Abstract
Antioxidants play an important role as Reactive Oxygen Species (ROS) chelating agents and, therefore, the screening for potent antioxidants from natural sources as potential protective agents is of great relevance. The main aim of this study was to obtain antioxidant-enriched fractions from the common seaweed Fucus spiralis and evaluate their activity and efficiency in protecting human cells (MCF-7 cells) on an oxidative stress condition induced by H2O2. Five fractions, F1–F5, were obtained by reversed-phase vacuum liquid chromatography. F3, F4 and F5 revealed the highest phlorotannin content, also showing the strongest antioxidant effects. The cell death induced by H2O2 was reduced by all fractions following the potency order F4 > F2 > F3 > F5 > F1. Only fraction F4 completely inhibited the H2O2 effect. To understand the possible mechanisms of action of these fractions, the cellular production of H2O2, the mitochondrial membrane potential and the caspase 9 activity were studied. Fractions F3 and F4 presented the highest reduction on H2O2 cell production. All fractions decreased both caspase-9 activity and cell membrane depolarization (except F1). Taken all together, the edible F. spiralis reveal that they provide protection against oxidative stress induced by H2O2 on the human MCF-7 cellular model, probably acting as upstream blockers of apoptosis.
Keywords: edible algae, oxidative stress, phlorotannins, marine natural products, MCF-7 cells, reactive oxygen species
1. Introduction
For thousands of years, edible seaweeds have been highly valued and widely consumed as a direct human food by Oriental communities [1]. According to several studies, these organisms contain a range of components that have potential health benefits. They are also good sources of dietary fiber, polyunsaturated fatty acids, minerals, vitamins and a wide range of phenolic compounds [2,3]. Marine brown algae include a wide range of edible seaweeds such as Laminaria spp., Undaria spp., Ecklonia spp., Sargassum spp., Fucus spp., etc., which are rich in a specific group of antioxidant compounds, the phlorotannins [1,4,5]. Phlorotannins are unique phenolic compounds that belong to a large class of marine secondary metabolites exclusively produced by brown algae. They are often considered to act as a chemical defense against herbivores and also possess primary functions, such as contributing to cell wall structure and reproduction [6]. These potent antioxidant compounds are oligomers or polymers of phloroglucinol (1,3,5-trihydroxybenzene), connected by aryl–aryl bonds (fucols), ether bonds (phlorethols, hydroxyphlorethols, fuhalols), or both (fucophlorethols), or with a dibenzodioxin linkage (eckols and carmalols) [7].
Due to the health benefits of phlorotannins, marine brown algae are considered a rich source of healthy food. In fact, in Occidental cultures, algae are highly valued for their nutritional content as well as antioxidant benefits [8,9].
Antioxidants act as sacrificial reducing agents, eliciting their benefits by preventing, delaying, or neutralizing the effects of oxidative change and suppression and/or scavenging of free radicals. Therefore, antioxidants can neutralize reactive free radicals in cells, reducing potential mutations and act as stabilizers in the food industry, in order to increase the shelf-life of food products [10,11]. Consequently, antioxidant compounds have attracted the interest of many researchers, mainly for recognition of their scavenging properties, and also for their preventive role in several diseases associated with oxidative stress. Oxidative stress occurs when the balance between antioxidants and reactive oxygen species (ROS) is disrupted because of either depletion of antioxidants or accumulation of ROS. ROS are produced by cellular metabolic activities and triggered by environmental factors, such as air pollutants or cigarette smoke. They are highly reactive and can react with several biological macromolecules in cells, such as carbohydrates, nucleic acids, lipids and proteins, altering their functions [12]. Generation and accumulation of ROS are, therefore, detrimental to cells and they are proven to be directly related to the occurrence of a vast range of disorders/diseases from neurodegenerative diseases, cancer to skin problems, and are even linked to the aging process [13,14,15].
One approach for preventing or treating these ROS-mediated disorders can be based on a diet rich in antioxidants. Nevertheless, while many antioxidants have revealed high efficiency in vitro, their efficiency is unclear in humans [15]. Therefore, the search for new molecules with antioxidant properties from natural sources is of great importance.
Fucus spiralis (spiral wrack) is an edible brown seaweed (Phaeophyceae) living on the Atlantic coasts of Europe and North America and has been previously identified for high antioxidant properties, mainly linked to its phlorotannin content [16,17,18]. The separation of seaweed’s phenolic compounds is usually highly challenging due to their instability, complex mixture, pH dependence and high affinity with other biomolecules. In this work, we wanted to obtain phlorotannin-enriched fractions from Fucus spiralis and to assess their antioxidant activity and efficiency in protecting human cells (MCF-7 cells) exposed to oxidative stress conditions.
2. Results
2.1. Antioxidant Activity
In order to describe the antioxidant potential of each fraction, the total phenolic content (TPC) was assessed as well as the evaluation of the Oxygen Radical Absorbance Capacity (ORAC), the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity and evaluation of the hydroxyl radical scavenging activity (•OH).
The total phenolic content was evaluated in all fractions by the Folin–Ciocalteu method, and the results are shown in Table 1. Fractions 4, 3 and 5 demonstrated the highest phenolic content with 419.00 ± 3.00, 379.00 ± 34.0 and 285.00 ± 12.00 mg·PE/g extract, respectively.
Table 1.
Total phenolic content and antioxidant activity of Fucus spiralis fractions.
| Fractions | TPC a | DPPH b | •OH c | ORAC d |
|---|---|---|---|---|
| 1 | 8.00 ± 1.10 | 182.90 (124.30–269.00) | 7.90 (5.60–11.60) | 2,988.00 ± 107.77 |
| 2 | 33.00 ± 18.00 | 44.40 (38.30–51.47) | 11.52 (8.03–16.52) | 5,411.00 ± 91.54 |
| 3 | 379.00 ± 34.0 | 15.58 (13.31–18.22) | 9.73 (6.51–14.55) | 6,877.00 ± 92.56 |
| 4 | 419.00 ± 3.00 | 13.94 (11.13–17.46) | 10.86 (5.90–19.95) | 34,893.68 ± 945.20 |
| 5 | 285.00 ± 12.00 | 9.74 (8.14–11.66) | 58.61 (41.32–83.14) | 30,691.00 ± 1172.90 |
| BHT | - | 40.55 (25.74–63.87) | >1000 | 320.49 ± 32.31 |
a mg Phloroglucinol equivalents/g extract; b 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (IC50 µg/mL); c hydroxyl (•OH) scavenging activity (IC50 µg/mL); d µmol TE/g extract; TPC = total phenolic content; ORAC = oxygen radical absorbance capacity; BHT = butylated hydroxytoluene.
