Abstract
Deficits in cognitive flexibility, the ability to modify behavior in response to changes in the environment, contribute to the onset and maintenance of stress-related neuropsychiatric illnesses, such as depression. Cognitive flexibility depends on medial prefrontal cortex (mPFC) function, and in depressed patients, cognitive inflexibility is associated with hypoactivity and decreased glutamate receptor expression in the mPFC. Rats exposed to chronic unpredictable stress (CUS) exhibit compromised mPFC function on the extradimensional (ED) set-shifting task of the attentional set-shifting test. Moreover, CUS-induced ED deficits are associated with dendritic atrophy and decreased glutamate receptor expression in the mPFC. This evidence suggests that impaired glutamate signaling may underlie stress-induced deficits in cognitive flexibility. To test this hypothesis, we first demonstrated that blocking NMDA or AMPA receptors in the mPFC during ED replicated CUS-induced deficits in naïve rats. Secondly, we found that expression of activity-regulated cytoskeleton-associated protein (Arc) mRNA, a marker of behaviorally induced glutamate-mediated plasticity, was increased in the mPFC following ED. We then showed that CUS compromised excitatory afferent activation of the mPFC following pharmacological stimulation of the mediodorsal thalamus (MDT), indicated by a reduced induction of c-fos expression. Subsequently, in vivo recordings of evoked potentials in the mPFC indicated that CUS impaired afferent activation of the mPFC evoked by MDT stimulation, but not the ventral hippocampus. Lastly, glutamate microdialysis, showed that CUS attenuated the acute stress-evoked increase in extracellular glutamate in the mPFC. Together, these results demonstrate that CUS-induced ED deficits are associated with compromised glutamate neurotransmission in the mPFC.
Keywords: attentional set-shifting, chronic unpredictable stress, cognitive flexibility, glutamate, medial prefrontal cortex, mediodorsal thalamus
INTRODUCTION
Deficits in cognitive function and emotional regulation play an integral role in the pathology of stress-related neuropsychiatric illnesses, such as depression and anxiety disorders. Specifically, impaired cognitive flexibility contributes to the onset and maintenance of these illnesses (Taylor Tavares et al., 2007, Disner et al., 2011, Millan et al., 2012). Cognitive flexibility, the ability to modify patterns of thought or behavior in response to feedback from the environment, is strongly associated with medial prefrontal cortical (mPFC) function. Imaging studies have shown that deficits in cognitive flexibility are associated with hypoactivity in the mPFC of depressed and chronically stressed individuals (Anand et al., 2005, Bermpohl et al., 2009, Koenigs and Grafman, 2009, Liston et al., 2009). Further, those suffering from depression also exhibit a decrease in glutamate/glutamine ratios, glutamate receptor expression, and markers of synaptic plasticity in the prefrontal cortex (Hasler et al., 2007, Feyissa et al., 2009). Moreover, acute low-dose administration of the N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine, has been shown to induce rapid antidepressant effects in treatment-resistant patients (Carlson et al., 2006, Zarate et al., 2006, Machado-Vieira et al., 2009). Evidence suggests that this therapeutic effect results in part from ketamine enhancing glutamate transmission in the mPFC, including elevated glutamate levels and increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor activation (Moghaddam et al., 1997, Li et al., 2010, Li et al., 2011). Accordingly, changes in glutamatergic signaling in mPFC may play a key role in the pathology of stress-related neuropsychiatric disorders, as well as their treatment.
Preclinical studies have demonstrated that acute stress enhances glutamate release in the mPFC, and that this response is neuronally mediated (Moghaddam, 1993, Moghaddam et al., 1994, Lupinsky et al., 2010, Musazzi et al., 2011). Furthermore, acute stress-evoked glutamate activity in the mPFC is associated with enhanced working memory (Yuen et al., 2009, Yuen et al., 2011), whereas blocking this activity by AMPA or NMDA receptor antagonism during behavioral testing impairs cognitive flexibility (Stefani et al., 2003, Stefani and Moghaddam, 2005, Dalton et al., 2011). This evidence suggests that acutely-evoked glutamate transmission in the mPFC facilitates cognitive function. In contrast, rodents exposed to chronic stress show reductions in glutamate receptor expression and markers of synaptic plasticity that mirror deficits in the prefrontal cortex of depressed patients (Lee and Goto, 2011, Li et al., 2011, Yuen et al., 2012). Chronic stress induces atrophy of pyramidal cell dendrites in the mPFC of rodents (Cook and Wellman, 2004, Radley et al., 2004, Liston et al., 2006). This detrimental effect of chronic stress may result from excessive stress-evoked glutamate release, as NMDA receptor antagonist treatment during chronic stress prevents changes in dendritic atrophy (Martin and Wellman, 2011). Hence, chronic stress-induced changes in dendritic morphology and glutamate receptor expression may be a compensatory response to protect the mPFC from excessive glutamate signaling, excitotoxicity and cell death (Bruno et al., 1993, Skaper et al., 2001). However, such compensatory modifications in glutamate transmission could have secondary consequences, such as attenuated mPFC activity and deficits in higher order cognitive function (e.g., cognitive inflexibility).
To assess cognitive flexibility and stress-induced prefrontal cortical dysfunction in rats, we have employed the attentional set-shifting test (AST) (Birrell and Brown, 2000). This cognitive assay was reverse translated from a human and non-human primate test of cognitive set-shifting (Keeler and Robbins, 2011). In the AST, rats are trained to dig for a food reward in small pots differentiated by cues in two stimulus dimensions: the material with which the pots are filled, and the odor with which they are scented. Thus, the rats must learn which of the two stimulus dimensions is informative for locating the reward, and which cue within that dimension signals the location of the reward. After mastering a given contingency, indicated by reaching a criterion of 6 consecutive correct trials, the rules are changed and the rat must then learn a new association in the next task. By proceeding through a series of such changes in which the same stimulus dimension remains informative, the rats form a “cognitive set”, a higher-order learning strategy by which they can more readily acquire the new rule when faced with a subsequent change. However, in the extra-dimensional (ED) set-shifting task, the informative dimension is switched, so the rat must abandon their cognitive set in order to acquire the new rule. This form of cognitive flexibility, called a cognitive set-shift, depends on the function of the mPFC. Lesioning the mPFC of rats induces a deficit on the ED task, similar to deficits seen with impairments in lateral prefrontal cortex function in humans and non-human primates (Owen et al., 1991, Dias et al., 1996, Birrell and Brown, 2000). Moreover, similar to depressed patients, rats exposed to chronic unpredictable stress (CUS) exhibit deficits in cognitive flexibility on the ED task (Taylor Tavares et al., 2007, Bondi et al., 2008).
