Abstract
The mitochondrial calcium uniporter (MCU)—a calcium uniporter on the inner membrane of mitochondria—controls the mitochondrial calcium uptake in normal and abnormal situations. Mitochondrial calcium is essential for the production of adenosine triphosphate (ATP); however, excessive calcium will induce mitochondrial dysfunction. Calcium homeostasis disruption and mitochondrial dysfunction is observed in many neurodegenerative disorders. However, the role and regulatory mechanism of the MCU in the development of these diseases are obscure. In this review, we summarize the role of the MCU in controlling oxidative stress-elevated mitochondrial calcium and its function in neurodegenerative disorders. Inhibition of the MCU signaling pathway might be a new target for the treatment of neurodegenerative disorders.
Keywords: MCU, neurodegenerative disorder, inflammation, iron overload, oxidative stress
1. Introduction
Calcium, which can be referred to as the “life and death signal”, plays an important role in multiple biological process [1]. Changes in calcium concentration in the cytoplasm or mitochondria are proven to be involved in many fundamental biological processes such as neuronal activation, adenosine triphosphate (ATP) production and cell death. Calcium homeostasis is critical for the function of the central nerve system. Disruption of calcium homeostasis in the brain is found in multiple neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington disease (HD), amyotrophic lateral sclerosis (ALS) and cerebral ischemic stroke [2,3,4,5]. Mitochondria are able to take up calcium rapidly and, therefore, play an essential role in regulating cellular calcium homeostasis [6,7,8]. The mitochondrial calcium level has been proven to be involved in a series of physiological and pathological process. Calcium flows into mitochondria and can activate three critical enzymes of the Krebs cycle (pyruvate, α-ketoglutarate, and isocitrate dehydrogenases) and increase mitochondrial reactive oxygen species (ROS) levels, which facilitates ATP production [9,10,11,12,13]. Calcium overload in mitochondria leads to an excess in the production of ROS, causing mitochondrial dysfunction, which triggers complement-dependent superoxide-mediated programmed cell death and other biological pathways [14].
To describe how mitochondria accomplish calcium influx and efflux, several channels and uniporters have been identified. Among those, the mitochondrial calcium uniporter (MCU) is one of the most important and highly selective calcium transporting complexes in mitochondrial calcium uptake and is located on the inner membrane of mitochondria [15]. Despite intensive studies on the MCU, its encoding genes were only recently identified. According to a recent study, the MCU is comprised of several parts, where Mcu functions as the pore-forming and calcium-conducting subunit, while MICU1 (calcium uptake protein 1, mitochondrial), MICU2 (calcium uptake protein 2, mitochondrial), EMRE (essential MCU regulator, mitochondrial), MCUR1 (mitochondrial calcium uniporter regulator 1) and MCUb (calcium uniporter regulatory subunit MCUb, mitochondrial) work as regulatory subunits [16]. Here, in our review, we summarize the function and regulatory mechanisms of the MCU in the brain and emphasize the perplexing questions on the MCU that need to be answered in future studies.
2. The Molecular Components and Calcium Transporting Mechanism of the MCU
The calcium uniporter is located on the inner membrane of mitochondria. Its function can be inhibited by some small molecules, such as ruthenium red and RU360. Recently, the composition of the mitochondrial calcium uniporter was identified [16,17,18,19]. MICU1 is encoded by Micu1, which is the first identified subunit. As an EF-hand domain-containing protein, MICU1 is located on the inner membrane of mitochondria and plays a regulatory role in mitochondrial calcium uptake [20]. Following the discovery of MICU1, the crucial pore-forming protein Mcu (encoded by MCU) was discovered using an integrative genomics approach [19]. Since then, the remaining subunits of the MCU have been identified: MICU2 (EFHA1), MICU3 (EFHA2), MCUb (CCDC109B), MCUR1 (CCDC90A) and EMRE (Smdt1) (Figure 1A) [21,22,23,24]. Those subunits interact with Mcu and MICU1 and form a complex that regulates the calcium homeostasis of mitochondria.
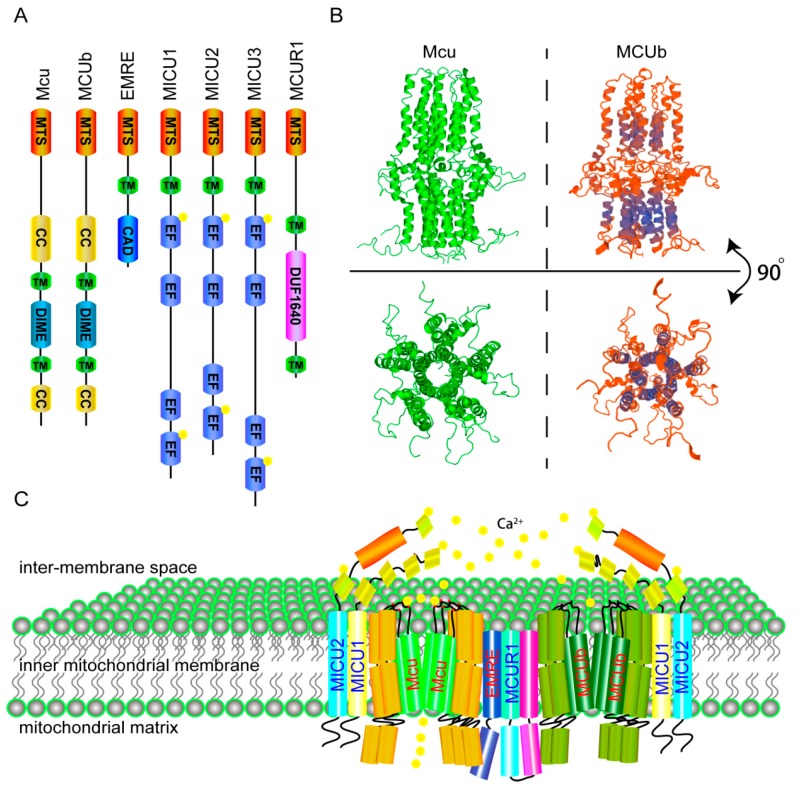
Figure 1.
The molecular components and structure of mitochondrial calcium uniporter (MCU). (A) The components of Homo sapiens MCU. From left to right, the schematic shows a linear overview of the predicted domain architecture of each of the seven Homo sapiens MCU components. MTS: mitochondrial targeting signal, TM: transmembrane domain, CC: coiled-coil domain, DIME: the conserved DIME motif, CAD: carboxy-terminal domain, EF: EF-hand domain, DUF1640: protein of unknown function (DUF1640); (B) The structure of Mcu-∆NTD (left) and MCUb-∆NTD (right). Upper: front view of Mcu and MCUb show both of them have three distinct layers, down: top view displays the calcium pore formed by five transmembrane domains of Mcu (left) or MCUb (right) (http://swissmodel.expasy.org/repository/) [25,28]; (C) Cartoon of Homo sapiens MCU in the inner membrane of mitochondria.