As regards the DPPH radical scavenging ability, all fractions presented a concentration-dependent effect and fraction 5 presented the highest scavenging activity with an IC50 of 9.74 µg/mL (8.14–11.66) followed by fractions 4 and 3, which presented an IC50 of 13.94 µg/mL (11.13–17.46) and 15.58 µg/mL (13.31–18.22), respectively.
Regarding the hydroxyl radical scavenging activity, all fractions presented a concentration-dependent effect, and fraction 1 presented the highest potential in this radical scavenging activity with an IC50 of 7.90 µg/mL (5.60–11.60) followed by fractions 3, 4 and 2, which presented an IC50 of 9.73 µg/mL (6.51–14.55), 10.86 µg/mL (5.90–19.95) and 11.52 µg/mL (8.03–16.52), respectively.
As regards ORAC, all fractions presented a high oxygen radical scavenging capacity, ranging from 2988.00 to 34,893.68, fraction 4 being the strongest scavenger, followed by fractions 5 and 3 with 30,691.00 and 6877.00 µmol·TE/g extract, respectively (Table 1).
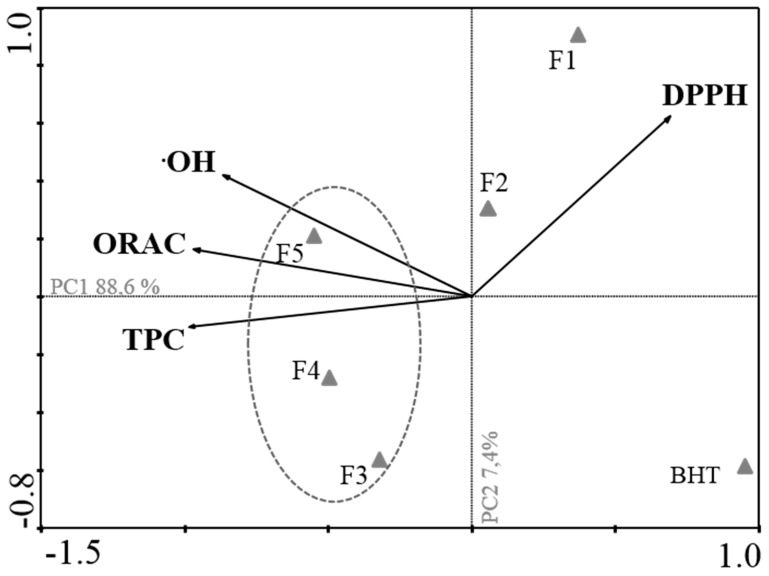
A principal components analysis (Figure 1) was performed in order to obtain an overview of the similarities and differences between all the studied fractions, as well as to investigate the relationship between the different methods used for evaluating the antioxidant activity.
Figure 1.
Principal component analysis (PCA) of total phenolic content (TPC) and antioxidant activities (DPPH, •OH and ORAC) of Fucus spiralis fractions. The vectors represent a variant existing among the antioxidant methods. The first principal component (PC1) and the second principal component (PC2) represent the total variance of the data. The circle defines a group of samples that contains the highest antioxidant activity. The triangles in the figure represent each sample distributed according to its variance.
The first two principal components, PC1 and PC2, explain 88.6% and 7.4% of the total variance of the data set, respectively. Through the analysis of PC1, it is possible to observe a strong negative correlation between TPC (on the left) and DPPH (on the right), indicating that fractions with the highest potency in scavenging DPPH radical (lower IC50 values) presented the highest TPC, namely, fractions 3, 4 and 5. On the other hand, TPC shows a positive correlation with ORAC, revealing that fractions with the highest Phloroglucinol-equivalent contents were also the ones presenting the highest ORAC activity (fractions 4, 5 and 3). All fractions presented a high •OH scavenging activity, being strongly correlated with ORAC and TPC. DPPH radical scavenging activity is not correlated with ORAC and •OH scavenging. This analysis confirmed that F3, F4 and F5 are the most potent antioxidant fractions.
2.2. Protective Effect of Seaweed Fractions on MCF-7 Cells Exposed to H2O2
The antioxidant screening allowed us to verify that all fractions presented potential as ROS scavengers. Therefore, it was important to access their cytotoxicity on MCF-7 cells. The cytotoxicity of all fractions was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method, and none of the fractions (1 mg/mL) presented cytotoxicity on MCF-7 cells.
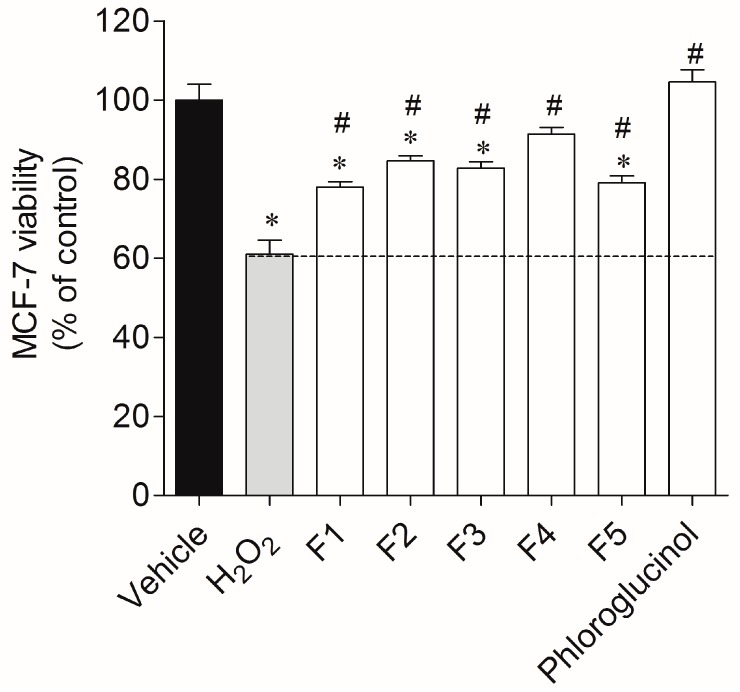
In the cytoprotection assay, MCF-7 cells were exposed to H2O2 (0.2 mM), which led to a 40% reduction of cell viability (61.1% ± 3.5%, Figure 2). On the other hand, in the presence of all fractions it was possible to verify a recovery of cell viability from 17%–30% after 24 h of treatment, with fraction 4 presenting the highest protective effect with 91.4% ± 1.7% of viable cells (30% recovery). Phloroglucinol was used as standard and fully blunted the H2O2 effect (100% ± 3.13% viable cells).
