1. Introduction
Meniere’s disease is a pathologic condition of the inner ear, clinically characterized by the presence of sudden bouts of vertigo, fluctuating hearing loss, tinnitus, and/or aural fullness.1 The concept of changes in the inner ear as the cause of those symptoms was first described by French physician Prosper Meniere, in 18612; however, that concept was considered controversial at the time. But further histopathologic studies in human temporal bones supported Prosper Meniere’s assumption: in 1938, Hallpike and Cairns3 and Yamakawa4 reported findings that were later considered hallmarks of the disease (dilation of the scala media of the cochlea, with displacement of Reissner’s membrane into the vestibular scala). Several other authors consistently reported similar findings in human temporal bones as well.5–8
Yet the idea that endolymphatic hydrops is the direct cause of the symptoms of Meniere’s disease has been questioned, and still is, because many temporal bones that showed unequivocal signs of hydrops were obtained from deceased donors who never experienced the characteristic symptoms when alive. Thus, today, endolymphatic hydrops is considered a histologic marker of the disease, rather than the causative agent.8,9
2. Histopathology and Pathophysiology
2.1. Anatomic considerations
The inner ear is composed of membranous structures (collectively known as the “membranous labyrinth”) encased by a bone shell (the “bony labyrinth”). The membranous structures are filled with a potassium-rich extracellular fluid called endolymph; the space between them and the bone shell contains a completely separate fluid called perilymph, which ionic composition is similar to extracellular fluid found elsewhere in the body.
The composition of endolymph is different from that of every other fluid in the human body. Endolymph has a very high concentration of potassium (K+), which creates an environment that allows a high electric potential in the scala media of the cochlea (+80 to +110 mV). The membranous labyrinth comprises the following structures: the scala media, scala vestibuli, and scala tympani of the cochlea; the ductus reuniens; the saccule; the utricle; the semicircular canals; the endolymphatic duct; and the endolymphatic sac. Those structures are divided into 2 separate compartments, connected by the ductus reuniens: the vestibular compartment (saccule, utricle, semicircular canals, and endolymphatic duct and sac) and the cochlear compartment (scala media, scala vestibuli, and scala tympani). The ductus reuniens is a tubular structure that runs from the early base of the scala media to the saccule.
In the cochlea, the scala media is filled with endolymph, whereas the scala tympani and the scala vestibuli contain perilymph. The utricle is connected, in its posterior area, with the semicircular canals. Two other tubular structures—1 coming from the saccule (the saccular duct) and the other from the utricle (the utricular duct)—join in a single fusiform membranous structure (the endolymphatic sinus) that lies in a groove on the posteriomedial surface of the vestibule. In the proximal opening of the utricular duct, the slit-shaped utriculoendolymphatic valve (commonly known as Bast’s valve, given its description by Theodore H. Bast in 1928) seems to control the inflow of endolymph from the utricle toward the endolymphatic duct and the endolymphatic sac. Bast’s valve seems to be passively activated in response to sudden decreases in the pressure of structures of the pars superior (utricle and semicircular canals), thereby preventing those structures from working properly.10
The inferior portion of the endolymphatic sinus connects to the endolymphatic duct, another membranous structure that runs inside of the petrous bone through a bony canal, the vestibular aqueduct. The endolymphatic duct ends in another membranous structure, the endolymphatic sac, which partially runs inside of the temporal bone and partially is an invagination of the dura mater, leaving the temporal bone in the level of the foveate fossa.
In the mid-20th century, the endolymphatic sac was studied with particular interest. The prevailing hypothesis then was that a decrease in the absorptive function of the sac could potentially cause endolymphatic hydrops.11 Intimately associated with layers of dura mater, the endolymphatic sac can be divided into 3 portions based on the cellular lining: (1) the proximal (rugose) portion, which lies within the vestibular aqueduct and is constituted by the same epithelia of the endolymphatic duct; (2) the intermediate portion, partly inside of the vestibular aqueduct and partly between layers of dura, which consists of cuboidal cells; and (3) the distal portion, which lies within layers of dura mater and is lined by cuboidal cells.12
2.2. Endolymph production and regulation
Endolymph is not only potassium-rich (150 to 180 mmol/L) but also nearly sodium-free— a unique composition seen only in the membranous labyrinth. Its composition is crucial in maintaining a constant, high endocochlear electric potential, which varies only slightly, from +80 to +110 mV.13,14 Evidence points toward the formation of endolymph from perilymph, rather than from plasma.15 The transepithelial K+ transportation from perilymph to endolymph through the sodium-potassium-adenosine triphosphate (Na+,K+-ATPase) ion pump is responsible for maintaining both the high endocochlear electric potential and the unique composition of endolymph.14
The stria vascularis is responsible for the secretion of endolymph, with small contributions from the planum semilunatum and from dark vestibular cells.16 The rate of endolymph secretion seems to be influenced by a number of factors and hormones, including aldosterone17 and vasopressin18; other substances such as cathecholamines, thyroid hormones, and somatostatin are also implicated in endolymph homeostasis.13 But the K+ in endolymph is absorbed by the sensory hair cells via apical transduction channels, from which it is transported back to perilymph.14
Endolymph flows within the membranous labyrinth thanks to 2 concurrent mechanisms: (1) radial (a rapid, ongoing process), which is important for energy metabolism and ion exchange around the sensory cell regions; and (2) longitudinal (slow) flow, which enables reabsorption of endolymph and disposal of high-molecular waste products and debris by the endolymphatic sac.16,19 Those 2 mechanisms occur simultaneously, in a continuous fashion.
