Abstract
Background
Single-center studies suggest that patients with cancer have similar outcomes after intracerebral hemorrhage (ICH) compared to patients without cancer. However, these studies were limited by small sample sizes and high rates of intratumoral hemorrhage. Our hypothesis was that systemic cancer patients without brain involvement fare worse after ICH than patients without cancer.
Methods
We identified all patients diagnosed with spontaneous ICH from 2002 through 2011 in the Nationwide Inpatient Sample. Our predictor variable was systemic cancer. Our primary outcome was discharge disposition, dichotomized into favorable discharge (home/self-care or rehabilitation) or unfavorable discharge (nursing facility, hospice, or death). We used logistic regression to compare outcomes and performed secondary analyses by cancer subtype (i.e., non-metastatic solid tumors, non-metastatic hematologic tumors, and metastatic solid or hematologic tumors).
Results
Among 597,046 identified ICH patients, 22,394 (3.8%) had systemic cancer. Stroke risk factors such as hypertension and diabetes were more common in patients without cancer, while anticoagulant use and higher Charlson comorbidity scores were more common among cancer patients. In multivariate logistic regression analysis adjusted for demographics, comorbidities, and hospital-level characteristics, patients with cancer had higher odds of death (OR 1.62, 95% CI 1.56–1.69) and lower odds of favorable discharge (OR 0.59, 95% CI 0.56–0.63) than patients without cancer. Amongst cancer groups, patients with non-metastatic hematologic tumors and those with metastatic disease fared the worst.
Conclusions
Patients with systemic cancer have higher mortality and less favorable discharge outcomes after ICH than patients without cancer. Cancer subtype may influence outcomes after ICH.
Keywords: Intracerebral hemorrhage, cancer, malignancy, clinical outcomes
Introduction
The incidence of cancer in patients with spontaneous intracranial hemorrhage ranges from 1 to 10%(1, 2), with the intracerebral compartment being the most frequently affected site.(3) Cohort studies on intracerebral hemorrhage (ICH) have suggested that patients with cancer have similar outcomes compared to patients without cancer.(4, 5) However, these studies were mostly small, single-center, and included patients with primary or metastatic brain tumors complicated by intratumoral hemorrhage—a condition that may not affect clinical outcomes to the same degree as other causes of ICH since patients with brain tumors often have preexisting disability from cerebral edema and/or herniation.(5) Therefore, we sought to better examine the relationship between systemic cancer and ICH outcomes by using data from a large, heterogeneous, nationally-representative research database, and by excluding patients with known primary intracranial tumors or systemic cancer with brain metastases. Our pre-specified hypothesis was that systemic cancer patients without known brain involvement fare worse after ICH than patients without cancer.
Methods
Study Design and Population
This was a retrospective cohort study using de-identified, inpatient discharge data from the Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project.(6) The NIS includes data from 1050 U.S. hospitals on approximately 8 million inpatient hospitalizations each year, representing a 20% stratified sample of all non-federal American hospitals.(6) As this database comprises anonymized, de-identified, publicly-available patient information, this study was exempt from our Institutional Review Board’s review.
We identified consecutive patients between January 1, 2002 and December 31, 2011 with a primary discharge diagnosis of ICH using the previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 431.(7, 8) In an effort to restrict our population to adults with spontaneous ICH from causes other than intratumoral hemorrhage(5) or vascular malformations(9), which may have different natural histories than other more typical causes of ICH, we excluded patients who were less than 18 years of age, had known primary intracranial tumors or systemic cancer with brain metastases, were diagnosed with traumatic brain injury or any cerebral vascular malformation, or were treated with aneurysm clipping or coiling during the index hospitalization. We also excluded patients who initially presented to one hospital but were subsequently transferred to a higher acuity hospital, in order to prevent double counting of the same patient, as performed in prior NIS studies.(10, 11)
Measurements
Our primary predictor was systemic cancer. Patients with cancer were divided into three categories based on the National Comprehensive Cancer Network classification: solid tumors without metastases (ICD-9-CM 140.xx- 190.xx, 193.xx-195.xx, 209.00–209.30), hematologic tumors without metastases (200.xx-208.xx, 238.7x), and metastatic solid or hematologic tumors (196.xx-198.xx, 209.7x).(12) The ICD-9 codes for individual cancers have been used in prior NIS studies(13, 14), and can be obtained using the Clinical Classification Software provided by the Healthcare Cost and Utilization Project.(15)
Palliative care, which we used as a surrogate for care limitation, was identified using the ICD-9 code V66.7.(16) The modified Charlson comorbidity index, in a cumulative score format, was used to adjust for underlying medical comorbidities.(17) This index, which incorporates 17 different comorbidities, has been previously validated as an effective outcome adjustment method for analyses using administrative data.(17) Coagulopathy, a known modifier of outcomes in ICH, was identified using ICD-9-CM diagnosis codes 286.5–286.9, 287.1, 287.3–287.5 and 289.82.(18)
Our primary outcome was discharge disposition, which was dichotomized as in previous studies using the NIS into favorable discharge (home/self-care or rehabilitation) and unfavorable discharge (nursing facility, hospice, or death). Discharge disposition has been validated as a surrogate for functional outcome.(19) Our secondary outcome was inpatient mortality.