Figure 2.
The effect of Fucus spiralis fractions (1 mg/mL) in an oxidative stress condition promoted by H2O2 (0.2 mM), after 24 h of treatment, on MCF-7 cells. Results were obtained by the MTT method. Values in each column represent the mean ± standard error of the mean (SEM) of eight independent experiments. Symbols represent statistically significant differences (p < 0.05, analysis of variance (ANOVA), Dunett’s test) when compared to: * vehicle and # to H2O2.
2.3. Study of the Cellular Mechanisms Involved in the Cytotoxicity Induced by H2O2 on MCF-7 Cells in the Presence or Absence of Fucus spiralis Fractions
2.3.1. Real-Time Quantification of H2O2 Production
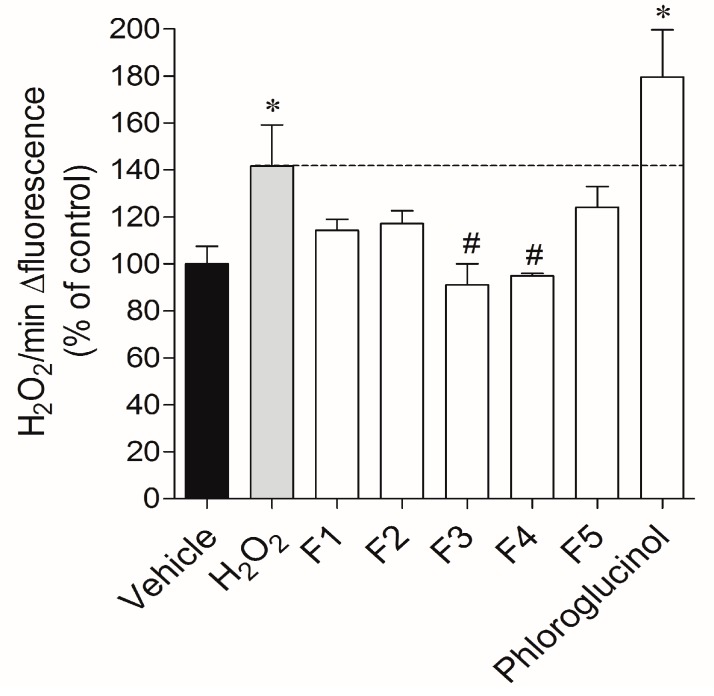
In order to understand whether the cytotoxicity induced by H2O2 and the protection evidenced by Fucus spiralis fractions were associated with oxidative stress, H2O2 production was quantified in real time. The exposure of MCF-7 cells to H2O2 (0.2 mM) during 24 h promoted an increase of H2O2 levels in about 42% when compared with the vehicle (Figure 3). This increase was also visible after the replacement of the H2O2 loading medium. On the other hand, when cells were incubated with H2O2 in the presence of fractions 3 and 4, the H2O2 production decreased by 50.64% (91.04% ± 8.96% of control) and 46.71% (94.97% ± 1.08% of control), respectively. Fractions 1, 2, 5 and phloroglucinol did not exhibit capacity to decrease the H2O2 production.
Figure 3.
Real-time H2O2 quantification on MCF-7 cells in the absence or presence of Fucus spiralis fractions (1 mg/mL; 24 h). Each column represents the mean ± standard error of the mean (SEM) of three or four independent experiments. Symbols represent statistically significant differences (p < 0.05, ANOVA, Dunett’s test) when compared to: * vehicle and # to H2O2.
2.3.2. Mitochondrial Membrane Potential (ΔΨm)
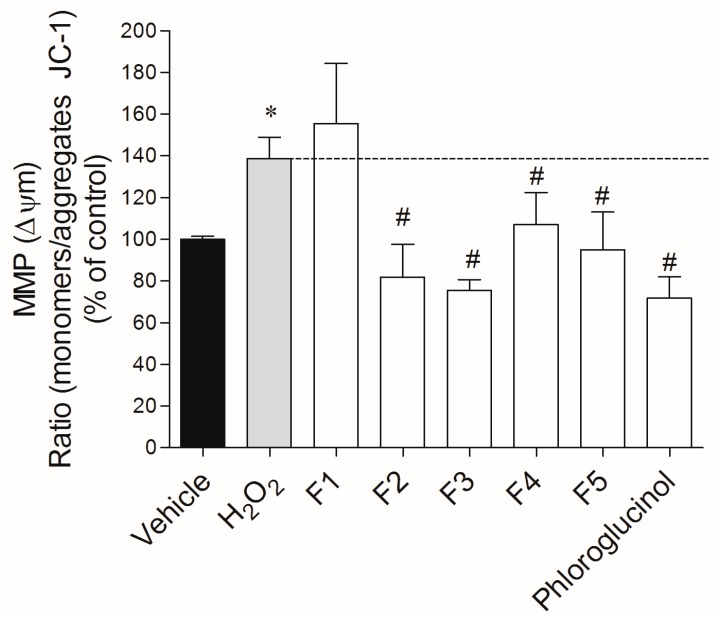
The incubation of MCF-7 cells with H2O2 (0.2 mM) induced a depolarization of the mitochondrial membrane potential (ΔΨm, MMP) in about 40% compared with vehicle, after 24 h of incubation (Figure 4). During the treatment, it was possible to observe a preventive effect of all fractions, with the exception of fraction 1. The H2O2-induced depolarization of MMP was reduced in 63.3%, 56.9%, 43.8% and 31.6% by fractions 3, 2, 5 and 4, respectively. Phloroglucinol was used as standard and presented a protective effect of about 67%.
Figure 4.
H2O2 (0.2 mM) effects on the mitochondrial membrane potential (MMP) of MCF-7 cells in the absence or presence of Fucus spiralis fractions (1 mg/mL) after 24 h of treatment. The results were obtained by the ratio between the monomers/aggregates of JC-1. The values in each column represent the mean ± standard error of the mean (SEM) of seven or eight independent experiments. Symbols represent statistically significant differences (p < 0.05, ANOVA, Dunett’s test) when compared to: * vehicle and # to H2O2.
