Abstract
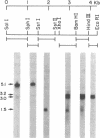
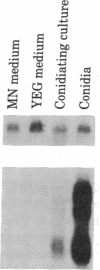
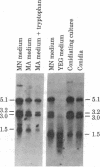
We have cloned the trifunctional trpC gene from Aspergillus nidulans by hybrid phage lambda complementation of an Escherichia coli trpC mutant lacking phosphoribosylanthranilate isomerase activity. Four different phages sharing a 4.3-kilobase region were obtained. Plasmid subclones containing this region also complemented the E. coli trpC mutant. We determined that a 1.8-kilobase DNA fragment was minimally required for complementation. The fragment hybridized with two poly(A)+ RNAs, 3.0 and 3.2 kilobases in length. We infer that these transcripts are Aspergillus trpC mRNAs and that the entire Aspergillus trpC gene is not required for complementation in E. coli. Levels of both trpC transcripts in poly(A)+ RNA are regulated by growth medium composition. They were highest when cells were grown in minimal medium containing nitrate as the nitrogen source and lowest when cells were grown in medium containing yeast extract. The concentrations of the transcripts are also regulated during conidiophore development. Conidiating cultures grown on medium containing yeast extract had significantly higher levels of both transcripts than did hyphae grown in minimal medium containing nitrate. Levels of the transcripts in mature spores were equivalent to those found in hyphae grown in minimal medium containing nitrate. Results from nutritional experiments with an A. nidulans trpC mutant suggest that developmental regulation of trpC mRNA levels may be related to a high requirement for tryptophan or a compound derived from tryptophan during conidiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. E., Gealt M., Pastushok M. Gene control of developmental competence in Aspergillus nidulans. Dev Biol. 1973 Sep;34(1):9–15. doi: 10.1016/0012-1606(73)90335-7. [DOI] [PubMed] [Google Scholar]
- Barbata G., Valdes L., Sermonti G. Complementation among developmental mutants in Aspergillus nidulans. Mol Gen Genet. 1973 Nov 12;126(3):227–232. doi: 10.1007/BF00267533. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. A mutational analysis of conidial development in Aspergillus nidulans. Genetics. 1969 Oct;63(2):317–327. doi: 10.1093/genetics/63.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. A variegated position effect in Aspergillus nidulans. Genet Res. 1970 Dec;16(3):303–316. doi: 10.1017/s0016672300002561. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Gealt M. A., Sheir-Neiss G., Morris N. R. The isolation of nuclei from the filamentous fungus Aspergillus nidulans. J Gen Microbiol. 1976 May;94(1):204–210. doi: 10.1099/00221287-94-1-204. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., DeMoss J. A. Subunit structure of anthranilate synthetase from Neurospora crassa. J Biol Chem. 1975 Sep 10;250(17):6648–6652. [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Enzyme analysis of the tryptophan pathway in Aspergillus nidulans. Genetics. 1967 Feb;55(2):241–247. doi: 10.1093/genetics/55.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käfer E. The anthranilate synthetase enzyme complex and the trifunctional trpC gene of Aspergillus. Can J Genet Cytol. 1977 Dec;19(4):723–738. doi: 10.1139/g77-079. [DOI] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli S. D., Clutterbuck A. J. A quantitative survey of conidiation mutants in Aspergillus nidulans. J Gen Microbiol. 1971 Dec;69(2):261–268. doi: 10.1099/00221287-69-2-261. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Orr W. C., Timberlake W. E. Clustering of spore-specific genes in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5976–5980. doi: 10.1073/pnas.79.19.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts C. F. Complementation analysis of the tryptophan pathway in Aspergillus nidulans. Genetics. 1967 Feb;55(2):233–239. doi: 10.1093/genetics/55.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Orr W. C., Timberlake W. E. Diversity and abundance of polyadenylated RNA from Achlya ambisexualis. Biochemistry. 1978 Feb 21;17(4):716–722. doi: 10.1021/bi00597a025. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Alterations in RNA and protein synthesis associated with steroid hormone-induced sexual morphogenesis in the water mold Achlya. Dev Biol. 1976 Jul 15;51(2):202–214. doi: 10.1016/0012-1606(76)90138-x. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Barnard E. C. Organization of a gene cluster expressed specifically in the asexual spores of A. nidulans. Cell. 1981 Oct;26(1 Pt 1):29–37. doi: 10.1016/0092-8674(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Shumard D. S., Goldberg R. B. Relationship between nuclear and polysomal RNA populations of Achlya: a simple eucaryotic system. Cell. 1977 Apr;10(4):623–632. doi: 10.1016/0092-8674(77)90095-2. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager L. N., Kurtz M. B., Champe S. P. Temperature-shift analysis of conidial development in Aspergillus nidulans. Dev Biol. 1982 Sep;93(1):92–103. doi: 10.1016/0012-1606(82)90242-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]