2.3. Histopathology
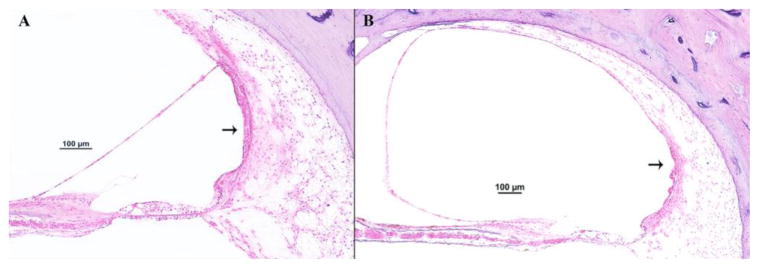
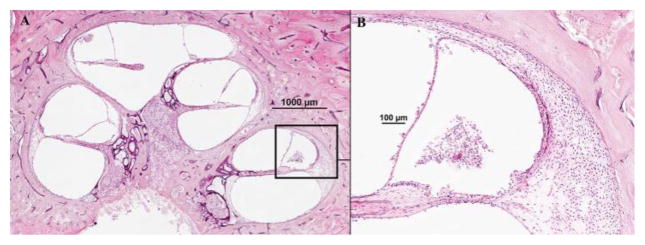
The first authors to describe (almost simultaneously, in 1938) the histopathologic findings that would be considered as markers of Meniere’s disease were Hallpike and Cairns3 and Yamakawa.4. Many authors, in all patients with the disease, consistently observed dilation of the scala media of the cochlea, with displacement of Reissner’s membrane into the vestibular scala (endolymphatic hydrops) (Figs. 1 and 2).6–9 Also a common, though less frequent, finding was saccular hydrops (Fig. 3); however, utricular hydrops was rarely observed.3
Fig. 1.
A representative human temporal bone section of a donor who had Meniere’s disease (hematoxylin and eosin): (A) Mid-modiolar section (4x); squared area = basal turn of the cochlea. (B) Basal turn of the cochlea (20x), showing distension of the Reissner’s membrane into the scala vestibuli.
Fig. 2.
A representative human temporal bone section of a donor without any ear diseases (hematoxylin and eosin): (A) Mid-modiolar section (4x); squared area = basal turn of the cochlea. (B) Basal turn of the cochlea (20x), without changes in any of the structures in the scala media
Fig. 3.
Two representative human temporal bone sections, showing the vestibular portion of the bony and membranous labyrinths, in a donor with Meniere’s disease (A) and a non-diseased bone (B) (hematoxylin and eosin, 2x). (A) 1 = saccule; 2 = utricle; 3 = lateral semicircular canal; 4 = posterior semicircular canal; 5 = footplate of the stapes (fractured); 6 = facial nerve; 7 = basal turn of the cochlea; 8 = internal auditory canal; and; 9 = vestibular aqueduct and endolymphatic duct; the arrows point to a severe saccular dilatation, bulging towards the footplate and the lateral semicircular canal. (B) 1 = saccule; 2 = utricle.
In severe cases of Meniere’s disease, Reissner’s membrane can bulge into the helicotrema, as well as through the saccule into the semicircular canals (especially the horizontal canal)7 and onto the footplate of the stapes (Fig. 3). Regarding the sensory elements of the inner ear, the reports in the literature conflict: the hair cells may or may not further degenerate in the organ of Corti and in the macula and crista ampullaris of the vestibular system (Fig. 4).20–23 However, Kariya et al.20 reported these findings: bilateral ischemia of the stria vascularis (Fig. 5); fibrous tissue proliferation within the vestibule; and focal loss of neurons, as well as degeneration of the dendrites, in the upper middle and apical turns of the cochlea (Fig. 6). Other findings with unclear significance have also been reported in temporal bones from donors who had Meniere’s disease, including blockage of the ductus reuniens16,20,28 (Figs. 7 and 8) and Cupulolithiasis16,28 (Fig. 9).
Fig. 4.
A 40x- magnification view of the organ of Corti of the temporal bones represented in figures 1(A) and 2 (B). (A) Organ of Corti in a bone from a donor who had Meniere’s disease; (1) tectorial membrane; (2) inner hair cell; (3) loss of the 3 rows of outer hair cells. (B) Organ of Corti from a non-diseased bone; (1) tectorial membrane; (2) inner hair cell; (3) outer hair cells.
Fig. 5.
Two representative human temporal bone sections showing the stria vascularis (arrow) in the basal turn of the cochlea (20x; hematoxylin and eosin). (A) Section of the middle cochlear scala in a non-diseased bone, demonstrating a normal stria vascularis; (B) Specimen from a donor with Meniere’s disease, showing atrophy of the stria vascularis.