Statistical Analysis
To obtain national estimates of inpatient hospitalizations for ICH, we used standard weights provided by the Healthcare Cost and Utilization Project. The Pearson Chi-square test was used to compare categorical variables between patients with and without cancer, while the Mann-Whitney U test was used for continuous variables (e.g., length of stay and resource utilization measures) since these data were not normally distributed. Bivariate and multivariate logistic regression was used to evaluate the association between systemic cancer and ICH outcomes. The analyzed covariates included demographic and socioeconomic factors such as age, sex, race, and insurance status; hospital-level characteristics such as geographic region, rural vs. urban location, teaching status, bed size, and annual ICH volume; and the following clinical variables known to affect outcomes in ICH: Charlson comorbidity index inserted as quartiles, stroke risk factors, diagnosis of coagulopathy, anticoagulant use, mechanical ventilation, infections (e.g., pneumonia and urinary tract infection), and relevant inpatient procedures performed (e.g., craniotomy, ventriculostomy, tracheostomy, and gastrostomy). All covariates associated with outcomes at the bivariate level with a p-value of <0.05 were inserted into the multivariate model. All analyses were two-tailed and were performed using Stata (version 14.0, College Station, TX); statistical significance was defined as a p-value of <0.05.
Results
Patient Characteristics
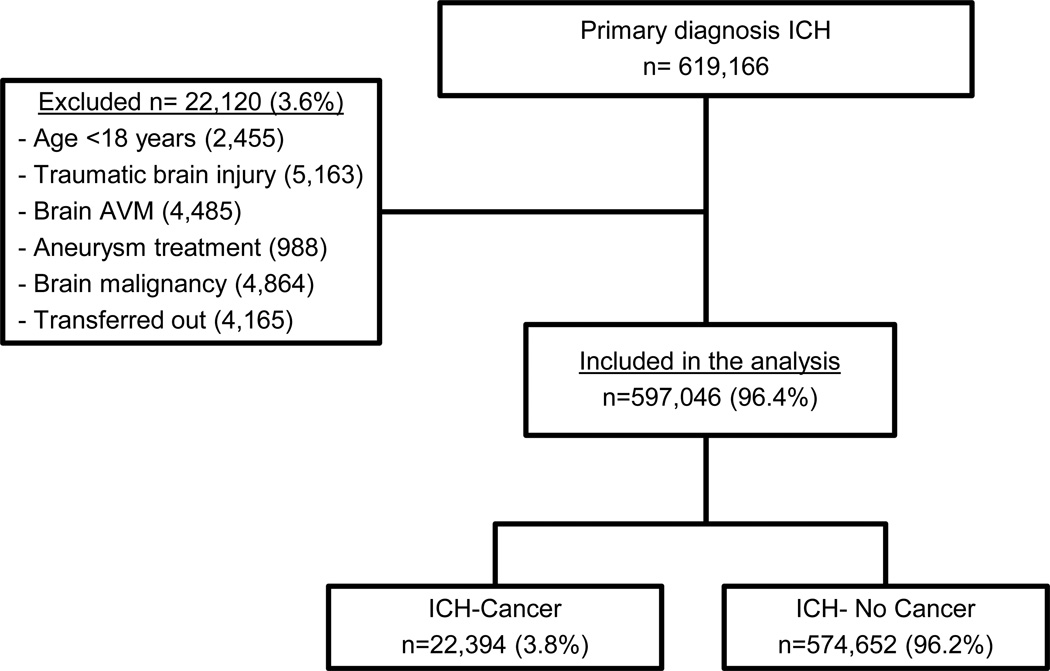
Among 619,166 patients with a primary diagnosis of ICH, 597,046 met the study eligibility criteria and were included in the final analysis (Figure 1). Of these patients, 23,034 (3.8%) had a diagnosis of systemic cancer, including 10,846 with non-metastatic solid tumors, 5,863 with non-metastatic hematologic tumors, and 5,695 with metastatic tumors (3,967 solid and 1,728 hematologic). As compared to patients without cancer, ICH patients with cancer were significantly older and more often male, Caucasian, had received anticoagulation before ICH, and had higher Charlson comorbidity scores (Table 1). Conversely, stroke risk factors such as hypertension and diabetes mellitus were significantly less common among the cancer cohort (Table 2). Patients with cancer were also less likely to be cared for at large, urban, teaching hospitals with high volumes of ICH and they averaged lower cost-of-care and hospital length-of-stay than patients without cancer.
