Abstract
Orthostatic changes in systolic blood pressure (SBP) impact cardiovascular outcomes. In this study, we aimed to determine the pattern of orthostatic systolic pressure changes in participants enrolled in the SBP Intervention Trial (SPRINT) at their baseline visit before randomization and sought to understand clinical factors predictive of these changes. Of the 9323 participants enrolled in SPRINT, 8662 had complete data for these analyses. The SBP after 1 minute of standing was subtracted from the mean value of the three preceding seated SBP values. At the baseline visit, medical history, medications, anthropometric measures, and standard laboratory testing were undertaken. The mean age of SPRINT participants was 68 years, two-thirds were male, with 30% black, 11% Hispanic, and 55% Caucasian. The spectrum of SBP changes on standing demonstrated that increases in SBP were as common as declines, and about 5% of participants had an increase, and 5% had a decrease of >20 mm Hg in SBP upon standing. Female sex, taller height, more advanced kidney disease, current smoking, and several drug classes were associated with larger declines in BP upon standing, while black race, higher blood levels of glucose and sodium, and heavier weight were associated with more positive values of the change in BP upon standing. Our cross-sectional results show a significant spectrum of orthostatic SBP changes, reflecting known (eg, age) and less well-known (eg, kidney function) relationships that may be important considerations in determining the optimal target blood pressure in long-term outcomes of older hypertensive patients.
Keywords: Cross-sectional, epidemiology, human, orthostasis, SBP
Introduction
Assuming a standing position initiates a series of compensatory changes on the part of the circulation to defend blood flow to vital organs. Standing promotes the pooling of blood in the legs,1 and the adaptive circulation increases venous tone through sympathetic stimulation, coordinated in the brainstem, to limit this pooling.2 Baroreceptors in the neck detect the decrease in arterial stretch as the cardiac output declines, and in addition to stimulating venous tone, a variable increase in heart rate is also recruited.2 The clinical findings in normal humans subjected to this orthostatic stress in the laboratory consist of a small decline in systolic blood pressure (SBP), often a small increase in diastolic blood pressure (DBP), and a small increase in heart rate.1 Aging, comorbidities like diabetes and Parkinson’s disease and a variety of drugs are known to exaggerate these changes.2,3 In some cases, the compensatory mechanisms underrespond, or are absent, resulting in a sustained fall in SBP of more than 20 mm Hg, a sustained fall in DBP of 10 mm Hg, or a combination of these events, which are typical criteria to diagnoses orthostatic intolerance.4 Orthostatic changes in blood pressure (BP) are important predictors for future cardiovascular events (eg, stroke, heart attack, heart failure, and death) in addition to being important components of falls and their resultant complications like fracture and long-term immobility.5–8
A less well-appreciated finding is an increase in BP on standing.9 Clinicians are encouraged to check on orthostatic reductions in BP, particularly in older patients,10 and are generally relieved when the BP does not fall in the upright position in the clinical encounter. However, the impact of an increase in BP on standing may represent a different set of physiologic adaptations whose overcompensation could be a significant contributor to future cardiovascular events. Less is known about the prevalence of significant increases in BP on standing or the factors that likely contribute to this finding. Consequently, we evaluated the orthostatic changes in SBP in subjects enrolled in the SBP Intervention Trial (SPRINT) at the time of randomization in treated hypertensives to determine demographic, comorbid, pharmacologic, and laboratory-based factors that were associated with orthostatic changes. In addition, we evaluated the same population for strictly defined (categorical) orthostatic hypotension, and orthostatic hypertension, which incorporated DBP values. We then sought to determine characteristics associated with such categorical changes.
Methods
The rationale and design of the SPRINT have been published.11 SPRINT was designed to examine two different SBP goals in older hypertensive patients in the USA and Puerto Rico. SPRINT was undertaken as a multicenter, randomized, controlled trial that compared two strategies for treating BP: one targeting SBP to <140 mm Hg and the other targeting SBP to <120 mm Hg.
Study Participants
Eligibility required age ≥50 years (no upper limit), an average screening SBP of at least 130 mm Hg and no more than 150–180 mm Hg depending on the number of antihypertensive medications (0–4), and increased cardiovascular disease (CVD) risk evidenced by prevalent or subclinical CVD, chronic kidney disease (CKD), 10-year Framingham CVD risk ≥15%,12 or age ≥75 years. SPRINT focused recruitment on three subgroups: patients with nondiabetic CKD, those with prior CVD, and patients 75 years of age and older. CKD at baseline was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 at baseline based on the four-variable MDRD equation13 and staged according to National Kidney Foundation guidelines.14 Prevalent CVD at baseline included a history of myocardial infarction; acute coronary syndrome, ECG changes on graded exercise test, or positive cardiac imaging study; coronary revascularization; carotid endarterectomy or stenting; peripheral arterial disease with revascularization; 50% stenosis of a coronary, carotid, or lower extremity artery; abdominal aortic aneurysm >5 cm with or without repair; coronary artery calcium score >400 Agatston units; ankle-brachial index <0.90; and left ventricular hypertrophy by computer ECG reading, echocardiogram report, or other cardiac imaging procedure. Screened subjects with prior stroke, diabetes, proteinuria >1 gram/d, eGFR <20 mL/min/1.73 m2, symptomatic heart failure or ejection fraction <35%, or with diagnosed dementia or nonadherence were excluded from SPRINT.