Fig. 6.

A representative human temporal bone section from a donor who had Meniere’s disease (4x; hematoxylin and eosin), showing loss of the ganglion cells and nerves in the middle and apical turns of the cochlea (squared area),
Fig. 7.
A representative human temporal bone section from a donor who had Meniere’s disease (hematoxylin and eosin), highlighting an open ductus reuniens, under 2x–(A - squared area) and 40x–(B) magnifications.
Fig. 8.
A representative human temporal bone section from a donor who had Meniere’s disease (hematoxylin and eosin), showing a blocked ductus reuniens, under 2x (A – squared area) and 40x (B) magnifications.
Fig. 9.

A representative human temporal bone section showing the ampulla of the posterior semicircular canal, in a 77-year old female patient who had profound hearing loss, otosclerosis, and Meniere’s disease (10x; hematoxylin and eosin). The arrow points to a dense deposit on the cupula of the crista ampullaris, characterizing cupulolithiasis of the posterior semicircular canal.
In several studies in the 1970s and 1980s of temporal bones from deceased donors who had Meniere’s disease (as compared with nondiseased controls), the membranous labyrinth showed some marked changes, including perisaccular fibrosis,24 loss of epithelial integrity and atrophy of the endolymphatic sac,25 and narrowing or complete obstruction of the lumen in the endolymphatic duct (Figs. 10 and 11).26 More recently, anatomic changes have been extensively reported not only in temporal bone studies involving deceased donors who had Meniere’s disease, but also in imaging studies of living patients with Meniere’s disease and healthy volunteers: hypoplasia of the vestibular aqueduct (Fig. 10); hypodevelopment of Trautmann’s triangle; an altered relationship between the position of the posterior fossa dural plate and the position the endolymphatic sac; and aberrant (lateral) displacement of the lateral venous sinus.27–29
Fig. 10.

Two representative horizontal sections of human temporal bones, showing the vestibular aqueduct and endolymphatic duct (hematoxylin and eosin; 2x). A: Specimen from a non-diseased donor; squared area = normal-sized vestibular aqueduct. B: Specimen from a deceased donor who had Meniere’s disease; squared area = hypoplastic vestibular aqueduct.
Fig. 11.
A representative horizontal section of a left human temporal bone from a 78 year-old female donor, who had otosclerosis and Meniere’s disease; the patient had a profound hearing loss on the left side (hematoxylin and eosin). (A) otosclerotic foci involving the bony areas around the cochlea and vestibule (2x); squared area: blockage of the vestibular aqueduct by an otosclerotic foci. (B) squared area seen under 10x magnification.
Meniere’s disease usually affects only 1 of the patient’s ears, but bilateral disease is not rare; furthermore, the incidence of bilateral involvement tends to increase with the duration of the disease, or with the length of follow-up.30–33 The contralateral ears often show signs of cochlear degeneration, including severe loss of cochlear hair cells, significant damage of the stria vascularis, and loss of spiral ganglion cells; 20% of them also have histologic endolymphatic hydrops.20
In addition, histopathologic studies have demonstrated the intimate association of Meniere’s disease with several other pathologic conditions of the ear, including otosclerosis (Figs. 11 and 12), autoimmune diseases (Fig. 13), congenital anomalies, tumors (Fig. 14), otitis media (Fig. 14), syphilis, and head trauma.7,16,20 Some of those associations seem to be coincidental (tumors and congenital anomalies), but some may well cause endolymphatic hydrops (otosclerosis, autoimmune diseases, syphilis, and head trauma).
Fig. 12.
A representative human temporal bone section of a 77 year-old female donor who had profound hearing loss, otosclerosis, and Meniere’s disease (hematoxylin and eosin). (A) Severe otosclerosis involving the inner ear structures, causing distortion of the anatomy of the cochlea (squared area) (2x). (B) Severe distortion of the anatomy of the cochlea; signs of endolymphatic hydrops, represented by dilation of the Reissner’s membrane into the scala vestibuli in all cochlear turns (4x).
Fig. 13.
A representative human temporal bone section, collected from a 63 year-old female donor who had signs of immune-mediated disease of the inner ear, and labyrinthitis ossificans in the basal turn (hematoxylin eosin). (A) mid-modiolar section, containing degeneration of the organ of Corti, focal areas of fibrous proliferation and new bone formation, and endolymphatic hydrops ; squared area = basal turn of the cochlea (4x); (B) basal turn of the cochlea, showing the presence of inflammatory cells in the scala media and scala vestibuli, and bulging of the Reissner’s membrane (20x).
Fig. 14.
A representative human temporal bone section, collected from a 56 year-old male donor who had chronic otitis media, Meniere’s disease, and leukemic infiltration of the middle ear (hematoxylin and eosin; 2x). 1 = retraction pocket; 2 = fibrous tissue; 3 = serous effusion; 4 = tumoral cells; small arrows in the cochlea = signs of endolymphatic hydrops.
