Torrance, CA) maintained at 19°C with an external CERA Column Cooler 250 (Cera Inc, Baldwin Park, CA). (R,S)-MDPV enantiomers were separated using isocratic conditions at a 1 ml/min flow rate. The mobile phase consisted of a 50:50 mixture of 5 mM ammonium acetate buffer (pH 8.9) and acetonitrile. The column eluent was diverted to waste for the first 2.5 min. The flow was then directed to the analyzer from 2.5 to 5.5 min with a total chromatographic run time of 10 min.
The column was interfaced to the mass spectrometer with an electrospray ionization probe operated in positive ion mode. The nitrogen desolvation gas was operated at 450°C and 700 L/hr. The source temperature was operated at 150°C, and the cone gas was operated at 75 L/hr. (R,S)-MDPV and (R,S)-MDPV-D8 internal standard positive ions were generated at cone voltages of 32 and 40 volts, respectively. Product ions for (R,S)-MDPV and (R,S)-MDPV-D8 were produced by argon collision-induced-disassociation at a collision energy of 25 and 20 volts eV, respectively, while maintaining a collision cell pressure of 1.9 × 10−3 mbar. Detection was achieved in the multiple-reaction-monitoring mode using the following transitions, m/z 276.2→134.8 (MDPV) and 283.7→204.8 (MDPV-D8). See Fig. 2 for MDPV and collision induced mass spectra. Only one transition was used for quantitation since the source of analyte was known, unlike situations such as forensic analysis where the source of the sample is not clearly defined and multiple transitions would be necessary to improve the reliability of accurate identification for analysis. The interchannel delay and interscan delay were 0.005 sec and the dwell time was 0.795 sec. The column was rinsed with 10 column volumes of 90/10 methanol/ethanol following each batch run.
Fig. 2.
Mass spectra of precursor and product ion. The m/z 276.2→134.8 transition ion was chosen for analysis.
Validation
Overall Design
The primary goal of the work was to develop and validate a new analytical method in preparation for a comprehensive serum pharmacokinetic evaluation of (R)-, (S)- and (R,S)-MDPV in rats. For this type of study, up to eight blood samples are collected over an approximate 4–6 hr time period. Due to the small size of male and female rats (~250–350 g), the serum sample volumes that would be available for analysis at each time point would be limited to only 5–50 µl of serum analyzed from early to late collection time points (respectively). This is approximately 10–100 times less volume than might be expected to be available for human pharmacokinetics samples.
With these considerations in mind, the LLOQ for these studies was defined as the lowest concentration showing accuracy of 70 – 130% and precision of ± 30%. All other calibration and QC standards above 1.5 ng/ml had to be within 80 – 120% of the nominal value and have a precision within ± 20%. Use of this method in human clinical studies or forensics would require additional development to attain the more stringent FDA recommended limits.16
Selectivity
Considering that the primary emphasis of this method is serum analysis, selectivity was analyzed in 4 types of rat serum (different suppliers and rat sex) and brain homogenate. These included: non-hemolyzed mixed sex NRS from Pel-Freeze (Roger, AR), mixed sex SD rat serum from Innovative Research (Novi, MI), male and female SD rat serum from BioreclamationIVT (Baltimore, MD), and homogenized SD rat brain (4× water added per g of brain). The whole brain tissue for the sensitivity experiment was collected from an (R,S)-MDPV naïve rat. The rat was under deep isoflurane anesthesia prior to sacrifice and tissue collection. With each tissue type, a blank and an (R,S)-MDPV standard containing 1 ng/ml (LLOQ) of each enantiomer was extracted and analyzed in triplicate.
Accuracy and Precision
Each QC standard, 1.5 ng/ml (low), 50 ng/ml (medium), and 800 ng/ml (high) of each enantiomer, was analyzed five times within an analytical run and in five separate runs. The validation criteria required that three out of five of each QC standard per concentration per enantiomer to be either within 30% (low) or 20% (medium and high) of the nominal concentration, as well as have a percent coefficient of variation within that range. The accuracy and precision validation process was considered complete after the five successful repetitions over time. As an additional validation, a standard with 3000 ng/ml of each enantiomer, which was outside the dynamic range of the assay (1—1000 ng/ml), was diluted four-fold (5 µl standard plus 15 µl NRS) so that this standard was within the dynamic range of the standard curve.
Matrix Effects, Process Efficiency, and Recovery
(R,S)-MDPV standards with low (1 and 3 ng/ml), medium (30 ng/ml), and high (300 ng/ml) concentrations of each enantiomer were prepared in serum prior to extraction, in post extraction serum, and in mobile phase. Matrix effect, process efficiency, and recovery were calculated as described by Matuszewski et al.17 Percent matrix effect was determined by dividing the average peak areas of the spiked blank extractions by that of the standard in mobile phase (× 100%). The percent process efficiency was determined by dividing the average peak area of the extracted standard by the average peak area of the standard in mobile phase (× 100%). Percent extraction recovery was determined by dividing the average peak area of the extracted standards by the average peak areas of the spiked extracted blank serum standard (× 100%). To test the effects of the acetonitrile volume on the extraction method, standards containing 3, 30, and 300 ng/ml of each enantiomer were extracted with 100, 200, and 300 µl volumes of acetonitrile to determine its effect on process efficiency.
