Abstract
Receptor tyrosine kinases (RTKs) belong to a family of transmembrane receptors that display tyrosine kinase activity and trigger the activation of downstream signalling pathways mainly involved in cell proliferation and survival. RTK amplification or somatic mutations leading to their constitutive activation and oncogenic properties have been reported in various tumour types. Numerous RTK-targeted therapies have been developed to counteract this hyperactivation. Alternative splicing of pre-mRNA has recently emerged as an important contributor to cancer development and tumour maintenance. Interestingly, RTKs are alternatively spliced. However, the biological functions of RTK splice variants, as well as the upstream signals that control their expression in tumours, remain to be understood. More importantly, it remains to be determined whether, and how, these splicing events may affect the response of tumour cells to RTK-targeted therapies, and inversely, whether these therapies may impact these splicing events. In this review, we will discuss the role of alternative splicing of RTKs in tumour progression and response to therapies, with a special focus on two major RTKs that control proliferation, survival, and angiogenesis, namely, epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor-1 (VEGFR1).
Keywords: alternative splicing, angiogenesis, cancer, EGFR, receptor tyrosine kinases, tumourigenesis, targeted therapies, VEGFR
1. Introduction
Growth factors and their receptors are the core components of signal transduction pathways. The majority of growth factor receptors contain extracellular, transmembrane, and cytoplasmic tyrosine kinase domains and transmit the activation signal across the plasma membrane. Binding of growth factors to the extracellular domain of their cognate receptors activates the cytoplasmic tyrosine kinase. Receptor tyrosine kinase (RTK) activation initiates a network of signalling pathways that relay information to the nucleus and other intracellular compartments [1,2]. RTKs are key regulators of critical cellular processes, such as proliferation and differentiation, cell survival and metabolism, organ morphogenesis, neovascularization, cell migration, and tissue repair and regeneration [3,4]. In normal cells, RTK activity is strictly regulated. Numerous diseases result from genetic changes or abnormalities that alter the activity, abundance, cellular distribution, or regulation of RTKs. Cancers frequently display deregulation or constitutive activation of RTKs, and abnormal RTK signalling is an important feature of tumour initiation and progression. Therefore, RTKs appear as promising molecular targets in cancer.
It is now well accepted that approximately 90% of human multi-exon genes are regulated by pre-mRNA alternative splicing [5,6]. In addition, an increasing number of studies have shown that misregulation of alternative splicing occurs in many pathologies, such as cancers ([7] for review). Target genes whose splicing is deregulated in tumours include those encoding various RTKs and their ligands (Table 1).
Table 1.
Examples of pre-mRNA alternative splicing (AS) of various receptor tyrosine kinases and functional consequences.
| RTK | Splicing Events | Functional Consequences | References |
|---|---|---|---|
| ALK | Skipping of exons 2–3 Skipping of exons 4–11 |
Truncated proteins with increased constitutive kinase activity and transformation potential in neuroblastoma | [8] |
| Skipping of exon 23 or exon 27 | Truncated proteins lacking the full kinase domain of ALK in Non Small Cell Lung Carcinoma | [9] | |
| AXL | Skipping of exon 10 | Shorter AXL protein with same transforming potential as full-length AXL | [10] |
| DDR | Exon skipping or inclusion | Distinct binding partners Differential activation by collagen |
[11] |
| EGFR | Inclusion of exon 10, 9a, 16 or 17 | Soluble receptors acting as negative regulators of EGFR signalling | [12] |
| Skipping of exons 2–7 | Constitutively active receptor | [13] | |
| Enhanced signalling, survival, and tumourigenicity | [14] | ||
| Skipping of exons 2–22 | Enhanced migration and invasion Cancer stem cells marker |
[15] | |
| ERB4 | N- and C-terminal alternative splicing generating four isoforms | Modulation of sub-cellular localization and partner binding | [16] |
| FGFR | Mutually exclusive exon 8 or 9 | Generation of distinct extracellular Ig-like domain III with distinct affinity for FGF ligands | [17] |
| Induction of Epithelial to Mesenchymal Transition (EMT), invasion and motility | [18] | ||
| INSR | Skipping or inclusion of exon 11 | Generation of INSR-A and INSR-B splice variants that respond differentially to IGF-II and insulin ligands and differentially activate the RAS/MAPK pathway | [19] |
| MET | Skipping of exon 14 | Activation of MET kinase activity Oncogenic transformation |
[20] |
| Increased sensitivity to MET inhibitors | [21] | ||
| RET | 3′-end alternative splicing generating multiple isoforms that differ in their C-terminal domain | Modulation of signalling partner binding Distinct sub-cellular localization and trafficking properties Transforming capacity |
[22] |
| RON | Skipping of exon 11 | Constitutively active receptor Enhanced signalling, invasion, motility |
[23] |
| Skipping of exons 15–19, 16–19, 16–17 and 16 | Truncated protein lacking active kinase domain Dominant negative isoforms in lung cancers. |
[24] | |
| NTRK | Skipping of exons 6, 7 and 9 | Constitutively active receptor Oncogenic function in neuroblastoma |
[25] |
| VEGFR | Intron retention followed by premature polyadenylation | Soluble decoy receptor acting as negative regulator of VEGFR signalling | [26,27] |
| Increased resistance to anti-angiogenic therapies | [28,29] |
ALK: Anaplastic Lymphoma Kinase; DDR: Discoidin Domain Receptor; FGFR: Fibroblast Growth Factor Receptor; INSR: Insulin Receptor; RON: Receptor d’Origine Nantaise; NTRK: Neurotrophic Tyrosine Kinase Receptor.
Alternative splicing of RTKs has several biological consequences. First, it can modify the subcellular distribution of RTKs, thereby affecting their activity. As an example, Vorlova and colleagues identified 31 decoy receptors produced by intron retention and activation of intronic poly(A) sites in 16/20 RTK family members [27]. These truncated RTKs are devoid of their transmembrane and intra-cytoplasmic tyrosine kinase domains. Therefore, they are considered dominant-negative receptors that act either by sequestering RTK ligands or by inhibiting other RTKs through heterodimerization [27]. Another example is the alternative splicing of ErbB4, which produces both juxtamembrane (JM-a and JM-b) and cytoplasmic (CYT-1 and CYT-2) isoforms that are differentially endocytosed [16]. Second, alternative splicing can modify the affinity of the RTKs for their ligands, providing a specific signalling for each splice variant. This is the case for the fibroblast growth factor receptor 2 (FGFR2) that is expressed as two different splice isoforms, FGFR2IIIb and FGFR2IIIc, depending on whether exon 8 or exon 9 is skipped [30]. These FGFR2 splice variants do not bind the same FGF ligands. Interestingly, FGFR2 exon switching from the IIIb to the IIIc isoform has been observed during epithelial cell tumour progression, notably in breast cancer [31]. Moreover, expression of FGFR2IIIc was associated with epithelial to mesenchymal transition [32]. Therefore, alternative splicing of RTKs may contribute to cellular reprogramming and the generation of cancer cells with more invasive features. This was also seen with a splice variant of Ron, the tyrosine kinase receptor for the macrophage-stimulating protein. This Ron isoform, named RonΔ65, is generated through the skipping of exon 11, leading to a kinase that is constitutively active in the cytoplasm, even in the absence of Ron ligand. This splicing event is controlled by the splicing factor SRSF1. Importantly, the accumulation of RonΔ65 is associated with a metastatic phenotype in human colorectal and breast carcinomas [33]. Although increasing evidence indicates that alternative splicing of RTKs may have a critical role during tumourigenesis, many questions still remain to be elucidated. For example, less is known about the upstream regulators and extracellular cues that control the splicing of these RTKs. In addition, as alternative splicing may be dependent on tumour types, it remains to be determined whether alternative splicing of RTKs may play distinct roles depending on the tumour context, and which specific signalling networks are activated by these splice variants in this setting. Last but not least, it is largely unknown whether, and how, alternative splicing of RTKs may be involved in the primary and/or acquired resistance of tumour cells to targeted therapies widely used in the clinic. As an example, the splice variant derived from exon 16 skipping of the HER2 receptor, called Δ16HER-2, has been associated with malignant transformation and resistance to trastuzumab, an anti-HER2 monoclonal antibody, in breast cancers [34]. Investigating these questions is crucial, as this could lead to the identification of new prognostic markers and help define new therapeutic strategies.
This review will focus on two key RTKs, namely, the epidermal growth factor receptor (EGFR) and the vascular endothelial receptor (VEGFR1), as an illustration of how alternative splicing of RTKs can be involved in both tumourigenesis and response to therapies. These RTKs were chosen because they are critical regulators of tumour proliferation, survival, and angiogenesis, and because they are targeted by pharmacological compounds widely used in the clinic to treat cancer patients.