2.4. Causative mechanisms
Several theories exist regarding the mechanisms leading to endolymphatic hydrops. It seems clear that hydrops reflects the changes in the anatomy of the membranous labyrinth as a consequence of overaccumulation of endolymph.3 Thus, possible causes of hydrops include overproduction of endolymph and/or a decrease in the absorption of endolymph. Extensive investigation suggests that the causes are multifactorial, involving a number of pathogenic mechanisms.16
The role of the endolymphatic sac as the biologically active structure responsible for endolymph absorption is acknowledged and supported by many authors. Several ion homeostasis mechanisms have been identified in the sac, such as active Na+,K+-ATPase, aquaporins, and adrenocorticosteroid receptors.34,35
Further evidence of the role of the endolymphatic sac in endolymph absorption was provided by Kimura and Schuknecht,36 who successfully created endolymphatic hydrops in animals by surgically ablating the sac. However, ablation completely destroyed the sac and created significant surgical stress and trauma, so it is not considered a trustworthy method to cause the changes observed in Meniere’s disease. Other studies have involved induction of variable degrees of endolymphatic hydrops in animals by administering substances such as vasopressin, aldosterone, and cholera toxin (either to increase endolymph production or to block endolymph absorption).17,35,37. Yet those models of endolymphatic hydrops failed to induce the clinical symptoms of Meniere’s disease; moreover, the hydrops went into remission within a few days. For those mechanisms to lead to hydrops in Meniere’s disease, either the stimuli should be continuous or other factors should be acting concomitantly to prevent adequate absorption of endolymph.
Apart from the absorptive role of the endolymphatic sac, evidence in the literature points to its participation in regulating endolymph pressure. A study published in 2009 found that systemic administration of isoproterenol (a β-adrenergic agonist) increased endolymph pressure and decreased the potential size of the sac’s lumen. When the sac was surgically destroyed, both of those effects were suppressed. Thus, through agents such as catecholamines, the sac might regulate the hydrostatic pressure in the endolymphatic system.38
One of the proposed theories to explain endolymphatic hydrops is that longitudinal blockage—in 1 of the structures responsible for drainage (such as the endolymphatic duct or Bast’s valve)—acts as a dam, increasing retrograde volume and endolymph pressure. Support for this theory has come from studies of temporal bones from deceased donors who had Meniere’s disease, showing that their endolymph drainage system was smaller in size and volume and that their endolymphatic duct and sac were blocked.
The endolymphatic sinus has also been implicated as participating in endolymph regulation (Fig. 15). Given the distensible nature of its walls, associated with its position at the entrance of the endolymphatic duct, the sinus might act as a reservoir39; another hypothesis is that a distended sinus could block the entrance to the endolymphatic duct, by compressing Bast’s valve.
Fig. 15.
Two representative horizontal sections of human temporal bones, showing the endolymphatic sinus and the Bast’s valve in bones from two donors who had Meniere’s disease (hematoxylin and eosin; 10x). (A) Open Bast’s valve (arrow); (B) closed Bast’s valve.
Bast’s valve (Fig. 15) seems to function as a physiologic mechanism to prevent the pars superior from collapsing in case of a sudden decrease in its volume.40 However, both animal models and temporal bone studies have also demonstrated that the valve could open in response to increased pressure in the endolymphatic sac and duct, allowing the excess of endolymph to flow backward.10,40 If the valve opens in that way, progression of Meniere’s disease and further impairment of the absorptive mechanisms of the sac could prevent it from closing; the sensorial epithelia could then be more vulnerable to pressure changes, leading to vestibular symptoms.40 Those symptoms could affect patients with hydrops even in the absence of clear hearing loss, a condition described by Paparella as vestibular Meniere’s disease.7,16 Severely enlarged saccules can also dislocate the utricular walls toward Bast’s valve, causing it to appear blocked when the temporal bones are examined.40
In light of the aforementioned observations that several hormones regulate endolymph pressure, production, and absorption, many studies have focused on the role of those hormones and their importance in the pathology of Meniere’s disease.41,44 Some studies have found an increase in the number and activity of V2 vasopressin receptors (V2Rs) and of aquaporin-2 channels, concluding that such an increase causes acute vertigo attacks in patients with Meniere’s disease.35 Furthermore, Bartoli et al.45 observed the presence of volume receptors in the inner ear of guinea pigs, demonstrating that the inner ear can regulate the release of vasopressin in a mechanism that is completely independent of what physiologically occurs when volume receptors in the thorax detect hypovolemia. Nonetheless, clinical data have been inconclusive: some authors have reported an increase in vasopressin levels during the acute phase of Meniere’s disease,42,46,47 but others have not observed such an increase.48,49 In addition, other stress-related hormones, such as prolactin, have been studied, but with no unequivocal results.