In this study we tested the hypothesis that CUS-induced ED deficits are associated with compromised glutamate transmission in the mPFC. First, we administered NMDA, AMPA, or metabotropic glutamate receptor (mGluR5) antagonists locally into the mPFC of naïve rats to test if directly compromising local glutamate transmission during the ED task would mimic the CUS-induced cognitive deficits reported previously. Of the mGluR receptor subtypes, we targeted the mGuR5 receptor because, like NMDA and AMPA receptors, it exhibits reduced expression in the prefrontal cortex of depressed patients, is associated with antidepressant efficacy, and modulates learning and memory (Naie and Manahan-Vaughan, 2004, Witkin et al., 2007, Deschwanden et al., 2011). Secondly, we investigated the effects of CUS on behaviorally-induced expression of the immediate early gene, Arc/Arg3.1 (activity-regulated cytoskeleton-associated protein), a marker of experience-dependent plasticity in glutamatergic (i.e., CaMKII-positive) cortical neurons (Shepherd and Bear, 2011). Induction of Arc expression in the mPFC during performance of the ED task was used to assess CUS-induced changes in glutamate-mediated plasticity. Next we evaluated if CUS-induced deficits in cognitive function are associated with changes in excitatory afferent-evoked activation of the mPFC by quantifying c-fos induction and local field potentials evoked by stimulation of major glutamatergic afferents to the mPFC, namely the mediodorsal thalamus (MDT) or the ventral hippocampus (vHipp)(Gigg et al., 1994, Pirot et al., 1994, Hoover and Vertes, 2007). Both of these regions are associated with the pathology of depression, and also modulate the stress response, emotional regulation, and cognitive flexibility (Floresco and Grace, 2003, Block et al., 2007, Godsil et al., 2013). Lastly, we used in vivo microdialysis to investigate whether the acute stress-evoked glutamate response in the mPFC is changed as a consequence of CUS. Together, the results demonstrate that CUS-induced cognitive deficits are associated with impaired glutamate neurotransmission in the mPFC. Portions of this work have been presented in abstract form (Jett et al., 2015b).
EXPERIMENTAL PROCEDURES
Animals
A total of 138 male Sprague-Dawley rats (Envigo, USA), weighing 220–300g upon arrival, were used for the present studies. Prior to initiating experimental procedures, rats were individually housed in 25 x 45 x 15 cm cages and maintained on a 12:12 hr light/dark cycle (lights on at 07:00). All experimental procedures were conducted during the light phase, and food and water was given ad libitum unless rats were food restricted for AST (Experiments 1 and 2). For the social defeat stressor in the CUS protocol, 12 Long-Evans retired male breeders were each pair-housed with an ovariectomized female (Charles River, USA) in large cages (63 x 63 x 40 cm) in a separate room. All procedures were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee and complied with National Institute of Health guidelines.
Stereotaxic surgery
Rats were anesthetized (ketamine 43 g/ml, acepromazine 1.4 g/ml, xylazine 8.6 mg/ml, 1.0 ml/kg i.m.; 25% supplement as needed) and guide cannulae were implanted by stereotaxic surgery. Rats used in the microinjection study were bilaterally implanted with 23 ga stainless steel cannulae terminating 1 mm above the mPFC (10° lateral approach; coordinates relative to bregma: AP +2.6 mm, ML +1.4 mm, DV −2.7 mm). Rats used for the c-fos study were unilaterally implanted with a guide cannula terminating 1 mm above the MDT (AP −2.5 mm, ML +0.9 mm, DV −4.6 mm; (Paxinos and Watson, 2007). Lastly, rats scheduled for microdialysis were unilaterally implanted with a microdialysis guide cannula (CMA Microdialysis, North Chelmsford, MA, USA) terminating 2 mm above the infralimbic/prelimbic boundary of the mPFC (10° approach; AP +2.6 mm, ML +1.4 mm, DV −1.7 mm). For studies with unilateral cannulae, placement was balanced between left and right hemispheres. Cannulae were anchored to the skull with jeweler screws and dental acrylic. Rats were treated prophylactically with antibiotic (penicillin G, 300,000 IU/ml, 1.0 ml/kg, s.c.), hydrated with saline (1.0 ml, s.c.), singly housed in fresh bedding and given one week of recovery.
Chronic unpredictable stress
CUS was conducted as previously described (Bondi et al., 2008), with minor modification. A different acute stressor was administered once daily for two weeks. For studies in which rats were chronically implanted with cannulae and exposed to CUS (Experiments 3 and 5), swim stressors were replaced with other CUS stressors to prevent infection (see Table 1B). Following each stressor, rats were placed in an isolated room to recover for 1 hr, then transferred to a clean cage and returned to housing. Unstressed controls remained in housing and were handled 1–2 min/day for 2 weeks.
Table 1.
CUS Schedulea
| A | |
|---|---|
| Day 1 | Restraint |
| Day 2 | Shaking and crowding |
| Day 3 | Social defeat |
| Day 4 | Warm swim |
| Day 5 | Wet bedding |
| Day 6 | Cold swim |
| Day 7 | Shaking and crowding |
| Day 8 | Foot shock |
| Day 9 | Social defeat |
| Day 10 | Warm swim |
| Day 11 | Foot shock |
| Day 12 | Tail pinch |
| Day 13 | Cold swim |
| Day 14 | Foot shock |
| B | |
|---|---|
| Day 1 | Restraint |
| Day 2 | Shaking and crowding |
| Day 3 | Social defeat |
| Day 4 | Tail pinch |
| Day 5 | Wet bedding |
| Day 6 | Social defeat |
| Day 7 | Shaking and crowding |
| Day 8 | Foot shock |
| Day 9 | Restraint |
| Day 10 | Social defeat |
| Day 11 | Foot shock |
| Day 12 | Tail pinch |
| Day 13 | Wet bedding |
| Day 14 | Foot shock |
Table 1A is the CUS schedule with swim stressors used in Experiment 2 and 4. For experiments 3 and 5, swim stressors were substituted with other stressors for rats that had surgery prior to CUS (Table 1B).
Attentional set-shifting test
The AST was conducted as described previously (Lapiz-Bluhm et al., 2008). All rats tested on the AST were food restricted to 14 g/day for one week prior to testing. In the testing arena (75 x 44 x 30 cm), a removable divider formed a start gate in the proximal third of the arena. A Plexiglas divider separated the distal third of the arena into two regions, into each of which was placed a terracotta digging pot (diameter 7 cm, depth 6 cm). The pots were differentiated by two stimulus dimensions: the texture of the digging medium that filled the pot, and the odor with which each pot was scented by applying an aromatic oil to the rim (Frontier Natural Brands, Boulder, CO, USA). The food reward, a ¼ piece of Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA), was buried in the bottom half of the “positive” pot. To prevent location of the reward by smell, the digging media in both pots was lightly dusted with Cheerio powder.
Day 1, Habituation
Rats were taught to dig for reward in pots filled with sawdust.
Day 2, Training
Rats first learned to make two simple discriminations (SD) in the arena. Reward was first associated with an odor (i.e., lemon vs. rosewood, pots filled with sawdust), then with a digging medium (i.e., shredded felt vs. shredded paper, pots unscented). All rats were trained using the same stimuli. The stimuli used during training were not used again during testing.
Day 3, Testing
Rats were tested on a series of discrimination tasks, in which the discriminative stimulus dimension and positive cue within that dimension were varied as shown in Table 2. The first task was a SD, similar to the training tasks. Half the rats discriminated between pots differentiated by odor, and half between digging media in unscented pots (for clarity, the following description will consider the example beginning with odor as the discriminating stimulus). The second task was a compound discrimination (CD), in which odor remained the informative dimension, and the second, irrelevant dimension (e.g., medium) was introduced as a distractor. The third task was a reversal (R1), in which the same odors and media were used, but the previously positive cue was now negative and the previously positive cue was negative. The fourth task was an intra-dimensional shift (ID); new media and odor were introduced, and odor remained informative. The fifth task was a second reversal (R2). The sixth task was the extra-dimensional (ED) set-shift; all new stimuli were again introduced, but this time the relevant dimension was switched to digging medium, and odor became the distractor. The dependent measure was the number of trials required to reach the criterion of six consecutive correct responses (Trials to Criterion, TTC) on the ED set-shifting task.
Table 2.