Each participating SPRINT site received Institutional Review Board approval for the SPRINT protocol and obtained written informed consent from each participant. The data we report here are based on information obtained and BP/heart rate measurements performed at the visit at which randomization to the SBP targets occurred, before subjects were treated to their goal BP, and while they were still taking their original medication regimen. SPRINT was registered at ClinicalTrials.gov as NCT01206062.
Standard demographic information was collected from SPRINT participants by trained coordinators at SPRINT sites. A medical history captured information related to prior CVD, cerebrovascular disease, or impaired kidney function. The participant’s usual medications were also recorded.
BP Protocol
The BP and heart rate were measured three times in the seated position, using appropriate cuff size, after the participant had rested quietly for 5 minutes in a chair with back support, and with feet on floor. Readings were separated by 1-minute intervals using a programmed Professional Digital Blood Pressure Monitor (Omron Healthcare, Lake Forest, IL) model 907XL. After collection of seated pressures, the participant was asked to stand with their arm supported and with a single measurement of SBP, DBP, and HR taken 1 minute after their feet touched the ground. While standing, participants were asked about symptoms of hypotension. The SPRINT protocol excluded people with a standing SBP <110 mm Hg during screening.
For each participant, we calculated the change in SBP upon standing (⊿SBP) by subtracting the average seated SBP from the single measurement of standing SBP. A negative value of ⊿SBP indicates that the SBP measured 1 minute after standing was lower than the average seated SBP (standing SBP < seated SBP), while a positive value indicates the standing pressure was higher than the average seated SBP (standing SBP > seated SBP). The change in DBP (⊿DBP) upon standing was calculated in a similar fashion.
We also classified participants into three mutually exclusive categories of BP change. Participants with ⊿SBP less than or equal to −20 mm Hg or ⊿DBP less than or equal to −10 mm Hg were classified as having orthostatic hypotension. Participants without orthostatic hypotension with ⊿SBP greater than or equal to +20 mm Hg or with ⊿DBP greater than or equal to +10 mm Hg were classified as having orthostatic hypertension. Participants with ⊿SBP between −20 mm Hg and +20 mm Hg, exclusive, and ⊿DBP between −10 mm Hg and +10 mm Hg, exclusive, were classified as having neither orthostatic hypotension nor orthostatic hypertension.
Other Assessments
Information on age, gender, race, ethnicity, and a detailed history of CVD was collected during screening. At the baseline visit, assessments included a fasting blood draw, urine collection, height, weight, BPs, a 12-lead electrocardiogram, and questionnaires assessing sociodemographics, medical history, concomitant medications, tobacco use, alcohol intake, education level, living with others, medication adherence, cognitive function, and quality of life. The Veterans Rand 12-item questionnaire,15 including a question on feeling faint, dizzy, or lightheaded within the past 3 months, was also administered during the baseline visit.
Statistical Methods
Categorical data are reported as N (%) and continuous data as mean (standard deviation) unless otherwise noted (Table 1). We used simple and multiple linear regression analysis to examine relationships between ⊿SBP and demographic factors as well as other variables considered to be informative regarding changes in BPs upon standing (a listing of covariates is presented in Table 2). Physical characteristics (age, BP, laboratory measures, smoking, drinking, and medical conditions) were assessed at the SPRINT baseline visit and medications reflected use prior to randomization. We restricted the analysis to a cohort of 8662 participants with complete data for BP and all covariates. A total of 457 participants were excluded for missing laboratories, 88 were excluded for missing data on medical conditions or medications; 63 were excluded for missing data on body mass, drinking, or smoking; and 53 were excluded for missing data on standing BPs or symptoms of dizziness. Multiple regression models were fit using a backward selection algorithm with the criteria for stepping out of the model set at P > .05. All variables listed in Table 2 were included in the starting model. Categorical variables with more than two categories (eg, eGFR categories) were coded using grouped indicator variables that were retained or stepped out of the model together. Standard regression diagnostics were used, and no important violations of model assumptions were noted. We define significance based on two-sided P-values <.05.
Table 1.