Figure 1.
Flow diagram of patient selection for the final cohort.
ICH: Intracerebral Hemorrhage, AVM: Arteriovenous Malformation
TABLE 1.
Demographic and Hospital-level Characteristics of Patients with Intracerebral Hemorrhage in the Nationwide Inpatient Sample Stratified by Presence of Cancer.
| Characteristic | ICH without Cancer (N= 574,652) |
ICH with Cancer (N= 22,394) |
P value |
|---|---|---|---|
| Age, years | <0.001 | ||
| 18–64 | 201,233 (35.0) | 5,119 (22.9) | |
| 65–79 | 192,458 (33.5) | 9,388 (41.9) | |
| 80 or more | 180,961 (31.5) | 7,887 (35.2) | |
| Gender | <0.001 | ||
| Male | 280,926 (48.9) | 12,546 (56.0) | |
| Female | 293,459 (51.1) | 9,847 (44.0) | |
| Race/ethnicity | <0.001 | ||
| Caucasian | 295,042 (51.3) | 13,303 (59.4) | |
| Black | 76,018 (13.2) | 2,168 (9.7) | |
| Hispanic | 43,213 (7.5) | 1,086 (4.8) | |
| Other | 37,424 (6.5) | 1,095 (4.9) | |
| Missing information | 122,955 (21.4) | 4,742 (21.2) | |
| Health insurance | <0.001 | ||
| Medicare | 356,993 (62.2) | 16,076 (71.8) | |
| Medicaid | 47,924 (8.4) | 1,102 (4.9) | |
| Private insurance | 115,733 (20.2) | 4,277 (19.1) | |
| Other | 53,031 (9.2) | 920 (4.1) | |
| Hospital geographic region | <0.001 | ||
| Northeast | 101,740 (17.7) | 4,409 (19.7) | |
| Midwest | 122,081 (21.2) | 5,314 (23.7) | |
| South | 228,884 (39.8) | 7,879 (35.2) | |
| West | 121,947 (21.3) | 4,792 (21.4) | |
| Hospital location | <0.001 | ||
| Rural | 42,453 (7.4) | 2,199 (9.9) | |
| Urban | 527,896 (92.6) | 20,054 (90.1) | |
| Hospital teaching status | <0.001 | ||
| Nonteaching | 241302 (42.3) | 10159 (45.7) | |
| Teaching | 329047 (57.7) | 12094 (54.3) | |
| Hospital bed size | <0.001 | ||
| Small | 41,768 (7.3) | 1,907 (8.6) | |
| Medium | 121,030 (21.2) | 4,904 (22.0) | |
| Large | 407,552 (71.5) | 15,443 (69.4) | |
| Hospital ICH case volume quartile | <0.001 | ||
| 1st (1–23 cases/year) | 136,624 (23.8) | 6,544 (29.2) | |
| 2nd (24–47 cases/year) | 14,489 (25.1) | 5,747 (25.7) | |
| 3rd (48–85 cases/year) | 147,576 (25.7) | 5,201 (23.2) | |
| 4th (>85 cases/year) | 145,962 (25.4) | 4,902 (21.9) |
Abbreviations: ICH: Intracerebral Hemorrhage
Data are presented as number (%) unless otherwise specified.