Representative example of stimulus pairings on the ASTa
| DISCRIMINATION STAGE | DIMENSIONS | EXAMPLE COMBINATIONS | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove | Nutmeg | |
|
| ||||
| Compound (CD) | Odor | Medium |
Clove/Raffia Clove/Yarn |
Nutmeg/Yarn Nutmeg/Raffia |
|
| ||||
| Reversal 1 (R1) | Odor | Medium |
Nutmeg/Raffia Nutmeg/Yarn |
Clove/Yarn Clove/Raffia |
|
| ||||
| Intradimensional Shift (ID) | Odor | Medium |
Rosemary/Wood balls Rosemary/Plastic Beads |
Cinnamon/Plastic Beads Cinnamon/Wood balls |
|
| ||||
| Reversal 2 (R2) | Odor | Medium |
Cinnamon/Wood balls Cinnamon/Plastic Beads |
Rosemary/Plastic Beads Rosemary/Wood balls |
|
| ||||
| Extradimensional Set-Shift (ED) | Medium | Odor |
Velvet/Citronella Velvet/Thyme |
Crepe/Thyme Crepe/Citronella |
Half the rats in each treatment started with odor as the initial discriminating dimension and shifted to medium, while the other half started with medium and shifted to odor. For each task, the positive stimulus is indicated in bold. Once a rat met criterion of six consecutive correct trials on a task, they proceeded to the next stage.
Experiment 1. Microinjections of glutamate receptor antagonists into the mPFC during performance on the ED set-shifting task
A total of 47 rats were used for this study. On the test day, after completing either the R2 task or the R1 task, the obdurators were removed and 30-gauge stainless steel microinjectors inserted into the mPFC. Bilateral infusions were made into the mPFC (0.5 μl/side at 0.2 μl/min) of one of the following: vehicle (0.66% sterile saline or 20% (2-hydroxypropyl)-β-cyclodextrin); the AMPA receptor antagonist, NBQX (3.0 μg/0.5 μl); the NMDA receptor antagonist, D-AP5 (5.0 μg/0.5 μl); or the mGluR5 receptor antagonist MPEP (1.5 μg/0.5 μl). Injectors were removed 2 min after completing the infusion, and the rat was returned to the arena. Testing resumed 5 min post-infusion. To verify that the observed deficits were specific to ED set-shifting and not attributable to non-specific changes in e.g., motivation, mobility, or the ability to smell, separate groups of rats were injected with NBQX or D-AP5 into the mPFC immediately prior to ID, a non mPFC-mediated task.
Experiment 2: Induction of Arc mRNA and protein expression in the mPFC after performance on the ED set-shifting task
Arc is almost exclusively expressed in cortical glutamatergic neurons (i.e., CaMKII-positive cells). It is induced by high neuronal activity, and has been implicated in several forms of synaptic plasticity and remodeling (Steward and Worley, 2001, Vazdarjanova et al., 2006, Shepherd and Bear, 2011). Thus, Arc expression was used as a marker of glutamate-mediated plasticity induced in the mPFC after completion of the ED task. A total of 31 rats were exposed to 14 days of CUS then divided into two groups differentiated by behavioral treatment (cage controls and AST). Cage controls were food restricted and transferred to the behavioral testing room in parallel with AST rats. They remained in their home cage while AST rats performed in the arena, and were given cheerios in parallel with the AST rats to prevent differences in caloric intake or receipt of reward from confounding results. Thirty minutes after completing the ED task, or at a comparable time for cage controls, rats were sacrificed and the mPFC dissected using a brain matrix on ice. A 2 mm coronal slab was cut between 2 and 4 mm caudal to the frontal apex. Cortex medial to the forceps minor was dissected, flash frozen and stored at −80°C. One hemisphere from each rat was used for quantitative RT-PCR, and the other was used for western blots.
Quantitative RT-PCR was performed as described previously (Girotti et al., 2011). Briefly, total RNA was extracted and purified using Trizol reagent (Invitrogen) and the PureLink RNA Mini Kit (Invitrogen Carlsbad, CA) with an additional on-column DNAse purification step. Primer sets were designed using the Integrated DNA Technology Primer Quest Freeware and were obtained from IDT (see Table 3). The primers were tested for optimal annealing temperatures by gradient PCR, and the absence of non-specific amplification was confirmed by running the melt-curve method at the end of each q-PCR. Real-time quantification of diluted cDNA and No Reverse Transcriptase controls was performed in triplicate. Reactions contained sample, SYBR green fluorescence (SsoFast EvaGreen Supermix, BioRad) and 400 nM of each forward and reverse primer on a BioRad CFX384 Real Time System. Conditions were one cycle at 95°C for 2 min then 40 cycles of denaturation (95°C, 5 sec), annealing and elongation (60°C, 10 sec). Relative gene expression was calculated using the 2−ΔΔCt method.
Table 3.
Primer sequences used for quantitative RT-PCR
For western blots, tissue was sonicated (12 sec, 50% power) in RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.5% Na-deoxycholate, 0.1% SDS, 1% Nonident- P40) containing protease and phosphatase inhibitors (Sigma). Homogenates were incubated on ice for 10 min with occasional mixing and centrifuged for 10 min at 18000 x g at 4°C. Protein content was determined using Bradford assay. Equal amounts of protein were subject to MOPS-SDS electrophoresis then transferred to nitrocellulose membrane via the dry iBlot2 transfer system (Novex, Life Technologies). Blots were first incubated with rabbit monoclonal anti-Arc antibody overnight at 4°C (1:5,000, Santa Cruz). Following, blots were incubated with a horseradish peroxidase-linked anti-rabbit secondary antibody (1:5,000) and Prime ECL reagent (GE Healthcare) for ECL detection. Subsequently, blots were stripped and re-probed with rabbit monoclonal anti-GAPDH antibody (1:20,000, Cell Signaling) and horseradish peroxidase-linked anti-rabbit secondary antibody (1:20,000). For quantitative RT-PCR and western blots, levels of Arc were expressed as a ratio of GAPDH for each animal, which were then normalized to the mean cage control value.
Experiment 3. Induction of c-fos mRNA expression, measured by in situ hybridization, to assess the mPFC response to excitatory afferent activation
To test the hypothesis that CUS-induced cognitive deficits are associated with changes in the mPFC response to excitatory afferent activation, we measured the induction of c-fos, an immediate early gene and indirect indicator of cell activation, in the mPFC of CUS and non-stressed rats following pharmacological stimulation of the mediodorsal thalamus (MDT), a major glutamatergic afferent to the mPFC. A total of 20 rats were used for this study. One week after surgery, rats started CUS or non-stress handling procedures. On Days 12–14 of stress treatment, all rats were habituated to the experimental room and to being handled for microinjections. Twenty-four hrs after the last stress procedure (Day 15), a 30 ga stainless steel microinjector extending 1 mm beyond the guide cannula was inserted into the MDT. Rats were then given a local infusion of saline vehicle (0.25 μl at a flow rate of 0.125 μl/min) or the GABAA receptor antagonist, 1(S),9(R)-( −)bicuculline-methiodide (BMI, 100 pmol/0.25 μl, Sigma), while in their home cage. This dose of BMI was chosen based on results of a pilot study testing the fos response to a range of doses. Following drug administration, the microinjector remained in place for 2 min to allow diffusion. Once the microinjector was removed, the rat was returned to its home cage for an additional 25 min before sacrifice. The brain was rapidly removed, frozen in 2-methylbutane on dry ice, and stored at −80°C.