Among the MCU subunits, Mcu is the critical pore-forming subunit. Structure analysis reveals that Mcu is a homo-oligomer with two transmembrane domains. The second transmembrane helixes from five Mcu form a hydrophilic pore in the inner membrane of mitochondria, and the DXXE motif forms the pore entrance in the inter-membrane space (Figure 1B, left and Figure 1C) [25]. Mutations of key acidic residues in the motif (D261A or E264A) abolish the calcium uptake activity of the MCU [19]. In addition, the S259A mutant form of Mcu completely blocks the effect of Ru360 [19]. However, the Mcu deficiency phenotype in mice is dependent on mouse strains. Mice from mixed genetic backgrounds are normal, while mice of inbred strains cannot live without Mcu [26,27]. MCUb is the negative regulatory subunit of the MCU. A recent study discovered that MCUb contains two transmembrane domains and forms a structure similar to the Mcu [22]. Homology modeling of MCUb by Swiss model shows MCUb has the same three-dimension-structure and hydrophilic pore with Mcu (Figure 1B, right) (http://swissmodel.expasy.org/repository/) [28]. However, substitutions of the amino acids in the pore forming region result in lost activity of calcium intake [22]. Overexpression of MCUb results in a decreased calcium uptake activity of the MCU, indicating that MCUb is a dominant-negative pore-forming subunit. MICU1 is an important gatekeeper of the MCU and is found to localize to the mitochondrial matrix side of the inner membrane of mitochondria. MICU1 controls MCU-mediated mitochondrial calcium influx by interacting with the coiled-coil domains of Mcu on its N-terminal [29]. Crystal structure analysis indicates that Ca2+-free MICU1 forms a hexamer. When binding with calcium, MICU1 reforms to oligomers and activates MCU [30]. Loss of MICU1 in cells leads to an adaptive mitochondrial matrix calcium accumulation and increased resting mitochondrial calcium level [31,32]. Deletion of MICU1 in mice causes significant perinatal mortality, marked ataxia and muscle weakness. In addition, patients with loss-of-function mutations in MICU1 exhibit an obvious phenotype with brain and muscle disorders. Individuals with MICU1 mutations display proximal myopathy, learning difficulties and a progressive extrapyramidal movement disorder [33]. MICU2 is another gatekeeper of the MCU and plays nonredundant roles with MICU1 in inhibiting the calcium intake activity of the MCU, when outside calcium is low and permitting MCU transport of calcium into mitochondria in response to a stimulus above the threshold [31]. MICU3 plays the same role as MICU2 but has a different expression pattern compare with MICU2. EMRE is a 10-kD single-pass transmembrane protein that functions as a positive regulator of MCU [24]. EMRE is first synthesized as a precursor, which is processed into mature form by general mitochondrial processing peptidase MPP (MPP) with the help of m-AAA protease interacting protein 1 (MAIP1). Mature EMRE inserts into mitochondrial inner membrane, while excess precursor EMRE is degraded [34]. According to studies in yeast, the tight interaction between EMRE and the MCU is essential for mitochondrial calcium uptake. Additionally, EMRE is required for the MCU to reconstitute the minimal uniporter activity in yeast [35]. Assembly assay further shows that loss of EMRE impairs the assembly of Mcu subunits, and overexpression of EMRE facilitates the formation of MCU complex. In addition, both precursor and mature EMRE are capable of forming active calcium uptake channel with Mcu, but only mature EMRE ensures the assembly of gatekeeper subunits to prevent calcium overload. The gatekeeper decreased form MCU is observed in m-AAA proteinase deficient neurons, which has increased calcium influx into mitochondria and more sensitivity to cell death [34]. Those results indicate that EMRE may function as a structural factor for the opening of the Mcu-forming pore and a recruiter for the gatekeeper. MCUR1 was first found to be required for MCU-mediated mitochondrial calcium uptake. One recent study shows that MCUR1 functions as a scaffold factor in the MCU complex by binding to Mcu and EMRE [36]. Cells with MCUR1 knockdown/overexpression display the same mitochondrial calcium uptake activities as the MCU knockdown/overexpression cells [23]. However, one study demonstrated that MCUR1 is a cytochrome c oxidase assembly factor and does not directly regulate the MCU [37]. Thus, the role of MCUR1 in the MCU needs further studies. Collectively, all those subunits form the MCU complex in the inner membrane of mitochondria to maintain mitochondrial calcium homeostasis (Figure 1C) [38].
3. The Role of the MCU in Excitotoxicity
Excitotoxicity is the pathological process in which neuronal cells are damaged or killed by overloaded stimulation by neurotransmitters [39]. Among the diverse transmitters in the mammalian central nervous system, glutamate is the major excitatory neurotransmitter and plays a critical role in the development of AD, PD and stroke [40,41,42,43]. To date, every glutamate receptor subtype discovered has been proven to mediate neurotoxicity [44,45,46]. An obvious phenomenon in glutamate-induced excitotoxicity is delayed calcium deregulation (DCD). A secondary, irreversible increase in calcium occurs after a variable latent period post glutamate stimulation, which precedes and predicts subsequent cell death [47]. According to recent studies, most glutamate receptor-mediated neurotoxicity has been proven to be calcium-dependent, and selective inhibition of glutamate receptors could block the prolonged elevation of calcium [48,49]. In addition, glutamate receptors have found to be capable of calcium uptake (Figure 2) [50,51]. Besides direct calcium transportation, some glutamate receptors were found to regulate inositol 1,4,5-trisphosphate (InsP3) receptor and L-type Ca2+ channel, and subsequently cause calcium increasing in cytosol (Figure 2) [52,53]. Except glutamate-induced excitotoxicity, calcium is also implicated in estrogenic chemical-induced neurotoxicity [54]. Excessive elevation in the intracellular calcium level and mitochondrial dysfunction have been observed in neurons in an excitotoxic condition, although the role of mitochondrial calcium in excitotoxicity has not been clearly described [55,56]. In addition, glutamate-induced mitochondrial dysfunction and loss of membrane potential could be suppressed by RU360, suggests that mitochondrial calcium uptake is essential for excitotoxicity (Figure 2) [57]. A recent study revealed that the MCU controls excitotoxicity and is implicated in N-methyl-D-aspartate (NMDA) receptor-mediated cell death [58]. In this study, authors found that the activation of the NMDA receptor leads to an increase in the mitochondrial calcium level and promotes mitochondrial depolarization. Overexpression of Mcu leads to an NMDA-mediated increase in the mitochondrial calcium level and causes a loss of the mitochondrial membrane potential. In contrast, knockdown of Mcu decreases NMDA-mediated mitochondrial calcium levels, therefore preventing the loss of the mitochondrial membrane potential and excitotoxicity cell death. Those results indicate that the MCU may play an essential role in excitotoxicity; however, more studies are needed to confirm the function of the MCU in vivo.
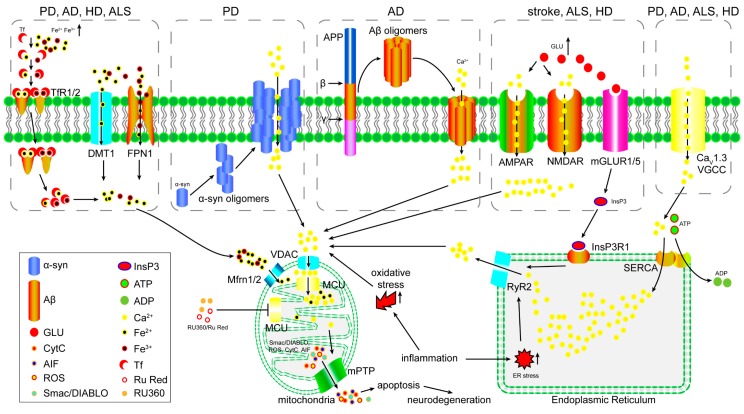
Figure 2.
The proposed model of mitochondrial calcium dysregulation in neurodegenerative disorders and the potentiality of MCU as therapeutic target. Iron over-imported by TfR1/2, DMT1 and FPN1 will be taken up by mitochondria through Mfrn1/2 and potentially MCU. Excess calcium gets into cytoplasm through Aβ oligomers or α-syn oligomers formed ion channel, or glutamate receptors in pathological tissues of neurodegenerative disorders. InsP3R1 activation induce calcium release from ER. Continuous activation of L-type calcium channel CaV1.3 VGCC in PD, AD, HD and ALS. Excess calcium and iron in cytosol induce mitochondrial dysfunction, mPTP opening and pro-apoptosis factors release through MCU-mediated calcium and iron uptake. (PD: Parkinson’s disease; AD: Alzheimer’s disease; HD: Huntington’s disease; ALS: Amyotrophic lateral sclerosis; TfR1/2: transferrin receptor protein 1/2; DMT1: divalent cation transporter 1; FPN1: ferroportin-1; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NMDAR: N-methyl-D-aspartate receptor; mGLUR1/5: metabotropic glutamate receptor 1/5; VGCC: voltage-dependent L-type calcium channel subunit β-2; VDAC: Voltage-dependent anion-selective channel; Mfrn1/2: mitoferrin 1/2: MCU: mitochondrial calcium uniporter; RyR2: ryanodine receptor 2; InsP3R1: inositol 1,4,5-trisphosphate receptor type 1; SERCA: Sarcoplasmic/endoplasmic reticulum calcium ATPase 2; GLU: glutamate; mPTP: mitochondrial permeability transition pore; CytC: cytochrome C; ROS: reactive oxygen species; AIF: apoptosis-inducing factor; Smac/DIABLO: Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI. ER: endoplasmic reticulum).