Lower Limit of Quantification (LLOQ) and Calibration Curve
The LLOQ for each enantiomer was determined from the lowest standard with a response at least five times greater than the background response. (R,S)-MDPV standards consisted of 1 (LLOQ), 3, 10, 30, 100, and 1000 ng/ml of (R)- and (S)-MDPV. The 1, 10, 100, and 1000 ng/ml standards were analyzed in duplicate. In addition, blank serum with and without internal standard was analyzed. Calibration curves were generated using a linear least squares fit (with 1/x weighting) to the response versus concentration data. A validation run passed if at least 75% of the standards for both enantiomers were within 20% of the nominal concentration and the LLOQ was within 30% of the nominal value. These curves were repeated five times. In the validation experiments, the QC measurements were used to determine accuracy and precision. The lower limit of detection (LLOD) was defined as the concentration producing a signal-to-noise of 3 and was extrapolated from data at 1 ng/ml.
Stability
An (R,S)-MDPV standard (3000 ng/ml of each enantiomer) was repeatedly analyzed after several freeze and thaw cycles to determine the stability over time (25 days). The standard curve samples were injected at the beginning and end of each run to determine stability in the sample manager (4°C).
To assure that the sample did not racemize in whole blood, aliquots of freshly collected SD rat whole blood were spiked with 100 ng/ml of each enantiomer, allowed each sample to clot for 1 hr, and centrifuged the sample at 20,817 rcf (4°C) for 7 min to collect serum for extraction. To test the enantiomeric stability of (R)- and (S)-MDPV in the sample manager (4°C), 1000 ng/ml standards of each enantiomer were prepared in mobile phase and injected at 0, 4, 6, 8, 18, 25, and 40 hrs. To test stability in an NRS matrix (Pel-Freeze), NRS spiked with each enantiomer (1000 ng/ml) was extracted immediately after spiking the sample. To test the stability of each enantiomer during the extraction, aliquots of frozen samples were thawed and extracted immediately, stored for 2 hrs prior to extraction at room temperature, and stored for 2 hrs prior to extraction at 4°C. Percent enantiomeric purity was defined as the percentage of (R)-MDPV present in an (S)-MDPV standard (or the reverse). To assess the effects of freezing and thawing the sample, separate 100 ng/ml standards of each enantiomer were made in male SD rat serum from BioreclamationIVT. Aliquots of each standard were frozen and thawed one, two, and three times prior to analysis.
In vivo Studies of SD Rat Serum Concentrations over time following administration of (R,S)-MDPV
All animal experiments were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences (Little Rock, AR). A dual jugular vein catheterized male Sprague-Dawley rat was purchased from Charles River Laboratories (Wilmington, MA). The rat was allowed two weeks to acclimate, and fed enough food pellets to maintain a 300–350 g body weight.
To test the analytical method in preparation for pharmacokinetic studies, 3 mg/kg (R,S)-MDPV was administered intravenously to the male SD rat through a venous jugular catheter and blood samples were collected from the other venous jugular catheter. The blood collection time points were 1, 5, 20, 60, 120, 180, and 240 min. The 1 min sample was diluted 4-fold, and the 5 and 20 min samples were diluted 2-fold with Pel-freeze NRS. A 20 µl serum aliquot was assayed for earlier time points and a 50 µl aliquot was assayed for the 240 min time point. After allowing time for the blood to clot, the samples were centrifuged at 20,817 rcf (4°C) for 7 min and the serum was collected. Serum samples were stored at −80°C until analyzed.
For the model-dependent pharmacokinetic analyses of (R)-MDPV, (S)-MDPV and (RS)-MDPV data sets, log concentration versus time curves were analyzed by model-dependent methods using Phoenix WinNonlin V6.4 (Certara USA, Princeton, NJ). (R,S)-MDPV serum concentrations were calculated by adding the (R)- and (S)-MDPV serum concentrations at each time point. Bi- and tri-exponential curves were fit successively to the individual data sets using no weighting, 1/y and 1/y2 weighting functions, where y was the predicted concentration. The best-fit line was chosen by statistical analysis, visual inspection, analysis of the residuals, and examination of the coefficients of variation for each pharmacokinetic parameter.