For in situ hybridization, frozen 16 μm sections were cut on a cryostat through the mPFC and MDT and thaw-mounted onto silanized slides. Adjacent sections were cresyl-stained for regional definition and histological verification. A 1.7kb 35S-labeled riboprobe generated from a linearized cDNA plasmid was used to detect c-fos mRNA expression. Slides were incubated with 107 cpm/ml fos antisense riboprobe in 50% formamide hybridization buffer at 55°C for 18h, post-treated with RNAase, washed in saline sodium citrate (SSC) solutions of increasing stringency, then exposed to x-ray film for 2 weeks. Digital images were captured, and the region of interest analyzed densitometrically using ImageJ. Mean integrated density was calculated from 4–8 sections for each rat.
Experiment 4. in vivo electrophysiological response of mPFC to MDT and vHipp afferent stimulation
Similar to the MDT, the vHipp is a glutamatergic afferent to the mPFC associated with cognitive function and the stress response (Floresco et al., 1997, McEwen et al., 1997, McEwen, 1999, Herry and Garcia, 2002, Block et al., 2007). Thus, we used in vivo electrophysiology to investigate if the effects of CUS on the mPFC response to excitatory afferent activation are specific to the MDT-mPFC pathway, or generalize across excitatory inputs to this region. A total of 15 naïve rats were randomly divided into two stress treatments (CUS or non-stress handling). Twenty-four hours after the last stress session (Day 15), rats were anesthetized with chloral hydrate (400 mg/kg, i.p.). A bipolar stainless steel stimulating electrode was lowered into the right MDT (from bregma; DV: −5.4, AP: −2.6, ML: +0.8 mm) and a glass recording electrode filled with saline was placed in the right mPFC (DV: −3.5 − 4.0, AP: 3.0, ML: +0.6 mm). Body temperature was maintained at 37°C. Following a 30 min equilibration period, local field potentials were recorded in the mPFC (low cutoff filter 0.3 Hz, high cutoff 100 Hz) and digitized (Power Lab; AD Instruments). A current-response curve was established by stimulating the MDT with 30 pulses (100–800 μA in 100 μA steps, 260 msec pulse width, 0.1 Hz). After completing the recording of field potentials evoked from the MDT, the stimulating electrode was withdrawn and relocated to the right vHipp (DV: −7.5, AP: −5.3, ML +5.0 mm). The recording electrode was also withdrawn and repositioned in the mPFC, 200 μm anterior to the initial placement. Field potentials evoked from the vHipp were then recorded as above. The magnitude of the first negative deflection occurring after the stimulus artifact was measured after stimulation in both sites. After completing the experiment, rats were sacrificed and electrode placements confirmed histologically.
Experiment 5: in vivo microdialysis to measure the acute stress-evoked glutamate response in the mPFC
A number of studies have shown that acute stress increases extracellular glutamate levels in the mPFC and that this response is neuronal in nature (Moghaddam, 1993, Moghaddam et al., 1994, Lupinsky et al., 2010, Musazzi et al., 2011). However, the effect of chronic stress on this acute response is unknown. Thus, we used in vivo microdialysis to compare acute stress-evoked glutamate responses in the mPFC of CUS-treated rats and non-stressed controls.
Twenty-five rats were exposed to CUS or non-stressed handling procedures beginning one week post-surgery. All rats were habituated to the buckets (60 cm height x 30 cm diameter) in which microdialysis sample collection would occur for 10min/day after the CUS or handling procedures on Days 12–14. Microdialysis was conducted 1 day after the end of CUS (Day 15). A 4mm microdialysis probe (CMA/12) with a 20 kDa MW cutoff was inserted into the mPFC and perfused with artificial cerebrospinal fluid (147 mM NaCl, 2.5 mM KCl, 1.3 mM CaCl2, 0.9 mM MgCl2, pH 7.4) at 1.0 μl/min. Rats were placed in a circular plastic bucket lined with bedding. After 4 hr equilibration, 3 baseline samples were collected at 20 min intervals, yielding 20 μl/sample. The fourth sample was collected during acute immobilization stress (IMB), a novel stimulus to which the rats had not been exposed during CUS. For IMB, the rat was held prone on a plastic rack large enough to support its body (26 x 13 cm), while its head, limbs and torso were taped gently but securely to the rack. After 20 min, the rat was released and returned to the bucket for two 20 min recovery samples. Levels of glutamate in dialysate were quantified using pre-column o-phthaldialdehyde/sulfate (OPA) derivatization and HPLC with coulometric detection (Coulochem II, ESA Inc., East Chelmsford, MA, USA). Mobile phase (0.1M phosphatate buffer in 20% methanol, pH 4.6) ran at a flow rate of 0.6 ml/min. Glutamate was measured against a calibration curve established daily.
Statistical analyses
Following the completion of experiments, cannulae and electrode placement were verified histologically. Rats with cannulae or electrodes outside of the targeted region were removed from analysis. Likewise, rats that failed to complete AST testing by not attempting to dig for a reward on six consecutive trials were eliminated. All datasets were tested for normality and homogeneity of variance before applying parametric analyses. One-way ANOVA was used to assess the effects of local glutamatergic antagonist treatment in the mPFC on ED performance, as well as the effects of AST and CUS on Arc expression. Two-way ANOVA was used for the c-fos data (Stress x Drug), and a two-way ANOVA with repeated measures for Sample was applied to the microdialysis data. For the microdialysis data, both the absolute levels of glutamate in the dialysate as well as values normalized to percent of mean baseline for each animal were analyzed. For the in vivo electrophysiology study, stimulus-response curves measuring evoked field potentials in the mPFC following MDT or vHipp stimulation were analyzed using an extra sum-of-squares F-test. In all cases, pairwise comparisons were made using the Newman-Keuls test. Significance was set at p< 0.05.
RESULTS
Experiment 1. Glutamatergic AMPA or NMDA receptor antagonists in mPFC impair ED set-shifting performance
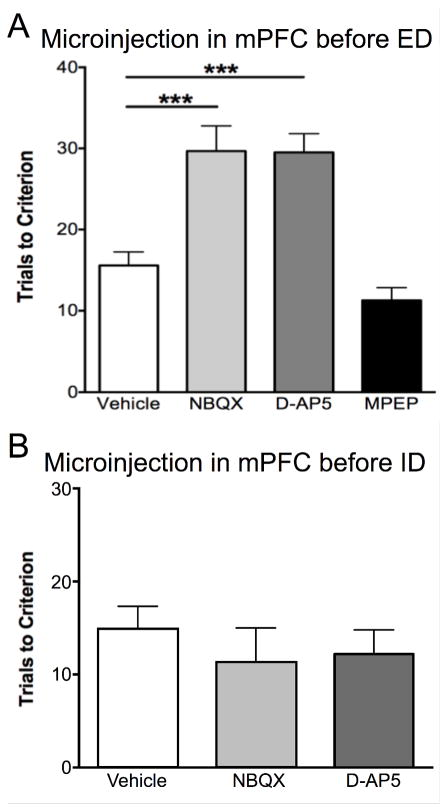
To investigate whether glutamate receptors in the mPFC modulate set-shifting behavior, we locally administered an AMPA receptor (NBQX), NMDA receptor (D-AP5), or mGluR5 receptor antagonist (MPEP) into the mPFC immediately prior to ED. One-way ANOVA revealed a main effect of Drug (F3,27 = 18.37, p<0.0001). Pairwise comparisons found that blocking NMDA or AMPA receptors significantly increased TTC on the ED task, indicating impaired cognitive flexibility (NBQX or D-AP5 vs vehicle, p < 0.005, see Figure 1A). In contrast, rats injected with MPEP were comparable to vehicle treated controls, suggesting that mGluR5 receptors do not modulate set-shifting behavior in the mPFC. The deficit induced by AMPA or NMDA receptor blockade in the mPFC was specific to performance on the ED task, as administration of the antagonists into mPFC prior to the ID task had no effect on ID performance (F2, 13 = 0.68, p = 0.52, n=5–6 per group; Figure 1B).