4. The Role of the MCU in Iron Overload-Induced Mitochondrial Dysfunction
Iron overload is an inducer of brain mitochondrial dysfunction, and excess accumulation of iron in the brain has been detected in various neurodegenerative disorders, such as PD, AD and HD (Figure 2) [59,60,61,62,63,64]. Iron is taken up by cells in transferrin-bound form or free form. In neuron, iron is imported in transferrin-iron (Tf-iron) form and is mediated by transferrin receptor protein 1 (TfR1) (Tf-Fe2+) or TfR2 (Tf-Fe3+). When Tf-iron-TfR complex was endocytosed, iron would be released in cytosol. In TfR1/2-deficient cells, iron is mainly taken up by metal transporters divalent metal transporter 1 (DMT1) and ferroportin-1 (FPN1) in free form (Figure 2) [65]. Overexpression of those transporters is sufficient to induce iron overload and cell death, in addition, increased expression of DMT1 had been observed in PD and AD murine model [66,67]. Accumulation of iron in cytoplasm will induce excessive iron influx into mitochondria through mitoferrin 1/2 (Mfrn1/2) and cause mitochondrial dysfunction [68]. So, individuals with iron overload usually display miscellaneous signs and symptoms. Brains from rats fed with excess iron show severe meningeal hemorrhage, congestion, edema and lipid peroxidation, whereas, 5-HT and DOPA are dramatically decreased [69]. These results indicate that iron overload is sufficient to induce neurodegenerative disease. Excess iron causes cell injury mainly by uncoupling oxidative-phosphonates and inducing mitochondrial dysfunction, resulting in increased reactive oxygen species (ROS) production and oxidative-stress [70,71]. Evidence from in vitro experiments suggests that the MCU may be involved in iron overload-induced mitochondrial dysfunction. Recent studies have shown that only the MCU blocker RU360 could completely prevent iron overload-induced cardiac or brain mitochondrial dysfunction by decreasing ROS production, mitochondrial depolarization and swelling. However, the mitochondrial permeability transition pore (mPTP) blocker cyclosporin A (CsA) merely inhibits ROS production [72,73,74]. These results indicate that the MCU could be a major portal for mitochondrial iron uptake; however, the mechanism demands further study because there is no direct evidence that the MCU could transport iron into mitochondria.
5. The Role of the MCU in Oxidative Stress-Induced Mitochondrial Dysfunction
Oxidative stress is an accelerator of neurodegenerative diseases and is a main factor in PD and stroke [75,76,77]. Cytosolic calcium overload is a common consequence of oxidative stress and indicates that excess cytosolic calcium may induce mitochondrial calcium overload and dysfunction [78,79]. An increase in the calcium level is reported to be an important indicator for oxidative stress-associated diseases. Patients with high serum calcium have been found to be more likely to suffer from a poor short-term prognosis and higher risk of long-term mortality after ischemic stroke [80]. Results from in vitro experiments indicated that inhibition of the activity of the MCU by the MCU inhibitor RU360 or siRNA or miRNA against Mcu rendered the cells resistant to oxidative stress. Consistently, overexpression of the MCU can cause higher sensitivity to the oxidative stress of cells [81,82]. In addition, inhibition of the MCU reduces oxygen glucose deprivation/reperfusion (OGD/RP)-induced autophagy and mitophagy, therefore improving cell viability after OGD/RP [83]. Results from in vivo studies also prove that in a middle cerebral artery occlusion and reperfusion (MCAO-R) model, compared with normal or spermine-treated rats, rats treated with RU360 show decreased total infarct volume, reduced water content, less neuronal damage and cell apoptosis, and lower aquaporin 4 expression [84]. However, another study demonstrated that oxidative stress does not increase mitochondrial calcium in the first few minutes, while cytosolic calcium rapidly rises [85]. These conflicts may be due to the fact that the increase in the mitochondrial calcium lags far behind the increase in the cytosolic calcium, which is similar to DCD (Figure 2).
6. The Function of the MCU in Inflammation
Inflammation is a critical factor in the development of neurodegenerative disorders, such as PD, AD, ALS, depression, and stroke [86,87,88]. Recent studies have shown that upon activation of the inflammatory response, the production of ROS increases, causing damage to the blood-brain barrier (BBB) and mitophagy activation, together with elevated expression of inflammatory cytokines [89,90,91,92]. Mitochondrial calcium homeostasis is reported to be disrupted in infectious diseases, indicating that mitochondrial calcium may be involved in inflammation-associated diseases (Figure 2) [93,94,95,96]. To date, evidence has indicated that the MCU plays an important role in the regulation of bacteria- and virus-induced activation of inflammation. In the Pseudomonas aeruginosa-dependent inflammatory response, the MCU-mediated increase in mitochondrial calcium is critical for the activation of NACHT, LRR and PYD domain-containing protein 3 (NLRP3) and interleukin 1 β (IL-1β) and interleukin 18 (IL-18) processing [97]. The MCU can integrate pro-inflammatory signals and relay the information to NLRP3 by increasing mitochondrial calcium uptake and inducing mitochondrial dysfunction. Another study showed that MCU-mediated calcium uptake is essential for the virus-induced increase in endoplasmic reticulum (ER) stress, which facilitates the amplification of retinoic acid-inducible gene I (RIG-I)-mediated activation of the type I interferon pathway and apoptosis [98]. Taken together, the MCU functions as an enhancer in inflammation by increasing the calcium concentration of mitochondria and upregulation of ER stress. However, the mechanism by which the MCU increases mitochondrial calcium uptake in inflammation is not clear.
7. The Potential of the MCU as a Target for Neurodegenerative Disorder Therapy
Calcium overload can be induced by a wide range of stimuli [99,100]. Mitochondrial dysfunction and calcium homeostasis disruption are observed in many other neurodegenerative disorders as well [2,3,4,5,101,102,103,104].
Alzheimer’s disease is a neurodegenerative disease with specific accumulation of Alzheimer’s β-amyloid (Aβ1–42) peptides in the brain tissue of AD patients [105]. Mitochondrial dysfunction is one of the major symptoms in AD. Studies show that Aβ1–42 peptides accumulate in the mitochondria of the brain in AD patients and APP-transgenic mice [106,107]. Aβ1–42 peptides are imported into the mitochondria via the translocase of the outer membrane import machinery and localizes to mitochondrial cristae [107]. The translocation of Aβ1–42 peptides into mitochondria has been linked to mitotoxic effects by in vitro and in vivo evidence, such as increased release of adenylate kinase and ROS, and this mitotoxicity is promoted by aging [108,109,110]. Calcium influx is one of the mitotoxic effects induced by Aβ1–42, and evidence suggests that oxidative stress and the subsequent neurodegeneration induced by accumulation of Aβ1–42 peptides are calcium influx-dependent (Figure 2) [111,112,113]. This is suitable to support the Aβ ion channel hypothesis. The Aβ ion channel hypothesis suggests that Aβ oligomers form a non-specific ion channel on cell membrane and mitochondrial membrane [114]. This hypothesis had been supported by some other evidences. Three-dimensional structure analysis revealed that Aβ oligomers could form a channel in bilayer, and another study showed the channel display a strong selective affinity for Calcium (Figure 2) [115,116]. Therefore, the “modulation of mitochondrial calcium as a pharmacological target for Alzheimer’s disease” has been recently proposed [117].