2. Splicing of EGFR: An Alternative Method to Control Tumour Progression
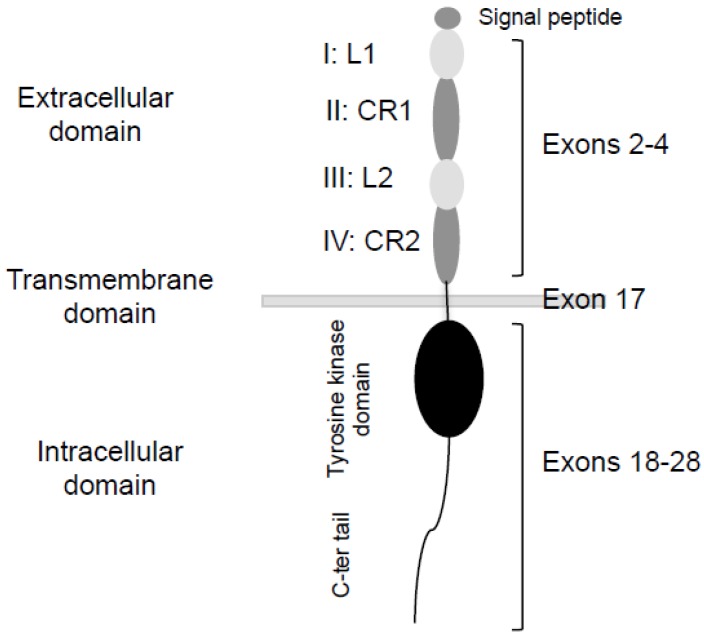
EGFR is a transmembrane protein with tyrosine kinase activity (Figure 1). Its structure includes an extracellular domain with four domains repeated two by two: L1 (I), CR1 (II), L2 (III), and CR2 (IV). The L1 and L2 domains are required for ligand fixation, and the CR1 and CR2 domains (cysteine-rich) increase the affinity of the ligand for its receptor and allow dimerization with the second receptor of the dimer [35,36]. Ligand binding to the extracellular domain triggers the dimerization of the receptor and induces its autophosphorylation. Dimerization of the receptor induces a cascade of phosphorylation leading to the activation of proliferation and survival pathways. Dysregulated EGFR signalling contributes to the formation of many epithelial malignancies in humans. Therefore, EGFR is an attractive candidate for targeted therapy, as it is often overexpressed on the surface of cancer cells.
Figure 1.
Schematic structure of the EGFR monomer. L: Ligand binding domain. CR: Cysteine-rich domain.
2.1. Soluble EGFR Variants
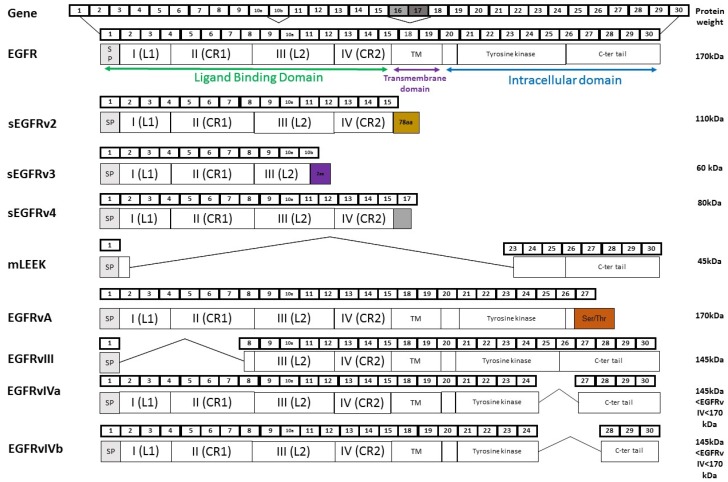
EGFR contains 30 exons and generates different mRNAs (Figure 2). Variant 1 mRNA is devoid of exons 16–17 and encodes the 170-kDa full-length EGFR. In addition to full length receptor, normal and tumour cells express soluble EGFR isoforms (sEGFR) that contain only the extracellular domain of EGFR. These sEGFR proteins can result either from alternative splicing or from proteolytic cleavage of the receptor [37,38]. Alternative splicing of the EGFR gene generates three variants that encode 110-kDa, 80-kDa, and 60-kDa sEGFR isoforms [39,40,41]. Soluble EGFR isoforms have been described in normal tissues, but they are highly expressed in human placenta; the 110-kDa isoform is the major one, suggesting a potential role in this tissue. Intra-tumoral and/or plasma/serum levels of sEGFR have been examined in tumour patients, but in most studies the data reflect overall sEGFR. Baron and colleagues were the first to report the presence of sEGFR in epidermal ovarian cancer (EOC) patients. They observed that the level of sEGFR is lower in patients harbouring EOC compared to healthy patients [42]. Similar results were reported in breast tumours [43] and non-small cell lung cancer (NSCLC) patients [44], suggesting that expression of sEGFR may have a physiological and protective role against cancer development. In this respect, sEGFR was proposed as a potential negative biomarker for the early detection in NSCLC [45]. On the other hand, other studies found significantly elevated sEGFR levels in cervical and gastric carcinoma compared to the healthy population [46], suggesting that sEGFR could also be a positive tumour marker. Moreover, clinical data regarding the potential of sEGFR as a prognostic biomarker in patients with various solid tumours remain controversial. Lower sEGFR levels were associated with reduced survival in advanced NSCLC patients [47] and in meningioma [12]. In agreement with an antiproliferative function, sEGFRs were reported to inhibit tumour cell proliferation and migration in NSCLC cell lines [48]. On the other hand, high levels of sEGFR were associated with a shorter survival rate in gastric cancer [46]. Therapeutic predictive values of sEGFR levels in blood have also been shown in some cancers. High levels of sEGFR may predict the response to tyrosine kinase inhibitors in colorectal cancer [49]. Low sEGFR levels are associated with shorter overall survival in patients with metastatic breast cancer treated with chemotherapy [50]. As a whole, these results highlight sEGFR as an interesting biomarker for diagnosis, prognosis, and response to therapy, but its overall role in cancer is still to be defined. Further investigations aiming at deciphering the identity (110-kDa, 80-kDa and/or 60-kDa EGFR isoforms) and origin (alternative splicing and/or proteolytic cleavage of EGFR) of the sEGFR isoforms expressed in these biological samples will probably help to understand the clinical data.
Figure 2.
EGFR and its splicing variants. Alternative splicing of EGFR generates eight variants including those that encode soluble isoforms, sEGFRv2, sEGFRv3, and sEGFRv4, and those can encode non-soluble isoforms, mLEEK, EGFRvA, EGFRvIII, EGFRvIVa, and EGFRvIVb. For each splice variant, the number of exons (upper) and functional domains of the protein (lower) are represented. L: ligand binding, CR: Cysteine-Rich.
2.2. EGFRvIII
EGFRvIII is the most common EGFR splice variant. It skips exons 2–7 and encodes a protein of 145 kDa that is devoid of the extracellular ligand-binding domain. As a consequence, EGFRvIII is unable to bind the soluble ligands of EGFR. Nevertheless, EGFRvIII shows constitutive tyrosine phosphorylation through basal dimerization, activates multiple downstream signalling pathways [51] and exhibits a high tumourigenic potential [52]. It is often co-expressed with wild-type EGFR, especially in tumours with EGFR amplification [53], and almost all published studies report the absence of EGFRvIII expression in normal tissues [54]. EGFRvIII has been mainly studied in high-grade brain tumours such as glioblastoma multiforme (GBM), in which EGFRvIII is detected at an overall frequency of 25%–64% [13]. EGFRvIII expression has also been reported in human tumours outside the central nervous system, including lung, breast and HNSCC [13], but its frequency and significance remain controversial. Some studies have identified EGFRvIII as a marker of poor prognosis in GBM patients [55], but others did not find an association between EGFRvIII and patients outcomes [56]. EGFRvIII expression has been implicated in the progression of breast cancer and could play a role in tumour metastasis [57]. EGFRvIII signalling has been reported to enhance the tumourigenicity of GBM, breast, lung and ovarian tumour cells, among others. The pro-tumourigenic effects of EGFRvIII seem to be mediated by pathways that are activated by wild-type EGFR, including RAS/RAF/mitogen-activated protein kinase (MAPK), phosphatidylinositol3-kinase (PI3K)/AKT, signal transducer and activator of transcription 3 (STAT3), and nuclear factor κB (NF-κB) [13], but there is emerging evidence that EGFRvIII can co-activate other cell-surface receptors [58]. Therefore, EGFRvIII signalling plays a role in tumourigenesis and tumour progression by mediating cell survival, proliferation, motility and invasion. Interestingly, a recent study also showed that EGFRvIII displays cancer stem cell-specific expression and can be used to specifically target this population [59].
The therapeutic potential of targeting EGFRvIII is becoming apparent, especially in brain tumours. A number of therapeutic approaches, some of them being commonly used to also target wild-type EGFR, have shown pre-clinical and/or clinical promise. Tyrosine kinase inhibitors showed good pre-clinical results with inhibition of tumour growth, angiogenesis, survival, and proliferation [60]. Response rates in glioblastoma patients were disappointing for many inhibitors [61]. Antibody-based therapies demonstrated efficacy in vitro, but their systemic injection failed to block tumour growth in vivo [62]. Various clinical trials that use conjugated antibodies with toxins or radioactive isotopes gave positive results with improvement of median survival [63]. Pre-clinical trials of RNA therapies based on the use of antisense oligonucleotide, RNA interference and ribozyme also yielded encouraging in vitro and in vivo results [64]. However, intra-tumoral heterogeneity of EGFR expression, development of resistance mechanisms by the tumour cells and low efficiency of therapeutic drugs to bypass the blood-brain barrier have limited the clinical utility of these therapies [61]. Immune therapy using vaccines is a promising treatment. Several laboratories have shown that a peptide vaccine targeting the EGFRvIII antigen can effectively reduce tumour progression in pre-clinical models [65]. Furthermore, several phase II clinical trials demonstrated improved survival and specific immune response to EGFRvIII in patients treated with the vaccine [66]. Clinical trials testing the efficiency of vaccine combined with EGFR-targeted therapy in brain tumour patients are underway. The role of anti-EGFRvIII therapy in other tumour types is still to be addressed.