Figure 1. AMPA and NMDA receptors in the mPFC modulate ED-set-shifting behavior.
A) Local infusion of the AMPA receptor antagonist, NBQX, or the NMDA receptor antagonist, D-AP5, but not the mGluR5 receptor antagonist, MPEP, into the mPFC immediately prior to the ED task compromised set-shifting performance, increasing trials to criterion compared to vehicle-injected controls (***p < 0.001, n = 6–12/group). B) In contrast to ED, blocking AMPA or NMDA receptors in the mPFC with NBQX or D-AP5 prior to the ID task had no effect on performance (n = 5–6/group). Data expressed as mean ± SEM.
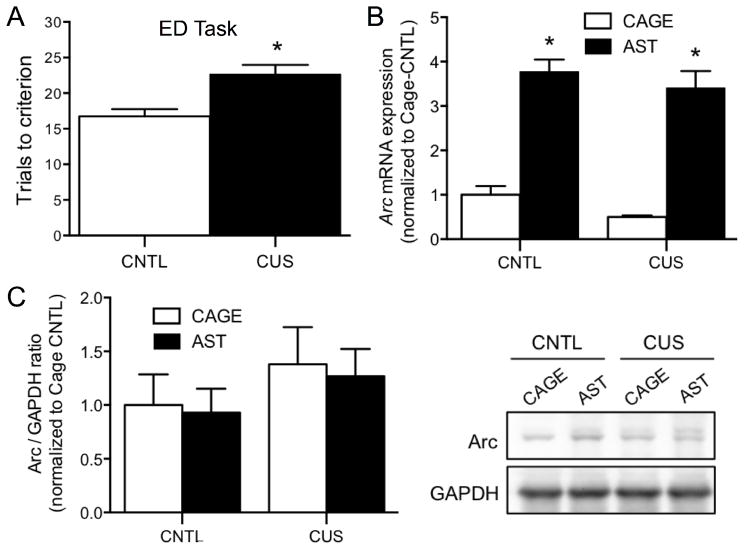
Experiment 2: Performance on the ED set-shifting task increases Arc mRNA expression in the mPFC
For this study we used Arc as a marker of glutamate-mediated plasticity induced by performance on the ED task. As shown previously, CUS compromised set-shifting, inducing a significant increase in trials to criterion on the ED task (Control-AST vs CUS-AST, t15 = 3.52, p<0.01, Figure 2A). There was a significant main effect of AST on Arc mRNA expression in the mPFC (F1,27 = 103.9, p < 0.001, Figure 2B, n = 6–9/group), but no effect of Stress (F1,27 = 2.40, p = 0.132) nor a Stress x AST interaction (F1,27 = 0.05, p = 0.82). In contrast to Arc mRNA expression, there was no effect of AST (F1,25 = 0.10, p = 0.76), Stress (F1,25 = 1.62, p = 0.21), or an AST x Stress interaction (F1,25 = 0.004, p = 0.94, Figure 2C) on Arc protein levels.
Figure 2. Effects of CUS on Arc expression induced in the mPFC by performance on the ED set-shifting task.
A) CUS compromised performance on the ED set-shifting task, inducing a significant increase in trials required to reach criterion (*p<0.01, n=8–9). B) Regardless of stress treatment, Arc mRNA expression was significantly increased in the mPFC 30 min after completing the ED task (*p <0.001, n=6–9). C) Conversely, there was no effect of either CUS or AST on the expression of Arc protein 30 min after completion of the ED task (p = 0.7, n=6–9). Representative western blot images are shown at right. Data expressed as mean ± SEM.
Experiment 3. CUS compromises induction of c-fos expression in the mPFC by excitatory afferent activation from the MDT
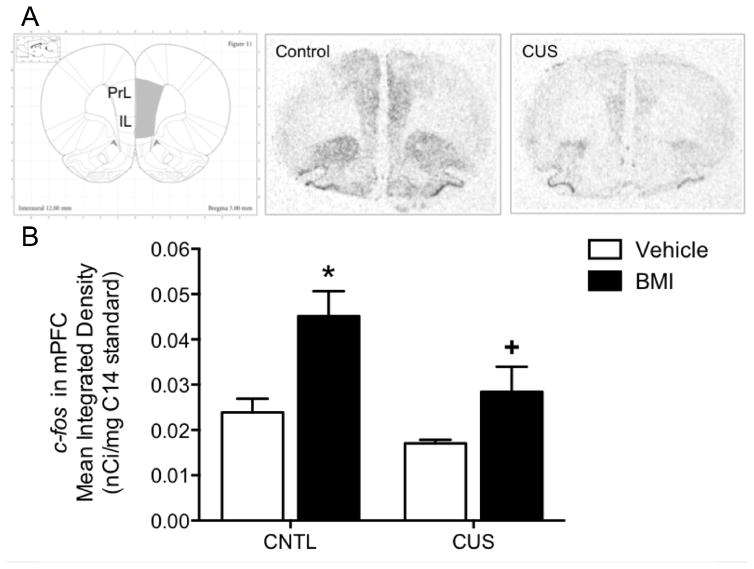
The purpose of this study was to determine if chronic stress-induced cognitive deficits are associated with changes in the mPFC response to glutamatergic afferent activation from the MDT. Figure 3A shows representative audioradiograms of the c-fos response induced in the mPFC of CUS and non-stressed control rats following microinjection of BMI into the MDT. There was a significant main effect of Stress (F1,16 = 6.34, p < 0.05), and of Drug (F1,16 = 12.28, p < 0.05), but no Stress x Drug interaction (F1,16 = 1.10, p = 0.3). In non-stressed control rats, BMI injection in the MDT significantly induced an increase in c-fos expression in the mPFC compared to vehicle injection (p < 0.05). Thus, c-fos induction in the mPFC was a specific response to activation of the MDT, not a non-specific response to the microinjection procedure itself. There was no difference in c-fos expression in the mPFC of CUS and non-stressed control rats in the absence of MDT activation (i.e., following vehicle injection in the MDT). However, specific induction of c-fos expression in the mPFC in response to activation of the MDT was significantly attenuated in CUS-treated rats compared to non-stressed controls (p < 0.05, n = 4–6/group, Figure 3B). To confirm that afferent activation in the two stress groups was equivalent, we measured c-fos expression at the injection site in the MDT. Expression was comparable in the MDT of CUS and non-stressed control rats infused with BMI (t9 = 1.07, p = 0.3, data not shown). Thus, CUS-induced deficits in afferent activation in the mPFC were likely the result of changes in glutamate signaling within the mPFC, rather than changes in the injection site in MDT.
Figure 3. Effects of CUS on glutamatergic afferent-induced activation of the mPFC.
A) Representative autoradiograms of c-fos induction in the mPFC following microinjections of the GABAa receptor antagonist, bicuculline (BMI), into the MDT of control (CNTL) and CUS treated rats. Schematic diagram reproduced with permission from Paxinos and Watson (2007). B) Local BMI injection into the MDT significantly increased c-fos expression in the mPFC of non-stressed control rats compared to vehicle injection (*p < 0.05). The c-fos response to MDT activation was significantly attenuated in the mPFC of CUS-treated rats compared to unstressed controls (+p < 0.05). CUS had no effect on c-fos expression in the mPFC in the absence of MDT activation, i.e., in vehicle-injected rats. Data expressed as mean ± SEM, n = 4–6/group.