Another controversial theory is that dysfunctional cochlear blood flow participates in the genesis of the symptoms. In mice, Takumida et al.50 reported collapse of the lumen of the endolymphatic sac and loss of balance secondary to intratympanic injection of epinephrine; they attributed those effects to a decrease in inner ear blood flow (mean reduction, 60%). In other studies of animals with endolymphatic hydrops, the same pattern of a decrease in cochlear blood flow has been reported.51,52 Andrews et al.53 demonstrated that increased blood viscosity can result in inner ear dysfunction, causing symptoms of hearing loss, tinnitus, and vertigo. But other studies did not report any differences when comparing hydropic ears with nondiseased controls.54,55
Several recent studies have genetically evaluated families with Meniere’s disease. Women are slightly more affected than men, accounting for 56% of the cases. Whites are the most affected ethnic group (83%); Meniere’s disease is rare in blacks.56 A genetic predisposition has been reported in 2.6% to 12% of patients with Meniere’s disease. Familial cases seem to involve an autosomal dominant inheritance with an incomplete penetrance (60%), plus evidence of the possibility of more severe clinical symptoms in offspring.57 Early investigations analyzed the possible association between human leukocyte antigens (HLAs)and susceptibility to Meniere’s disease, but conflicting evidence was found.58 However, in a specific population in Europe, López-Escámez et al.59 found a possible association between the HLA-DRB1*1101 allele and bilateral Meniere’s disease.
Chromosomal studies involving a Swedish family with several cases of Meniere’s disease demonstrated linkage with several markers on chromosome 12; further studies narrowed the locus to 12p12.3. The only known gene in that region encodes phosphatidylinositol 3-kinase class 2 gamma (PIK3C2G), whose activation was demonstrated to regenerate cells in the utricular macula of rats.60 Two other studies showed an association between Meniere’s disease and single-nucleotide polymorphisms. One found a variation in the heat shock protein HSP70-1, possibly involved in the cellular stress response61; the other, a variation in adducing (Gly460Trp), which was associated with changes in the metabolism of sodium and in the activity of Na+,K+-ATPase.62
Autoimmunity has also been implicated in the pathophysiology of Meniere’s disease, mostly because of the high incidence of autoimmune diseases associated with Meniere’s disease. Gazquez et al.63 found a higher prevalence (as compared with what is expected in the general population) of rheumatoid arthritis, systemic lupus erythematosus, and ankylosing spondylitis in patients with Meniere’s disease. Further supporting evidence includes these findings: the presence of alleles of the DRB1 gene of the major histocompatibility complex (MHC)59 and elevated levels of autoantibodies in patients with Meniere’s disease,64 as well as the experimental induction of hydrops, in a guinea pig model, by injecting antigens or monoclonal antibodies.65 Hornibrook et al.66 hypothesized 3 possible mechanisms through which the autoimmune response could lead to changes in the absorptive capacity of the endolymph drainage system: (1) direct damage, caused by autoantibodies, to the tissue cells; (2) deposition of antigen-antibody complexes, resulting in activation of the complement cascade and in tissue destruction; or (3) an inflammatory reaction mediated by sensitized T lymphocytes.
The possible linkage between allergies and the symptoms of Meniere’s disease has been extensively investigated; however, an unequivocal cause-effect relationship has never been found. Some authors have reported airborne or food allergies in patients with Meniere’s, with an incidence ranging from 40.3% to 59.2%.67 According to published research, the small vessels of the endolymphatic sac could hypothetically allow antigen entry, stimulating an immune allergic response that damages the sac’s filtering capability.67
3. Conclusion
The histopathologic and pathophysiologic changes observed in Meniere’s disease have been extensively documented. Yet to date, no explanation for the genesis of clinical symptoms has been universally accepted. Further molecular studies in human tissues might shed some light.
Footnotes
Financial disclosure:
This project was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD), grant number U24 DC011968; the International Hearing Foundation; the Starkey Hearing Foundation; and the 5M Lions International.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol-Head Neck Surg. 1995;113(3):181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RW. Prosper Ménière and his disease. Arch Neurol. 2001;58(7):1151–6. doi: 10.1001/archneur.58.7.1151. [DOI] [PubMed] [Google Scholar]