SPRINT cohort with no missing data (n = 8662)
| Characteristic | Baseline Value |
|---|---|
| Age and sex mean (SD) or N (%) | |
| Mean age (all) | 67.9 (9.4) |
| Age <75 | 6221 (71.8) |
| Age 75–79 | 1367 (15.8) |
| Age ≥80 | 1074 (12.4) |
| Women | 3070 (35.4) |
| Race/ethnicity, N (%) | |
| White | 4944 (57.1) |
| Black | 2629 (30.4) |
| Hispanic | 930 (10.7) |
| Other ethnicity | 159 (1.8) |
| Clinical center networks, N (%) | |
| Ohio CCN | 1449 (16.7) |
| SE CCN | 1900 (21.9) |
| Utah CCN | 1925 (22.2) |
| UAB CCN | 1858 (21.5) |
| VAMC CCN | 1530 (17.7) |
| Biochemistry N (%) or mean (SD) | |
| eGFR >60 | 6190 (71.5) |
| eGFR 46–60 | 1639 (18.9) |
| eGFR ≤45 | 833 (9.6) |
| ACR <30 | 6991 (80.7) |
| Microalbuminuria | 1436 (16.6) |
| Macroalbuminuria | 235 (2.7) |
| BUN mg/dL | 18.8 (6.7) |
| Glucose mg/dL | 98.9 (13.6) |
| Sodium mmol/L | 140.2 (2.4) |
| Potassium mmol/L | 4.2 (0.4) |
| Anthropometrics mean (SD) | |
| Height, inches | 66.9 (4) |
| Weight, lbs | 190.7 (41.6) |
| BMI, kg/m2 | 29.9 (5.8) |
| Medical history, N (%) | |
| Alcohol user | 5617 (64.8) |
| Never smoker | 3800 (43.9) |
| Former smoker | 3693 (42.6) |
| Current smoker | 1169 (13.5) |
| Prevalent cardiovascular disease | 1765 (20.4) |
| Heart failure | 306 (3.5) |
| Peripheral arterial disease | 471 (5.4) |
| Atrial fibrillation | 684 (7.9) |
| Dizzy during examination | 368 (4.2) |
| Dizzy since last visit | 1008 (11.6) |
| Blood pressure and heart rate mean (SD) | |
| Seated SBP, mm Hg | 139.7 (15.6) |
| Seated DBP, mm Hg | 78.2 (12) |
| Seated HR, beats/min | 66.3 (11.5) |
| Standing SBP, mm Hg | 140.3 (17.9) |
| Standing DBP, mm Hg | 81.8 (12.9) |
| Standing HR, beats/min | 71.5 (12.9) |
| Medication usage mean (SD) or N (%) | |
| Number of BP med classes | 1.8 (1) |
| Beta blocker | 2696 (31.1) |
| Calcium channel blocker | 3032 (35) |
| Ace-I | 3188 (36.8) |
| ARB | 1830 (21.1) |
| Diuretic | 3863 (44.6) |
| Direct vasodilator | 130 (1.5) |
| Centrally acting | 192 (2.2) |
| Alpha blocker | 385 (4.4) |
| Alpha-beta blocker | 387 (4.5) |
| Nitrates | 280 (3.2) |
| Narcotics | 757 (8.7) |
| Phosphodiesterase-5 inhibitors | 553 (6.4) |
| Antidepressant | 1120 (12.9) |
ACR, albumin to creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CCN, clinical center network; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; SBP, systolic blood pressure; SD, standard deviation; SE, Southeast; SPRINT, SBP Intervention Trial; UAB, University of Alabama at Birmingham; VAMC, Veterans Administration Medical Center.
Table 2.
Univariate relationship between SPRINT participant characteristics and change in SBP upon standing
| Characteristic | SBP Change (mm Hg) |
|
|---|---|---|
| Beta | P Value | |
| Age and sex | ||
| Age, y | −0.0614 | <.0001 |
| Age categories | ||
| Age <75 | REF | |
| Age 75–79 | −1.4482 | <.0001 |
| Age ≥80 | −1.0959 | .0047 |
| Women | −0.5349 | .0427 |
| Race/ethnicity | ||
| White | REF | |
| Black | 1.9642 | <.0001 |
| Hispanic | 0.0774 | .85 |
| Other ethnicity | −0.9187 | .33 |
| Biochemistry | ||
| eGFR categories | ||
| eGFR >60 | REF | |
| eGFR 46–60 | −1.6158 | <.0001 |
| eGFR ≤45 | −3.6069 | <.0001 |
| ACR categories | ||
| ACR <30 | REF | |
| Microalbuminuria | −0.442 | .19 |
| Macroalbuminuria | −1.3625 | .08 |
| BUN, mg/dL | −0.1088 | <.0001 |
| Glucose, mg/dL | 0.0196 | .0343 |
| Sodium, mmol/L | 0.1269 | .0148 |
| Potassium, mmol/L | −0.3463 | .22 |
| Anthropometrics | ||
| Height, inches | 0.0005 | .99 |
| Weight, lbs | 0.0355 | <.0001 |
| BMI, kg/m2 | 0.2879 | <.0001 |
| Medical history | ||
| Prevalent cardiovascular disease | −0.7796 | .0129 |
| Alcohol user | 0.466 | .07 |
| Smoking history | ||
| Never smoker | REF | |
| Former smoker | 0.352 | .19 |
| Current smoker | −1.0221 | .0093 |
| Heart failure | −1.0239 | .13 |
| Peripheral arterial disease | −1.5809 | .0045 |
| Atrial fibrillation | −1.2779 | .0063 |
| Blood pressure and heart rate | ||
| Seated SBP, mm Hg | −0.1241 | <.0001 |
| Seated DBP, mm Hg | −0.0460 | <.0001 |
| Seated HR, beats/min | 0.0152 | .17 |
| Standing SBP, mm Hg | 0.3351 | <.0001 |
| Standing DBP, mm Hg | 0.2452 | <.0001 |
| Standing HR, beats/min | −0.0060 | .54 |
| Medication usage | ||
| # BP med classes used | −0.4175 | .0007 |
| Beta blocker | −0.7532 | .0057 |
| Using calcium channel blocker | −1.0285 | .0001 |
| Using Ace-I | −0.0092 | .97 |
| Using ARB | −0.0202 | .95 |
| Using diuretic | 0.4488 | .08 |
| Using direct vasodilator | 0.8655 | .4 |
| Using centrally acting | −1.0391 | .23 |
| Using alpha blocker | −0.8419 | .17 |
| Using alpha-beta blocker | −2.3203 | .0001 |
| Using nitrates | −1.187 | .1 |
| Using narcotics | −0.9237 | .0388 |
| Using phosphodiesterase-5 inhibitors | 1.7514 | .0007 |
| Using antidepressant | −1.5099 | <.0001 |
ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCN, clinical center network; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SPRINT, SBP Intervention Trial.