Results
Validation
Selectivity
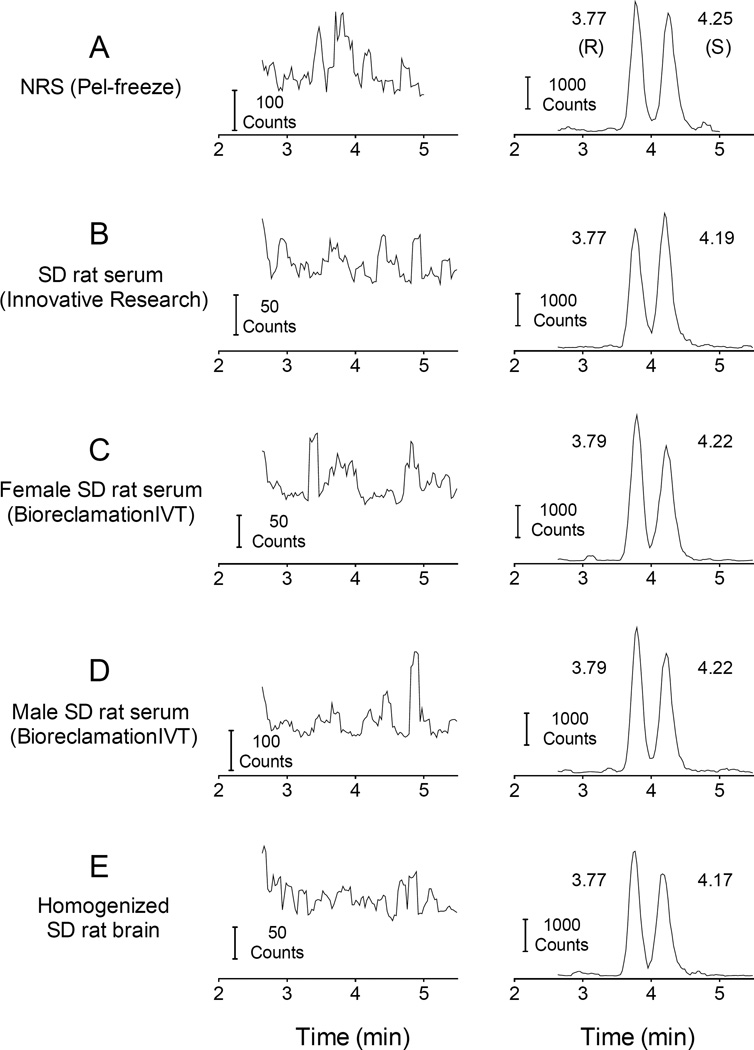
Fig. 3 shows representative chromatograms for extracted blank serum and brain samples compared to representative MDPV-spiked serum and brain chromatograms. The average peaks in the blank samples (left column of plots) at the nearest retention time to the analyte was ≤1.4% of the average peak for the 1 ng/ml (R)- and (S)-MDPV samples (right column).
Fig. 3.
LC-MS/MS chromatograms of extracted blank serum and brain matrix samples from different sources (left column) and chromatograms of extracted 1 ng/ml of (R)- and (S)-MDPV in each serum type and brain (right column).
Accuracy and Precision
In the five validation runs, the (R)- and (S)-MDPV QC standards met our accuracy and precision requirements. Indeed, 158 out of 162 QC standards (97.5%) were within the targeted accuracy range and all QC standards met the established precision requirements (Table 1). The four-fold diluted 3000 ng/ml (R)- and (S)-MDPV values remained within ± 20% accuracy after eight freeze/thaw cycles (Fig. 4). The pooled average predicted concentration of (R)- and (S)-MDPV was 3132 (± 153) and 3017 (± 215) ng/ml, respectively. The CV% of these average (R)- and (S)-MDPV concentrations was 4.9% and 7.1%, respectively.
Table 1.
Average predicted values for QC standards (n=5, with CV%), which consisted of five separate serum extractions per concentration per validation run.