- 3.Hallpike CS, Cairns H. Observations on the Pathology of Ménière’s Syndrome. Proc R Soc Med. 1938;31(11):1317–36. doi: 10.1177/003591573803101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamakawa K. Temporal Bone Histopathology of Meniere’s Patient. Paper presented at the Annual Meeting Oto-Rhino-Laryngol Society; Japan. 1938. [Google Scholar]
- 5.Rauch SD, Merchant SN, Thedinger BA. Meniere’s syndrome and endolymphatic hydrops. Double-blind temporal bone study. Ann Otol Rhinol Laryngol. 1989;98(11):873–83. doi: 10.1177/000348948909801108. [DOI] [PubMed] [Google Scholar]
- 6.Salvinelli F, Greco F, Trivelli M, Linthicum FH. Ménière’s disease. Histopathological changes: a post mortem study on temporal bones. Eur Rev Med Pharmacol Sci. 1999;3(4):189–93. [PubMed] [Google Scholar]
- 7.Paparella MM. Pathogenesis of Meniere’s disease and Meniere’s syndrome. Acta Oto-Laryngol Suppl. 1984;406:10–25. doi: 10.3109/00016488309122996. [DOI] [PubMed] [Google Scholar]
- 8.Foster CA, Breeze RE. Endolymphatic hydrops in Ménière’s disease: cause, consequence, or epiphenomenon? Otol Neurotol. 2013;34(7):1210–4. doi: 10.1097/MAO.0b013e31829e83df. [DOI] [PubMed] [Google Scholar]
- 9.Merchant SN, Adams JC, Nadol JB. Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26(1):74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hofman R, Segenhout JM, Buytaert JAN, Dirckx JJJ, Wit HP. Morphology and function of Bast’s valve: additional insight in its functioning using 3D-reconstruction. Eur Arch Otorhinolaryngol. 2008;265(2):153–7. doi: 10.1007/s00405-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altmann F, Fowler E. Histological findings in Meniere’s symptom complex. Ann Otol Rhinol Laryngol. 1943;52:52–80. [Google Scholar]
- 12.Gibson WP, Arenberg IK. Pathophysiologic theories in the etiology of Meniere’s disease. Otolaryngol Clin North Am. 1997;30(6):961–7. [PubMed] [Google Scholar]
- 13.Harris JP. Meniere’s disease. Kugler Publications; The Hague, The Netherlands: 1999. [Google Scholar]
- 14.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165(1–2):1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 15.Konishi T, Hamrick PE. Ion transport in the cochlea of guinea pig. II. Chloride transport. Acta Otolaryngol (Stockh) 1978;86(3–4):176–84. doi: 10.3109/00016487809124734. [DOI] [PubMed] [Google Scholar]
- 16.Paparella MM. Pathogenesis and Pathophysiology of Meniere’s Disease. Acta Otolaryngol (Stockh) 1991;111(sup485):26–35. doi: 10.3109/00016489109128041. [DOI] [PubMed] [Google Scholar]
- 17.Takumida M, Akagi N, Anniko M. A new animal model for Ménière’s disease. Acta Otolaryngol (Stockh) 2008;128(3):263–71. doi: 10.1080/00016480701497436. [DOI] [PubMed] [Google Scholar]
- 18.Degerman E, In’t Zandt R, Pålbrink A-K, Magnusson M. Vasopressin induces endolymphatic hydrops in mouse inner ear, as evaluated with repeated 9. 4 T MRI. Hear Res. 2015;330(Pt A):119–24. doi: 10.1016/j.heares.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Lundquist PG. Aspects on endolymphatic sac morphology and function. Arch Otorhinolaryngol. 1976;212(4):231–40. doi: 10.1007/BF00453671. [DOI] [PubMed] [Google Scholar]
- 20.Kariya S, Cureoglu S, Fukushima H, et al. Histopathologic changes of contralateral human temporal bone in unilateral Ménière’s disease. Otol Neurotol. 2007;28(8):1063–8. doi: 10.1097/MAO.0b013e31815a8433. [DOI] [PubMed] [Google Scholar]
- 21.Nadol JB, Adams JC, Kim J-R. Degenerative Changes in the Organ of Corti and Lateral Cochlear Wall in Experimental Endolymphatic Hydrops and Human Meniere’s Disease. Acta Otolaryngol (Stockh) 1995;115(sup519):47–59. doi: 10.3109/00016489509121870. [DOI] [PubMed] [Google Scholar]
- 22.Schuknecht HF. Symposium: Meniere’s disease. Meniere’s disease: A correlation of symptomatology and pathology. The Laryngoscope. 1963;73(6):651–65. doi: 10.1288/00005537-196306000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Schuknecht HF. The pathophysiology of Meniere’s disease. Am J Otol. 1984;5(6):526–7. [PubMed] [Google Scholar]
- 24.Saito H, Kitahara M, Yazawa Y, Matsumoto M. Histopathologic findings in surgical specimens of endolymphatic sac in Meniere’s disease. Acta Otolaryngol (Stockh) 1977;83(5–6):465–9. doi: 10.3109/00016487709128872. [DOI] [PubMed] [Google Scholar]
- 25.Arenberg IK, Marovitz WF, Shambaugh GE. The role of the endolymphatic sac in the pathogenesis of endolymphatic hydrops in man. Acta Oto-Laryngol Suppl. 1970;275:1–49. [PubMed] [Google Scholar]