We also examined univariable associations between baseline characteristics and orthostatic hypotension and orthostatic hypertension using general linear models for continuous characteristics and chi-square tests for categorical characteristics. For the general linear models, we used estimate statements weighted by sample counts to compare participants with and without orthostatic hypotension and to compare participants with and without orthostatic hypertension. Similar comparisons were performed for categorical characteristics.
Results
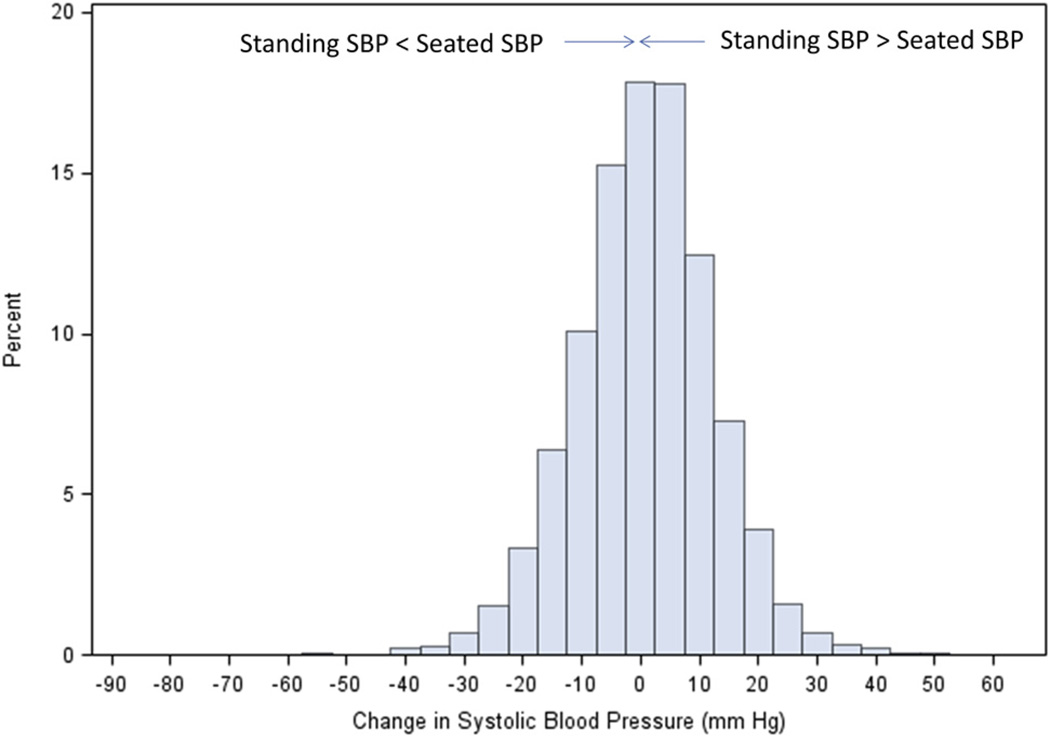
Table 1 portrays the main demographic findings of the 8662 subjects with complete data and other variables of interest. The mean age of SPRINT participants was 68 years, about 2/3 were men, and most were white (57%) or black race (30%). Figure 1 shows the distribution of the standing minus the mean seated SBP. The double arrow shows the point where no change in BP occurred.
Figure 1.
Shown is the distribution of changes in seated-to-standing SBP in 8662 SPRINT participants at their baseline visit determined by subtracting the seated from the standing SBP values. Negative numbers indicate a lower SBP on standing compared with sitting. SBP, systolic blood pressure; SPRINT, SBP Intervention Trial.
Of the 8662 participants, 4.7% had a SBP drop of 20 mm Hg or more (4.0% had a drop of more than 20 mm Hg) and 4.6% had an SBP increase of 20 mm Hg or more upon standing. The changes in SBP ranged from −88 mm Hg to +42 mm Hg as shown in Figure 1.
Changes in SBP on Standing
Table 2 shows the univariate relationship between each exposure variable and the outcome of ⊿SBP. For continuous exposure variables (eg, age), the beta coefficients represent slopes. For example, the relationship between change in SBP and age has a slope of −0.0614 mm Hg systolic/y of age. Because we defined the outcome variable as the standing SBP value minus the seated value, a negative change implies standing SBP < seated SBP. The negative beta for age versus SBP change implies a lower standing pressure for a given seated pressure in older participants compared to younger ones. For categorical data, the beta coefficients represent a difference in means between the specified category and the reference. For example, the beta for SBP change in African-Americans is 2.0 mm Hg, indicating that the expected standing pressure of African Americans is 2.0 mm Hg greater than that for whites (the reference group) at the same level of seated SBP.