2.3. EGFRvIV
The carboxyl terminal deletion mutants collectively called EGFRvIV lack either exons 25–27 (EGFRvIVa) or exons 25–26 (EGFRvIVb) [67]. The deletion initiates immediately downstream of the kinase domain. Little is known about the oncogenic potential of these EGFR mutants. It has been shown that internal deletions of EGFRvIV enhance basal kinase activity and confer tumourigenic growth in animals [68]. Furthermore, like EGFRvIII, EGFRvIV mutants display basal dimerization, enhanced basal kinase activity and increased stability due to association with HSP90. Constitutive downstream signalling by the EGFRvIV mutants includes activation of STAT3, MAPK, and AKT pathways, but it is suggested that the mutants activate different cellular programs and that distinct pathways are recruited by EGFRvIII and the EGFRvIV mutants [68]. The EGFRvIV mutants have been identified in glioblastoma multiforme, and to date there is no evidence of their expression in other cancer types.
2.4. EGFRvA
Structurally, EGFRvA is characterized by the substitution of exon 28 of EGFR with a serine/threonine-rich sequence [69]. Compared to wild-type EGFR, EGFRvA is more stable because of its decreased binding to c-Cbl [70], and it promotes cancer migration and invasion more significantly through activation of the STAT3 pathway and autocrine production of heparin-binding EGF [69]. EGFRvA is highly expressed in placenta and only slightly expressed in other normal tissues. Many cancer cell lines and tissues have EGFRvA, suggesting a role in tumour development. The detection of EGFRvA is positively correlated with tumour grade and with adverse prognosis in glioma patients, more significantly than EGFR [69]. Thus, EGFRvA plays a critical role in the tumour progression of gliomas and might be a good therapeutic target for cancer treatment.
2.5. mLEEK
Recently, a new alternative splicing variant called mLEEK was identified [15]. This protein lacks the extra-cytoplasmic, transmembrane and ATP binding site of the tyrosine kinase domain of EGFR. mLEEK is widely expressed in normal tissues and is overexpressed in human tumours, including those from ovary, skin, and lung. Interestingly, this variant localizes in the nucleus and co-regulates target gene expression that controls the unfolded protein response (UPR). Thus, mLEEK is able to favour cell growth in unfavourable conditions, making it an interesting target for future therapeutic development.
3. Alternative Splicing of VEGFR1: From Anti-Angiogenic to Pro-Angiogenic Factors
The VEGF (vascular endothelial growth factor) family is composed of seven glycoproteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF (placental growth factor), which exert different biological functions, such as angiogenesis and lymphangiogenesis, by binding to three VEGF receptors (VEGFRs): VEGFR1, VEGFR2, and VEGFR3 [71]. It has been shown that the entire family of VEGF ligands and VEGFRs display alternative splice variants. For instance, alternative splicing of VEGF-A generates multiple splice variants known as VEGFxxx (where xxx is the total number of amino acids in the mature protein) (see [72,73] for reviews). At the functional level, these VEGFxxx splice variants differentially bind heparin-containing proteoglycans that are present at the cell surface or in the extracellular matrix. As a consequence, VEGFxxx splice variants are more or less diffusible [74]. In addition, they display different binding affinities for their VEGFR1/VEGFR2 receptors and their co-receptors, neuropilins, thereby being more or less potent activators of VEGFR signalling pathways [75]. Interestingly, in 2002, a new family of VEGF-A splice variants, termed VEGFxxxb, was discovered [76]. VEGFxxxb isoforms result from the selection of a distal splice site in the last exon of VEGF-A and the inclusion of a new exon called exon 8b. At the protein level, VEGFxxxb share 94%–98% homology with VEGFxxx and differ only at the level of six amino acids in the C-terminal end. VEGFxxxb can bind VEGFR receptors with the same affinity than VEGFxxx, but they are unable to bind neuropilins and to fully activate VEGFR-dependent signalling pathways [77,78]. As a consequence, while VEGFxxx splice variants are angiogenic factors overexpressed in many tumours [74], VEGFxxxb appear to act as anti-angiogenic factors competing with and inhibiting all the effects (proliferation, migration, survival) of VEGFxxx on endothelial cells. In addition to VEGF-A, VEGFRs are also subjected to alternative splicing. Here, we will discuss only the role of the VEGFR1 splice variants, but it is interesting to note that a soluble VEGFR2 splice variant has also been identified and shown to act as an inhibitor of lymphatic vessel growth [79].
3.1. VEGFR1 Splice Variants
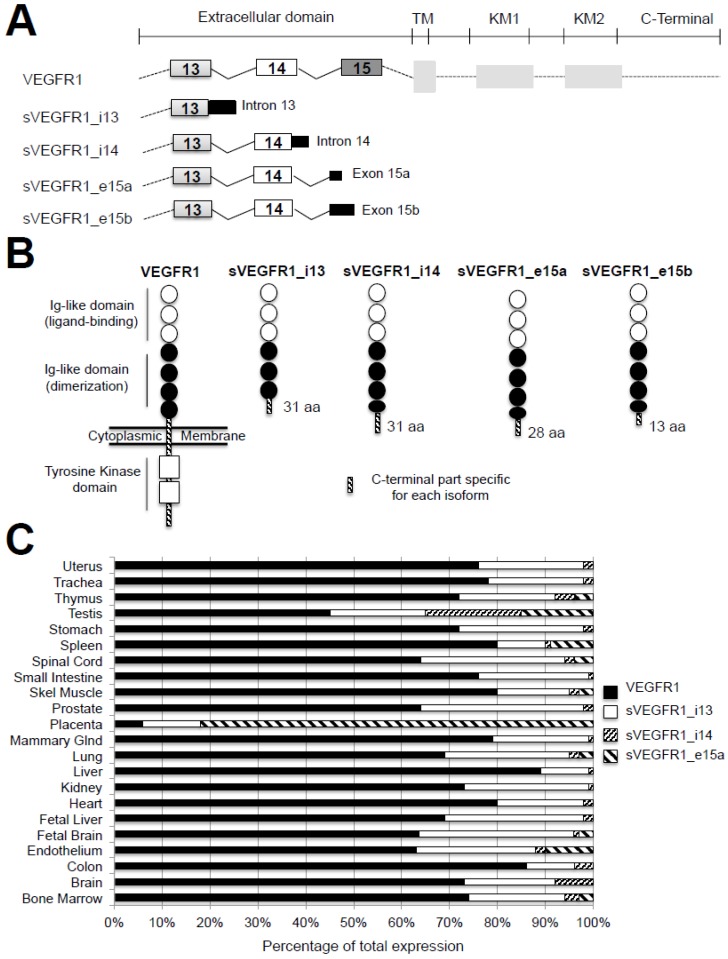
To date, four alternative splice variants of VEGFR1, named sVEGFR1-i13, sVEGFR1-i14, sVEGFR1-e15a, and sVEGFR1-e15b, have been described [26] (Figure 3A). These variants have the same transcription start site as Vegfr1. However, while Vegfr1 mRNA contains 30 exons, sVegfr1 mRNAs share only the first 13–15 exons, depending on the variant, and encode truncated proteins that are devoid of their transmembrane and intra-cytoplasmic tyrosine kinase domains. This is the reason why they are considered soluble decoy receptors and annotated as sVEGFR1. The sVEGFR-1_v1 or sVEGFR1-i13 was initially discovered in 1993 as a 100-kDa protein highly expressed in endothelium [26]. It results from an inclusion of intron 13 followed by a premature polyadenylation. This splice variant is the shortest and the most studied among sVEGFR1s. The sVEGFR-1_v2 or sVEGFR1-e15a contains the first 14 exons and a new terminal exon (denoted exon 15a) derived from an intronic sequence [80], while the sVEGFR-1_v3 or sVEGFR1-e15b contains a new 3′-end denoted exon 15b distinct from the variant v2 [81]. The sVEGFR-1_v4 or sVEGFR-1-i14 results from skipping a splice site, leading to the extension of exon 14 followed by a polyadenylation [81].
Figure 3.