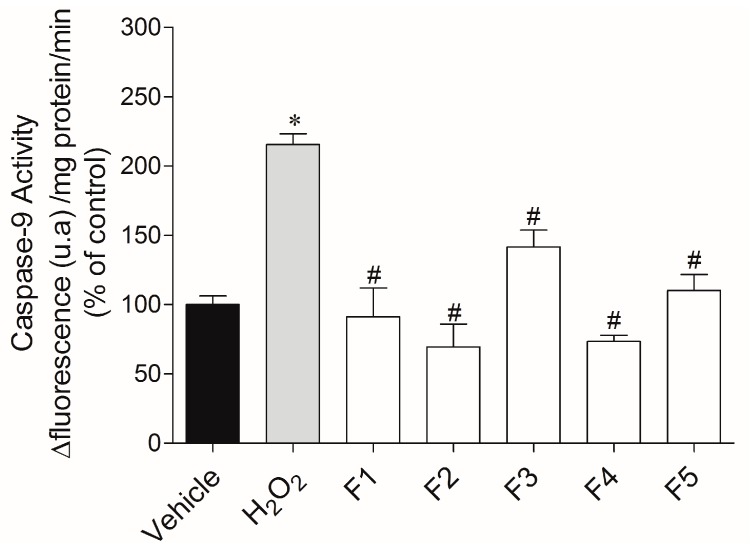
2.3.3. Caspase-9 Activity
In order to understand whether the cell death induced by H2O2 was linked to apoptosis, the caspase-9 activity was evaluated (Figure 5). When MCF-7 cells were exposed to H2O2, we observed an increase of caspase-9 activity in more than two times (215.50% ± 7.78%) when compared to the vehicle (100.00% ± 6.24%). When cells were incubated with H2O2 and fractions (1 mg/mL; 24 h), it was possible to detect a reduction of caspase-9 activity for all fractions when compared to the H2O2 situation.
Figure 5.
H2O2 (0.2 mM) effects on caspase-9 activity of MCF-7 cells in the presence or absence of Fucus spiralis fractions (1 mg/mL) after 24 h of treatment. The activity was quantified by the slope of the linear phase accumulation of 7-amino-4-(trifluoromethyl) coumarin (between 25 and 75 min). The results are presented in arbitrary units of fluorescence per mg of protein (% of control). The values in each column represent the mean ± standard error of the mean (SEM) from three independent experiments. Symbols represent statistically significant differences (p < 0.05, ANOVA, Dunett’s test) when compared to: * vehicle and # to H2O2.
3. Discussion
Oxidative stress has been associated with the occurrence of numerous diseases [14,19]. Although different defense mechanisms against ROS exist, the aging process associated with an unhealthy lifestyle can trigger ROS production and induce their effects. Therefore, preventing ROS formation as well as fortifying the natural defense mechanisms can be beneficial in the prevention and/or treatment of several metabolic disorders [20].
During the last decade, marine organisms have been targeted for their ability to produce bioactive compounds with high antioxidant potential. Within marine organisms, algae are known to produce important bioactive compounds such as antimicrobial, antitumor and antioxidant compounds. Nevertheless, the purification of bioactive compounds, especially antioxidant compounds of a phenolic nature, continues to be a challenging task. In this work, we fractionated the chemical extract of the seaweed Fucus spiralis, which was previously identified as a producer of compounds with high antioxidant activity [16,21].
In order to access the antioxidant activity of the fractions, different approaches were undertaken, namely DPPH radical scavenging activity, the oxygen radical absorbent capacity (ORAC), the hydroxyl radical scavenging activity and the quantification of the total phenolic content (TPC) was also accessed. All fractions presented high antioxidant activity; however, it was important to compare all the data to identify the most potent fractions. Through principal component analysis (PCA), it was possible to verify that fractions F4, F5 and F3 are the most bioactive fractions as regards the antioxidant activity, and therefore the most promising fractions for protecting from ROS effects. Nevertheless, since all of the fractions presented high antioxidant activity, they were all tested for cytotoxicity and for their ability to revert an oxidative stress condition induced by H2O2 on a cellular model, the MCF-7 cells.
Although H2O2 is a relatively weak oxidant compared to other ROS such as •OH, it has emerged as an important signaling molecule based on its unique biochemical properties. It is generated from nearly all sources of oxidative stress. Exogenous H2O2 can also enter cells readily due to its high membrane permeability, inducing cytotoxicity. It is ubiquitously present in the biological system with a relatively long half-life and it is soluble in both lipid and aqueous media [22,23]. Moreover, H2O2 can form highly reactive and detrimental •OH species through the metal-catalyzed Fenton reaction, which could involve numerous targets, ultimately causing oxidative injury in cells [24]. Therefore, H2O2 was used to induce oxidative stress in the model with MCF-7 cells in the present study.
Our results clearly demonstrated that all fractions presented capacity to revert a stress situation promoted by the H2O2. On the other hand, through the analysis of the real-time H2O2 production, only fractions 3 and 4 were able to decrease the production of H2O2 when compared to the control. Despite its toxicity, H2O2 is normally produced by healthy cells in response to activation of various cell surface receptors and it plays a key role as an intracellular messenger in mammalian cells. For instance, cells are known to produce H2O2 to signal the immune system to heal wounds and mediate cell-to-cell communication [23]. Nevertheless, cancer cells keep producing H2O2 and do not regulate its production. Our results highlight the potential of Fucus spiralis phlorotannin-enriched fractions as ROS protectors, by increasing cell viability in the presence of the H2O2 radical and, on the other hand, by decreasing the cellular production of H2O2. Interestingly, phloroglucinol did not reduce H2O2 production, suggesting that this antioxidant molecule can be involved in the detoxification of other reactive molecules resulting from H2O2 transformation. Other investigations support the potential of phlorotannins as ROS protectors in H2O2-induced oxidative stress. Kang and co-workers [25] evaluated the cytoprotective effect of eckol, a phlorotannin isolated from Ecklonia cava (a brown seaweed) on an oxidative stress condition induced by H2O2 on lung fibroblast cells (V79-4) and they could verify an effective cellular protection by decreasing ROS levels. On the other hand, a recent study on Ecklonia cava also reported the protective effects of phlorotannins against H2O2-induced oxidative stress in murine hippocampal HT22 cells [26]. Heo and Jeon [27] reported the protective effect of a phlorotannin (diphlorethohydroxycarmalol) isolated from Ishige okamura on a monkey kidney fibroblast cells line (Vero). The results clearly indicated that diphlorethohydroxycarmalol exerted profound protective effects against H2O2-mediated cell damage. These works corroborate our data showing clear evidence of phlorotannins cytoprotective potential in oxidative stress situations. Furthermore, several pathologies are associated with oxidative stress conditions, such as cancer. This idea put in incidence the F. spiralis protection against oxidative stress, mainly through the phlorotannin-enriched fractions. This view is supported by the study of Kim et al. (2015) [28], which demonstrated that the phlorotannin dieckol derived from Ecklonia cava inhibited the MCF-7 cell migration through inhibition of matrix metalloprotease (MMP)-9 and vascular endothelial growth factor (VEGF).