PD is another major neurodegenerative disease with extensive mitochondrial dysfunction and oxidative stress [118]. The pathological hallmark of PD is Lewy bodies, of which the primary structural component is abnormal aggregated α-synuclein (SNCA) [119]. In vivo experiments indicate that oligomeric SNCA is sufficient to induce mitochondrial calcium overload and mitochondrial dysfunction (Figure 2) [120,121]. Similar to Aβ oligomers, SNCA oligomers could also form an ion channel on lipid membrane and lead to calcium dyshomeostasis (Figure 2) [122]. Mitochondrial dysfunction and oxidative stress is also observed in genetic forms of PD. Mutations in PINK1, DJ1 and LRRK2 are the main genetic factors of familial PD [123,124,125,126]. All three genes have been demonstrated to have an effect on mitochondrial function. Deficiencies in these genes make mitochondria more vulnerable to oxidative stress [127,128,129,130]. A recent study demonstrated that MCU is involved in PD. Inhibition of MCU could rescue PINK1 deficiency-induced dopaminergic neurons loss [131]. These studies shine light on calcium channels as a target for PD therapy. A clinical trial carried out in Denmark showed that L-type calcium channel blockers are able to reduce the risk of PD (Table 1) [132]. This research suggests that targeting the MCU as a therapy for PD is hopeful.
Table 1.
Calcium/calcium channel antagonist is now used in clinical trials for neurodegenerative diseases therapy.
| Neurodegenerative Disorders | Drug | Target | Clinical Trial ID | Status |
|---|---|---|---|---|
| AD | Losartan/amlodipine | Angiotensin/calcium | NCT02913664 | Phase II |
| SAM-531 | Calcium | NCT00745576 | Phase I | |
| Nilvadipine/Placebo | Calcium | NCT02017340 | Phase III | |
| PD | Isradipine | Calcium channel | NCT00909545 | Phase II |
| Isradipine/Placebo | Calcium channel | NCT02168842 | Phase III | |
| ALS | Fasudil | Calcium | NCT01935518 | Phase II |
AD: Alzheimer’s disease; PD: Parkinson’s disease; ALS: amyotrophic lateral sclerosis.
Stroke is the third prevalent and acute neurodegenerative disorder. Glutamate-induced excitotoxicity and mitochondrial calcium overload have been proven to be the main pathogenic factors in stroke patients and stroke model animals [133,134,135]. High levels of glutamate and calcium in plasma generally indicate a poor prognosis for acute ischemic stroke patients [80,136]. Strategies targeting glutamate and calcium channels have been carried out for decades. Glutamate is an important transmitter that plays an essential role in multiple biological processes, and inhibition of glutamate release and blocking glutamate receptors returns a significant improvement in stroke, although the undesired side effects induced by blockade of the glutamate pathway hold back its therapeutic process [137,138,139]. As calcium overload is the consequence of excitotoxicity and the high serum calcium level in stroke, targeting calcium channels as a therapy seems to be a strong possibility. Calcium antagonists and calcium channel antagonists have been proven to reduce the injury induced by acute ischemic stroke in animal models and patients [140,141,142]. However, a meta-analysis recently indicated that none of the calcium and calcium channel antagonists currently developed display significant performance in acute ischemic stroke patients so far [143]. Severe side effects of calcium antagonists may be the reason for their poor effectiveness in clinical treatment, as calcium is a critical secondary messenger in mammalian cells. The discovery of the MCU provides a new target for treatment, as it is a mitochondria-specific calcium uniporter and, most importantly, mice with an outbred background can live without Mcu. According to recent studies in the rat, the MCU antagonist RU360 displays protective effects in an acute cardiac ischemic model [144]. Whether MCU antagonists can prevent stroke-induced injury is worthy of further study.
8. Conclusions and Future Perspectives
In the brain systems, especially in neurons, calcium overload-induced metabolic derangements and eventual cell death are not only the end of these cells but also the beginning of neuronal injury and neurodegeneration in the brain. Accumulation of excessive calcium in mitochondria causes mitochondrial dysfunction, which is found in most neurodegenerative diseases. Therefore, drugs targeting calcium or calcium channel have strong potentiality in neurodegenerative diseases therapy. A search of ClinicalTrials.gov (see: https://clinicaltrials.gov) with the keywords “calcium antagonist” and “neurodegenerative” revealed several ongoing clinical trials for AD, PD and ALS (Table 1). The MCU, the calcium uniporter on the inner membrane of mitochondria, by controlling the mitochondrial calcium uptake, is implicated in excitotoxicity, iron overload, inflammation and oxidative stress-induced mitochondrial dysfunction and cell death. The involvement of the MCU in those stress-induced cell deaths indicates that the MCU may play an essential role in neurodegenerative disorders as well, suggesting that the MCU could be a new target for disease therapy. For example, a series of studies on glutamate-induced excitotoxicity have commenced in clinical trials; however, a diverse set of drugs targeting glutamate release or glutamate receptors have so far failed due to strong side effects. Inhibition of the MCU in vitro displays significant attenuation of NMDA receptor-mediated excitotoxicity, while loss of the MCU in mice can be tolerated, suggesting that the MCU antagonist is a great potential therapy [58]. On account of this, further in vivo studies are needed to better understand the role of the MCU in neurodegeneration and the application of the MCU antagonist in neurodegenerative disorder therapy.
Targeting mitochondrial calcium has been proposed for the clinical treatment of some neurodegenerative diseases. The MCU is a better target, although further studies are still required. For example, future studies must find an effective MCU antagonist and investigate the effects of the antagonist in disease models. We believe that there will be a detailed description of the role of the MCU and the molecular signaling of MCU proteins in the pathophysiology of central nervous system diseases with more extensive studies in the future. Moreover, it would not be surprising to discover a new therapeutic strategy for neurological diseases through targeting the MCU complex or its subunits.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (Grant No. 81400987).