The different VEGFR1 splice variants, proteins and expression in tissues. (A) Schematic representation of full-length VEGFR1, sVEGFR1_i13, sVEGFR1_i14, sVEGFR1-e15a, and sVEGFR1-e15b mRNAs. Exons and introns are shown. TM: Transmembrane domain, KM1: ATP-binding domain, KM2: phosphotransferase domain; (B) Schematic representation of full-length VEGFR1 and sVEGFR1s proteins. Each splice variant isoform contains the first six extracellular Ig-like domains of VEGFR1, with (sVEGFR1-i14, sVEGFR1-e15a, sVEGFR1-e15b) or without (sVEGFR1-i13) a part of the last Ig-like domain, followed by a specific C-terminal end represented as a hatched box in the figure (adapted from [82]). aa represents the number of amino acids contained in the specific C-terminal part; (C) Percentage of expression of VEGFR1, sVEGFR1-i13, sVEGFR1-i14, and sVEGFR1-e15a mRNAs as indicated, according to the tissue type. sVEGFR1-e15b mRNA is undetectable in most of these tissues (adapted from [84]).
At the protein level, sVEGFR1-i13 protein contains the first six Ig-like domains of the full-length VEGFR1 that correspond to 657 amino acids. Therefore, it possesses the ligand binding domain but lacks the seventh Ig-like domain, as well as the transmembrane and the tyrosine kinase regions of VEGFR1 (Figure 3B). In contrast, it has a unique C-terminal sequence composed of 31 amino acids. This specific sequence is highly conserved in mammals and differs only at the level of two amino acids between mouse and human [82]. The molecular weight of sVEGFR1-i13 varies depending on the cell type, being 110 kDa in HUVEC (human umbilical vein endothelial cells) and HDMEC (human dermal microvascular endothelial cells) and 120–130 kDa in COLO-800 melanoma cells [83]. These differences might reflect various glycosylation levels. For sVEGFR1-i14, sVEGFR1-e15a, and sVEGFR1-e15b, the extension of the open reading frame is low (93, 84, and 33 bp, respectively), and the protein is modified after amino acid 706, leading to a truncated protein between the two last Ig-like domains. These variants contain specific C-terminal extremities of 31, 28, and 13 amino acids, respectively [82] (Figure 3B).
3.2. Expression and Regulation of sVEGFR1 in Tissues
The sVEGFR1 isoforms are secreted by several cell types, including endothelial cells [85], smooth muscle cells [86], monocytes and macrophages [85], trophoblasts of the placenta [85], and proximal tubular cells of the renal epithelium [87], as well as by various cancer cells [88]. Each cell type expresses different levels of sVEGFR1 variants. For example, smooth muscle cells predominantly express sVEGFR1-i14, while endothelial cells mainly express sVEGFR1-i13 [86]. sVEGFR1s are also differentially expressed according to the organs (Figure 3C). For example, the level of sVEGFR1-i13 is forty times greater in the placenta than in the heart, kidneys, and lungs. In addition, the sVEGFR1-e15a is the most abundant isoform in the placenta [83]. Taken together, these data suggest that the role of each sVEGFR1 might be highly dependent on the tissue and/or the cell type. Although extracellular signals that control sVEGFR1 expression are largely unknown, endogenous stimuli, such as growth factors [89,90], cytokines [91], hypoxia [92], and miRNAs [93], have been shown to induce the expression of sVEGFR1-i13. Moreover, a cooperative role between the arginine demethylase and lysine hydroxylase JMJD6 (JuMonJi Domain containing-protein 6) and the splicing factor U2AF65 [94], as well as inhibition of the NOTCH1 signalling pathway [95], have been reported to up-regulate the expression of sVEGFR1-i13. These studies have been performed in endothelial cells, dendritic cells, macrophages, cytotrophoblasts, placenta, or retina. To date, the upstream signals that control sVEGFR1 expression in cancer cells remain unknown.
3.3. Role of Vascular Functions of sVEGFR1s in Physiological and Pathological Conditions
It was first shown that sVEGFR1s, which are devoid of tyrosine kinase domains (Figure 3B), exert anti-angiogenic functions through inhibition of VEGF-A/VEGFR signalling. Two mechanisms of action were proposed: the sequestration of VEGF-A ligand and/or its heterodimerization with VEGFR2 receptor [96,97]. Based on its vascular effects, both physiological and pathological functions have been attributed to sVEGFR1. Physiologically, the non-vascularization of the cornea supports optical clarity. Among many anti-angiogenic molecules (angiostatin, endostatin, etc.), sVEGFR1 is the only one required to inhibit the pro-angiogenic effects of VEGF-A in the cornea [98,99]. During normal pregnancy, it was also proposed that sVEGFR1 maintains the vascular integrity of the placenta by sequestering the excess of VEGF-A [100]. In addition, sVEGFR1 has an anti-oedema role through its ability to interfere with the vascular permeability function of VEGF-A [101,102]. Last but not least, sVEGFR1 displays protective anti-inflammatory functions because it prevents the activation and migration of monocytes and macrophages [103]. Conversely, sVEGFR1 has also been implicated in many vascular pathologies. The most described is preeclampsia, a pregnancy-specific disorder characterized by hypertension and proteinuria occurring in the second half of pregnancy and resulting in neonatal or maternal morbidity and mortality. It has been shown that plasma levels of both sVEGFR1-i13 and sVEGFR1-e15a proteins increase five weeks before the onset of preeclampsia symptoms [104]. However, the sVEGFR1-e15a variant appears to be mainly involved in this pathology [82,86]. In preeclampsia, various studies have shown that hypoxia, oxidative stress, and an excess of VEGF-A in the endometrium regulate the production of sVEGFR1 [100,104,105]. Moreover, increased expression of sVEGFR1 was observed in patients with wound healing defects [106], as well as in patients with idiopathic pulmonary arterial hypertension [107] or adult respiratory distress syndrome [108]. Both pathologies are associated with abnormal vascular permeability.
3.4. Role of sVEGFR1 in Tumour Progression
Tumour neo-angiogenesis is a prerequisite for tumour progression in most solid cancers. In different xenografted tumours in mice (melanoma, lung cancer, fibrosarcoma, glioblastoma), exogenous administration of sVEGFR1 through different approaches (transfection, adenovirus infection, or use of recombinant protein) was found to inhibit tumour growth and neo-angiogenesis and to increase the survival rate [109,110,111,112]. However, contrasting results were also reported. Expression of sVEGFR1 was able to rescue the aberrant morphogenesis of embryonic vessels that occurs in VEGFR1 knock-out mice through stimulation of vascular sprouting and endothelial cell migration [113]. In addition, sVEGFR1-i13 was shown to induce the adhesion and migration of endothelial cells through interaction with α5β1 integrin and activation of rac1 and radixin, a substrate of PKC [114]. Taken together, these data extended the previous view regarding the anti-angiogenic functions of sVEGFR1 by providing evidence that sVEGFR1 can act as both a positive and a negative regulator of the angiogenic process.
Other studies demonstrated that sVEGFR1 can also act on tumour cells themselves. sVEGFR1 was recently reported to induce non-apoptotic death in ovarian or colorectal cancer cell lines and to promote tumour regression in an ovarian carcinoma mouse model [115]. Conversely, Ruffini and collaborators showed that sVEGFR1-i13 is produced in the extracellular matrix of melanoma cells and induces cell adhesion by activating the VEGF-A/VEGFR2 signalling pathway [116]. In addition, involvement of sVEGFR1 in metastatic processes was highlighted. Indeed, high levels of VEGF-A and sVEGFR1 were reported in metastatic breast cancer compared to non-metastatic cancers [117]. In melanomas, high levels of sVEGFR1-i13 are depicted with respect to human melanocytes, but there is a lower level of sVEGFR1i-13 in skin metastases compared to primary tumours [116].
3.5. sVEGFR1 as a Prognostic Biomarker in Cancer
Several studies have analysed intra-tumoral and/or plasma/serum levels of sVEGFR1 in tumours. However, few of them have examined which sVEGFR1 splice variant is expressed, and most of the data reflect overall sVEGFR1. It has been shown that sVEGFR1 protein is overexpressed in many types of cancer, including glioblastoma, leukaemia, melanoma, colorectal, breast, renal, hepatocellular, head and neck, and lung carcinoma [88,116,118,119,120,121,122,123,124]. In this setting, high levels of sVEGFR1 are often correlated with poor prognosis. In addition, the levels of both sVEGFR1 and VEGF-A have been previously used to determine the prognosis of cancer patients, but the results remain controversial. As an example, in lung cancer patients, high levels of both sVEGFR1 and VEGF-A are correlated with poor prognosis and with very advanced stages [124]. In glioma, leukaemia, breast, or pancreatic cancer, high levels of VEGF-A combined with low levels of sVEGFR1 in the serum, plasma or tumour extracts are correlated with high grade, reduced survival rate and/or poorer response to therapy [118,125,126,127]. In contrast, Toi et al. reported that when the level of sVEGFR1 is 10 times higher than that of VEGF-A, this correlates with a better prognosis in breast cancer [120]. Therefore, the balance between sVEGFR1 and VEGF-A levels appear to be important for clinical outcome.