Experiment 4. CUS-induced attenuation of the mPFC response to excitatory afferent activation is specific to the MDT-mPFC pathway
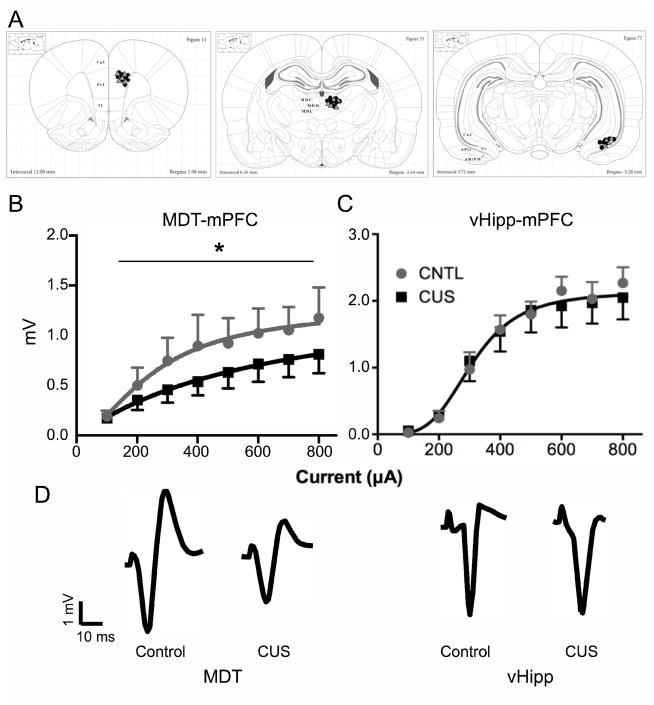
The application of in vivo electrophysiological techniques allowed multiple glutamatergic pathways to be assessed within the same animal (i.e., MDT-mPFC and vHipp-mPFC). Figure 4A shows electrode placements for both treatments. In agreement with our c-fos results, CUS significantly reduced local field potentials evoked in the mPFC by electrical stimulation of the MDT (F3,114 = 3.17, p < 0.05, Figure 4B). In contrast, there was no significant effect of CUS on the mPFC response to vHipp stimulation (F3,90 = 0.24, p = 0.87, Figure 4C, n = 5–10/group). Together, these data suggest that the deleterious effects of CUS on glutamatergic neurotransmission in the mPFC may be specific to the MDT-mPFC pathway.
Figure 4. Impaired excitatory afferent activation of the mPFC is specific to MDT.
A) Placement of recording electrodes in the mPFC and stimulating electrodes in the MDT and vHipp, respectively. Gray triangles and black circles indicate electrode placements for non-stressed control rats and CUS-treated rats, respectively. Schematic diagrams reproduced with permission from (Paxinos and Watson, 2007). B) The mPFC response to MDT stimulation was significantly attenuated in CUS-treated rats compared to non-stressed controls (CNTL, *p < 0.05). C) Conversely, there was no effect of CUS on the mPFC response to vHipp stimulation (p = 0.87), suggesting that the deleterious effects of CUS on excitatory afferent activation of the mPFC may be specific to the MDT-mPFC pathway. D) Representative field potential traces recorded in the mPFC, evoked by stimulation at 800 μA in the MDT (left) or vHipp (right) of rats from each stress condition (CUS and control). Data expressed as mean ± SEM, n = 5–10/group.
Experiment 5. CUS attenuates the acute stress-evoked increase in extracellular glutamate in the mPFC
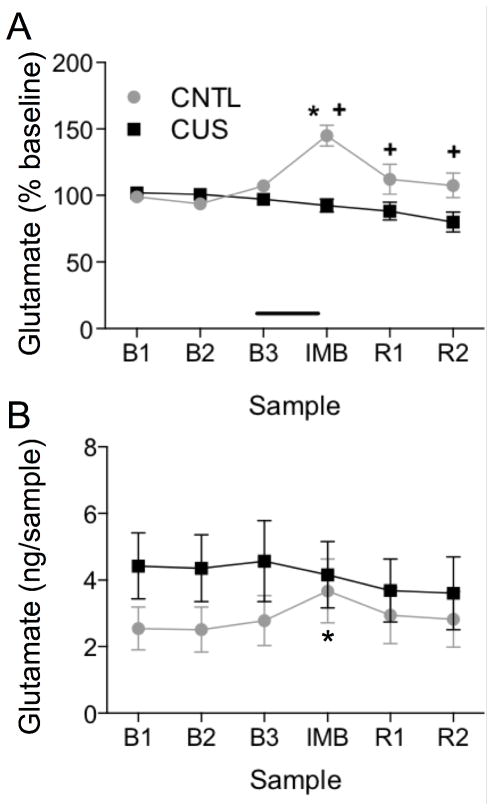
In this experiment, in vivo microdialysis was used to compare acute stress-evoked increases in extracellular glutamate levels in the mPFC of CUS-treated and non-stressed control rats. For data normalized to percent baseline, two-way ANOVA with repeated measures for Sample revealed a main effect of Stress (F1,22 = 12.13, p < 0.01), Sample (F5,110 = 5.07, p < 0.001), and a Stress x Sample interaction (F5,110 = 8.49, p < 0.0001; Figure 5A). Pairwise comparisons revealed that IMB significantly elevated extracellular glutamate in the mPFC of non-stressed control rats compared to their baselines (p < 0.001). By contrast, the IMB-induced response in CUS-treated rats was attenuated compared to unstressed controls (p < 0.001), and was not different from baseline. In the analysis of absolute glutamate levels in the dialysate, there was a slight but non-significant increase in overall glutamate levels in CUS rats (Figure 5B), although a direct comparison of baselines revealed no difference (Control: 2.6 ± 0.7 ng/sample, CUS: 4.8 ± 1.0 ng/sample). Nonetheless, there was no main effect of Stress (p=0.35) or Sample (p=0.10), but there was a significant interaction (F5,110 = 3.00, p < 0.05). Pairwise comparisons again showed that IMB elevated extracellular glutamate in the mPFC of non-stressed control rats (p<0.05), but not in CUS rats (Figure 5B). One rat was removed from this analysis due to probe misplacement.
Figure 5. CUS attenuates the acute stress-evoked increase in extracellular glutamate in the mPFC.
A) Data normalized to percent baseline for each subject show that acute immobilization stress (IMB) significantly increased glutamate levels in dialysate collected in the mPFC of unstressed control rats (CNTL) (*p < 0.001, IMB compared to baseline), and the IMB-induced glutamate response in the mPFC of CUS treated rats was significantly attenuated compared to controls (+p < 0.001, CUS compared to control).. B) Data analyzed as the absolute amount of glutamate collected in the dialysate samples (ng/sample) without normalizing also show that IMB increased extracellular glutamate levels in control rats, but not after CUS (*p < 0.05, IMB compared to baseline). There were no significant baseline differences between groups. In both cases, data are expressed as mean ± SEM, n = 11–13/group.