In order to understand the mechanisms that can be involved in cellular protection, the mitochondrial membrane potential (ΔΨm) and caspase-9 activity were examined. Mitochondria plays a key role in the apoptotic process due to being involved in two distinct signaling pathways: (1) maintenance of ATP production, and (2) mitochondrial membrane potential and mitochondrial membrane permeability. The mitochondrial membrane potential results from the difference in the electrical potential generated by the electrochemical gradient across the inner membrane, which is critical for maintaining the physiological function of the respiratory chain to generate ATP. Therefore, changes in the ΔΨm have been originally postulated to be early and obligate events in the apoptotic signaling pathway [29,30]. The H2O2 treatments promoted a membrane depolarization, which, consequently, may be involved in the apoptotic events, which led to the cell death verified. In the presence of Fucus spiralis fractions, the depolarization induced by H2O2 was completely prevented by all fractions, except F1. Taking into account that apoptosis-signaling cascades can be initiated by mitochondria, the maintenance of the membrane potential and mitochondrial functionality in H2O2-exposed cells by the tested fractions can explain the increased cells survival. Supporting our hypothesis of phlorotannins as mitochondrial membrane protectors, Zhang and co-workers [31] also verified that a phlorotannin isolated from Ecklonia cava (triphlorethol-A) prevented cellular damage by restoring the mitochondrial membrane potential of hamster lung fibroblast (V79-4) cells, which were previously distressed with formaldehyde.
The apoptotic process is triggered by a sequential activation of caspases. Caspase-9 is activated in the upstream of the apoptotic process by the release of cytochrome c by the mitochondria as a response to an apoptotic stimulus. Once activated, caspase-9 then cleaves and activates other downstream caspases such as caspase-3 [32].Our results show that all fractions decreased caspase-9 activity. In accordance with this, Chia and co-workers [33] analyzed the antioxidant and cytotoxic activities of three species of tropical seaweed extracts, and after 24 h of incubation, Turbinaria ornata, a brown macroalgae of the order Fucales, was able to decrease the caspase-9 activity also on MCF-7 cells. Another work with phlorotannins (2-phloroeckol and eckol) from Ecklonia cava revealed their protective effects on oxidative stress induced by Tacrine on HepG2 cells, by inhibiting the activation of caspase-3 [34]. Taken altogether, our results suggest that the cell death promoted by H2O2 is mainly mediated by apoptosis, and that Fucus spiralis fractions have the capacity to inhibit cellular damage promoted by ROS. These protective effects can be very useful in the prevention and treatment of free radical-related diseases.
4. Materials and Methods
4.1. Chemicals and Reagents
Methanol, n-hexane, water and dichloromethane of analytical grade were purchased from Fisher Scientific (Loughborough, Leicestershire, UK). All of the other chemicals and reagents were purchased from Sigma (Sigma-Aldrich GmbH, Steinheim, Germany).
4.2. Collection, Preparation and Extraction of Fucus spiralis
Fucus spiralis specimens were collected between April and July of 2014, in Marques Neves beach (39°37′03.53″ N 9°38′87.59″ W), Peniche (Portugal) and immediately transferred to laboratory. The samples were then soaked in seawater to remove epibionts, and finally freeze-dried (Scanvac Cool Safe, LaboGene, Lynge, Denmark). Dry samples were extracted overnight with constant stirring in a 1:40 biomass:solvent ratio with methanol. The organic extract was subjected to a liquid-liquid partition with n-hexane to remove fats. The methanol fraction was then concentrated in a rotary evaporator at 40 °C and the biomass was stored at −20 °C until further use.
4.3. Fractionation of Fucus spiralis Methanolic Extract by Vacuum Liquid Chromatography (VLC)
In order to concentrate the antioxidant metabolites, a reversed-phase VLC was performed with the following solvent system (400 mL each): 100% H2O (F1); 1:1 H2O:CH3OH (F2); 100% CH3OH (F3); 3:1 CH3OH:CH2Cl2 (F4) and 100% CH2Cl2 (F5) on a pad of C18 bulk (Polygoprep 60–50 Macherey-Nagel, GmbH, Düren, Germany). The dried fractions were then stored at −20 °C and used in the experimental assays.
4.4. Analysis of Total Phenolic Content (TPC)
The total phenolic content of Fucus spiralis fractions was determined using the Folin–Ciocalteu method adapted to microscale [35] with minor modifications. Phloroglucinol was used as standard. Briefly, 2 µL of extract was added to 158 µL of distilled water in a 96-well microplate, followed by 10 µL of Folin-Ciocalteu reagent. The reaction mixture was pre-incubated for 2 min at room temperature and then 30 µL of 20% Na2CO3 (w/v) was added and mixed. After one hour of reaction in the dark, the absorbance was measured at 755 nm (Synergy H1 Multi-Mode Microplate Reader, BioTek® Instruments, Winooski, VT, USA) against blank solution (prepared by the same procedure described above, but replacing Folin-Ciocalteu reagent for the same amount of water) and used to calculate the phenolic content. The TPC is expressed as milligrams of phloroglucinol equivalents per gram of dry extract (mg·PE/g).
4.5. Evaluation of Antioxidant Activities
4.5.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Radical Scavenging Activity
DPPH radical scavenging activity was performed according to Brand–Williams and co-workers [36] and adapted to microscale with slight modifications. DPPH radical was dissolved in absolute ethanol. Various concentrations (10–1000 μg/mL) of 2 µL of sample solution were added to 198 µL of the DPPH radical solution (0.1 mM). The mixture was vortexed for 1 min and allowed to stand at room temperature in the dark for 30 min, at which time the decrease in absorbance at 517 nm was measured. The radical solution was freshly prepared each day. BHT (butylated hydroxytoluene) was used as a standard. The ability of samples to scavenge the DPPH radical was calculated using the follow equation:
| (1) |
where the Acontrol is the absorbance of the control (DPPH solution with dimethyl sulfoxide), the Asample is the absorbance of the test sample (DPPH solution plus test sample), and the Asample blank is the absorbance of the sample in ethanol (sample without DPPH solution). Results are expressed as mean ± SEM (standard error of the mean). IC50 values (μg/mL) were also determined for the fractions that scavenged DPPH radical over 50%.