3.6. sVEGFR1 as a Biomarker of Tumour Response to Therapies
sVEGFR1 has also been investigated for its potential as a determinant of response to anti-angiogenic therapies, and many studies have quantified its plasma and/or serum levels before and/or after treatment [128]. Interestingly, high basal plasma levels of sVEGFR1 were often inversely correlated with response to bevacizumab or VEGFR-TKI in colorectal cancer (vandetanib plus cetuximab/irinotecan), hepatocellular carcinoma (cediranib), sarcoma (sorafenib), lung or renal cancer (bevacizumab), or triple-negative breast cancer [28,121,129,130]. In breast cancers, the resistance to bevacizumab was directly correlated with pericyte coverage, a marker of vascular normalization, thereby suggesting that the vascular functions of sVEGFR1 may account for its negative impact on tumour response to anti-angiogenic therapies [129]. However, and making things slightly more complicated, it was also shown that sVEGFR1 expression level either decreased or increased upon treatment in triple-negative breast, rectal, or metastatic colorectal cancers [28,129,131,132]. These data indicated that these therapies regulate sVEGFR1 expression level in opposite ways depending on the tumour type. In addition, combination of sVEGFR1 levels with those of other angiogenic regulators has been used to predict response. As an example, in patients with advanced colorectal cancer treated with bevacizumab and chemoradiation, high levels of sVEGFR1 and low levels of VEGF-A correlated with abnormal vascularity and poor response despite fewer side effects [133]. In advanced non-squamous cell lung carcinoma, high levels of PlGF and sVEGFR1 before treatment were associated with a poor response. After treatment, although the level of PIGF remained high, the level of sVEGFR1 transiently decreased [29]. Overall, these data highlight the potential role of sVEGFR1 as a biomarker of resistance to anti-angiogenic therapies, mainly through vascular effects [131,134,135]. As sVEGFR1 also acts on tumour cells themselves, it remains to be determined whether this autocrine function also contributes to the resistant phenotype.
Finally, and based on the anti-angiogenic functions of sVEGFR1, different therapeutic strategies have been elaborated in tumours to design “sVEGFR1-like” therapies, among them the VEGF-Grab. This soluble receptor comprises the second and third Ig-like domains of VEGFR-1 that are combined to the Fc fragment. The Ig-like domain 3 of VEGFR1 is glycosylated because the positive charges induce non-specific binding to the extracellular matrix. Interestingly, VEGF-Grab demonstrated anti-angiogenic, anti-tumour and anti-metastatic effects, suggesting that its clinical use could result in promising anti-angiogenic effects [136]. In addition, it was shown that morpholino constructs targeting the Vegfr1 mRNA exon13/intron13 junction promote the production of sVEGFR1 over membrane-bound VEGFR1 and decrease tumour neovascularization in vivo [137]. Manipulating sVEGFR1 expression could offer translational potential for therapy, although it is important to keep in mind that overexpressing sVEGFR1 could also trigger deleterious effects, depending on the context and/or the tumour type.
4. Conclusions
Enhanced RTK signalling is a driving force in many human malignancies. Although much work has been completed concerning the identification and functional consequences of RTK mutations or copy number variations in cancer, less is known about the role of RTK splice variants. Management of tumour patients with primary/acquired resistance to targeted therapies remains a significant challenge with therapeutic, social, and economic impacts. Thorough understanding of the biological significance of alternative splicing of RTKs and components of RTK signalling pathways could allow for the identification of new prognosis biomarkers, as well as the definition of alternative therapeutic strategies. Drugs targeting the spliceosome machinery are currently being tested in pre-clinical trials and have already demonstrated anti-tumour efficacy. As an example, spliceostatin A, or its analogue meayamycin B (MAMB), slows down the growth of vemurafenib-resistant tumours by decreasing the amount of the resistant BRAF3-9 splice variant [138,139]. In addition, treatment with the spliceosome inhibitor E7107 results in substantial reductions in leukaemic burden, specifically in patient-derived xenograft AMLs carrying mutations of spliceosomal components [140]. These results provide a rationale for targeting RNA splicing in cancer in combination or not with RTK-targeted therapies.
Acknowledgements
This work was supported by the Institute National de la Santé et de la Recherche Médicale, the Fondation ARC pour la recherche sur le cancer, la Ligue Nationale contre Le Cancer Comités de Savoie et de l’Isère and Agir pour les Maladies Chroniques. Anne-Sophie Hatat was supported by the French Research Ministry, and Cherine Abou-Fayçal was supported by la Ligue Nationale contre Le Cancer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Brivanlou A.H., Darnell J.E., Jr. Signal transduction and the control of gene expression. Science. 2002;295:813–818. doi: 10.1126/science.1066355. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen P., Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-K. [DOI] [PubMed] [Google Scholar]
- 5.Pan Q., Shai O., Lee L.J., Frey B.J., Blencowe B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 6.Sultan M., Schulz M.H., Richard H., Magen A., Klingenhoff A., Scherf M., Seifert M., Borodina T., Soldatov A., Parkhomchuk D., et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- 7.David C.J., Manley J.L. Alternative pre-mRNA splicing regulation in cancer: Pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G., Wang J., Liu Z., Kornmann M. Exon III splicing of fibroblast growth factor receptor 1 is modulated by growth factors and cyclin D1. Pancreas. 2008;37:159–164. doi: 10.1097/MPA.0b013e31816618a4. [DOI] [PubMed] [Google Scholar]
- 9.De Figueiredo-Pontes L.L., Wong D.W., Tin V.P., Chung L.P., Yasuda H., Yamaguchi N., Nakayama S., Janne P.A., Wong M.P., Kobayashi S.S., et al. Identification and characterization of ALK kinase splicing isoforms in non-small-cell lung cancer. J. Thorac. Oncol. 2014;9:248–253. doi: 10.1097/JTO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Bryan J.P., Frye R.A., Cogswell P.C., Neubauer A., Kitch B., Prokop C., Espinosa R., Le Beau M.M., Earp H.S., Liu E.T. AXL, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 1991;11:5016–5031. doi: 10.1128/MCB.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel W., Gish G.D., Alves F., Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1997;1:13–23. doi: 10.1016/S1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 12.Guillaudeau A., Durand K., Bessette B., Chaunavel A., Pommepuy I., Projetti F., Robert S., Caire F., Rabinovitch-Chable H., Labrousse F. EGFR soluble isoforms and their transcripts are expressed in meningiomas. PLoS ONE. 2012;7:e37204. doi: 10.1371/journal.pone.0037204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan H.K., Cvrljevic A.N., Johns T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013;280:5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 14.Padfield E., Ellis H.P., Kurian K.M. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front. Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccione E.C., Lieu T.J., Gentile C.F., Williams T.R., Connolly A.J., Godwin A.K., Koong A.C., Wong A.J. A novel epidermal growth factor receptor variant lacking multiple domains directly activates transcription and is overexpressed in tumors. Oncogene. 2012;31:2953–2967. doi: 10.1038/onc.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundvall M., Korhonen A., Paatero I., Gaudio E., Melino G., Croce C.M., Aqeilan R.I., Elenius K. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc. Natl. Acad. Sci. USA. 2008;105:4162–4167. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P., Greendorfer J.S., Jiao J., Kelpke S.C., Thompson J.A. Alternatively spliced FGFR-1 isoforms differentially modulate endothelial cell activation of c-YES. Arch. Biochem. Biophys. 2006;450:50–62. doi: 10.1016/j.abb.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Eswarakumar V.P., Lax I., Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Malakar P., Chartarifsky L., Hija A., Leibowitz G., Glaser B., Dor Y., Karni R. Insulin receptor alternative splicing is regulated by insulin signaling and modulates β cell survival. Sci. Rep. 2016;6:31222. doi: 10.1038/srep31222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frampton G.M., Ali S.M., Rosenzweig M., Chmielecki J., Lu X., Bauer T.M., Akimov M., Bufill J.A., Lee C., Jentz D., et al. Activation of met via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to met inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 21.Zheng D., Wang R., Ye T., Yu S., Hu H., Shen X., Li Y., Ji H., Sun Y., Chen H. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget. 2016;7:41691–41702. doi: 10.18632/oncotarget.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson D.S., Rodrigues D.M., Hyndman B.D., Crupi M.J., Nicolescu A.C., Mulligan L.M. Alternative splicing results in RET isoforms with distinct trafficking properties. Mol. Biol. Cell. 2012;23:3838–3850. doi: 10.1091/mbc.E12-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghigna C., de Toledo M., Bonomi S., Valacca C., Gallo S., Apicella M., Eperon I., Tazi J., Biamonti G. Pro-metastatic splicing of ron proto-oncogene mrna can be reversed: Therapeutic potential of bifunctional oligonucleotides and indole derivatives. RNA Biol. 2010;7:495–503. doi: 10.4161/rna.7.4.12744. [DOI] [PubMed] [Google Scholar]