Greater reductions in SBP on standing were associated with older age, female sex, progressively worse National Kidney Foundation eGFR stage, current smoking, a history of heart failure, atrial fibrillation, or peripheral arterial disease, and several antihypertensive drug classes including beta blockers, calcium channel blockers, and combined alpha-beta blockers.
Smaller reductions in SBP on standing were associated with black race, higher body weight, and body mass index (BMI), increasing glucose, and usage of phosphodiesterase-5 inhibitors.
Using multivariable regression analysis (data not shown), we observed that age >74 years, female sex, taller height, progressively worse eGFR stage, current smoking, and several drug classes including beta blockers, calcium channel blockers, and combined alpha-beta blockers were independently associated with greater reductions in SBP on standing. Black race, higher blood levels of sodium and potassium, and increasing weight were independently associated with lesser reductions in standing SBP.
Orthostatic Hypotension or Hypertension
We also determined the prevalence of categorical orthostatic hypotension (decline in SBP of 20 mm Hg or more or a decline in DBP of 10 mm Hg or more) and orthostatic hypertension (increase in SBP of 20 mm Hg or more or an increase in DBP of 10 mm Hg or more) in the SPRINT participants as shown in Table 3. Among the 8662 participants, 634 (7%) had a drop in standing BP that met criteria for orthostatic hypotension. Of the participants classified as having orthostatic hypotension, 294 met the SBP criteria only, 227 met the DBP criteria only, and 113 met both the SBP and DBP criteria. In contrast, 1819 (21%) had an increase in standing BP that met criteria for orthostatic hypertension, including 132 that met the SBP criteria only, 1418 that met the DBP criteria only, and 269 that met both SBP and DBP criteria. Orthostatic hypotension was more frequent in older participants, women, whites, and those with CKD (eGFR < 60 mL/min/1.73 m2), albuminuria, higher BUN, lower BMI, and higher seated BP. Orthostatic hypotension was also more frequent in participants with a history of PAD, atrial fibrillation, or heart failure; symptoms of dizziness since the last visit; and treatment with sympathoadrenergic (alpha2-agonist, alpha/beta blocker, beta blocker) or antidepressant drugs. In contrast, orthostatic hypertension was again more frequent in women and participants of black race, higher BMI and lower seated BP, but was not significantly associated with age, albuminuria, BUN, CVD history, or symptoms of dizziness. Modest but significant associations also were found between orthostatic hypertension and the absence of CKD (eGFR > 60 mL/min/1.73 m2) and nonuse of alpha blockers and antidepressants.
Table 3.
Baseline characteristics by orthostatic category
| Characteristic | Participants With | P Value | ||||
|---|---|---|---|---|---|---|
| (A) Orthostatic Hypotension, N = 634 |
(B) Neither, N = 6209 |
(C) Orthostatic Hypertension, N = 1819 |
A vs. (B or C) | (A or B) vs. C | ||
| Age and sex mean (SD) or N (%) | ||||||
| Mean age, y | 69.6 (9.5) | 67.7 (9.4) | 67.8 (9.4) | <.001 | .83 | |
| Age categories | ||||||
| Age <75 | 407 (64.2) | 4506 (72.6) | 1308 (71.9) | REF | REF | |
| Age 75–79 | 128 (20.2) | 942 (15.2) | 297 (16.3) | <.001 | .36 | |
| Age ≥80 | 99 (15.6) | 761 (12.3) | 214 (11.8) | <.001 | .68 | |
| Women | 254 (40.1) | 2070 (33.3) | 746 (41) | .012 | <.001 | |
| Race/ethnicity, N (%) | ||||||
| White | 404 (63.7) | 3630 (58.5) | 910 (50) | REF | REF | |
| Black | 143 (22.6) | 1780 (28.7) | 706 (38.8) | .017 | <.001 | |
| Hispanic | 77 (12.1) | 681 (11) | 172 (9.5) | .12 | .11 | |
| Other ethnicity | 10 (1.6) | 118 (1.9) | 31 (1.7) | .67 | .55 | |
| Biochemistry N (%) or mean (SD) | ||||||
| eGFR categories | ||||||