4.5.2. Oxygen Radical Absorbance Capacity (ORAC)
Oxygen Radical Absorbance Capacity (ORAC-fluorescein) assay was performed as described by Dávalos and co-workers (2004). This method is based on the decrease of the fluorescence of a fluorescent probe (β-phycoerythrin) by reacting with peroxyl radicals generated by the decomposition of 2,2’-azobis(2-methylpropionamidine) dihydrochloride (AAPH). In the presence of antioxidants, the peroxyl radicals are scavenged and the decay on the fluorescence is delayed. The difference between the area under the fluorescence decay curve (AUC) corresponding to the mixture with antioxidant, and the AUC corresponding to the mixture without antioxidant, is then converted into a Trolox standard calibration. The antioxidant activity is expressed in µmol of Trolox equivalents/g of dry extract (µmol·TE/g).
The reaction was carried out in 75 mM phosphate buffer (pH 7.4) on a total volume of 200 µL. Briefly to 20 µL of sample was added 120 µL of fluorescein (70 nM in phosphate buffer). After being pre-incubated for 15 min at 37 °C, an AAPH solution (60 µL; 12 mM, final concentration) was added. The fluorescence (λexcitation = 458 nm, λemission = 520 nm) was recorded every minute for 240 min. A blank using phosphate buffer instead of the fluorescein, and eight calibration solutions using Trolox (1–8 µM), were also carried out in each assay. All reactions were prepared in duplicate, and at least three independent assays were performed for each sample.
4.5.3. Hydroxyl Radical Scavenging Activity (•OH)
Hydroxyl radical (•OH) scavenging activity was measured by the capacity of the seaweed fractions to scavenge the •OH generated by the Fe3+–ascorbate–EDTA–H2O2 system (Fenton reaction) [37,38]. The ability to eliminate hydroxyl radicals was measured by the method adapted from Halliwell and co-workers (1987) and Kunchandy and Rao (1990) [39]. The reaction mixture consisted in 200 µL EDTA (1.04 mM), 200 µL FeCl3 (0.2 mM) (1:1 v/v), 100 µL of H2O2 (1.0 mM), 100 µL of ascorbic acid (1.0 mM), 100 µL of 2-deoxy-d-ribose (28 mM in 20 mM KH2PO4-KOH buffer, pH 7.4) and 500 µL of the sample. After 1 h incubation at 37 °C, 1.0 mL of thiobarbituric acid (1%) and 1.0 mL of trichloroacetic acid (2.8%) are added and the solution incubated at 100 °C for 20 min. After cooling, absorbance is measured at 532 nm, against a blank sample.
The ability of test samples to scavenge the •OH was calculated using the follow equation:
| (2) |
where the Acontrol is the absorbance of the control (without sample), the Asample is the absorbance in the presence of the sample, and the Asample blank is the absorbance of the solution without 2-deoxy-d-ribose. Results are expressed as mean ± SEM. IC50 values (μg/mL) were also determined for the fractions that scavenged •OH over 50%.
4.6. In Vitro Assay of Oxidative Stress Prevention
4.6.1. Cell Maintenance Culture Conditions
Human breast adenocarcinoma model (MCF-7 cells-ACC 115) was acquired from the DSMZ—German collection of microorganisms and cell cultures. The cells were cultured in RPMI 1640 medium supplemented with 10% of fetal bovine serum (FBS), 1% of antibiotic and antimicotic solution (100 U/mL penicillin G, 0.25 µg/mL amphotericin B and 100 µg/mL streptomycin), 1% of MEM non-essential amino acids, 1 mM of sodium pyruvate, 10 µg/mL of human insulin. Cells medium was changed every three days, and the cells reached confluence after 5–6 days of initial seeding. For subculture, cells were dissociated with trypsin-EDTA, split 1:3 and subculture in Petri dishes with 25 cm2 growth area. Cells were maintained at 37 °C, 95% of humidity and 5% of CO2 (CO2 Unitherm, Planegg, Munich, Germany).
4.6.2. Fucus spiralis Fractions Cytotoxicity Evaluation
The cytotoxicity of seaweed fractions with major antioxidant activity was determined. Cell viability studies were performed after cells reached the total confluence in 96-well plates (Thermo Fisher Scientific, Seoul, Korea). The selected fractions were dissolved in culture medium without FBS (1 mg/mL) and sterile filtered (0.2 µm, Whatman, Buckinghamshire, UK). Cells were incubated during 24 h and cell viability evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method [40].
4.6.3. Evaluation of the Protective Effect of Seaweed Fractions in an Oxidative Stress Condition Induced by H2O2 on MCF-7 Cells
Seaweed fractions with high antioxidant activity and without cytotoxicity were evaluated for their potential in preventing an oxidative stress condition promoted on MCF-7 cells by the addition of H2O2 (0.2 mM). The concentration of the extracts and H2O2 and the assay conditions were defined in preliminary tests, and established as follows: after cells reached total confluence in 96-well plates, previously filtered (0.2 µm) seaweed fractions (1 mg/mL on culture medium without FBS) were added together with H2O2 (0.2 mM). The incubation occurred along 24 h. The protective effects of seaweed fractions were considered as MCF-7 cells viability comparing with the control (cells incubated with H2O2 (0.2 mM) during 24 h without seaweed fractions).
4.6.4. Real-Time Quantification of H2O2 Production
Quantification of H2O2 was performed using the “AmplexTM Red hydrogen peroxide Assay” Kit A22188 (ThermoFisher Scientific, Waltham, MA, USA). The amplex red is a fluorophore that evidences a low basal fluorescence, which reacts with H2O2 in a 1:1 ratio. This reaction is initiated with horseradish peroxidase, leading to the appearance of a highly fluorescent product, designated resofurin [41]. H2O2 production was quantified on MCF-7 cells after 24 h of treatment with H2O2 (0.2 mM) in the absence or presence of seaweed fractions (1 mg/mL). The variation of H2O2 production was accompanied in real-time along 60 min at room temperature. The fluorescence intensity was measured at wavelengths of 590 nm (excitation) and 530 nm (emission). The levels of H2O2 were calculated by the slope of the linear phase of fluorescence curve and the results were expressed in percentage of control.
4.6.5. Mitochondrial Membrane Potential (ΔΨm)
Mitochondrial membrane potential was determined using the fluorescent probe, JC-1 (Molecular Probes, Eugene, OR, USA). MCF-7 cells were treated with H2O2 (0.2 mM) in the absence or presence of seaweed fractions (1 mg/mL) throughout 24 h. The medium was then removed, the cells washed with Hank’s buffer (medium composition in mM: NaCl 137, KCl 5, MgSO4 0.8, Na2HPO4 0.33, KH2PO4 0.44, CaCl2 0.25; MgCl2 1.0 and Tris HCl 0.15, pH = 7.4), and incubated with JC-1 (3 µM) during 15 min at 37 °C. The JC-1 probe was removed and cells washed with Hank’s buffer. The formation of JC-1 aggregates (490 nm of excitation and 590 nm of emission) and the monomeric form of JC-1 (490 nm of excitation and 530 nm of emission) were measured simultaneously throughout 30 min. Results were expressed as the ratio of the monomers/aggregates of JC-1 in percentage of control.