- 24.Krishnaswamy S., Mohammed A.K., Amer O.E., Tripathi G., Alokail M.S., Al-Daghri N.M. Novel splicing variants of recepteur d’origine nantais (RON) tyrosine kinase involving exons 15–19 in lung cancer. Lung Cancer. 2016;92:41–46. doi: 10.1016/j.lungcan.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Vaishnavi A., Le A.T., Doebele R.C. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendall R.L., Wang G., Thomas K.A. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 27.Vorlova S., Rocco G., Lefave C.V., Jodelka F.M., Hess K., Hastings M.L., Henke E., Cartegni L. Induction of antagonistic soluble decoy receptor tyrosine kinases by intronic polyA activation. Mol. Cell. 2011;43:927–939. doi: 10.1016/j.molcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerhardt J.A., Ancukiewicz M., Abrams T.A., Schrag D., Enzinger P.C., Chan J.A., Kulke M.H., Wolpin B.M., Goldstein M., Blaszkowsky L., et al. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PLoS ONE. 2012;7:e38231. doi: 10.1371/journal.pone.0038231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heist R.S., Duda D.G., Sahani D.V., Ancukiewicz M., Fidias P., Sequist L.V., Temel J.S., Shaw A.T., Pennell N.A., Neal J.W., et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl. Acad. Sci. USA. 2015;112:1547–1552. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Ibrahimi O.A., Olsen S.K., Umemori H., Mohammadi M., Ornitz D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan G., Fukabori Y., McBride G., Nikolaropolous S., McKeehan W.L. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol. Cell. Biol. 1993;13:4513–4522. doi: 10.1128/MCB.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha J.Y., Lambert Q.T., Reuther G.W., Der C.J. Involvement of fibroblast growth factor receptor 2 isoform switching in mammary oncogenesis. Mol. Cancer Res. 2008;6:435–445. doi: 10.1158/1541-7786.MCR-07-0187. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y.Q., He C., Chen Y.Q., Wang D., Wang M.H. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: Generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 34.Mitra D., Brumlik M.J., Okamgba S.U., Zhu Y., Duplessis T.T., Parvani J.G., Lesko S.M., Brogi E., Jones F.E. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol. Cancer Ther. 2009;8:2152–2162. doi: 10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- 35.Jorissen R.N., Walker F., Pouliot N., Garrett T.P., Ward C.W., Burgess A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003;284:31–53. doi: 10.1016/S0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson K.M. Structure-based view of epidermal growth factor receptor regulation. Annu. Rev. Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Torres M., Valle B.L., Maihle N.J., Negron-Vega L., Nieves-Alicea R., Cora E.M. Shedding of epidermal growth factor receptor is a regulated process that occurs with overexpression in malignant cells. Exp. Cell Res. 2008;314:2907–2918. doi: 10.1016/j.yexcr.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson M.P., Keller S., Alonso A., Riedle S., Dempsey P.J., Altevogt P. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J. Cell. Biochem. 2008;103:1783–1797. doi: 10.1002/jcb.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter J.L., Maihle N.J. A 1.8 kb alternative transcript from the human epidermal growth factor receptor gene encodes a truncated form of the receptor. Nucleic Acids Res. 1996;24:4050–4056. doi: 10.1093/nar/24.20.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter J.L., Threadgill D.W., Eley G.D., Strunk K.E., Danielsen A.J., Sinclair C.S., Pearsall R.S., Green P.J., Yee D., Lampland A.L., et al. Comparative genomic sequence analysis and isolation of human and mouse alternative EGFR transcripts encoding truncated receptor isoforms. Genomics. 2001;71:1–20. doi: 10.1006/geno.2000.6341. [DOI] [PubMed] [Google Scholar]
- 41.Flickinger T.W., Maihle N.J., Kung H.J. An alternatively processed mRNA from the avian c-erbB gene encodes a soluble, truncated form of the receptor that can block ligand-dependent transformation. Mol. Cell. Biol. 1992;12:883–893. doi: 10.1128/MCB.12.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baron A.T., Cora E.M., Lafky J.M., Boardman C.H., Buenafe M.C., Rademaker A., Liu D., Fishman D.A., Podratz K.C., Maihle N.J. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2003;12:103–113. [PubMed] [Google Scholar]
- 43.Asgeirsson K.S., Agrawal A., Allen C., Hitch A., Ellis I.O., Chapman C., Cheung K.L., Robertson J.F. Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients. Breast Cancer Res. 2007;9:R75. doi: 10.1186/bcr1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maramotti S., Paci M., Manzotti G., Rapicetta C., Gugnoni M., Galeone C., Cesario A., Lococo F. Soluble epidermal growth factor receptors (sEGFRS) in cancer: Biological aspects and clinical relevance. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemos-Gonzalez Y., Rodriguez-Berrocal F.J., Cordero O.J., Gomez C., de la Cadena M.P. Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br. J. Cancer. 2007;96:1569–1578. doi: 10.1038/sj.bjc.6603770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi J.H., Oh J.Y., Ryu S.K., Kim S.J., Lee N.Y., Kim Y.S., Yi S.Y., Shim K.S., Han W.S. Detection of epidermal growth factor receptor in the serum of gastric carcinoma patients. Cancer. 1997;79:1879–1883. doi: 10.1002/(SICI)1097-0142(19970515)79:10<1879::AID-CNCR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 47.Jantus-Lewintre E., Sirera R., Cabrera A., Blasco A., Caballero C., Iranzo V., Rosell R., Camps C. Analysis of the prognostic value of soluble epidermal growth factor receptor plasma concentration in advanced non-small-cell lung cancer patients. Clin. Lung Cancer. 2011;12:320–327. doi: 10.1016/j.cllc.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 48.Lococo F., Paci M., Rapicetta C., Rossi T., Sancisi V., Braglia L., Cavuto S., Bisagni A., Bongarzone I., Noonan D.M., et al. Preliminary evidence on the diagnostic and molecular role of circulating soluble EGFR in non-small cell lung cancer. Int. J. Mol. Sci. 2015;16:19612–19630. doi: 10.3390/ijms160819612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zampino M.G., Magni E., Santoro L., Zorzino L., Dell’Orto P., Sonzogni A., Fazio N., Monfardini L., Chiappa A., Biffi R., et al. Epidermal growth factor receptor serum (sEGFR) level may predict response in patients with EGFR-positive advanced colorectal cancer treated with gefitinib? Cancer Chemother. Pharmacol. 2008;63:139–148. doi: 10.1007/s00280-008-0722-x. [DOI] [PubMed] [Google Scholar]
- 50.Muller V., Witzel I., Pantel K., Krenkel S., Luck H.J., Neumann R., Keller T., Dittmer J., Janicke F., Thomssen C. Prognostic and predictive impact of soluble epidermal growth factor receptor (sEGFR) protein in the serum of patients treated with chemotherapy for metastatic breast cancer. Anticancer Res. 2006;26:1479–1487. [PubMed] [Google Scholar]
- 51.Huang P.H., Xu A.M., White F.M. Oncogenic EGFR signaling networks in glioma. Sci. Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 52.Nagane M., Coufal F., Lin H., Bogler O., Cavenee W.K., Huang H.J. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 53.Ekstrand A.J., James C.D., Cavenee W.K., Seliger B., Pettersson R.F., Collins V.P. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 54.Moscatello D.K., Holgado-Madruga M., Godwin A.K., Ramirez G., Gunn G., Zoltick P.W., Biegel J.A., Hayes R.L., Wong A.J. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 55.Feldkamp M.M., Lala P., Lau N., Roncari L., Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 56.Nieto Y., Nawaz F., Jones R.B., Shpall E.J., Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J. Clin. Oncol. 2007;25:4405–4413. doi: 10.1200/JCO.2006.09.8822. [DOI] [PubMed] [Google Scholar]
- 57.Ge H., Gong X., Tang C.K. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int. J. Cancer. 2002;98:357–361. doi: 10.1002/ijc.10224. [DOI] [PubMed] [Google Scholar]
- 58.Pillay V., Allaf L., Wilding A.L., Donoghue J.F., Court N.W., Greenall S.A., Scott A.M., Johns T.G. The plasticity of oncogene addiction: Implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. doi: 10.1593/neo.09230. 442 p following 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emlet D.R., Gupta P., Holgado-Madruga M., del Vecchio C.A., Mitra S.S., Han S.Y., Li G., Jensen K.C., Vogel H., Xu L.W., et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014;74:1238–1249. doi: 10.1158/0008-5472.CAN-13-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halatsch M.E., Gehrke E.E., Vougioukas V.I., Botefur I.C., A-Borhani F., Efferth T., Gebhart E., Domhof S., Schmidt U., Buchfelder M. Inverse correlation of epidermal growth factor receptor messenger RNA induction and suppression of anchorage-independent growth by OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in glioblastoma multiforme cell lines. J. Neurosurg. 2004;100:523–533. doi: 10.3171/jns.2004.100.3.0523. [DOI] [PubMed] [Google Scholar]