- 26.Ikeda M, Sando I. Endolymphatic duct and sac in patients with Meniere’s disease. A temporal bone histopathological study. Ann Otol Rhinol Laryngol. 1984;93(6 Pt 1):540–6. doi: 10.1177/000348948409300603. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Huang W, Meng X, Wang Z, Liu X, Chen Q. Comparison of noninvasive evaluation of endolymphatic hydrops in Meniere’s disease and endolymphatic space in healthy volunteers using magnetic resonance imaging. Acta Otolaryngol (Stockh) 2012;132(3):234–40. doi: 10.3109/00016489.2011.637232. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu S, Cureoglu S, Yoda S, Suzuki M, Paparella MM. Blockage of longitudinal flow in Meniere’s disease: A human temporal bone study. Acta Otolaryngol (Stockh) 2011;131(3):263–8. doi: 10.3109/00016489.2010.532155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebbar GK, Rask-Andersen H, Linthicum FH. Three-dimensional analysis of 61 human endolymphatic ducts and sacs in ears with and without Menière’s disease. Ann Otol Rhinol Laryngol. 1991;100(3):219–25. doi: 10.1177/000348949110000310. [DOI] [PubMed] [Google Scholar]
- 30.Balkany TJ, Sires B, Arenberg IK. Bilateral aspects of Meniere’s disease: an underestimated clinical entity. Otolaryngol Clin North Am. 1980;13(4):603–9. [PubMed] [Google Scholar]
- 31.Rosenberg S, Silverstein H, Flanzer J, Wanamaker H. Bilateral Menière’s disease in surgical versus nonsurgical patients. Am J Otol. 1991;12(5):336–40. [PubMed] [Google Scholar]
- 32.Palaskas CW, Dobie RA, Snyder JM. Progression of hearing loss in bilateral meniere’s disease. The Laryngoscope. 1988;98(3):287–90. doi: 10.1288/00005537-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 33.House JW, Doherty JK, Fisher LM, Derebery MJ, Berliner KI. Meniere’s disease: prevalence of contralateral ear involvement. Otol Neurotol. 2006;27(3):355–61. doi: 10.1097/00129492-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Wackym PA, Glasscock ME, Linthicum FH, Friberg U, Rask-Andersen H. Immunohistochemical localization of Na+, K+-ATPase in the human endolymphatic sac. Arch Otorhinolaryngol. 1988;245(4):221–3. doi: 10.1007/BF00463931. [DOI] [PubMed] [Google Scholar]
- 35.Kumagami H, Loewenheim H, Beitz E, et al. The effect of anti-diuretic hormone on the endolymphatic sac of the inner ear. Pflüg Arch Eur J Physiol. 1998;436(6):970–5. doi: 10.1007/s004240050731. [DOI] [PubMed] [Google Scholar]
- 36.Kimura RS, Schuknecht HF. Membranous Hydrops in the Inner Ear of the Guinea Pig after Obliteration of the Endolymphatic Sac. Pract Otorhinolaryngol (Basel) 1965;27:343–54. [Google Scholar]
- 37.Roheim PS, Brusilow SW. Effects of cholera toxin on cochlear endolymph production: model for endolymphatic hydrops. Proc Natl Acad Sci U S A. 1976;73(5):1761–4. doi: 10.1073/pnas.73.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inamoto R, Miyashita T, Akiyama K, Mori T, Mori N. Endolymphatic sac is involved in the regulation of hydrostatic pressure of cochlear endolymph. Am J Physiol. 2009;297(5):R1610–4. doi: 10.1152/ajpregu.00073.2009. [DOI] [PubMed] [Google Scholar]
- 39.Gibson WPR. Hypothetical mechanism for vertigo in Meniere’s disease. Otolaryngol Clin North Am. 2010;43(5):1019–27. doi: 10.1016/j.otc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Schuknecht HF, Belal AA. The utriculo-endolymphatic valve: its functional significance. J Laryngol Otol. 1975;89(10):985–96. doi: 10.1017/s0022215100081305. [DOI] [PubMed] [Google Scholar]
- 41.Aoki M, Ando K, Kuze B, Mizuta K, Hayashi T, Ito Y. The association of antidiuretic hormone levels with an attack of Meniere’s disease. Clin Otolaryngol. 2005;30(6):521–5. doi: 10.1111/j.1749-4486.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 42.Aoki M, Hayashi H, Kuze B, Mizuta K, Ito Y. The association of the plasma vasopressin level during attacks with a prognosis of Meniere’s disease. Int J Audiol. 2010;49(1):1–6. doi: 10.3109/14992020903160850. [DOI] [PubMed] [Google Scholar]
- 43.Ishiyama G, López IA, Ishiyama A. Aquaporins and Meniere’s disease. Curr Opin Otolaryngol Head Neck Surg. 2006;14(5):332–6. doi: 10.1097/01.moo.0000244191.51560.22. [DOI] [PubMed] [Google Scholar]
- 44.Katagiri Y, Takumida M, Hirakawa K, Anniko M. Long-term administration of vasopressin can cause Ménière’s disease in mice. Acta Otolaryngol (Stockh) 2014;134(10):990–1004. doi: 10.3109/00016489.2014.902989. [DOI] [PubMed] [Google Scholar]
- 45.Bartoli E, Satta A, Melis F, et al. Volume receptors in guinea pig labyrinth: relevance with respect to ADH and Na control. Am J Physiol. 1989;257(3 Pt 2):F341–6. doi: 10.1152/ajprenal.1989.257.3.F341. [DOI] [PubMed] [Google Scholar]