| Predicted Concentration (CV%) | ||||||
| 1.5 ng/ml | 50 ng/ml | 800 ng/ml | ||||
|
Within Day |
(R)-MDPV | (S)-MDPV | (R)-MDPV | (S)-MDPV | (R)-MDPV | (S)-MDPV |
| #1 | 1.7 (8.7) | 1.6 (19.2) | 57.6 (2.0) | 59.2 (4.2) | 915.8 (3.3) | 944.6 (3.1) |
| #2 | 1.5 (7.4) | 1.9 (2.9) | 51.7 (3.4) | 52.1 (2.6) | 790.7 (5.8) | 810.4 (6.4) |
| #3 | 1.4 (6.6) | 1.3 (6.3) | 48.7 (3.3) | 50.1 (5.5) | 794.5 (6.3) | 863.2 (4.8) |
| #4 | 1.6 (9.7) | 1.4 (7.9) | 47.0 (5.2) | 53.7 (7.0) | 775.0 (2.1) | 849.1 (5.9) |
| #5 | 1.5 (3.8) | 1.4 (9.2) | 54.9 (3.0) | 56.1 (2.3) | 840.0 (1.4) | 854.4 (3.4) |
|
Between Days |
1.5 (10.9) | 1.5 (15.9) | 52.0 (8.2) | 54.2 (7.3) | 823.2 (7.4) | 864.3 (6.8) |
| Percent nominal value (CV%) | ||||||
| 1.5 ng/ml | 50 ng/ml | 800 ng/ml | ||||
|
Within Days |
(R)-MDPV | (S)-MDPV | (R)-MDPV | (S)-MDPV | (R)-MDPV | (S)-MDPV |
| #1 | 116.7 (9.4) | 105.7 (17.4) | 115.1 (2.0) | 118.3 (4.1) | 114.5 (3.3) | 118.1 (3.1) |
| #2 | 101.7 (8.2) | 123.9 (1.8) | 103.4 (3.3) | 104.1 (2.7) | 98.8 (5.8) | 101.3 (6.4) |
| #3 | 91.1 (6.5) | 86.6 (7.6) | 97.5 (3.3) | 100.2 (5.5) | 99.3 (6.2) | 107.9 (4.8) |
| #4 | 105.6 (10.0) | 96.7 (8.5) | 94.0 (5.2) | 107.4 (7.0) | 96.9 (2.0) | 106.1 (5.9) |
| #5 | 96.7 (4.5) | 94.8 (9.2) | 109.8 (3.0) | 112.2 (2.3) | 105.0 (1.4) | 106.8 (3.4) |
|
Between Days |
102.4 (11.5) | 101.5 (15.8) | 104.0 (8.2) | 108.4 (7.3) | 102.9 (7.4) | 108.0 (6.8) |
Fig. 4.
Four-fold diluted 3000 ng/ml (R)-MDPV and (S)-MDPV plotted as percent of the nominal value over the course of repeated freeze/thaw storage cycles over 25 days.
Matrix Effects, Process Efficiency and Recovery
The method did not show significant interference from the serum matrix and the extraction efficiency was sufficient to quantitate (R)- and (S)-MDPV in a reproducible manner. Table 2 summarizes matrix effects of 20 µl of extracted NRS on 3, 30, and 300 ng/ml concentrations of enantiomer and 50 µl of extracted NRS on a 1 ng/ml concentration of enantiomer. The process efficiency and extraction recovery are also shown. Increasing the volume of acetonitrile produced progressive decreases in recovery with the 200 µl and 300 µl extraction resulting in only 84% and 71% of the recovery found with a 100 µl volume of solvent.
Table 2.
Matrix effects (ME%), process efficiency (PE%), and recovery (RE%) ±SD for (R)- and (S)-MDPV.
| ME% (±SD) | PE% (±SD) | RE% (±SD) | ||||
|---|---|---|---|---|---|---|
| Concentration (volume) |
(R) | (S) | (R) | (S) | (R) | (S) |
| 1 ng/ml (50 µl) | 103 (7) | 99 (7) | 72 (3) | 68 (2) | 69 (3) | 69 (2) |
| 3 ng/ml (20 µl) | 101 (7) | 106 (11) | 68 (3) | 69 (6) | 67 (3) | 65 (6) |
| 30 ng/ml (20 µl) | 94 (2) | 102 (6) | 69 (5) | 70 (3) | 73 (5) | 69 (3) |
| 300 ng/ml (20 µl) | 99 (1) | 99 (2) | 66 (1) | 66(1) | 67 (1) | 67 (1) |
Matrix effect (ME) refers to the degree of which non-MDPV components in the sample affect the quantitation of MDPV (i.e., ion suppression or enhancement). ME% = 100%(peak area of analyte in extracted blank matrix/peak area of analyte in mobile phase).
Recovery (RE) and Process efficiency (PE) describe how much analyte is recovered with the extraction compared to the same concentration analyte in extracted blank matrix or in mobile phase, respectively. RE%= 100%(peak area of extracted sample/peak area of analyte in extracted blank matrix). PE% = 100%(peak area of extracted sample/peak area of analyte in mobile phase). Values are the means (±SD). N = 3 replicates per concentration.
Lower Limit of Quantitation and Calibration Curve
The mean (± SD) retention times between runs for (R)-MDPV, (S)-MDPV, (R)-MDPV-D8 and (S)- MDPV-D8 were 3.62 (± 0.11), 4.05 (± 0.13), 3.34 (± 0.12) and 3.67 (± 0.13) min, respectively. The value for the retention time standard deviation was low within runs and was less than ± 0.02 min for each compound. The average resolution between (R)- and (S)-MDPV peaks in these validation runs was 1.18 ± 0.04 (based on 100 ng/ml peaks).