Author Contributions
Yajin Liao conceived the review topic, reviewed the literature, wrote the manuscript, and prepared the figures; Yuan Dong and Jinbo Cheng reviewed the manuscript; Yajin Liao and Jinbo Cheng performed a comprehensive review of the literature.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Berridge M.J., Bootman M.D., Lipp P. Calcium—A life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill C., Cowburn R.F., Bonkale W.L., Ohm T.G., Fastbom J., Carmody M., Kelliher M. Dysfunctional intracellular calcium homoeostasis: A central cause of neurodegeneration in Alzheimer’s disease. Biochem. Soc. Symp. 2001;67:177–194. doi: 10.1042/bss0670177. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer R.F. Parkinson disease: Calcium channel blockers and Parkinson disease. Nat. Rev. Neurol. 2010;6:188–189. doi: 10.1038/nrneurol.2010.31. [DOI] [PubMed] [Google Scholar]
- 4.Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 5.Tradewell M.L., Cooper L.A., Minotti S., Durham H.D. Calcium dysregulation, mitochondrial pathology and protein aggregation in a culture model of amyotrophic lateral sclerosis: Mechanistic relationship and differential sensitivity to intervention. Neurobiol. Dis. 2011;42:265–275. doi: 10.1016/j.nbd.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Deluca H.F., Engstrom G.W. Calcium uptake by rat kidney mitochondria. Proc. Natl. Acad. Sci. USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mraz F.R. Strontium and Calcium Uptake by Rat Liver and Kidney Mitochondria. Hw-76000. US At. Energy Comm. 1963;86:95–97. [PubMed] [Google Scholar]
- 8.Vasington F.D. Calcium ion uptake by fragments of rat liver mitochondria and its dependence on electron transport. J. Biol. Chem. 1963;238:1841–1847. [PubMed] [Google Scholar]
- 9.Browning M., Baudry M., Lynch G. Evidence that high frequency stimulation influences the phosphorylation of pyruvate dehydrogenase and that the activity of this enzyme is linked to mitochondrial calcium sequestration. Prog. Brain Res. 1982;56:317–337. doi: 10.1016/S0079-6123(08)63782-6. [DOI] [PubMed] [Google Scholar]
- 10.Budde R.J., Fang T.K., Randall D.D. Regulation of the phosphorylation of mitochondrial pyruvate dehydrogenase complex in situ: Effects of respiratory substrates and calcium. Plant Physiol. 1988;88:1031–1036. doi: 10.1104/pp.88.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz F., Komuniecki R. Characterization of the α-ketoglutarate dehydrogenase complex from Fasciola hepatica: Potential implications for the role of calcium in the regulation of helminth mitochondrial metabolism. Mol. Biochem. Parasitol. 1996;81:243–246. doi: 10.1016/0166-6851(96)02705-3. [DOI] [PubMed] [Google Scholar]
- 12.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Nichols B.J., Denton R.M. Towards the molecular basis for the regulation of mitochondrial dehydrogenases by calcium ions. Mol. Cell. Biochem. 1995;149–150:203–212. doi: 10.1007/BF01076578. [DOI] [PubMed] [Google Scholar]
- 14.Irigoin F., Inada N.M., Fernandes M.P., Piacenza L., Gadelha F.R., Vercesi A.E., Radi R. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem. J. 2009;418:595–604. doi: 10.1042/BJ20081981. [DOI] [PubMed] [Google Scholar]
- 15.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 16.Reed K.C., Bygrave F.L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem. J. 1974;140:143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthra R., Olson M.S. The inhibition of calcium uptake and release by rat liver mitochondria by ruthenium red. FEBS Lett. 1977;81:142–146. doi: 10.1016/0014-5793(77)80947-2. [DOI] [PubMed] [Google Scholar]
- 18.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plovanich M., Bogorad R.L., Sancak Y., Kamer K.J., Strittmatter L., Li A.A., Girgis H.S., Kuchimanchi S., de Groot J., Speciner L., et al. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PLoS ONE. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raffaello A., de Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabo I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallilankaraman K., Cardenas C., Doonan P.J., Chandramoorthy H.C., Irrinki K.M., Golenar T., Csordas G., Madireddi P., Yang J., Muller M., et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE Is an Essential Component of the Mitochondrial Calcium Uniporter Complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxenoid K., Dong Y., Cao C., Cui T.X., Sancak Y., Markhard A.L., Grabarek Z., Kong L.L., Liu Z.J., Ouyang B., et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan X., Liu J., Nguyen T., Liu C.Y., Sun J.H., Teng Y.J., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy E., Pan X., Nguyen T., Liu J., Holmstrom K.M., Finkel T. Unresolved questions from the analysis of mice lacking MCU expression. Biochem. Biophys. Res. Commun. 2014;449:384–385. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwede T., Kopp J., Guex N., Peitsch M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman N.E., Chandramoorthy H.C., Shamugapriya S., Zhang X.Q., Rajan S., Mallilankaraman K., Gandhirajan R.K., Vagnozzi R.J., Ferrer L.M., Sreekrishnanilayam K., et al. MICU1 Motifs Define Mitochondrial Calcium Uniporter Binding and Activity. Cell Rep. 2013;5:1576–1588. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Yang X., Li S., Wang Z., Liu Y., Feng J., Zhu Y., Shen Y. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J. 2014;33:594–604. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csordas G., Golenar T., Seifert E.L., Kamer K.J., Sancak Y., Perocchi F., Moffat C., Weaver D., de la Fuente Perez S., Bogorad R., et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J.C., Liu J., Holmstrom K.M., Menazza S., Parks R.J., Fergusson M.M., Yu Z.X., Springer D.A., Halsey C., Liu C., et al. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep. 2016;16:1561–1573. doi: 10.1016/j.celrep.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan C.V., Szabadkai G., Sharpe J.A., Parry D.A., Torelli S., Childs A.M., Kriek M., Phadke R., Johnson C.A., Roberts N.Y., et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 2014;46:188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- 34.Konig T., Troder S.E., Bakka K., Korwitz A., Richter-Dennerlein R., Lampe P.A., Patron M., Muhlmeister M., Guerrero-Castillo S., Brandt U., et al. The m-AAA Protease Associated with Neurodegeneration Limits MCU Activity in Mitochondria. Mol. Cell. 2016;64:148–162. doi: 10.1016/j.molcel.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T., Yamagoshi R., Harada K., Kawano M., Minami N., Ido Y., Kuwahara K., Fujita A., Ozono M., Watanabe A., et al. Analysis of the structure and function of EMRE in a yeast expression system. Biochim. Biophys. Acta. 2016;1857:831–839. doi: 10.1016/j.bbabio.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Tomar D., Dong Z., Shanmughapriya S., Koch D.A., Thomas T., Hoffman N.E., Timbalia S.A., Goldman S.J., Breves S.L., Corbally D.P., et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paupe V., Prudent J., Dassa E.P., Rendon O.Z., Shoubridge E.A. CCDC90A (MCUR1) Is a Cytochrome c Oxidase Assembly Factor and Not a Regulator of the Mitochondrial Calcium Uniporter. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Kamer K.J., Mootha V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 39.Budd S.L., Nicholls D.G. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Mallory M., Alford M., Tanaka S., Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Revett T.J., Baker G.B., Jhamandas J., Kar S. Glutamate system, amyloid β peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013;38:6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caudle W.M., Zhang J. Glutamate, excitotoxicity, and programmed cell death in Parkinson disease. Exp. Neurol. 2009;220:230–233. doi: 10.1016/j.expneurol.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 43.DeLorenzo R.J., Sun D.A., Blair R.E., Sombati S. An in vitro model of stroke-induced epilepsy: Elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int. Rev. Neurobiol. 2007;81:59–84. doi: 10.1016/S0074-7742(06)81005-6. [DOI] [PubMed] [Google Scholar]