- 61.Gan H.K., Kaye A.H., Luwor R.B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009;16:748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Eller J.L., Longo S.L., Hicklin D.J., Canute G.W. Activity of anti-epidermal growth factor receptor monoclonal antibody c225 against glioblastoma multiforme. Neurosurgery. 2002;51:1005–1014. doi: 10.1097/00006123-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 63.Li L., Quang T.S., Gracely E.J., Kim J.H., Emrich J.G., Yaeger T.E., Jenrette J.M., Cohen S.C., Black P., Brady L.W. A phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010;113:192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 64.Kang C.S., Zhang Z.Y., Jia Z.F., Wang G.X., Qiu M.Z., Zhou H.X., Yu S.Z., Chang J., Jiang H., Pu P.Y. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13:530–538. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- 65.Moscatello D.K., Ramirez G., Wong A.J. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57:1419–1424. [PubMed] [Google Scholar]
- 66.Sampson J.H., Aldape K.D., Archer G.E., Coan A., Desjardins A., Friedman A.H., Friedman H.S., Gilbert M.R., Herndon J.E., McLendon R.E., et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekstrand A.J., Sugawa N., James C.D., Collins V.P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl. Acad. Sci. USA. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pines G., Huang P.H., Zwang Y., White F.M., Yarden Y. EGFRvIV: A previously uncharacterized oncogenic mutant reveals a kinase autoinhibitory mechanism. Oncogene. 2010;29:5850–5860. doi: 10.1038/onc.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou M., Wang H., Zhou K., Luo X., Pan X., Shi B., Jiang H., Zhang J., Li K., Wang H.M., et al. A novel EGFR isoform confers increased invasiveness to cancer cells. Cancer Res. 2013;73:7056–7067. doi: 10.1158/0008-5472.CAN-13-0194. [DOI] [PubMed] [Google Scholar]
- 70.Song F., Zhou M., Wang B., Shi B., Jiang H., Zhang J., Li Z. Weak binding to E3 ubiquitin ligase c-Cbl increases EGFRvA protein stability. FEBS Lett. 2016;590:1345–1353. doi: 10.1002/1873-3468.12166. [DOI] [PubMed] [Google Scholar]
- 71.Eichmann A., Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 2012;24:188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arcondeguy T., Lacazette E., Millevoi S., Prats H., Touriol C. VEGF-A mRNA processing, stability and translation: A paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 2013;41:7997–8010. doi: 10.1093/nar/gkt539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oltean S., Bates D.O. Hallmarks of alternative splicing in cancer. Oncogene. 2014;33:5311–5318. doi: 10.1038/onc.2013.533. [DOI] [PubMed] [Google Scholar]
- 74.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 75.Roskoski R., Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit. Rev. Oncol. Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Bates D.O., Cui T.G., Doughty J.M., Winkler M., Sugiono M., Shields J.D., Peat D., Gillatt D., Harper S.J. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 77.Cebe Suarez S., Pieren M., Cariolato L., Arn S., Hoffmann U., Bogucki A., Manlius C., Wood J., Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell. Mol. Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawamura H., Li X., Harper S.J., Bates D.O., Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 79.Albuquerque R.J., Hayashi T., Cho W.G., Kleinman M.E., Dridi S., Takeda A., Baffi J.Z., Yamada K., Kaneko H., Green M.G., et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas C.P., Andrews J.I., Raikwar N.S., Kelley E.A., Herse F., Dechend R., Golos T.G., Liu K.Z. A recently evolved novel trophoblast-enriched secreted form of fms-like tyrosine kinase-1 variant is up-regulated in hypoxia and preeclampsia. J. Clin. Endocrinol. Metab. 2009;94:2524–2530. doi: 10.1210/jc.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heydarian M., McCaffrey T., Florea L., Yang Z., Ross M.M., Zhou W., Maynard S.E. Novel splice variants of sFlt1 are upregulated in preeclampsia. Placenta. 2009;30:250–255. doi: 10.1016/j.placenta.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Szalai G., Romero R., Chaiworapongsa T., Xu Y., Wang B., Ahn H., Xu Z., Chiang P.J., Sundell B., Wang R., et al. Full-length human placental sFlt-1-e15a isoform induces distinct maternal phenotypes of preeclampsia in mice. PLoS ONE. 2015;10:e0119547. doi: 10.1371/journal.pone.0119547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hornig C., Barleon B., Ahmad S., Vuorela P., Ahmed A., Weich H.A. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab. Investig. 2000;80:443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 84.Jebbink J., Keijser R., Veenboer G., van der Post J., Ris-Stalpers C., Afink G. Expression of placental FLT1 transcript variants relates to both gestational hypertensive disease and fetal growth. Hypertension. 2011;58:70–76. doi: 10.1161/HYPERTENSIONAHA.110.164079. [DOI] [PubMed] [Google Scholar]
- 85.Karumanchi S.A., Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: The “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 86.Sela S., Itin A., Natanson-Yaron S., Greenfield C., Goldman-Wohl D., Yagel S., Keshet E. A novel human-specific soluble vascular endothelial growth factor receptor 1: Cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ. Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 87.Kim N.H., Oh J.H., Seo J.A., Lee K.W., Kim S.G., Choi K.M., Baik S.H., Choi D.S., Kang Y.S., Han S.Y., et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 88.Yamaguchi T., Bando H., Mori T., Takahashi K., Matsumoto H., Yasutome M., Weich H., Toi M. Overexpression of soluble vascular endothelial growth factor receptor 1 in colorectal cancer: Association with progression and prognosis. Cancer Sci. 2007;98:405–410. doi: 10.1111/j.1349-7006.2007.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmad S., Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 90.Ahmad S., Hewett P.W., Al-Ani B., Sissaoui S., Fujisawa T., Cudmore M.J., Ahmed A. Autocrine activity of soluble FLT-1 controls endothelial cell function and angiogenesis. Vasc. Cell. 2011;3:15. doi: 10.1186/2045-824X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia L., Dong Z., Zhang Y., Zhang X., Song X., Sun M., Hu Y., Liu S., Wang K., Qu X., et al. Interleukin-4 and granulocyte-macrophage colony-stimulating factor mediates the upregulation of soluble vascular endothelial growth factor receptor-1 in RAW264.7 cells-a process in which p38 mitogen-activated protein kinase signaling has an important role. J. Microbiol. Immunol. Infect. 2016;49:344–351. doi: 10.1016/j.jmii.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Xiong Y., Liebermann D.A., Tront J.S., Holtzman E.J., Huang Y., Hoffman B., Geifman-Holtzman O. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J. Cell. Physiol. 2009;220:632–639. doi: 10.1002/jcp.21800. [DOI] [PubMed] [Google Scholar]
- 93.Anton L., Olarerin-George A.O., Hogenesch J.B., Elovitz M.A. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS ONE. 2015;10:e0122707. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boeckel J.N., Guarani V., Koyanagi M., Roexe T., Lengeling A., Schermuly R.T., Gellert P., Braun T., Zeiher A., Dimmeler S. Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc. Natl. Acad. Sci. USA. 2011;108:3276–3281. doi: 10.1073/pnas.1008098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kangsamaksin T., Murtomaki A., Kofler N.M., Cuervo H., Chaudhri R.A., Tattersall I.W., Rosenstiel P.E., Shawber C.J., Kitajewski J. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2015;5:182–197. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu F.T., Stefanini M.O., Mac Gabhann F., Kontos C.D., Annex B.H., Popel A.S. A systems biology perspective on sVEGFR1: Its biological function, pathogenic role and therapeutic use. J. Cell. Mol. Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ambati B.K., Nozaki M., Singh N., Takeda A., Jani P.D., Suthar T., Albuquerque R.J., Richter E., Sakurai E., Newcomb M.T., et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ambati B.K., Patterson E., Jani P., Jenkins C., Higgins E., Singh N., Suthar T., Vira N., Smith K., Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan X., Rai A., Kambham N., Sung J.F., Singh N., Petitt M., Dhal S., Agrawal R., Sutton R.E., Druzin M.L., et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Investig. 2014;124:4941–4952. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 102.Kumai Y., Ooboshi H., Ibayashi S., Ishikawa E., Sugimori H., Kamouchi M., Kitazono T., Egashira K., Iida M. Postischemic gene transfer of soluble FLT-1 protects against brain ischemia with marked attenuation of blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2007;27:1152–1160. doi: 10.1038/sj.jcbfm.9600420. [DOI] [PubMed] [Google Scholar]
- 103.Tsao P.N., Chan F.T., Wei S.C., Hsieh W.S., Chou H.C., Su Y.N., Chen C.Y., Hsu W.M., Hsieh F.J., Hsu S.M. Soluble vascular endothelial growth factor receptor-1 protects mice in sepsis. Crit. Care Med. 2007;35:1955–1960. doi: 10.1097/01.CCM.0000275273.56547.B8. [DOI] [PubMed] [Google Scholar]