TABLE 2.
Medical Comorbidities, Inpatient Complications and Relevant Performed Procedures in Patients with Intracerebral Hemorrhage Stratified by Presence of Cancer.
| Comorbidities | ICH without Cancer (N= 574,652) |
ICH with Cancer (N= 22,394) |
P value | |
|---|---|---|---|---|
| Modified Charlson comorbidity index |
<0.001 | |||
| 0–2 | 509,681 (88.7) | 7,385 (33.0) | ||
| ≥3 | 65,124 (11.2) | 14,973 (67.0) | ||
| Hypertension | 444,402 (77.3) | 14,221 (63.6) | <0.001 | |
| Diabetes mellitus | 122,597 (21.3) | 3,928 (17.6) | <0.001 | |
| Coagulopathy | 30,836 (5.4) | 3,163 (14.1) | <0.001 | |
| Anticoagulant use | 39,662 (6.9) | 1,808 (8.1) | <0.001 | |
| Medical complications | ||||
| 3M APR-DRG risk of mortality |
<0.001 | |||
| Minor to Moderate | 299,734 (52.2) | 9,077 (40.6) | ||
| Major | 117,539 (20.5) | 6,815 (30.5) | ||
| Extreme | 157,358 (27.3) | 6,467 (28.9) | ||
| Hydrocephalus | 38,134 (6.6) | 826 (3.7) | <0.001 | |
| Venous Thromboembolism | 6,837 (1.2) | 407 (1.8) | <0.001 | |
| Urinary tract infection | 78,411 (13.6) | 2,448 (10.9) | <0.001 | |
| Pneumonia | 40,741 (7.1) | 1,594 (7.1) | 0.992 | |
| Sepsis | 18,770 (3.3) | 602 (2.7) | <0.001 | |
| Seizures | 55,173 (9.6) | 2,221 (9.9) | 0.117 | |
| Inpatient procedures | ||||
| Palliative Care | 37,781 (6.6) | 2,189 (9.8) | <0.001 | |
| Craniotomy | 4,849 (0.8) | 142 (0.6) | <0.001 | |
| Ventriculostomy | 37421 (6.5) | 769 (3.4) | <0.001 | |
| Mechanical ventilation | 165,463 (28.8) | 5,424 (24.2) | <0.001 | |
| Tracheostomy | 2,800 (0.5) | 34 (0.2) | <0.001 | |
| Gastrostomy | 51,704 (9.0) | 1,246 (5.6) | <0.001 | |
| Survival >48 hours | 574,651 (87.1) | 18,229 (81.4) | <0.001 | |
|
Resource Utilization Measures |
Median (IQR) | Median (IQR) | ||
| Cost of care | 10,247 (5,561– 20,911) |
9,019 (4,724– 17,827) |
<0.001 | |
| Length of stay (days) | 5 (2– 9) | 4 (2–8) | <0.001 | |
Abbreviations: ICH: Intracerebral Hemorrhage, IQR: Inter Quartile Range
Data are presented as number (%) unless otherwise specified.
Primary Analysis
Rate of inpatient death was 37.3% in ICH patients with cancer compared to 29.2% in ICH patients without cancer (p<0.001); conversely, rate of favorable discharge was 23.5% in the cancer group and 33.9% in the non-cancer group (p<0.001). In multivariate logistic regression analysis adjusting for demographics, comorbidities, and hospital-level characteristics, patients with cancer had higher odds of death (OR 1.62, 95% CI 1.56–1.69, p=<0.001) and lower odds of favorable discharge (OR 0.59, 95% CI 0.56–0.63, p=<0.001) than patients without cancer (Table 3). These results were similar but slightly attenuated in a pre-specified sensitivity analysis that attempted to minimize the effects of early care limitation (i.e., self-fulfilling prophecy in ICH) by excluding patients who had palliative care codes and died within 48 hours of admission (OR for death 1.54, 95% CI 1.46–1.61, p=<0.001; OR for favorable discharge 0.65, 95% CI 0.61–0.71, p=<0.001).
TABLE 3.