- 44.Riederer P., Lange K.W., Kornhuber J., Jellinger K. Glutamate receptor antagonism: Neurotoxicity, anti-akinetic effects, and psychosis. J. Neural Transm. Suppl. 1991;34:203–210. doi: 10.1007/978-3-7091-9175-0_26. [DOI] [PubMed] [Google Scholar]
- 45.Akaike A., Katsuki H., Kume T., Maeda T. Reactive oxygen species in NMDA receptor-mediated glutamate neurotoxicity. Park. Relat. Disord. 1999;5:203–207. doi: 10.1016/S1353-8020(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 46.Iihara K., Joo D.T., Henderson J., Sattler R., Taverna F.A., Lourensen S., Orser B.A., Roder J.C., Tymianski M. The influence of glutamate receptor 2 expression on excitotoxicity in Glur2 null mutant mice. J. Neurosci. 2001;21:2224–2239. doi: 10.1523/JNEUROSCI.21-07-02224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castilho R.F., Hansson O., Ward M.W., Budd S.L., Nicholls D.G. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 1998;18:10277–10286. doi: 10.1523/JNEUROSCI.18-24-10277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attucci S., Clodfelter G.V., Thibault O., Staton J., Moroni F., Landfield P.W., Porter N.M. Group I metabotropic glutamate receptor inhibition selectively blocks a prolonged Ca2+ elevation associated with age-dependent excitotoxicity. Neuroscience. 2002;112:183–194. doi: 10.1016/S0306-4522(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 49.Vergun O., Keelan J., Khodorov B.I., Duchen M.R. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J. Physiol. 1999;519(Pt 2):451–466. doi: 10.1111/j.1469-7793.1999.0451m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaethling J.M., Klein D.M., Singh P., Meaney D.F. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J. Neurotrauma. 2008;25:1207–1216. doi: 10.1089/neu.2008.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqui F., Iqbal Z. Regulation of N-methyl-d-aspartate receptor-mediated calcium transport and norepinephrine release in rat hippocampus synaptosomes by polyamines. Neurochem. Res. 1994;19:1421–1429. doi: 10.1007/BF00972471. [DOI] [PubMed] [Google Scholar]
- 52.Kato H.K., Kassai H., Watabe A.M., Aiba A., Manabe T. Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L-type Ca2+ channel in mouse CA1 pyramidal cells. J. Physiol. 2012;590:3019–3034. doi: 10.1113/jphysiol.2012.232942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Grillner S., Wallen P. Calcium dynamics during NMDA-induced membrane potential oscillations in lamprey spinal neurons—Contribution of L-type calcium channels (Cav1.3) J. Physiol. 2013;591:2509–2521. doi: 10.1113/jphysiol.2012.248526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pretorius E., Bornman M.S. Calcium-mediated aponecrosis plays a central role in the pathogenesis of estrogenic chemical-induced neurotoxicity. Med. Hypotheses. 2005;65:893–904. doi: 10.1016/j.mehy.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Ward M.W., Rego A.C., Frenguelli B.G., Nicholls D.G. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 2000;20:7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parihar M.S., Brewer G.J. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J. Neurosci. Res. 2007;85:1018–1032. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- 57.Abramov A.Y., Duchen M.R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim. Biophys. Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Qiu J., Tan Y.W., Hagenston A.M., Martel M.A., Kneisel N., Skehel P.A., Wyllie D.J.A., Bading H., Hardingham G.E. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 2013 doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zecca L., Casella L., Albertini A., Bellei C., Zucca F.A., Engelen M., Zadlo A., Szewczyk G., Zareba M., Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J. Neurochem. 2008;106:1866–1875. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y., Connor J.R. Iron and ER stress in neurodegenerative disease. Biometals. 2012;25:837–845. doi: 10.1007/s10534-012-9544-8. [DOI] [PubMed] [Google Scholar]
- 61.Thompson K.J., Shoham S., Connor J.R. Iron and neurodegenerative disorders. Brain Res. Bull. 2001;55:155–164. doi: 10.1016/S0361-9230(01)00510-X. [DOI] [PubMed] [Google Scholar]
- 62.Shoham S., Youdim M.B. Iron involvement in neural damage and microgliosis in models of neurodegenerative diseases. Cell. Mol. Biol. 2000;46:743–760. [PubMed] [Google Scholar]
- 63.Hagemeier J., Geurts J.J., Zivadinov R. Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev. Neurother. 2012;12:1467–1480. doi: 10.1586/ern.12.128. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida K. [Iron accumulation and neurodegenerative diseases] Nihon Rinsho. Jpn. J. Clin. Med. 2016;74:1161–1167. [PubMed] [Google Scholar]
- 65.Urrutia P.J., Mena N.P., Nunez M.T. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front. Pharmacol. 2014;5:38. doi: 10.3389/fphar.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chew K.C., Ang E.T., Tai Y.K., Tsang F., Lo S.Q., Ong E., Ong W.Y., Shen H.M., Lim K.L., Dawson V.L., et al. Enhanced autophagy from chronic toxicity of iron and mutant A53T α-synuclein: Implications for neuronal cell death in Parkinson disease. J. Biol. Chem. 2011;286:33380–33389. doi: 10.1074/jbc.M111.268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong X.-H., Gao W.-J., Shao T.-M., Xie H.-L., Bai J.-T., Zhao J.-Y., Chai X.-Q. Age-related changes of brain iron load changes in the frontal cortex in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2015;30:118–123. doi: 10.1016/j.jtemb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Paradkar P.N., Zumbrennen K.B., Paw B.H., Ward D.M., Kaplan J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garringer H.J., Irimia J.M., Li W., Goodwin C.B., Richine B., Acton A., Chan R.J., Peacock M., Muhoberac B.B., Ghetti B., et al. Effect of Systemic Iron Overload and a Chelation Therapy in a Mouse Model of the Neurodegenerative Disease Hereditary Ferritinopathy. PLoS ONE. 2016;11:e0161341. doi: 10.1371/journal.pone.0161341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harley A., Cooper J.M., Schapira A.H. Iron induced oxidative stress and mitochondrial dysfunction: Relevance to Parkinson’s disease. Brain Res. 1993;627:349–353. doi: 10.1016/0006-8993(93)90341-J. [DOI] [PubMed] [Google Scholar]
- 71.Vatassery G.T., DeMaster E.G., Lai J.C., Smith W.E., Quach H.T. Iron uncouples oxidative phosphorylation in brain mitochondria isolated from vitamin E-deficient rats. Biochim. Biophys. Acta. 2004;1688:265–273. doi: 10.1016/j.bbadis.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 72.Yan H.Y., Hao S.Y., Sun X.Y., Zhang D.D., Gao X., Yu Z., Li K.Y., Hang C.H. Blockage of mitochondrial calcium uniporter prevents iron accumulation in a model of experimental subarachnoid hemorrhage. Biochem. Biophys. Res. Commun. 2015;456:835–840. doi: 10.1016/j.bbrc.2014.12.073. [DOI] [PubMed] [Google Scholar]
- 73.Sripetchwandee J., KenKnight S.B., Sanit J., Chattipakorn S., Chattipakorn N. Blockade of mitochondrial calcium uniporter prevents cardiac mitochondrial dysfunction caused by iron overload. Acta Physiol. 2014;210:330–341. doi: 10.1111/apha.12162. [DOI] [PubMed] [Google Scholar]
- 74.Sripetchwandee J., Sanit J., Chattipakorn N., Chattipakorn S.C. Mitochondrial calcium uniporter blocker effectively prevents brain mitochondrial dysfunction caused by iron overload. Life Sci. 2013;92:298–304. doi: 10.1016/j.lfs.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Navarro A., Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J. Bioenerg. Biomembr. 2009;41:517–521. doi: 10.1007/s10863-009-9250-6. [DOI] [PubMed] [Google Scholar]
- 76.Onyango I.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Neurochem. Res. 2008;33:589–597. doi: 10.1007/s11064-007-9482-y. [DOI] [PubMed] [Google Scholar]
- 77.Mattson M.P. Secretases, oxidative stress, and perturbed calcium homeostasis in AD and stroke. J. Gen. Physiol. 2008;132:14a. [Google Scholar]
- 78.Klonowski-Stumpe H., Schreiber R., Grolik M., Schulz H.U., Haussinger D., Niederau C. Effect of oxidative stress on cellular functions and cytosolic free calcium of rat pancreatic acinar cells. Am. J. Physiol. 1997;272:G1489–G1498. doi: 10.1152/ajpgi.1997.272.6.G1489. [DOI] [PubMed] [Google Scholar]