- 46.Aoki M, Ando K, Kuze B, Mizuta K, Hayashi T, Ito Y. The association of antidiuretic hormone levels with an attack of Meniere’s disease. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg. 2005;30(6):521–5. doi: 10.1111/j.1749-4486.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 47.Kitahara T, Doi K, Maekawa C, et al. Meniere’s attacks occur in the inner ear with excessive vasopressin type-2 receptors. J Neuroendocrinol. 2008;20(12):1295–300. doi: 10.1111/j.1365-2826.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 48.Hornibrook J, George P, Gourley J. Vasopressin in definite Meniere’s disease with positive electrocochleographic findings. Acta Otolaryngol (Stockh) 2011;131(6):613–7. doi: 10.3109/00016489.2010.541940. [DOI] [PubMed] [Google Scholar]
- 49.Lim JS, Lange ME, Megerian CA. Serum antidiuretic hormone levels in patients with unilateral Meniere’s disease. The Laryngoscope. 2003;113(8):1321–6. doi: 10.1097/00005537-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Takumida M, Akagi N, Anniko M. Effect of inner ear blood flow changes in Ménière’s model mice. Acta Otolaryngol (Stockh) 2009;129(3):244–53. doi: 10.1080/00016480802241980. [DOI] [PubMed] [Google Scholar]
- 51.Brechtelsbauer PB, Ren TY, Miller JM, Nuttall AL. Autoregulation of cochlear blood flow in the hydropic guinea pig. Hear Res. 1995;89(1–2):130–6. doi: 10.1016/0378-5955(95)00130-4. [DOI] [PubMed] [Google Scholar]
- 52.Miller JM, Ren TY, Laurikainen E, Golding-Wood D, Nuttall AL. Hydrops-induced changes in cochlear blood flow. Ann Otol Rhinol Laryngol. 1995;104(6):476–83. doi: 10.1177/000348949510400611. [DOI] [PubMed] [Google Scholar]
- 53.Andrews JC, Hoover LA, Lee RS, Honrubia V. Vertigo in the hyperviscosity syndrome. Otolaryngol-- Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1988;98(2):144–9. doi: 10.1177/019459988809800208. [DOI] [PubMed] [Google Scholar]
- 54.Larsen HC, Albers F, Jansson B, Angelborg C, Veldman J. Cochlear Blood Flow in Endolymphatic Hydrops. Acta Otolaryngol (Stockh) 1988;106(5–6):404–8. doi: 10.3109/00016488809122263. [DOI] [PubMed] [Google Scholar]
- 55.Muñoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol (Stockh) 2001;121(1):10–5. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 56.Chiarella G, Petrolo C, Cassandro E. The genetics of Ménière’s disease. Appl Clin Genet. 2015;8:9–17. doi: 10.2147/TACG.S59024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birgerson L, Gustavson KH, Stahle J. Familial Menière’s disease: a genetic investigation. Am J Otol. 1987;8(4):323–6. [PubMed] [Google Scholar]
- 58.López-Escámez JA, López-Nevot A, Cortes R, Ramal L, López-Nevot MA. Expression of A, B, C and DR antigens in definite Meniere’s disease in a Spanish population. Eur Arch Otorhinolaryngol. 2002;259(7):347–50. doi: 10.1007/s00405-002-0463-0. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Escamez JA, Vilchez JR, Soto-Varela A, et al. HLA-DRB1*1101 allele may be associated with bilateral Ménière’s disease in southern European population. Otol Neurotol. 2007;28(7):891–5. doi: 10.1097/MAO.0b013e3180dca1cc. [DOI] [PubMed] [Google Scholar]
- 60.Montcouquiol M, Corwin JT. Intracellular signals that control cell proliferation in mammalian balance epithelia: key roles for phosphatidylinositol-3 kinase, mammalian target of rapamycin, and S6 kinases in preference to calcium, protein kinase C, and mitogen-activated protein kinase. J Neurosci. 2001;21(2):570–80. doi: 10.1523/JNEUROSCI.21-02-00570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawaguchi S, Hagiwara A, Suzuki M. Polymorphic analysis of the heat-shock protein 70 gene (HSPA1A) in Ménière’s disease. Acta Otolaryngol (Stockh) 2008;128(11):1173–7. doi: 10.1080/00016480801901675. [DOI] [PubMed] [Google Scholar]
- 62.Teggi R, Lanzani C, Zagato L, et al. Gly460Trp alpha-adducin mutation as a possible mechanism leading to endolymphatic hydrops in Ménière’s syndrome. Otol Neurotol. 2008;29(6):824–8. doi: 10.1097/MAO.0b013e318180a4b1. [DOI] [PubMed] [Google Scholar]
- 63.Gazquez I, Soto-Varela A, Aran I, et al. High prevalence of systemic autoimmune diseases in patients with Menière’s disease. PloS One. 2011;6(10):e26759. doi: 10.1371/journal.pone.0026759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derebery MJ, Rao VS, Siglock TJ, Linthicum FH, Nelson RA. Menière’s disease: an immune complex-mediated illness? The Laryngoscope. 1991;101(3):225–9. doi: 10.1288/00005537-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Yoo TJ, Yazawa Y, Tomoda K, Floyd R. Type II collagen-induced autoimmune endolymphatic hydrops in guinea pig. Science. 1983;222(4619):65–7. doi: 10.1126/science.6623056. [DOI] [PubMed] [Google Scholar]
- 66.Hornibrook J, George P, Spellerberg M, Gourley J. HSP70 antibodies in 80 patients with “clinically certain” Meniere’s disease. Ann Otol Rhinol Laryngol. 2011;120(10):651–5. doi: 10.1177/000348941112001004. [DOI] [PubMed] [Google Scholar]
- 67.Derebery MJ, Berliner KI. Allergy and its relation to Meniere’s disease. Otolaryngol Clin North Am. 2010;43(5):1047–58. doi: 10.1016/j.otc.2010.05.004. [DOI] [PubMed] [Google Scholar]