Multivariate Logistic regression Analysis of Discharge Outcomes in ICH Patients with Cancer.
| Outcome | ICH without Cancer N= 574,652 |
ICH with Cancer N= 22,394 |
Adj. OR (95% CI) |
P value |
|---|---|---|---|---|
| All Cases Included | ||||
| Inpatient Mortality | 16,779 (29.2) | 8,361 (37.3) | 1.62 (1.56– 1.69) | <0.001 |
| Favorable Discharge | 165,059 (33.9) | 4,393 (23.5) | 0.59 (0.56– 0.63) | <0.001 |
| Unfavorable Discharge | 253,924 (52.2) | 11,829 (63.2) | 1.74 (1.57– 1.89) | <0.001 |
| Palliative Care and Early Death (<48 hours) Excluded | ||||
| Inpatient Mortality | 77,246 (16.3) | 3,382 (20.1) | 1.54 (1.46– 1.61) | <0.001 |
| Unfavorable Discharge | 170,058 (42.4) | 7,304 (51.8) | 1.46 (1.37– 1.52) | <0.001 |
| Favorable Discharge | 164,393 (41.0) | 4,350 (30.8) | 0.65 (0.61– 0.71) | <0.001 |
Abbreviations: Adj: adjusted, CI: Confidence Interval, OR: Odds Ratio
Favorable discharge = home/self-care or rehabilitation
Unfavorable discharge = skilled nursing facility, hospice, or death.
Sum of favorable and unfavorable discharge rates is not 100% as other less common specified (such as home health care, law enforcement, intermediate care center) and unspecified discharge dispositions were not included in the definitions.
Secondary Analysis
In subgroup multivariate analyses by broad cancer type, patients with cancer had uniformly worse outcomes than patients without cancer (Table 4). Among cancer patients, patients with metastatic solid or hematologic tumors fared the worst compared to patients without cancer (OR for death 2.09, 95% CI 1.96–2.23, p=<0.001; OR for favorable discharge 0.42, 95% CI 0.39–0.45, p=<0.001), followed by patients with non-metastatic hematologic tumors (OR for death 1.98, 95% CI 1.86–2.12, p=<0.001; OR for favorable discharge 0.62, 95% CI 0.57–0.66, p=<0.001), and patients with non-metastatic solid tumors (OR for death 1.25, 95% CI 1.19–1.32, p=<0.001; OR for favorable discharge 0.69, 95% CI 0.65–0.73, p=<0.001)
TABLE 4.
Multivariate Logistic Regression Analysis of Discharge Outcomes in ICH patients Stratified by Cancer Subtype.
| Reference Group: No Cancer |
Non-metastatic Solid Malignancies |
Non-metastatic Hematologic Malignancies |
Systemic Metastases | |||
|---|---|---|---|---|---|---|
| N= 10,846 (1.8%) | N= 5,863 (1.0%) | N= 5,695 (0.9%) | ||||
| Adj. OR | P value | Adj. OR | P value | Adj. OR | P value | |
| All Cases Included | ||||||
| Inpatient Mortality | 1.25 (1.19– 1.32) | <0.001 | 1.98 (1.86– 2.12) | <0.001 | 2.09 (1.96– 2.23) | <0.001 |
| Favorable Discharge | 0.69 (0.65– 0.73) | <0.001 | 0.62 (0.57– 0.66) | <0.001 | 0.42 (0.39– 0.45) | <0.001 |
| Unfavorable Discharge | 1.34 (1.27 –1.40) | <0.001 | 1.62 (1.52– 1.73) | <0.001 | 2.32 (2.17– 2.49) | <0.001 |
| Palliative Care and Early Death (<48 hours) Excluded | ||||||
| Inpatient Mortality | 1.25 (1.17– 1.33) | <0.001 | 1.72 (1.58–1.87) | <0.001 | 2.04 (1.87– 2.23) | <0.001 |
| Favorable Discharge | 0.71 (0.67– 0.75) | <0.001 | 0.73 (0.67– 0.79) | <0.001 | 0.48 (0.44– 0.52) | <0.001 |
| Unfavorable Discharge | 1.29 (1.23– 1.56) | <0.001 | 1.33 (1.23– 1.43) | <0.001 | 2.04 (1.90– 2.25) | <0.001 |
Abbreviations: Adj: adjusted, CI: Confidence Interval, OR: Odds Ratio
Discussion
In a large, heterogeneous, nationally representative cohort of patients with ICH, systemic cancer without brain involvement was independently associated with inpatient mortality and unfavorable discharge disposition. Furthermore, among cancer groups, patients with known metastatic tumors and those with non-metastatic hematologic cancers had the worst outcomes after ICH, with a two-fold increased odds of death as compared to ICH patients without cancer. These results remained materially unchanged when excluding patients who may have had early care limitation as indicated by palliative care codes and death within 48 hours of hospitalization.