- 79.Gutierrez-Martin Y., Martin-Romero F.J., Henao F., Gutierrez-Merino C. Alteration of cytosolic free calcium homeostasis by SIN-1: High sensitivity of L-type Ca2+ channels to extracellular oxidative/nitrosative stress in cerebellar granule cells. J. Neurochem. 2005;92:973–989. doi: 10.1111/j.1471-4159.2004.02964.x. [DOI] [PubMed] [Google Scholar]
- 80.Appel S.A., Molshatzki N., Schwammenthal Y., Merzeliak O., Toashi M., Sela B., Tanne D. Serum Calcium Levels and Long-Term Mortality in Patients with Acute Stroke. Cerebrovasc. Dis. 2011;31:93–99. doi: 10.1159/000321335. [DOI] [PubMed] [Google Scholar]
- 81.Liao Y.J., Hao Y.M., Chen H., He Q., Yuan Z.Q., Cheng J.B. Mitochondrial calcium uniporter protein MCU is involved in oxidative stress-induced cell death. Protein Cell. 2015;6:434–442. doi: 10.1007/s13238-015-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan L., Huang B.J., Ma X.E., Wang S.Y., Feng J., Lv F., Liu Y., Liu Y., Li C.M., Liang D.D., et al. MiR-25 protects cardiomyocytes against oxidative damage by targeting the mitochondrial calcium uniporter. Int. J. Mol. Sci. 2015;16:5420–5433. doi: 10.3390/ijms16035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu S.S., Zheng S.F., Leng J., Wang S.L., Zhao T., Liu J. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci. Lett. 2016;628:24–29. doi: 10.1016/j.neulet.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Li L.L., Wang S.L., Luan H.H. Effects of the mitochondrial calcium uniporter on cerebral edema in a rat model of cerebral ischemia reperfusion injury. Neural Regen. Res. 2011;6:1720–1724. [Google Scholar]
- 85.Barsukova A.G., Bourdette D., Forte M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur. J. Neurosci. 2011;34:437–447. doi: 10.1111/j.1460-9568.2011.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baune B.T. Inflammation and neurodegenerative disorders: Is there still hope for therapeutic intervention? Curr. Opin. Psychiatry. 2015;28:148–154. doi: 10.1097/YCO.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 87.Galimberti D., Fenoglio C., Scarpini E. Inflammation in neurodegenerative disorders: Friend or foe? Curr. Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 88.Whitney N.P., Eidem T.M., Peng H., Huang Y., Zheng J.C. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merrill J.E., Murphy S.P. Inflammatory events at the blood brain barrier: Regulation of adhesion molecules, cytokines, and chemokines by reactive nitrogen and oxygen species. Brain Behav. Immun. 1997;11:245–263. doi: 10.1006/brbi.1997.0496. [DOI] [PubMed] [Google Scholar]
- 90.Yang D., Elner S.G., Bian Z.M., Till G.O., Petty H.R., Elner V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kielland A., Blom T., Nandakumar K.S., Holmdahl R., Blomhoff R., Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic. Biol. Med. 2009;47:760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 92.Lin C., Chao H., Li Z., Xu X., Liu Y., Hou L., Liu N., Ji J. Melatonin attenuates traumatic brain injury-induced inflammation: A possible role for mitophagy. J. Pineal Res. 2016;61:177–186. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 93.Huang S.H., Lien J.C., Chen C.J., Liu Y.C., Wang C.Y., Ping C.F., Lin Y.F., Huang A.C., Lin C.W. Antiviral Activity of a Novel Compound CW-33 against Japanese Encephalitis Virus through Inhibiting Intracellular Calcium Overload. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hui B., Li J., Geng M.Y. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome drug candidate, decreased vulnerability of PC12 cells to human immunodeficiency virus tat protein through attenuating calcium overload. J. Neurosci. Res. 2008;86:1169–1177. doi: 10.1002/jnr.21566. [DOI] [PubMed] [Google Scholar]
- 95.Xu H., Heath M.C. Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell. 1998;10:585–598. doi: 10.1105/tpc.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright K.A., Olsen R.G. In vitro infection of cell lines with HTLV-I and SIVmac results in altered intracellular free calcium concentration and increased membrane polarization. Int. J. Cancer. 1989;44:753–756. doi: 10.1002/ijc.2910440433. [DOI] [PubMed] [Google Scholar]
- 97.Rimessi A., Bezzerri V., Patergnani S., Marchi S., Cabrini G., Pinton P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 2015;6:6201. doi: 10.1038/ncomms7201. [DOI] [PubMed] [Google Scholar]
- 98.Cheng J.B., Liao Y.J., Zhou L.J., Peng S.Y., Chen H., Yuan Z.Q. Amplified RLR signaling activation through an interferon-stimulated gene-endoplasmic reticulum stress-mitochondrial calcium uniporter protein loop. Sci. Rep. 2016;6 doi: 10.1038/srep20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen J., Liao W., Gao W., Huang J., Gao Y. Intermittent hypoxia protects cerebral mitochondrial function from calcium overload. Acta Neurol. Belg. 2013;113:507–513. doi: 10.1007/s13760-013-0220-8. [DOI] [PubMed] [Google Scholar]
- 100.Ishida H., Hirota Y., Genka C., Nakazawa H., Nakaya H., Sato T. Opening of mitochondrial K(ATP) channels attenuates the ouabain-induced calcium overload in mitochondria. Circ. Res. 2001;89:856–858. doi: 10.1161/hh2201.100341. [DOI] [PubMed] [Google Scholar]
- 101.Kawahara M., Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Res. Bull. 2000;53:389–397. doi: 10.1016/S0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 102.Marongiu R., Spencer B., Crews L., Adame A., Patrick C., Trejo M., Dallapiccola B., Valente E.M., Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J. Neurochem. 2009;108:1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J.Q., Chen Q., Wang X., Wang Q.C., Wang Y., Cheng H.P., Guo C., Sun Q., Chen Q., Tang T.S. Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease. J. Biol. Chem. 2013;288:3070–3084. doi: 10.1074/jbc.M112.407726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim H.J., Magrane J., Starkov A.A., Manfredi G. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain J. Neurol. 2012;135:2865–2874. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon B.A., Blazey T., Su Y., Fagan A.M., Holtzman D.M., Morris J.C., Benzinger T.L. Longitudinal β-Amyloid Deposition and Hippocampal Volume in Preclinical Alzheimer Disease and Suspected Non-Alzheimer Disease Pathophysiology. JAMA Neurol. 2016;73:1192–1200. doi: 10.1001/jamaneurol.2016.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosales-Corral S., Acuna-Castroviejo D., Tan D.X., Lopez-Armas G., Cruz-Ramos J., Munoz R., Melnikov V.G., Manchester L.C., Reiter R.J. Accumulation of exogenous amyloid-β peptide in hippocampal mitochondria causes their dysfunction: A protective role for melatonin. Oxidative Med. Cell. Longev. 2012;2012:843649. doi: 10.1155/2012/843649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I., Leinonen V., Ito A., Winblad B., Glaser E., et al. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tummala H., Li X., Homayouni R. Interaction of a novel mitochondrial protein, 4-nitrophenylphosphatase domain and non-neuronal SNAP25-like protein homolog 1 (NIPSNAP1), with the amyloid precursor protein family. Eur. J. Neurosci. 2010;31:1926–1934. doi: 10.1111/j.1460-9568.2010.07248.x. [DOI] [PubMed] [Google Scholar]
- 109.Tillement L., Lecanu L., Papadopoulos V. Further evidence on mitochondrial targeting of β-amyloid and specificity of β-amyloid-induced mitotoxicity in neurons. Neuro-Degener. Dis. 2011;8:331–344. doi: 10.1159/000323264. [DOI] [PubMed] [Google Scholar]
- 110.Sinha M., Behera P., Bhowmick P., Banerjee K., Basu S., Chakrabarti S. Aging promotes amyloid-β peptide induced mitochondrial dysfunctions in rat brain: A molecular link between aging and Alzheimer’s disease. J. Alzheimer’s Dis. JAD. 2011;27:753–765. doi: 10.3233/JAD-2011-110686. [DOI] [PubMed] [Google Scholar]
- 111.Cha M.Y., Han S.H., Son S.M., Hong H.S., Choi Y.J., Byun J., Mook-Jung I. Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE. 2012;7:e34929. doi: 10.1371/journal.pone.0034929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ekinci F.J., Malik K.U., Shea T.B. Activation of the L voltage-sensitive calcium channel by mitogen-activated protein (MAP) kinase following exposure of neuronal cells to β-amyloid—Map kinase mediates β-amyloid-induced neurodegeneration. J. Biol. Chem. 1999;274:30322–30327. doi: 10.1074/jbc.274.42.30322. [DOI] [PubMed] [Google Scholar]