- 104.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 105.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eming S.A., Lauer G., Cole M., Jurk S., Christ H., Hornig C., Krieg T., Weich H.A. Increased levels of the soluble variant of the vascular endothelial growth factor receptor VEGFR-1 are associated with a poor prognosis in wound healing. J. Invest. Dermatol. 2004;123:799–802. doi: 10.1111/j.0022-202X.2004.23310.x. [DOI] [PubMed] [Google Scholar]
- 107.Malhotra R., Paskin-Flerlage S., Zamanian R.T., Zimmerman P., Schmidt J.W., Deng D.Y., Southwood M., Spencer R., Lai C.S., Parker W., et al. Circulating angiogenic modulatory factors predict survival and functional class in pulmonary arterial hypertension. Pulm. Circ. 2013;3:369–380. doi: 10.4103/2045-8932.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wada T., Jesmin S., Gando S., Yanagida Y., Mizugaki A., Sultana S.N., Zaedi S., Yokota H. The role of angiogenic factors and their soluble receptors in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) associated with critical illness. J. Inflamm. 2013;10:6. doi: 10.1186/1476-9255-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goldman C.K., Kendall R.L., Cabrera G., Soroceanu L., Heike Y., Gillespie G.Y., Siegal G.P., Mao X., Bett A.J., Huckle W.R., et al. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc. Natl. Acad. Sci. USA. 1998;95:8795–8800. doi: 10.1073/pnas.95.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verrax J., Defresne F., Lair F., Vandermeulen G., Rath G., Dessy C., Preat V., Feron O. Delivery of soluble VEGF receptor 1 (sFlt1) by gene electrotransfer as a new antiangiogenic cancer therapy. Mol. Pharm. 2011;8:701–708. doi: 10.1021/mp100268t. [DOI] [PubMed] [Google Scholar]
- 111.Takayama K., Ueno H., Nakanishi Y., Sakamoto T., Inoue K., Shimizu K., Oohashi H., Hara N. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000;60:2169–2177. [PubMed] [Google Scholar]
- 112.Shiose S., Sakamoto T., Yoshikawa H., Hata Y., Kawano Y., Ishibashi T., Inomata H., Takayama K., Ueno H. Gene transfer of a soluble receptor of VEGF inhibits the growth of experimental eyelid malignant melanoma. Investig. Ophthalmol. Vis. Sci. 2000;41:2395–2403. [PubMed] [Google Scholar]
- 113.Kearney J.B., Kappas N.C., Ellerstrom C., DiPaola F.W., Bautch V.L. The VEGF receptor Flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 114.Orecchia A., Lacal P.M., Schietroma C., Morea V., Zambruno G., Failla C.M. Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the α5β1 integrin. J. Cell Sci. 2003;116:3479–3489. doi: 10.1242/jcs.00673. [DOI] [PubMed] [Google Scholar]
- 115.Miyake T., Kumasawa K., Sato N., Takiuchi T., Nakamura H., Kimura T. Soluble VEGF receptor 1 (sFlt1) induces non-apoptotic death in ovarian and colorectal cancer cells. Sci. Rep. 2016;6:24853. doi: 10.1038/srep24853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruffini F., Failla C.M., Orecchia A., Bani M.R., Dorio A.S., Fortes C., Zambruno G., Graziani G., Giavazzi R., D’Atri S., et al. Expression of the soluble vascular endothelial growth factor receptor-1 in cutaneous melanoma: Role in tumour progression. Br. J. Dermatol. 2011;164:1061–1070. doi: 10.1111/j.1365-2133.2010.10200.x. [DOI] [PubMed] [Google Scholar]
- 117.Thielemann A., Baszczuk A., Kopczynski Z., Kopczynski P., Grodecka-Gazdecka S. Clinical usefulness of assessing VEGF and soluble receptors sVEGFR-1 and sVEGFR-2 in women with breast cancer. Ann. Agric. Environ. Med. 2013;20:293–297. [PubMed] [Google Scholar]
- 118.Lamszus K., Ulbricht U., Matschke J., Brockmann M.A., Fillbrandt R., Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin. Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- 119.Wierzbowska A., Robak T., Wrzesien-Kus A., Krawczynska A., Lech-Maranda E., Urbanska-Rys H. Circulating VEGF and its soluble receptors sVEGFR-1 and sVEGFR-2 in patients with acute leukemia. Eur. Cytokine Netw. 2003;14:149–153. [PubMed] [Google Scholar]
- 120.Toi M., Bando H., Ogawa T., Muta M., Hornig C., Weich H.A. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int. J. Cancer. 2002;98:14–18. doi: 10.1002/ijc.10121. [DOI] [PubMed] [Google Scholar]
- 121.Harris A.L., Reusch P., Barleon B., Hang C., Dobbs N., Marme D. Soluble Tie2 and Flt1 extracellular domains in serum of patients with renal cancer and response to antiangiogenic therapy. Clin. Cancer Res. 2001;7:1992–1997. [PubMed] [Google Scholar]
- 122.Nagaoka S., Yoshida T., Akiyoshi J., Akiba J., Hisamoto T., Yoshida Y., Abe M., Koga H., Toirimura T., Ueno T., et al. The ratio of serum placenta growth factor to soluble vascular endothelial growth factor receptor-1 predicts the prognosis of hepatocellular carcinoma. Oncol. Rep. 2010;23:1647–1654. doi: 10.3892/or_00000807. [DOI] [PubMed] [Google Scholar]
- 123.Kulapaditharom B., Boonkitticharoen V., Sritara C. Plasma vascular endothelial growth factor dysregulation in defining aggressiveness of head and neck squamous cell carcinoma. J. Oncol. 2012;2012:687934. doi: 10.1155/2012/687934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ilhan N., Ilhan N., Deveci F. Functional significance of vascular endothelial growth factor and its receptor (receptor-1) in various lung cancer types. Clin. Biochem. 2004;37:840–845. doi: 10.1016/j.clinbiochem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 125.Chang Y.T., Chang M.C., Wei S.C., Tien Y.W., Hsu C., Liang P.C., Tsao P.N., Jan I.S., Wong J.M. Serum vascular endothelial growth factor/soluble vascular endothelial growth factor receptor 1 ratio is an independent prognostic marker in pancreatic cancer. Pancreas. 2008;37:145–150. doi: 10.1097/MPA.0b013e318164548a. [DOI] [PubMed] [Google Scholar]
- 126.Bando H., Weich H.A., Brokelmann M., Horiguchi S., Funata N., Ogawa T., Toi M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br. J. Cancer. 2005;92:553–561. doi: 10.1038/sj.bjc.6602374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aref S., El Sherbiny M., Goda T., Fouda M., Al Askalany H., Abdalla D. Soluble VEGF/sFlt1 ratio is an independent predictor of aml patient out come. Hematology. 2005;10:131–134. doi: 10.1080/10245330500065797. [DOI] [PubMed] [Google Scholar]
- 128.Lambrechts D., Lenz H.J., de Haas S., Carmeliet P., Scherer S.J. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 129.Tolaney S.M., Boucher Y., Duda D.G., Martin J.D., Seano G., Ancukiewicz M., Barry W.T., Goel S., Lahdenrata J., Isakoff S.J., et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. USA. 2015;112:14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu A.X., Finn R.S., Mulcahy M., Gurtler J., Sun W., Schwartz J.D., Dalal R.P., Joshi A., Hozak R.R., Xu Y., et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin. Cancer Res. 2013;19:6614–6623. doi: 10.1158/1078-0432.CCR-13-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Willett C.G., Duda D.G., di Tomaso E., Boucher Y., Ancukiewicz M., Sahani D.V., Lahdenranta J., Chung D.C., Fischman A.J., Lauwers G.Y., et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J. Clin. Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomas-Schoemann A., Blanchet B., Boudou-Rouquette P., Golmard J.L., Noe G., Chenevier-Gobeaux C., Lebbe C., Pages C., Durand J.P., Alexandre J., et al. Soluble VEGFR-1: A new biomarker of sorafenib-related hypertension (i.e., sorafenib-related is the compound adjective?) J. Clin. Pharmacol. 2015;55:478–479. doi: 10.1002/jcph.429. [DOI] [PubMed] [Google Scholar]
- 133.Duda D.G., Willett C.G., Ancukiewicz M., di Tomaso E., Shah M., Czito B.G., Bentley R., Poleski M., Lauwers G.Y., Carroll M., et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu A.X., Sahani D.V., Duda D.G., di Tomaso E., Ancukiewicz M., Catalano O.A., Sindhwani V., Blaszkowsky L.S., Yoon S.S., Lahdenranta J., et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kopetz S., Hoff P.M., Morris J.S., Wolff R.A., Eng C., Glover K.Y., Adinin R., Overman M.J., Valero V., Wen S., et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J. Clin. Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee J.E., Kim C., Yang H., Park I., Oh N., Hua S., Jeong H., An H.J., Kim S.C., Lee G.M., et al. Novel glycosylated VEGF decoy receptor fusion protein, VEGF-Grab, efficiently suppresses tumor angiogenesis and progression. Mol. Cancer Ther. 2015;14:470–479. doi: 10.1158/1535-7163.MCT-14-0968-T. [DOI] [PubMed] [Google Scholar]
- 137.Owen L.A., Uehara H., Cahoon J., Huang W., Simonis J., Ambati B.K. Morpholino-mediated increase in soluble Flt-1 expression results in decreased ocular and tumor neovascularization. PLoS ONE. 2012;7:e33576. doi: 10.1371/journal.pone.0033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salton M., Kasprzak W.K., Voss T., Shapiro B.A., Poulikakos P.I., Misteli T. Inhibition of vemurafenib-resistant melanoma by interference with pre-mRNA splicing. Nat. Commun. 2015;6:7103. doi: 10.1038/ncomms8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salton M., Misteli T. Small molecule modulators of pre-mRNA splicing in cancer therapy. Trends Mol. Med. 2016;22:28–37. doi: 10.1016/j.molmed.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee S.C., Dvinge H., Kim E., Cho H., Micol J.B., Chung Y.R., Durham B.H., Yoshimi A., Kim Y.J., Thomas M., et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat. Med. 2016;22:672–678. doi: 10.1038/nm.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]