An unweighted least squares linear fit to the response versus concentration data was compared to a fit with a 1/x weighted function. The function producing the lowest predicted value within the acceptable limits of ± 30% the nominal concentration was chosen for the calibration curve fit. The unweighted, linear fit resulted in an LLOQ of 10 ng/ml. The 1/x weighting function produced a LLOQ of 1 ng/ml. After considering the heteroscedastic nature of the analytical response (see Figure S1) and the improved LLOQ with a 1/x weighted function, the 1/x weighting function was chosen.18 The 1/x weighted calibration curves for both (R)- and (S)-MDPV are shown in Figure S1 of the Supplemental material. For (R)- and (S)-MDPV, the average least squares equations for the enantiomers for the five validation runs were y=0.024 (±0.002)x+0.002 (±0.001) and y=0.024 (±0.002)x + 0.001 (±0.008), respectively.
In the five validation runs, (R)- and (S)-MDPV calibration standards met the accuracy requirements in 198 out of 200 samples (99%). The LLOD was 0.06 ng/ml and 0.11 ng/ml for (R)- and (S)-MDPV, respectively.
Stability
The 3000 ng/ml (R)- and (S)-MDPV concentration samples were within ± 20% the nominal value for up to 8 freeze/thaw cycles over 25 days (see Accuracy and Precision section and Fig. 4). Extracted standard curve samples (1, 3, 10, 30, 100, 1000 ng/ml) of (R)- and (S)-MDPV injected at the end of an approximately 8 hr run was found on average to be 105.1 (± 8.3)% and 102.6 (± 12)%, respectively, of the values measured at the beginning of the run. At the LLOQ, the (R)- and (S)-MDPV concentrations at the end of the run were found to be 114.7 (± 3.2)% and 96.1 (± 6.6)%, respectively, of the values at the beginning of the run.
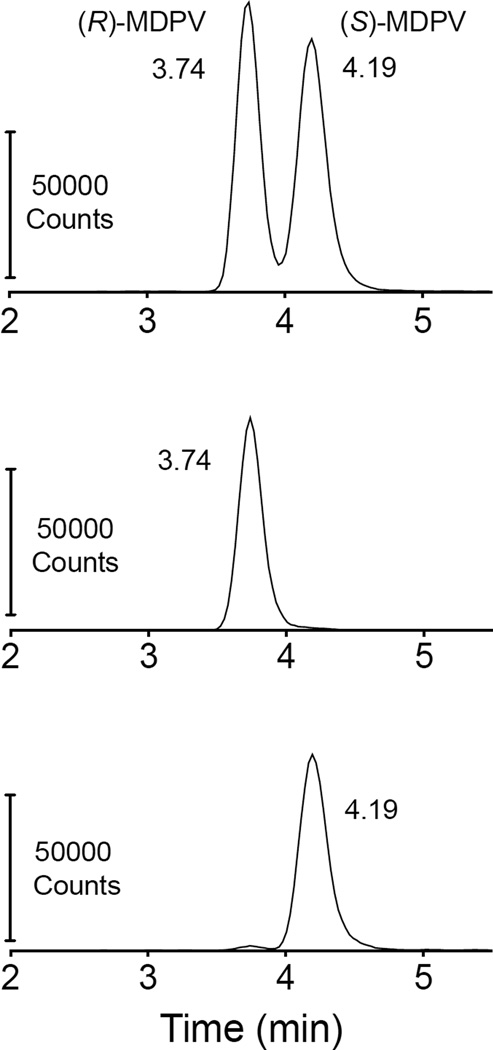
The (R)-enantiomer in mobile phase was stable for up to 40 hrs after dilution to 1000 ng/ml. The data showed 0.88% of the (S)-MDPV stock was (R)-MDPV, and this increased slightly to 1.15% (R)-MDPV after 40 hrs. Enantiomeric stability of 1000 ng/ml (R)- and (S)-MDPV in serum under different handling conditions is presented in Table 3. Also, there was no substantive difference in the degree of enantiomeric impurity of (R)- and (S)-MDPV after 1, 2, and 3 freeze/thaw cycles and in a standard derived from spiked whole blood. The freeze thaw cycle analysis was run with a standard curve, and despite the individual enantiomer stock not being 100% pure, the spiked NRS was within ± 20% of the 100 ng/ml value in seven of eight (R)-MDPV extractions, and all eight (S)-MDPV extractions (85 ± 4% and 110 ± 6% for (R)- and (S)-MDPV, respectively). See Fig. 5 for representative peaks of each enantiomer compared to (R,S)-MDPV.
Table 3.
Stereochemical stability of each enantiomer using different extraction conditions.
| Condition | % Enantiomeric Impurity* | |
|---|---|---|
| (R)-MDPV | (S)-MDPV | |
| Extract immediately | 0.1 | 1.51 |
| Extract after 2 hr at room temperature |
1.38 | 3.32 |
| Freeze-thaw 1× and extract immediately |
0.04 | 1.33 |
| Freeze (2 hrs)-thaw 1× and extract immediately |
0.13 | 1.29 |
| Extract 2 hrs after thaw at 4° C |
0.29 | 1.81 |
The value for percent extracted (R)-MDPV was calculated by dividing the area of the (S)-MDPV peak by the area of the (R)-MDPV peak and multiplying by 100%. The value for percent extracted (S)-MDPV was calculated by dividing the area of (R)-MDPV peak by the area of (S)-MDPV peak and multiplying by 100%.