DISCUSSION
In this study, microinjecting either an AMPA or NMDA receptor antagonist into the mPFC replicated CUS-induced cognitive deficits in ED set-shifting, suggesting that impaired glutamate transmission could underlie the detrimental effects of CUS on cognitive flexibility. In contrast, mGluR5 receptor blockade had no effect on ED performance, indicating the receptor specificity of this effect. We then investigated potential changes in glutamate transmission associated with CUS-induced cognitive impairment, and found that ED deficits in CUS treated rats were associated with attenuated afferent activation of the mPFC, manifest as reduced c-fos induction in the mPFC in response to pharmacological activation of the MDT. Expression of c-fos in the MDT injection site was comparable in CUS rats and non-stressed controls, indicating that the attenuated c-fos response after CUS likely results from changes in glutamate transmission in the mPFC. We also observed an attenuation of local field responses evoked in the mPFC by excitatory afferent activation after CUS. This effect appeared to be specific to the MDT-mPFC pathway, as CUS had no effect on the mPFC response to vHipp stimulation. Lastly, we found that acute stress-evoked glutamate efflux in the mPFC was significantly decreased in CUS-treated rats compared to non-stressed controls. Collectively, these results demonstrate that CUS dysregulates glutamate transmission in the mPFC.
Clinical and preclinical evidence has implicated compromised NMDA and AMPA receptor function in the pathophysiology of prefrontal cortical cognitive impairments and stress-related neuropsychiatric disorders. In agreement with our results, others have demonstrated that AMPA and NMDA receptors are necessary for cognitive flexibility (Stefani et al., 2003, Stefani and Moghaddam, 2005). Microinjection of AMPA or NMDA receptor antagonists into the mPFC of stress-naïve rats induced set-shifting deficits similar to those induced by CUS, indicating that compromised glutamate signaling in the mPFC after CUS could contribute to the resulting ED deficit. However, the deficits induced by NMDA or AMPA antagonists were greater than that after CUS, suggesting that CUS attenuates but does not completely inhibit glutamate signaling in the mPFC.
In contrast to NMDA and AMPA receptors, the role of mGluR5 receptors in cognitive flexibility is less clear. Previous studies have shown that mGluR5 antagonist treatment impairs working memory and spatial learning (Balschun and Wetzel, 2002, Homayoun et al., 2004). In the current study, administration of the mGluR5 antagonist, MPEP, into the mPFC had no effect on the ED task. Systemic administration of MPEP or the mGluR5 receptor positive allosteric modulator, CDPPB, also have been shown to have no effect on set-shifting (Darrah et al., 2008). However, under conditions in which glutamate transmission was compromised (e.g., NMDA receptor antagonist treatment), administration of a mGluR5 receptor antagonist augmented, and a mGluR5 receptor agonist attenuated deficits in cognitive flexibility (Homayoun et al., 2004, Darrah et al., 2008). Thus, whereas mGluR5 antagonists may have no effect in basal conditions, modulatory effects of mGluR5 receptors on cognitive flexibility might be evident under conditions in which glutamate transmission has been dysregulated.
Arc is commonly used as a neuronal marker for experience-driven activity, and is predominantly expressed in excitatory neurons (Vazdarjanova et al., 2006, Shepherd and Bear, 2011). It is perhaps surprising then that the increase in Arc mRNA expression in mPFC following completion of the set-shifting task was comparable in rats exposed to CUS and in unstressed controls, despite the increased difficulty of the task after CUS. Others have shown that rats less proficient at acquiring a learning task expressed more Arc mRNA, indicating a potential accumulation over time (Kelly and Deadwyler, 2003). Because CUS rats required more trials, hence more time, to complete the ED task, it is possible that by the time they completed the task, the cumulative induction of Arc was comparable to that in controls, as tissue was collected at an equivalent time point relative to completion of the task, i.e., 30 min after reaching criterion. Arc mRNA may also have accumulated throughout the entire sequence of tasks comprising the AST, rather than being induced after ED specifically. However, in experiment 1, injection of glutamate antagonists into the mPFC prior to the ID task did not disrupt performance on that task, suggesting that glutamate neurotransmission in the mPFC is not involved in the task immediately preceding ED. Hence, Arc was unlikely to have been induced during ID. Given the results of our subsequent studies showing that glutamate function in the mPFC was compromised after CUS, it is also unlikely that the comparable induction of Arc mRNA indicates that glutamate-mediated plasticity is resistant to the detrimental effects of CUS. It is important to note, however, that while set-shifting was compromised in the CUS rats, both groups eventually completed the task successfully, even if it required more trials for the CUS rats to do so. Thus, in this experiment, Arc mRNA induction appeared to reflect successful completion of the task, rather than the increased difficulty in completing the task after CUS.
By contrast with Arc mRNA, there was no change in Arc protein after completion of the set-shifting task. This could result from the early time point at which tissue was collected (30 min) in order to capture rapid changes in Arc mRNA, but increases in rapidly induced proteins can be observed after 30 min. A more likely explanation is that Arc transcription and translation can be regulated independently within this 30 min time frame. Whereas rapid transcription of Arc mRNA is induced by NMDA receptor or voltage-sensitive calcium channel activity, rapid Arc protein translation is associated with mGluR1/5 receptor activity (Rao et al., 2006, Lonergan et al., 2010, Shepherd and Bear, 2011). Thus, the selective induction of Arc mRNA may be consistent with the results of experiment 1, showing that NMDA and AMPA receptors, but not mGluR5 receptors in the mPFC are involved directly in modulating cognitive set-shifting. Another potential explanation is that Arc protein is localized to synaptic spines and to the post-synaptic density (Chowdhury et al., 2006). Thus, rapid plasticity accompanying successful completion of the ED set-shifting task may have recruited existing Arc protein to act locally in synapses activated during the task, whereas the observed induction of Arc transcription may be in anticipation of a later role for Arc in mediating longer-lasting plasticity (Plath et al., 2006).
In depressed patients, cognitive dysfunction is associated with hypoactivity and compromised glutamate transmission in the PFC (Anand et al., 2005, Bermpohl et al., 2009, Koenigs and Grafman, 2009, Disner et al., 2011). Similarly, we found that CUS-induced ED deficits are associated with attenuated excitatory afferent activation of the mPFC in rats. Preclinical studies have shown that chronic stress decrease glutamate receptor expression and induced dendritic atrophy in mPFC pyramidal cells (Cook and Wellman, 2004, Radley et al., 2004, Li et al., 2011, Yuen et al., 2012). Such postsynaptic changes may account for the attenuated mPFC response to afferent activation. c-fos is expressed in multiple cell types, including pyramidal cells, interneurons, and glia (Bing et al., 1992, Bubser et al., 1998, Edling et al., 2007, Yuan et al., 2010), although glial Fos expression is minimal in vivo (Bing et al., 1992, Yuan et al., 2010). There is evidence that chronic stress induces hypertrophy of interneurons that target the apical dendrites of pyramidal cells in layers II/III of the mPFC (Gilabert-Juan et al., 2013). Hence, enhanced inhibitory control of the mPFC could potentially reduce pyramidal cell activation and overall c-fos induction (Kuroda et al., 2004, Gilabert-Juan et al., 2013). However, interneurons only account for ~8% of the total Fos-positive cells in the mPFC following pharmacological stimulation of the MDT (Bubser et al., 1998). Thus, it is likely that CUS-induced changes in pyramidal cell response accounted for the CUS-induced attenuation of afferent-evoked c-fos expression in the present study.