| eGFR >60 | 380 (59.9) | 4459 (71.8) | 1351 (74.3) | REF | REF | |
| eGFR 46–60 | 149 (23.5) | 1169 (18.8) | 321 (17.6) | .78 | .57 | |
| eGFR ≤45 | 105 (16.6) | 581 (9.4) | 147 (8.1) | <.001 | .041 | |
| ACR categories | ||||||
| ACR <30 | 477 (75.2) | 5028 (81) | 1486 (81.7) | REF | REF | |
| Microalbuminuria | 129 (20.3) | 1019 (16.4) | 288 (15.8) | .95 | .78 | |
| Macroalbuminuria | 28 (4.4) | 162 (2.6) | 45 (2.5) | .026 | .88 | |
| Mean BUN mg/dL | 20.4 (8.5) | 18.7 (6.6) | 18.6 (6.4) | <.001 | .24 | |
| Mean glucose mg/dL | 97.9 (12.2) | 99 (13.8) | 98.7 (13.4) | .071 | .57 | |
| Mean sodium mmol/L |
140.2 (2.5) | 140.1 (2.4) | 140.2 (2.4) | .92 | .15 | |
| Mean potassium mmol/L |
4.24 (0.48) | 4.2 (0.44) | 4.19 (0.43) | .016 | .21 | |
| Anthropometrics | ||||||
| Mean height, inches | 66.9 (4) | 67 (4) | 66.6 (4.1) | .86 | <.001 | |
| Mean weight, lbs | 185.9 (38.8) | 190.7 (40.8) | 192.3 (45.1) | .003 | .06 | |
| Mean BMI, kg/m2 | 29.2 (5.6) | 29.8 (5.6) | 30.4 (6.5) | .002 | <.001 | |
| Medical history, N (%) | ||||||
| Alcohol user | 381 (60.1) | 4055 (65.3) | 1181 (64.9) | .019 | .84 | |
| Smoking history | ||||||
| Never smoker | 265 (41.8) | 2718 (43.8) | 817 (44.9) | REF | REF | |
| Former smoker | 288 (45.4) | 2632 (42.4) | 773 (42.5) | .59 | .66 | |
| Current smoker | 81 (12.8) | 859 (13.8) | 229 (12.6) | .17 | .20 | |
| Prevalent | 145 (22.9) | 1261 (20.3) | 359 (19.7) | .11 | .45 | |
| cardiovascular | ||||||
| disease | ||||||
| Heart failure | 42 (6.6) | 202 (3.3) | 62 (3.4) | <.001 | .75 | |
| Peripheral arterial disease |
57 (9) | 317 (5.1) | 97 (5.3) | <.001 | .82 | |
| Atrial fibrillation | 74 (11.7) | 476 (7.7) | 134 (7.4) | <.001 | .35 | |
| Dizzy during examination |
32 (5) | 245 (3.9) | 91 (5) | .30 | .073 | |
| Dizzy since last visit | 96 (15.1) | 704 (11.3) | 208 (11.4) | .004 | .76 | |
| Blood pressure and heart rate mean (SD) | ||||||
| Seated SBP, mm Hg | 145.2 (16.2) | 139.6 (15.3) | 138.2 (16.1) | <.001 | <.001 | |
| Seated DBP, mm Hg | 80.2 (12.2) | 78.7 (11.9) | 75.7 (12) | <.001 | <.001 | |
| Seated HR, beats/min | 66.2 (12.9) | 66.4 (11.5) | 65.8 (11.3) | .88 | .074 | |
| Standing SBP, mm Hg | 126 (18) | 139.2 (16.6) | 148.8 (18.3) | <.001 | <.001 | |
| Standing DBP, mm Hg | 71.9 (12.8) | 80.6 (12.1) | 89.1 (12.2) | <.001 | <.001 | |
| Mean standing HR, beats/min |
71.5 (14.7) | 71.2 (12.8) | 72.5 (12.6) | .96 | <.001 | |
| Mean BP med classes | 1.9 (1) | 1.8 (1) | 1.8 (1) | .012 | .29 | |
| Medication usage, N (%) | ||||||
| Beta blocker | 231 (36.4) | 1886 (30.4) | 579 (31.8) | .003 | .46 | |
| Calcium channel blocker |
237 (37.4) | 2185 (35.2) | 610 (33.5) | .19 | .14 | |
| Ace-I | 226 (35.6) | 2316 (37.3) | 646 (35.5) | .53 | .20 | |
| ARB | 153 (24.1) | 1295 (20.9) | 382 (21) | .054 | .88 | |
| Diuretic | 260 (41) | 2773 (44.7) | 830 (45.6) | .059 | .32 | |
| Direct vasodilator | 9 (1.4) | 90 (1.4) | 31 (1.7) | .86 | .42 | |
| Centrally acting | 24 (3.8) | 112 (1.8) | 56 (3.1) | .005 | .005 | |
| Alpha blocker | 26 (4.1) | 303 (4.9) | 56 (3.1) | .66 | .001 | |
| Alpha-beta blocker | 46 (7.3) | 273 (4.4) | 68 (3.7) | <.001 | .09 | |
| Nitrates | 25 (3.9) | 203 (3.3) | 52 (2.9) | .29 | .31 | |
| Narcotics | 69 (10.9) | 537 (8.6) | 151 (8.3) | .047 | .46 | |
| Phosphodiesterase-5 inhibitors |
25 (3.9) | 406 (6.5) | 122 (6.7) | .009 | .53 | |
| Antidepressant | 98 (15.5) | 809 (13) | 213 (11.7) | .049 | .081 | |
BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SD, standard deviation.
Data in columns are generally the number of participants and the column-based percentage. For example, in the orthostatic hypotension column, 407 of the 634 participants (64.2%) were less than age 75 years. For categorical variables with more than 2 categories (eg, eGFR), we present a single P-value corresponding to a test of any difference among the categories.