In our study, inpatient mortality (37.3% vs. 29.2%) and unfavorable discharge disposition (63.2% vs. 52.2%) after ICH were significantly higher in patients with cancer as compared to patients without cancer. In contrast, prior studies have not reported differences in ICH outcomes between cancer and non-cancer patients.(4, 5) This discrepancy may be explained by differences in patient characteristics and cancer types between studies. In particular, in contrast to other studies, our study excluded patients with known brain tumors. In a detailed series of 208 cancer patients with intracerebral or subarachnoid hemorrhage at a tertiary-care cancer center, 91 (44%) had a known primary or metastatic brain tumor at the time of hemorrhage and intratumoral hemorrhage was the most common cause of ICH.(5) We believe that the exclusion of patients with known brain tumors—thereby limiting the number of patients with intratumoral hemorrhage—and our study’s large sample size enhanced our ability to analyze the effects of systemic cancer on ICH outcomes.
Previous studies have reported that coagulopathy is a common underlying cause of ICH in cancer patients.(3, 20) Additionally, coagulopathy is a well-known predictor of hematoma expansion and poor outcomes in ICH.(21, 22) In our study, patients with cancer were more often diagnosed with coagulopathy than patients without cancer (14.1% vs. 5.4%, p<0.001). Furthermore, patients with non-metastatic hematologic tumors (26.4%) and those with metastatic solid or hematologic tumors (16.5%) had higher rates of coagulopathy than patients with non-metastatic solid tumors (6.3%). Although the administrative nature of our dataset prevented us from determining the mechanisms or characteristics of ICH in our cohort, it is possible that the different rates of coagulopathy between the patients with and without cancer, as well as among the different cancer groups, contributed to the differences in mortality seen in our study. We also observed that patients with cancer had fewer procedures performed and lower resource utilization measures, as well as increased death within 48 hours. Therefore, it is possible that less aggressive care in cancer patients, particularly those with metastatic disease, contributed to the worse outcomes in these patients.
This study should be interpreted in the context of several limitations. First and foremost, since our study relied on administrative data from the NIS, we lacked information on well-validated ICH severity measures such as the Glasgow Coma Scale score and ICH volume and location. In addition, data on specific cancer type, stage, and treatments such as chemotherapy and radiation, which could also affect the type and severity of ICH, were similarly not available. We also lacked information on the timing and method of coagulopathy reversal, as well as other standard medical treatments for ICH such as aggressive blood pressure control and osmotic agents for cerebral edema. Second, our reliance on ICD-9-CM diagnostic codes could have resulted in misclassification of some ICH diagnoses, as well as other measurement errors. However, the ICD-9-CM code 431, which we used to diagnose ICH, has been previously validated to have high specificity and positive predictive value for identifying ICH in administrative datasets.(7, 8) Similarly, the ICD-9-CM codes used to identify different cancer types in this study are believed to have high accuracy and have been used in other NIS studies.(13, 23) Third, we were unable to differentiate between primary and recurrent ICH. However, given the low annual rate of ICH recurrence (~2%)(24, 25), this is unlikely to have significantly affected our results.
In conclusion, patients with systemic cancer have higher mortality and less favorable discharge outcomes after ICH than patients without cancer. Amongst cancer subtypes, hematological and metastatic tumors appear to have the worst outcomes. Future studies with detailed clinical data are needed to determine the mechanisms responsible for the worse outcomes after ICH in cancer patients.
Acknowledgments
Dr. Murthy is supported by the American Brain Foundation and American Academy of Neurology.
Dr. Hanley is supported by NIH grants 5U01NS062851 for Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR-IVH) III and 1U01NS08082 for Minimally Invasive Surgery Plus r-tPA for Intracerebral Hemorrhage Evacuation (MISTIE) III.
Dr. Iadecola is supported by NIH grants R37NS089323-02, R01NS034179-21, R01NS037853-19, and R01 NS073666-04.