Fig. 5.
Representative peaks and retention times (in min) of extracted 100 ng/ml MDPV enantiomers compared to extracted (R,S)-MDPV. Differences in peak height are due to a lack of correction for recovery. The (R)- and (S)-MDPV concentrations determined by these peaks were 84.1 and 102.4 ng/ml respectively. Note that the apparent minor (R)-MDPV peak in the bottom panel present in the (S)-enantiomer analysis was likely due to a small amount of impurity of (R)-MDPV in the (S)-MDPV standard. The vendor only reported a ≥ 95% purity.
In vivo Serum Concentration Analysis
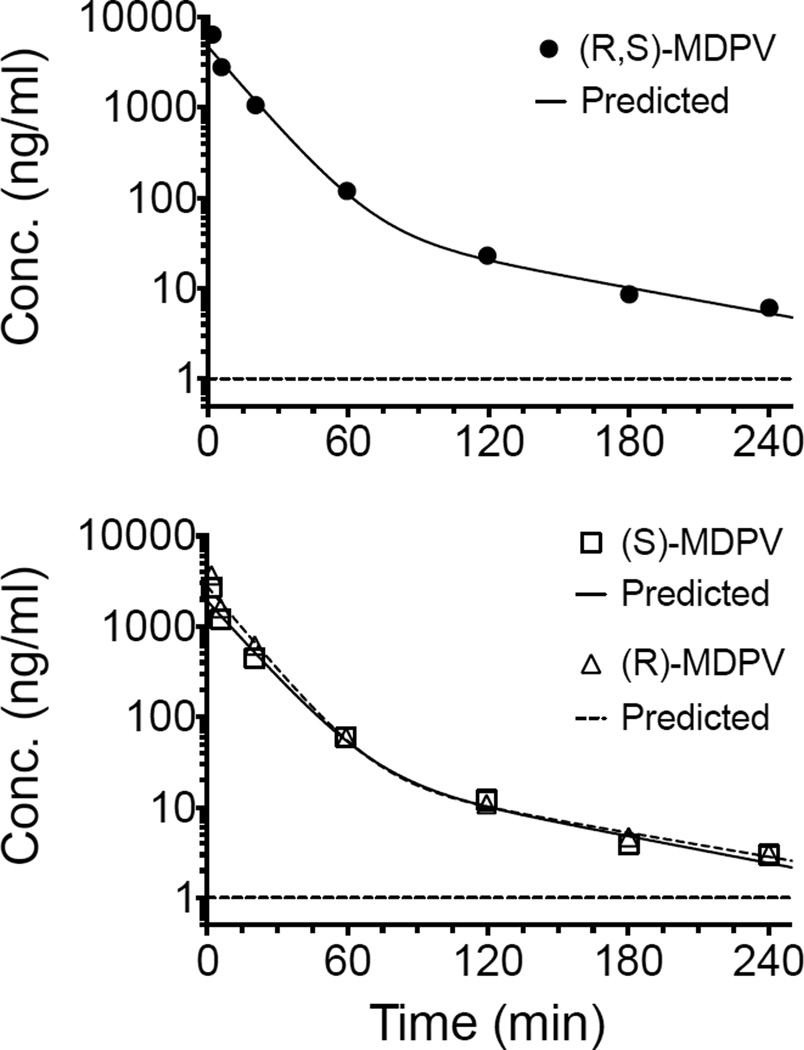
The concentration-time data sets for (R,S)-MDPV, (R)-MDPV, (S)-MDPV were best described by a 2-compartment pharmacokinetic model with 1/y2 weighting. Dilution of the 1 and 5 min time points after a 3 mg/kg IV (R,S)-MDPV dose allowed for determination of (R,S)-, (R)-, and (S)-MDPV concentration values well within the dynamic range of the assay. The terminal elimination half-life for each compound was; (R,S)-MDPV 1.1 h, (R)-MDPV 1.2 hr, and (S)-MDPV 1.0 hr. Note that this was proof of application data from a single male rat. Additional time points and multiple rats will be needed to properly characterize the pharmacokinetic parameters. The concentration data collected from extracted rat serum is depicted in Fig. 6 and Table 4.
Fig. 6.
(RS)-MDPV (upper graph) and (R)- and (S)-MDPV enantiomers (lower graph) serum concentrations over time after an IV administration of 3 mg/ml (R,S)-MDPV. Predicted non-linear best-fit lines were based on a 2-comparment pharmacokinetic model with 1/y2 weighting. The horizontal dashed lines depict the LLOQ (i.e., 1 ng/ml).
Table 4.