4.6.6. Caspase-9 Activity
Caspase-9 activity was assessed using “Caspase-9 Assay kit” K118 (Biovision, Milpitas, CA, USA). Cells were cultured in 6-well plates and treated with H2O2 (0.2 mM) in the presence or absence of Fucus spiralis VLC fractions (1 mg/mL) during 24 h. The cells were then washed twice with Hank’s buffer and collected by centrifugation at 5000 rpm during 10 min at 4 °C. The pellets were resuspended in 50 µL of lysis buffer and incubated on ice during 20 min. In order to separate the content of intracellular cytoplasmic organelles and cell membrane, centrifugation took place at 13,000 rpm during 20 min at 4 °C. Thereafter, 50 μL of supernatant was placed into a 96-well plate, to which was added 50 μL of reaction buffer containing DTT (10 mM) and 5 μL of substrate. This reaction was followed at wavelengths of 400 nm (excitation) and 505 nm (emission) along 90 min at room temperature. Caspase-9 activity was calculated by the slope of the fluorescence resulting from 7-amino-4-(trifluoromethyl) coumarin accumulation and expressed in % of control (Δ fluorescence (u.a)/mg of protein/min).
4.7. Statistical Analysis
Results were expressed as mean ± SEM. The IC50 concentration was calculated from nonlinear regression analysis using the GraphPad Prism software with the equation: Y = 100/(1 + 10(X – LogIC50)). All data were checked for normality and homoscedasticity using the Shapiro–Wilk and Levene’s test, respectively. Therefore, comparisons concerning variables, which did not meet variance or distributional assumptions, were carried out with Kruskal–Wallis non-parametric tests [42].
A two-step analysis was performed. The first step was a principal component analysis (PCA) for all antioxidant assays (TPC, DPPH, ORAC and •OH), using CANOCO version 4.5 (Biometris–Plant Research International, Wageningen, The Netherlands) [43]. The PCA allowed for the detection of similarities between samples and for identifying the main associations among variables that are responsible for the total variability of the studied data. The PCA model was built on the average of the measured data, and full cross-validation was used to validate the model. All the components were analyzed. Also, one-way analysis of variance (ANOVA) was carried out in order to appraise the seaweed fractions effects (1 mg/mL) in an oxidative stress condition. The statistical comparisons among the groups were assessed through Newman–Keuls multiple comparison test [42]. Where applicable, results are presented as mean ± standard error of the mean (SEM). Differences were considered statistically significant at a level of 0.05 (that is, p < 0.05).
All calculations were performed with GraphPad InStat v. 3.5 (GraphPad Software, La Jolla, CA, USA).
5. Conclusions
Mitochondrial dysfunction and oxidative stress have been implicated in the pathophysiology of many diseases and, therefore, it is of great importance to promote the organism defenses by respecting an active lifestyle with a healthy diet. In this work, it was shown that the edible Fucus spiralis produces compounds that act on oxidative stress mechanisms, for instance, by reducing the H2O2 negative effects on cells, by maintaining the normal membrane potential and, also, by decreasing the caspase-9 activity which is involved in cell death mechanisms.
On the basis of these results, Fucus spiralis reveals to be an excellent source of natural antioxidant compounds with protective effects against oxidative stress.
Acknowledgments
This work was supported through funding provided by the European Commission Collaborative Project FP7 KBBE 2010–4 BAMMBO (project number 265896), by the FCT—Fundação para a Ciência e Tecnologia Strategic Project UID/MAR/04292/2013 granted to MARE, and by the FCT—Fundação para a Ciência e Tecnologia R&D Project PTDC/MAR-BIO/6149/2014. Susete Pinteus, Joana Silva and Celso Alves are financial supported by a Grant from FCT—Fundação para a Ciência e Tecnologia (SFRH/BD/96203/2013, SFRH/BD/103255/2014 and SFRH/BD/977641/2013, respectively).
Author Contributions
The authors Susete Pinteus and Joana Silva did the main experiments (extraction and fractionation, antioxidant activity, cytotoxicity, protective assay, evaluation of the apoptotic mechanisms, mitochondrial membrane potential and evaluation of H2O2 production). Susete Pinteus, Joana Silva, Celso Alves, and André Horta have been involved in the seaweeds collection, antioxidant activity, cytotoxicity and protective assays. Susete Pinteus, Joana Silva and Celso Alves drafted the manuscript. Olivier P. Thomas designed and coordinated the fractionation and purification of Fucus spiralis methanolic extract by Vacuum Liquid Chromatography (VLC). Rui Pedrosa designed and coordinated the antioxidant activity, cytotoxicity, protective assay, apoptotic mechanisms, mitochondrial membrane potential and H2O2 production and conceived the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hasan M.R., Chakrabarti R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture—A Review. Volume 531. FAO; Rome, Italy: 2009. p. 123. [Google Scholar]
- 2.Nwosu F., Morris J., Lund V.A., Stewart D., Ross H.A., McDougall G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011;126:1006–1012. doi: 10.1016/j.foodchem.2010.11.111. [DOI] [Google Scholar]
- 3.Burtin P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003;2:498–503. [Google Scholar]
- 4.Skibola C.F. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: A case report. BMC Complement. Altern. Med. 2004;4:10. doi: 10.1186/1472-6882-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Gutierrez C.M., Guerra-Rivas G., Soria-Mercado I.E., Ayala-Sánchez N.E. Chapter 3—Marine edible algae as disease preventers. Adv. Food Nutr. Res. 2011;64:29–39. doi: 10.1016/B978-0-12-387669-0.00003-X. [DOI] [PubMed] [Google Scholar]
- 6.Julia K., Sarah E.L., William F., Mark E.H. Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar. Ecol. Prog.Ser. 2004;277:79–93. [Google Scholar]
- 7.Balboa E.M., Conde E., Moure A., Falqué E., Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138:1764–1785. doi: 10.1016/j.foodchem.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Li Y.-X., Wijesekara I., Li Y., Kim S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011;46:2219–2224. doi: 10.1016/j.procbio.2011.09.015. [DOI] [Google Scholar]
- 9.Cornish M.L., Garbary D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae. 2010;25:155–171. doi: 10.4490/algae.2010.25.4.155. [DOI] [Google Scholar]