Discussion
Our data show several important findings in the SPRINT participants. First is the remarkable spectrum of SBP change comparing the seated with the standing values in a large, mostly (~90%) treated, hypertensive population (Figure 1). Second is the proportion of seemingly stable outpatients who have a decline of more than 20 mm Hg systolic pressure (4.7%) on standing. Third is the presence of similarities, and a few differences, when changes in SBP are modeled as a continuous variable, or considered in the categories of “ orthostatic hypotension” and “ orthostatic hypertension” where DBP is also used. We observed that a decline in SBP on standing, and the presence of orthostatic hypotension, was more commonly seen in women, those with lower baseline kidney function, and among those taking sympathoadrenergic blocking medications known to contribute to BP falls on standing. On the other hand, we observed that less reduction in SBP on standing, and even orthostatic hypertension, was more commonly noted in black race participants and higher body weight or BMI. Finally, we observed that 4.6% of patients had an increase of 20 mm Hg or more in SBP on standing. Our observations regarding orthostatic declines of SBP by more than 20 mm Hg are particularly noteworthy as SPRINT excluded potential participants with diabetes or whose standing SBP fell to <110 mm Hg.
The observed distribution of ⊿SBP (Figure 1) is broad and relatively symmetrical. This shape is generally consistent with that reported for other large cohorts, including the Atherosclerosis Risk in Communities study,16 the Hypertension Detection and Follow-up Program,17 and the Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial.18 Small differences in the proportion of participants with a decrease in SBP upon standing among the cohorts are likely explained by differences in measurement protocols or selection criteria. In this regard, the exclusion of participants with a standing BP less than 110 mm Hg at screening in SPRINT does not appear to have dramatically shifted the distribution of ⊿SBP observed at the baseline visit relative to these other cohorts.
Standing upright relocates as much as 0.8 L of blood to the legs and splanchnic vasculature.1,4 This reduction in volume is mirrored by a reduced venous return to the heart and a decline in BP.19 In health, the baroreceptors in the aortic arch and carotid bifurcation are activated by these changes and shortly restore BP to/toward baseline values.19 Failure of these systems to compensate for blood relocation upon standing results in a longer and larger drop in the SBP in particular, referred to as orthostatic hypotension, and typically defined by a standing SBP that is at least 20 mm Hg lower than the seated or supine value, within 3 minutes of standing.4
Orthostatic hypotension is more common with age due to age-related decreases in baroreceptor function.20 It is also more common in diabetes and autonomic neuropathies which directly impair the effector arm of the regulatory systems.2 When orthostatic hypotension is present, it predicts a higher likelihood of stroke, heart attack, heart failure, and death.7,8 Orthostatic hypotension is also an important component of falls and their resultant complications like fracture and long-term immobility.5–8 In addition to the commonly recognized risks for orthostatic hypotension, our observation suggests that women tend to have a larger decline in standing SBP compared with men after having the same seated BP. We also observed that reductions in renal function when categorized by the National Kidney Foundation eGFR staging were also associated with greater declines in SBP. Although orthostatic hypotension was found to predict incident CKD in African-Americans in the Atherosclerosis Risk in Communities Study, there is little known about the prevalence of orthostatic changes in SBP in patients with CKD and our observations are novel in this regard and robust given the number of participants (n = 2472) with an eGFR less than 60 mL/min/1.73 m2 in SPRINT. Finally, we observed that antidepressants and narcotics were associated with standing BP reductions. Mechanisms by which antidepressants promote an orthostatic decline in BP include negative inotropism partly through inhibition of cardiac calcium currents.21 Narcotics may reduce BP by direct effects on the vessel22 or through mediating histamine release.23
A recent review of orthostatic hypertension notes that few studies have sought to characterize the prevalence of this disorder and also noted heterogeneity in the definition with increases of 5, 10, and 20 mm Hg in SBP on standing used as criteria.24 Unlike orthostatic hypotension, there is no agreed upon threshold of SBP increase on standing to define orthostatic hypertension, as there is for orthostatic hypertension.
Given the paucity of studies of orthostatic hypertension, there is little known that predicts an increase in SBP on standing upright. Our observation that participants of black race and those with higher body weight were associated with lesser reductions in standing SBP and also show greater likelihood of orthostatic hypertension by SBP or DBP criteria and represent reasonably novel observations in this understudied area of clinical hypertension.
Limited longitudinal data about orthostatic hypertension suggests a predisposition to peripheral arterial disease and stroke, particularly in those not treated for hypertension.25 In the Atherosclerosis Risk in Communities Study, there was a U-shaped association between the change in SBP on standing and stroke risk. In particular, those who had an increase in systolic pressure of more than 10 mm Hg on standing had an increased hazard for lacunar stroke.26
Overall, we observed a different set of factors associated with undercompensation compared with overcompensation in SBP when treated hypertensive subjects assume the standing position. Our observations support current recommendations about measuring BP in the standing position in hypertensive patients, particularly the elderly.10 The importance of these changes in SBP on standing in the SPRINT participants may be of interest when interpreting outcomes of this important clinical trial.