Serum concentration values after administration of iv 3 mg/kg (R,S)-MDPV in a male SD rat.
| Concentration (ng/ml) | |||
|---|---|---|---|
| Time (min) | (R)-MDPV | (S)-MDPV | (R,S)-MDPV |
| 1.78 | 3682.7 | 2715.6 | 6398.3 |
| 5.77 | 1601.5 | 1199.7 | 2801.2 |
| 20.28 | 619.1 | 444.8 | 1063.9 |
| 59.40 | 59.7 | 60.1 | 119.8 |
| 119.47 | 10.9 | 12.1 | 23.0 |
| 180.28 | 4.7 | 3.9 | 8.6 |
| 240.22 | 3.1 | 3.0 | 6.1 |
Volume of serum analyzed: 5 µl at 1.78 min, 10 µl at 5.77 – 20.28 min, 20 µl at 59.40 – 180.28 min, and 50 µl at 240.22 min.
Discussion
A key point in the development of this new method was the choice of a chiral column and initial chromatography conditions for separation of (R)- and (S)-MDPV. The column and the starting chromatography conditions were selected based on initial suggestions from the Phenomenex Phenologix application development laboratory following their analysis of (R,S)-MDPV separation by various columns and conditions. This service suggested a Lux 5 µm Amylose-2 100 × 4.6 mm column with an isocratic mobile phase of a 50:50 mixture of 5 mM ammonium bicarbonate buffer (pH 8.5) and acetonitrile at a flow rate of 1 ml/min. These conditions were modified and improved for the final approach as described in this manuscript. These changes were made to maintain a consistent and reproducible ion signal, to improve chiral separation, and to avoid the volatile ammonium bicarbonate salt in the mobile phase which over time fouled the sample cone on the Quattro Premier system. The initial extraction method attempted was based on the method of Wang et al10, which was intended for analysis of large volumes of equine plasma (1 mL). The liquid-liquid extraction method was successfully scaled down fifty-fold to account for the considerably smaller rat serum volumes. Unfortunately, the modified version of the Wang method required two extraction steps to attain an acceptable recovery (~80%) which led to a longer sample preparation time.
Of the previously published methods for the chiral separation of MDPV enantiomers, the Silva et al. work (2016)14 was most similar to the one described herein. We used a different column (the Silva group used an in-house column) that was operated in reversed-phase mode rather than normal phase mode. Reversed-phase chromatography is better suited and more compatible with electrospray ionization and is a major enabling factor toward improving the sensitivity and LLOQ over previously published work. This was important for the present method as it used tandem mass spectrometry for detection for quantitation of low concentrations of each enantiomer in small serum sample volumes. This low level of detection was not required in the Silva method which used UV absorbance for the detection of the percent enantiomer present in illicit products, analysis of a rat cell culture hepatotoxicity assay, and semi-preparative isolation of larger quantities of (R)- and (S)-MDPV for use in other experiments. The Silva method achieved better chromatographic resolution of enantiomers (Rs = 3.1) than our method (Rs = 1.18). The Silva group achieved this separation of (R)- and (S)-MDPV at a 0.5 ml/min flow rate rather than the 1 mg/ml flow rate used by our lab. A lower flow rate may improve separation of enantiomers and ion production in the electrospray source, and we are exploring this possibility for future applications. However, a decrease in flow rate will also slow the analysis time. While our method also does not attain the level of chiral resolution seen in the similar Silva et al.14 described method, it did achieve a 400-fold improvement in the LLOQ compared to the quantitative Baciu method.15
A six-point standard curve ranging from 1–1,000 ng/ml was used to analyze unknown samples. A 1/x weighted linear least squares fit to the response versus concentration data provided the best analytical recovery across the large dynamic range of the calibration curve while maintaining accuracy. While an unweighted fit is a simpler model for a calibration curve, the 1/x weighting was justified here given the fact that the absolute error in analytical response with MS detection increases with increasing concentration, while the relative error is relatively constant across the large dynamic range of the calibration curve. In a case like this, a 1/x weighted fit is justified to improve the analytical recovery and confidence at lower analyte concentrations.18 QC standards extracted in duplicate were injected just before and just after the samples analyzed in each run. Accurate and reproducible measurements of concentrations greater than the highest concentration standard (1000 ng/ml) was accomplished by sample dilution with NRS to bring the response back into the dynamic range of the standard curve (Fig. 4). This was very important for analyzing early, high serum concentrations from pharmacokinetic studies of 3 mg/kg (R,S)-MDPV in rat (see Fig. 6), and it allowed collection of less blood at earlier sampling times when concentrations of (R)- and (S)-MDPV were above the dynamic range of the standard curve (i.e., >1000 ng/ml).
Prior to the initial accuracy and precision validation runs, the analysis of samples was performed at room temperature. There was a shift in retention times and a decrease in peak response associated with fluctuations in the room temperature. Cooling the column temperature to a constant 19°C corrected this issue.