- 10.Devi K.P., Suganthy N., Kesika P., Pandian S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008;8:38. doi: 10.1186/1472-6882-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray P.M., Moane S., Collins C., Beletskaya T., Thomas O.P., Duarte A.W.F., Nobre F.S., Owoyemi I.O., Pagnocca F.C., Sette L.D., et al. Sustainable production of biologically active molecules of marine based origin. New Biotechnol. 2013;30:839–850. doi: 10.1016/j.nbt.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative Stress and Antioxidant Defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi D.-Y., Choi H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015;38:139–170. doi: 10.1007/s12272-014-0503-5. [DOI] [PubMed] [Google Scholar]
- 14.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 15.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 16.Pintéus S., Azevedo S., Alves C., Mouga T., Cruz A., Afonso C., Sampaio M., Rodrigues A., Pedrosa R. High antioxidant potential of Fucus spiralis extracts collected from Peniche coast (Portugal) New Biotechnol. 2009;25:S296. doi: 10.1016/j.nbt.2009.06.676. [DOI] [Google Scholar]
- 17.Cerantola S., Breton F., Ar Gall E., Deslandes E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006;49:347–351. doi: 10.1515/BOT.2006.042. [DOI] [Google Scholar]
- 18.Tierney M., Soler-Vila A., Croft A., Hayes M. Antioxidant activity of the brown macroalga Fucus spiralis Linnaeus harvested from the West Coast of Ireland. Curr. Res. J. Biol. Sci. 2013;5:81–90. [Google Scholar]
- 19.Dasgupta A., Klein K. Chapter 10—Role of Oxidative Stress in Neurodegenerative Diseases and Other Diseases Related to Aging. In: Klein A.D., editor. Antioxidants in Food, Vitamins and Supplements. Elsevier; San Diego, CA, USA: 2014. pp. 167–184. [Google Scholar]
- 20.Kim Y.R., Lee J.S., Lee K.R., Kim Y.E., Baek N.I., Hong E.K. Effects of mulberry ethanol extracts on hydrogen peroxide-induced oxidative stress in pancreatic β-cells. Int. J. Mol. Med. 2014;33:128–134. doi: 10.3892/ijmm.2013.1534. [DOI] [PubMed] [Google Scholar]
- 21.Pinteus S., Silva J., Alves C., Horta A., Fino N., Inês Rodrigues A., Mendes S., Pedrosa R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal) Food Chem. 2017;218:591–599. doi: 10.1016/j.foodchem.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 22.Ryter S.W., Kim H.P., Hoetzel A., Park J.W., Nakahira K., Wang X., Choi A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 23.Rhee S.G., Chang T.-S., Jeong W., Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 24.Jia N., Li T., Diao X., Kong B. Protective effects of black currant (Ribes nigrum L.) extract on hydrogen peroxide-induced damage in lung fibroblast MRC-5 cells in relation to the antioxidant activity. J. Funct. Foods. 2014;11:142–151. [Google Scholar]
- 25.Kang K.A., Lee K.H., Chae S., Zhang R., Jung M.S., Lee Y., Kim S.Y., Kim H.S., Joo H.G., Park J.W. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005;579:6295–6304. doi: 10.1016/j.febslet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Kang S.-M., Cha S.-H., Ko J.-Y., Kang M.-C., Kim D., Heo S.-J., Kim J.-S., Heu M.S., Kim Y.-T., Jung W.-K. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharm. 2012;34:96–105. doi: 10.1016/j.etap.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Heo S.-J., Jeon Y.-J. Evaluation of diphlorethohydroxycarmalol isolated from Ishige okamurae for radical scavenging activity and its protective effect against H2O2-induced cell damage. Process Biochem. 2009;44:412–418. doi: 10.1016/j.procbio.2008.12.005. [DOI] [Google Scholar]
- 28.Kim E.-K., Tang Y., Kim Y.-S., Hwang J.-W., Choi E.-J., Lee J.-H., Lee S.-H., Jeon Y.-J., Park P.-J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs. 2015;13:1785. doi: 10.3390/md13041785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh T., Enokido Y., Aoshima H., Uchiyama Y., Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress—Induced apoptosis in PC12 cells. J. Neurosci. Res. 1997;50:413–420. doi: 10.1002/(SICI)1097-4547(19971101)50:3<413::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Cook S.A., Sugden P.H., Clerk A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes association with changes in mitochondrial membrane potential. Circ. Res. 1999;85:940–949. doi: 10.1161/01.RES.85.10.940. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R., Lee I.K., Kang K.A., Piao M.J., Kim K.C., Kim B.J., Lee N.H., Choi J.-Y., Choi J., Hyun J.W. Cytoprotective Effects of triphlorethol-A against formaldehyde-induced oxidative damage and apoptosis: Role of mitochondria-mediated caspase-dependent pathway. J. Toxicol. Environ. Health Part A. 2010;73:1477–1489. doi: 10.1080/15287394.2010.511564. [DOI] [PubMed] [Google Scholar]
- 32.Kong C.-S., Kim J.-A., Yoon N.-Y., Kim S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009;47:1653–1658. doi: 10.1016/j.fct.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Chia Y., Kanthimathi M., Khoo K., Rajarajeswaran J., Cheng H., Yap W. Antioxidant and cytotoxic activities of three species of tropical seaweeds. BMC Complement. Altern. Med. 2015;15:339. doi: 10.1186/s12906-015-0867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee M.-S., Shin T., Utsuki T., Choi J.-S., Byun D.-S., Kim H.-R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012;60:5340–5349. doi: 10.1021/jf300157w. [DOI] [PubMed] [Google Scholar]
- 35.Singleton V., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vit. 1965;16:144–158. [Google Scholar]
- 36.Brand-Williams W., Cuvelier M., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 37.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliwell B., Gutteridge J.M.C., Aruoma O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1980;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 39.Kunchandy E., Rao M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990;58:237–240. doi: 10.1016/0378-5173(90)90201-E. [DOI] [Google Scholar]
- 40.Yuan Y.V., Walsh N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Mohanty J., Jaffe J.S., Schulman E.S., Raible D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods. 1997;202:133–141. doi: 10.1016/S0022-1759(96)00244-X. [DOI] [PubMed] [Google Scholar]
- 42.Zar J.H. Biostatistical Analysis. Prentice Hall; Upper Saddle River, NJ, USA: 2010. [Google Scholar]
- 43.Ter Braak C., Smilauer P. CANOCO Reference Manual and User’s Guide to Canoco for Windows—Software for Canonical Community Ordination. Microcomputer Power; New York, NY, USA: 1998. Version 4. [Google Scholar]