Limitations
Several limitations in our data need to be pointed out when considering our observations. First, we assessed standing pressure only once and only after 1 minute of standing. Second, SPRINT was not designed to pursue mechanisms of orthostatic BP regulation. Our data were obtained in a clinic scenario, not a controlled research setting with tilt table capability, and our findings are not directly generalizable to such studies. Finally, our findings are cross-sectional and supplemental to the longitudinal outcome data in SPRINT.
Conclusions
The large sample size, older age, and presence of comorbidities in SPRINT guarantees a substantial incidence of important health outcomes in this important trial. Our cross-sectional results show a significant spectrum of orthostatic SBP changes, reflecting known (eg, age) and less well-known (sex, race, electrolyte levels, kidney function) relationships which may be important considerations in determining the optimal target BP in long-term outcomes of older hypertensives.
Acknowledgments
The Systolic Blood Pressure Intervention Trial was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list:
SPRINT Acknowledgment: The authors also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134 and UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073, and UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN 268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001.
W.C.C. receives Institutional grants from Eli Lilly and Boehringer-Ingelheim; uncompensated consulting and steering committee Takeda Pharmaceuticals. G.W.E. receives Institutional grant from AstraZeneca.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Footnotes
Conflict of interest: All other authors have no conflicts of interest.
References
- 1.Sjostrand T. Volume and distribution of blood and their significance in regulating the circulation. Physiol Rev. 1953;33(2):202–228. doi: 10.1152/physrev.1953.33.2.202. [DOI] [PubMed] [Google Scholar]
- 2.Shibao C, Lipsitz LA, Biaggioni I. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens. 2013;7(4):317–324. doi: 10.1016/j.jash.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briasoulis A, Silver A, Yano Y, Bakris GL. Orthostatic hypotension associated with baroreceptor dysfunction: treatment approaches. J Clin Hypertens (Greenwich) 2014;16(2):141–148. doi: 10.1111/jch.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24(2):135–144. doi: 10.1038/ajh.2010.146. [DOI] [PubMed] [Google Scholar]
- 6.Jones CD, Loehr L, Franceschini N, Rosamond WD, Chang PP, Shahar E, et al. Orthostatic hypotension as a risk factor for incident heart failure: the atherosclerosis risk in communities study. Hypertension. 2012;59(5):913–918. doi: 10.1161/HYPERTENSIONAHA.111.188151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibao C, Biaggioni I. Orthostatic hypotension and cardiovascular risk. Hypertension. 2010;56(6):1042–1044. doi: 10.1161/HYPERTENSIONAHA.110.162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults: The Cardiovascular Health Study. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 9.Robertson D. Orthostatic hypertension: the last hemodynamic frontier. Hypertension. 2011;57(2):158–159. doi: 10.1161/HYPERTENSIONAHA.110.163485. [DOI] [PubMed] [Google Scholar]
- 10.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037–2114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, De Jong PE, Coresh J, El NM, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 15.Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA, et al. Updated U.S. population standard for the veterans RAND 12-item Health Survey (VR-12) Qual Life Res. 2009;18(1):43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 16.Nardo CJ, Chambless LE, Light KC, Rosamond WD, Sharrett RA, Tell GS, et al. Descriptive epidemiology of blood pressure response to change in body position: The ARIC Study. Hypertension. 1999;33(5):1123–1129. doi: 10.1161/01.hyp.33.5.1123. [DOI] [PubMed] [Google Scholar]
- 17.Davis BR, Langford HG, Blaufox MD, Curb JD, Polk F, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the hypertension detection and follow-up program experience. Circulation. 1987;75(2):340–346. doi: 10.1161/01.cir.75.2.340. [DOI] [PubMed] [Google Scholar]
- 18.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, et al. Orthostatic hypotension in the ACCORD (action to control cardiovascular risk in diabetes) blood pressure trial: prevalence, incidence and prognostic significance. Hypertension. 2016;68:888–895. doi: 10.1161/HYPERTENSIONAHA.116.07474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streeten DH, Auchincloss JHJ, Anderson GHJ, Richardson RL, Thomas FD, Miller JW. Orthostatic hypertension. Pathogenetic studies. Hypertension. 1985;7(2):196–203. doi: 10.1161/01.hyp.7.2.196. [DOI] [PubMed] [Google Scholar]
- 20.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952–958. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10(20):2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugghins SY, Champion HC, Cheng G, Kadowitz PJ, Jeter JR., Jr Vasorelaxant responses to endomorphins, nociceptin, albuterol, and adrenomedullin in isolated rat aorta. Life Sci. 2000;67(4):471–476. doi: 10.1016/s0024-3205(00)00631-7. [DOI] [PubMed] [Google Scholar]
- 23.Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci. 1993;53(18):1391–1399. doi: 10.1016/0024-3205(93)90581-m. [DOI] [PubMed] [Google Scholar]
- 24.Kario K. Orthostatic hypertension-a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9(12):726–738. doi: 10.1038/nrneph.2013.224. [DOI] [PubMed] [Google Scholar]
- 25.Fan XH, Wang Y, Sun K, Zhang W, Wang H, Wu H, et al. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens. 2010;23(8):829–837. doi: 10.1038/ajh.2010.76. [DOI] [PubMed] [Google Scholar]
- 26.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57(2):167–173. doi: 10.1161/HYPERTENSIONAHA.110.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]