In the development of the extraction method, increasing the ratio of acetonitrile volume to serum volume progressively decreased the process efficiency. An acetonitrile extraction volume five times greater than the sample volume was best. The sample cleanup methodology yielded results that were noticeably absent of substantive matrix effects (Table 2). This allowed for a reproducible and consistent LLOQ in sera from four different sources and brain tissue (Fig. 3). The recovery and process efficiency (Table 2) validation showed accurate and reproducible determination of QC standard concentrations (Table 1).
Even after repeated freeze-thaw cycles, the extracted 3000 ng/ml specimen stayed within ± 20% of the target value over the course of 25 days. Due to the slight difference in concentration found in samples injected at the beginning and end of the run, a single extraction of the calibration standard curve was injected before and after samples and QC standards. While the stability of racemic MDPV in whole blood and serum has been established,11,19 less is known about the enantiomeric stability of the (R) and (S) enantiomers over time. The individual (R)-MDPV and (S)-MPDV enantiomers diluted in mobile phase are stereochemically stable in mobile phase at 4°C for up to 40 hrs. Some racemization occurs if the samples are allowed to remain in NRS at room temperature for an extended duration of time (≥2 hrs), but this was prevented by thawing and storing samples at 4°C prior to extraction (Table 3). Samples containing a single enantiomer remained stereochemically and chemically stable up to at least three freeze-thaw cycles. The enantiomers also were stable after collecting serum from spiked whole blood. Considering these findings, pharmacokinetic samples should be thawed and stored under refrigeration prior to extraction. Additionally, blood should be stored at 4–8°C after collection, while clotting, and during centrifugation.
Another advantage of the method is that (R,S)-MDPV concentrations can be calculated by adding the concentrations (R)- and (S)-MDPV at each time point (Fig. 6). Thus, quantitation of (R)-, (S), and (R,S)-MDPV is possible in a single analysis.
Conclusions
This study developed and validated a simple, reproducible method for accurate and selective analysis of (R)- and (S)-MDPV in small volumes of rat serum. The method accurately and reproducibly determined the concentration of the QC standards in multiple runs over time (Table 1). The methodologies described herein were fit for the purpose of the determination of the serum concentrations of (R)-, (S)-, and (R,S)-MDPV over time in the same analytical run in male SD rats after a 3 mg/kg IV dose of (R,S)-MDPV (Table 4 and Fig. 6). Furthermore, this method is suitable for pharmacokinetic studies of (R,S)-MDPV and its enantiomers.
Supplementary Material
Acknowledgments
Funding for this work was provided by the NIH National Institute on Drug Abuse (Grant R01 DA039195); the National Center for Advancing Translational Sciences (Grant ULITR000039) and the Arkansas Biosciences Institute (the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000).
Notes and references
- 1.Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, Mckeown NJ, Rosenbaum CD, Young AC, Rusyniak DE. ACMT Toxicology Investigators Consortium (ToxIC) J Med Toxicol. 2015;11:185–194. doi: 10.1007/s13181-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck O, Franzen L, Bäckberg M, Signell P, Helander A. Clin Toxicol (Phila) 2015;53:865–873. doi: 10.3109/15563650.2015.1089576. [DOI] [PubMed] [Google Scholar]
- 3.Murray BL, Murphy CM, Beuhler MC. J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. J. Forensic Sci. 2013;58:1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- 5.Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. J Anal Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- 6.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu L-H, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Br. J. Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA. ACS Chem Neurosci. 2015;6:771–777. doi: 10.1021/acschemneuro.5b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. J. Pharmacol. Exp. Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CC, Hartmann-Fischbach P, Krueger TR, Wells TL, Feineman AR, Compton JC. J Anal Toxicol. 2012;36:327–333. doi: 10.1093/jat/bks033. [DOI] [PubMed] [Google Scholar]
- 11.Anizan S, Ellefsen K, Concheiro M, Suzuki M, Rice KC, Baumann MH, Huestis MA. Anal. Chim. Acta. 2014;827:54–63. doi: 10.1016/j.aca.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA. Addict Biol. 2016;21:339–347. doi: 10.1111/adb.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M, Deschamps JR, Jacobson AE, Rice KC. Chirality. 2015;27:287–293. doi: 10.1002/chir.22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva B, Fernandes C, Tiritan ME, Pinto MMM, Valente MJ, Carvalho M, de Pinho PG, Remião F. Forensic Toxicol. 2016;34:372–385. doi: 10.1007/s11419-016-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baciu T, Borrull F, Calull M, Aguilar C. Electrophoresis. 2016;37:2352–2362. doi: 10.1002/elps.201600149. [DOI] [PubMed] [Google Scholar]
- 16.FDAUS Department of Health and Human Services. 2013:1–34. [Google Scholar]
- 17.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 18.Almeida AM, Castel-Branco MM, Falcão AC. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;774:215–222. doi: 10.1016/s1570-0232(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RD, Botch-Jones SR. J Anal Toxicol. 2013;37:51–55. doi: 10.1093/jat/bks138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.