- 113.Ekinci F.J., Linsley M.D., Shea T.B. β-Amyloid-induced calcium influx induces neurodegeneration in culture by oxidative stress rather than tau phosphorylation. Mol. Biol. Cell. 1999;10:61A. doi: 10.1016/s0169-328x(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 114.Durell S.R., Guy H.R., Arispe N., Rojas E., Pollard H.B. Theoretical models of the ion channel structure of amyloid β-protein. Biophys. J. 1994;67:2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lal R., Lin H., Quist A.P. Amyloid β ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta. 2007;1768:1966–1975. doi: 10.1016/j.bbamem.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jang H., Zheng J., Nussinov R. Models of β-amyloid ion channels in the membrane suggest that channel formation in the bilayer is a dynamic process. Biophys. J. 2007;93:1938–1949. doi: 10.1529/biophysj.107.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hung C.H., Ho Y.S., Chang R.C. Modulation of mitochondrial calcium as a pharmacological target for Alzheimer’s disease. Ageing Res. Rev. 2010;9:447–456. doi: 10.1016/j.arr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Bose A., Beal M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016;139(Suppl. S1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 119.Jellinger K.A. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front. Neurol. Neurosci. 2009;24:114–125. doi: 10.1159/000197890. [DOI] [PubMed] [Google Scholar]
- 120.Ying Z., Lin F., Gu W., Su Y., Arshad A., Qing H., Deng Y. α-Synuclein increases U251 cells vulnerability to hydrogen peroxide by disrupting calcium homeostasis. J. Neural Transm. 2011;118:1165–1172. doi: 10.1007/s00702-011-0596-7. [DOI] [PubMed] [Google Scholar]
- 121.Melachroinou K., Xilouri M., Emmanouilidou E., Masgrau R., Papazafiri P., Stefanis L., Vekrellis K. Deregulation of calcium homeostasis mediates secreted α-synuclein-induced neurotoxicity. Neurobiol. Aging. 2013;34:2853–2865. doi: 10.1016/j.neurobiolaging.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt F., Levin J., Kamp F., Kretzschmar H., Giese A., Botzel K. Single-channel electrophysiology reveals a distinct and uniform pore complex formed by α-synuclein oligomers in lipid membranes. PLoS ONE. 2012;7:e42545. doi: 10.1371/journal.pone.0042545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarimon J., Eerola J., Hellstrom O., Peuralinna T., Tienari P.J., Singleton A.B. Assessment of PINK1 (PARK6) polymorphisms in Finnish PD. Neurobiol. Aging. 2006;27:906–907. doi: 10.1016/j.neurobiolaging.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 124.Healy D.G., Abou-Sleiman P.M., Jain S., Ahmadi K.R., Wood N.W. Assessment of a DJ-1 (PARK7) polymorphism in Finnish PD. Neurology. 2004;62:2335. doi: 10.1212/WNL.62.12.2335-a. [DOI] [PubMed] [Google Scholar]
- 125.Brockmann K., Apel A., Schulte C., Schneiderhan-Marra N., Pont-Sunyer C., Vilas D., Ruiz-Martinez J., Langkamp M., Corvol J.C., Cormier F., et al. Inflammatory profile in LRRK2-associated prodromal and clinical PD. J. Neuroinflamm. 2016;13:122. doi: 10.1186/s12974-016-0588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dodson M.W., Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Curr. Opin. Neurobiol. 2007;17:331–337. doi: 10.1016/j.conb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 127.Pilsl A., Winklhofer K.F. Parkin, PINK1 and mitochondrial integrity: Emerging concepts of mitochondrial dysfunction in Parkinson’s disease. Acta Neuropathol. 2012;123:173–188. doi: 10.1007/s00401-011-0902-3. [DOI] [PubMed] [Google Scholar]
- 128.Liu W., Vives-Bauza C., Acin-Perez R., Yamamoto A., Tan Y., Li Y., Magrane J., Stavarache M.A., Shaffer S., Chang S., et al. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and α-synuclein aggregation in cell culture models of Parkinson’s disease. PLoS ONE. 2009;4:e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Nottia M., Masciullo M., Verrigni D., Petrillo S., Modoni A., Rizzo V., Di Giuda D., Rizza T., Niceta M., Torraco A., et al. DJ-1 modulates mitochondrial response to oxidative stress: Clues from a novel diagnosis of PARK7. Clin. Genet. 2016 doi: 10.1111/cge.12841. [DOI] [PubMed] [Google Scholar]
- 130.Pereira C., Miguel Martins L., Saraiva L. LRRK2, but not pathogenic mutants, protects against H2O2 stress depending on mitochondrial function and endocytosis in a yeast model. Biochim. Biophys. Acta. 2014;1840:2025–2031. doi: 10.1016/j.bbagen.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 131.Soman S., Keatinge M., Moein M., Da Costa M., Mortiboys H., Skupin A., Sugunan S., Bazala M., Kuznicki J., Bandmann O. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons in pink1−/− zebrafish. Eur. J. Neurosci. 2016 doi: 10.1111/ejn.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ritz B., Rhodes S.L., Qian L., Schernhammer E., Olsen J.H., Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010;67:600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shirotani T., Shima K., Iwata M., Kita H., Chigasaki H. Calcium Accumulation Following Middle Cerebral-Artery Occlusion in Stroke-Prone Spontaneously Hypertensive Rats. J. Cereb. Blood Flow Metab. 1994;14:831–836. doi: 10.1038/jcbfm.1994.104. [DOI] [PubMed] [Google Scholar]
- 134.Vila N., Chamorro A., Castillo J., Davalos A. Glutamate, interleukin-6, and early clinical worsening in patients with acute stroke. Stroke. 2001;32:1234–1237. doi: 10.1161/01.STR.32.5.1234-a. [DOI] [PubMed] [Google Scholar]
- 135.Davalos A., Shuaib A., Wahlgren N.G. Neurotransmitters and pathophysiology of stroke: Evidence for the release of glutamate and other transmitters/mediators in animals and humans. J. Stroke Cerebrovasc. Dis. 2000;9:2–8. doi: 10.1053/jscd.2000.18908. [DOI] [PubMed] [Google Scholar]
- 136.Meng X.E., Li N., Guo D.Z., Pan S.Y., Li H., Yang C. High plasma glutamate levels are associated with poor functional outcome in acute ischemic stroke. Cell. Mol. Neurobiol. 2015;35:159–165. doi: 10.1007/s10571-014-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krivonos O.V., Amosova N.A., Smolentseva I.G. Use of the glutamate NMDA receptor antagonist PK-Merz in acute stroke. Neurosci. Behav. Physiol. 2010;40:529–532. doi: 10.1007/s11055-010-9292-6. [DOI] [PubMed] [Google Scholar]
- 138.Grotta J., Clark W., Coull B., Pettigrew L.C., Mackay B., Goldstein L.B., Meissner I., Murphy D., LaRue L. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke. 1995;26:602–605. doi: 10.1161/01.STR.26.4.602. [DOI] [PubMed] [Google Scholar]
- 139.Elting J.W., Sulter G.A., Kaste M., Lees K.R., Diener H.C., Hommel M., Versavel M., Teelken A.W., de Keyser J. AMPA antagonist ZK200775 in patients with acute ischemic stroke: Possible glial cell toxicity detected by monitoring of S-100B serum levels. Stroke. 2002;33:2813–2818. doi: 10.1161/01.STR.0000043823.37955.FB. [DOI] [PubMed] [Google Scholar]
- 140.Higashino H., Suzuki A. A calcium antagonist, NB-818, improves blood flow distribution in the brain and other major organs of stroke-prone spontaneously hypertensive rats (SHRSP) J. Brain Sci. 1997;23:79–95. [Google Scholar]
- 141.Shinyama H., Kawamura T., Iwamoto M., Egi Y., Tanaka H., Kawabata Y., Nakamura N., Kagitani Y. Effects of the calcium antagonist AE0047 on the development of neurological deficit and infarction after middle cerebral artery occlusion in stroke-prone spontaneously hypertensive rats. J. Pharm. Pharmacol. 1997;49:919–924. doi: 10.1111/j.2042-7158.1997.tb06136.x. [DOI] [PubMed] [Google Scholar]
- 142.Sahlen A., Hamid N., Amanullah M.R., Fam J.M., Yeo K.K., Lau Y.H., Lam C.S., Ding Z.P. Impact of aortic root size on left ventricular afterload and stroke volume. Eur. J. Appl. Physiol. 2016;116:1355–1365. doi: 10.1007/s00421-016-3392-0. [DOI] [PubMed] [Google Scholar]
- 143.Zhang J., Yang J., Zhang C.F., Jiang X.Q., Zhou H.Q., Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD001928.pub2. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S.Z., Gao Q., Cao C.M., Bruce I.C., Xia Q. Involvement of the mitochondrial calcium uniporter in cardioprotection by ischemic preconditioning. Life Sci. 2006;78:738–745. doi: 10.1016/j.lfs.2005.05.076. [DOI] [PubMed] [Google Scholar]