Dr. Kamel is supported by NIH grant K23NS082367 and the Michael Goldberg Stroke Research Fund.
Dr. Navi is supported by NIH grant K23NS091395 and the Florence Gould Endowment for Discovery in Stroke.
Footnotes
Disclosures
The authors have no conflict of interests to report.
References
- 1.McCormick WF, Rosenfield DB. Massive brain hemorrhage: a review of 144 cases and an examination of their causes. Stroke. 1973;4(6):946–954. doi: 10.1161/01.str.4.6.946. [DOI] [PubMed] [Google Scholar]
- 2.Schrader B, Barth H, Lang EW, Buhl R, Hugo HH, Biederer J, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta neurochirurgica. 2000;142(9):979–985. doi: 10.1007/s007010070052. [DOI] [PubMed] [Google Scholar]
- 3.Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14(4):373–381. doi: 10.1007/s11883-012-0250-3. [DOI] [PubMed] [Google Scholar]
- 4.Licata B, Turazzi S. Bleeding cerebral neoplasms with symptomatic hematoma. J Neurosurg Sci. 2003;47(4):201–210. [PubMed] [Google Scholar]
- 5.Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74(6):494–501. doi: 10.1212/WNL.0b013e3181cef837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healthcare Cost and Utilization Project (HCUP) [Accessed on August 5, 2015];Overview of the Nationwide Inpatient Sample (NIS). Agency for Research Healthcare and Quality website. http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 7.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke; a journal of cerebral circulation. 2005;36(8):1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 8.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke; a journal of cerebral circulation. 1999;30(12):2523–2528. doi: 10.1161/01.str.30.12.2523. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Mast H, Sciacca RR, Hartmann A, Khaw AV, Mohr JP, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006;37(5):1243–1247. doi: 10.1161/01.STR.0000217970.18319.7d. [DOI] [PubMed] [Google Scholar]
- 10.Moradiya Y, Murthy SB, Newman-Toker DE, Hanley DF, Ziai WC. Intraventricular thrombolysis in intracerebral hemorrhage requiring ventriculostomy: a decade-long real-world experience. Stroke. 2014;45(9):2629–2635. doi: 10.1161/STROKEAHA.114.006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy SB, Moradiya Y, Shah J, Hanley DF, Ziai WC. Incidence, Predictors, and Outcomes of Ventriculostomy-Associated Infections in Spontaneous Intracerebral Hemorrhage. Neurocrit Care. 2015 doi: 10.1007/s12028-015-0199-5. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) [Accessed on August 5,2015];The difference between solid and liquid tumors. NCCN website. http://www.Nccn.Com/component/content/article/54-cancer-basics/1042-liquid-versus-solid-tumors.Html.
- 13.Murthy SB, Karanth S, Shah S, Shastri A, Rao CP, Bershad EM, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke. 2013;44(12):3573–3576. doi: 10.1161/STROKEAHA.113.003058. [DOI] [PubMed] [Google Scholar]
- 14.Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22(7):1146–1150. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Healthcare Cost and Utilzation Project. [Accessed on January 5, 2016];Clinical Classification Software. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixASingleDX.txt.
- 16.Murthy SB, Moradiya Y, Hanley DF, Ziai WC. Palliative Care Utilization in Nontraumatic Intracerebral Hemorrhage in the United States. Crit Care Med. 2016;44(3):575–582. doi: 10.1097/CCM.0000000000001391. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Mujib M, Khanna N, Mazumder NK, Aronow WS, Kolte D, Khera S, et al. Pretransplant coagulopathy and in-hospital outcomes among heart transplant recipients: a propensity-matched nationwide inpatient sample study. Clin Cardiol. 2015;38(5):300–308. doi: 10.1002/clc.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93(8):1408 e1–1413 e1. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64(1):16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63(6):1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 23.Murthy SB, Moradiya Y, Shah S, Shastri A, Bershad EM, Suarez JI. In-hospital outcomes of thrombolysis for acute ischemic stroke in patients with primary brain tumors. J Clin Neurosci. 2015;22(3):474–478. doi: 10.1016/j.jocn.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56(6):773–777. doi: 10.1212/wnl.56.6.773. [DOI] [PubMed] [Google Scholar]
- 25.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31(1):123–127. doi: 10.1161/01.str.31.1.123. [DOI] [PubMed] [Google Scholar]