In line with the c-fos results, our in vivo electrophysiology results indicate that CUS impaired the maximal response of mPFC to MDT stimulation. This reduction in response magnitude suggests that the capacity of the MDT-mPFC circuit was altered by CUS, potentially through changes in spine density or receptor number post-synaptically, and/or pre-synaptic release capacity. The MDT and mPFC work in concert to facilitate cognitive flexibility during set-shifting behaviors (Monchi et al., 2001, Floresco and Grace, 2003, Block et al., 2007). Attenuating MDT activity increased perseverative errors without affecting acquisition, indicating that the ability to disengage from a previously learned contingency was impaired (Block et al., 2007, Parnaudeau et al., 2013, Parnaudeau et al., 2015). Thus, MDT activity signals the mPFC when it is necessary to shift from a learned contingency based on negative feedback from the environment, and CUS-induced deficits in MDT-mPFC activity may impair the ability to disengage from a learned cognitive set. In contrast to the MDT input, CUS had no effect on the mPFC response to vHipp stimulation, another major glutamatergic afferent to the mPFC. Although these findings suggest that CUS may selectively impair the MDT-mPFC pathway, others have shown that chronic stress decouples vHipp connectivity with the mPFC (Zheng and Zhang, 2015). These conflicting results may be due to procedural differences, as we used a 2-week stress procedure rather than 3 weeks in the previous study. While this difference in duration may seem trivial, it has been shown that 21 days of CUS induces dendritic atrophy in the hippocampus, but not 10–14 days (Magarinos and McEwen, 1995, Vyas et al., 2002, Bessa et al., 2009). Thus, if stress-induced changes in the vHipp contribute to impairments in mPFC response, perhaps our 2-week CUS paradigm was of insufficient duration to compromise the vHipp-mPFC pathway.
Acute stress exposure increases glutamate efflux in the mPFC, and this response is neuronally mediated (Moghaddam, 1993, Moghaddam et al., 1994, Bagley and Moghaddam, 1997, Lupinsky et al., 2010). To examine potential changes in this response after CUS, we used in vivo microdialysis to compare acute stress-evoked glutamate levels in the mPFC of control rats (i.e., response to first stress exposure) and CUS-treated rats (i.e., response to the 15th in a series of varied acute stress exposures). In agreement with others, we found that acute stress increased glutamate levels in the mPFC of non-stressed control rats. Conversely, the acute stress-evoked glutamate response in CUS-treated rats was attenuated compared to controls. Using the same chronic and acute stress protocol, we previously showed that the evoked release of norepinephrine in the mPFC was equivalent in control and CUS rats (Jett and Morilak, 2013). Therefore, habituation to the CUS paradigm is unlikely to account for the attenuated glutamate response. Others have shown that acute tail pinch increased glutamate in the mPFC following chronic restraint stress (Luczynski et al., 2015). However, unlike CUS, chronic restraint is a homotypic and predictable stressor, thus this discrepancy with our results may reflect differences in the nature of the chronic stress paradigms. Further, in vivo microdialysis measures net changes in extracellular levels of neurotransmitters, not glutamate release per se (Timmerman and Westerink, 1997, Hascup et al., 2010). Thus, the altered response to acute stress after CUS may reflect an increase in glutamate uptake, decrease in terminal release, or changes in glutamate synthesis and/or metabolism. Regardless of the mechanism, a decrease in glutamate response evoked by a potent physiological stimulus, together with reduced mPFC response to excitatory afferent activation, reflect a compromise in glutamate neurotransmission induced by CUS that could account for the deficit in cognitive flexibility mediated in the mPFC.
CONCLUSION
In conclusion, we examined the effects of CUS on molecular, circuit-level, and behavioral processes associated with glutamatergic signaling in the mPFC. Our findings indicate that chronic stress-induced deficits in cognitive flexibility are associated with compromised glutamatergic function in the mPFC. CUS attenuated the mPFC response to activation of the excitatory afferent from the MDT, and attenuated the acute stress-evoked increase in extracellular glutamate levels in the mPFC. Directly blocking AMPA or NMDA receptors in the mPFC induced deficits in cognitive set-shifting that mimic those induced by CUS, suggesting that reduced glutamate function in the mPFC could account for chronic stress-induced impairment on this task. By contrast, mGluR5 receptor blockade had no effect, indicating that ionotropic but not metabotropic glutamate receptors in mPFC are involved in set-shifting. The impairment induced by glutamate receptor antagonists was specific to set-shifting, as there was no effect when glutamate receptors were blocked prior to testing on the ID task. Further, successful performance of cognitive flexibility on the ED task increased Arc mRNA expression in the mPFC of both stressed and non-stressed rats, suggesting that neural plasticity is involved in this cognitive process once it is mastered, even if it is more difficult to master after stress.
The NMDA receptor antagonist, ketamine, has rapid antidepressant effects when given acutely in low doses to treatment-resistant depressed patients (Carlson et al., 2006, Zarate et al., 2006, Machado-Vieira et al., 2009). Previously, we showed that acute ketamine administration 24 hrs prior to AST testing reversed CUS-induced set-shifting deficits (Jett et al., 2015a). Acute ketamine administration increases glutamate signaling through AMPA receptors in the mPFC, which is necessary for its therapeutic-like effects after chronic stress (Moghaddam et al., 1997, Li et al., 2010). Thus, our current results, together with previous preclinical and clinical evidence suggest that ketamine may facilitate cognitive function in CUS treated rats, and in depressed patients, by restoring compromised glutamate neurotransmission in the mPFC and related circuitry. Accordingly, elucidating the mechanisms by which chronic stress compromises glutamate function in the mPFC may inform the development of more efficacious strategies for the treatment or prevention of stress-related psychiatric disorders.
Highlights.
Depression is associated with cognitive flexibility deficits and mPFC hypoactivity
Chronic unpredictable stress (CUS) induces cognitive flexibilty deficits
CUS attenuated mPFC response to afferent activation and acute increases in glutamate
NMDA or AMPA, but not mGlur5, antagonists in mPFC compromised set-shifting
Thus glutamate dysregulation in mPFC could underly cognitive deficits after CUS
Glutamate signaling is a viable therapeutic target for cognitive deficits in depression
Acknowledgments
We thank Michael Patton and Denisse Paredes for excellent technical assistance, and thank Dr. Milena Girotti for expert advice with the western blot and RT-PCR assays.
FUNDING SOURCE: This work was supported by research grant MH053851 from the National Institute of Mental Health, NIH; by a Translational Sciences Training fellowship awarded through grant TL1 TR001119 from the National Center for Advancing Translational Sciences, National Institutes of Health; and by the Blueprint Program for Enhancing Neuroscience Diversity through Undergraduate Research Education Experiences (BP-ENDURE) R25 grant NS080684. These funding sources had no role or influence in study design, data collection, analysis or interpretation, nor in the preparation or decision to submit this paper for publication.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- D-AP5
D-2-amino-5-phosphonopentanoate
- Arc/Arg3.1
Activity-regulated cytoskeleton-associated protein
- AST
Attentional set-shifting test
- BMI
Bicuculline methiodide
- CD
Compound discrimination
- CUS
Chronic unpredictable stress
- ED
Extradimensional set-shift
- ID
Intra-dimensional shift
- IMB
Immobility stress
- MDT
Mediodorsal thalamus
- MPEP
2-Methyl-6-(phenylethynyl)pyridine
- mPFC
Medial prefrontal cortex
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione
- NMDA
N-methyl-D-aspartic acid
- R1
First reversal
- R2
Second reversal
- SD
Simple discrimination
- TTC
Trials to criterion
- vHipp
Ventral hippocampus
Footnotes
CONTRIBUTORS: Dr. Jett participated in experimental design and planning, conduct of the experiments, data analysis and interpretation, writing and editing the manuscript.
Ms. Hatherall participated in conduct of the experiments, data analysis, and editing of the manuscript.
Ms. McCarley participated in conduct of the experiments, data analysis, and editing of the manuscript.
Dr. Bulin participated in experimental design, conduct of the experiments, data analysis, and editing of the manuscript.
Dr. Morilak participated in experimental design and planning, data analysis and interpretation, writing and editing the manuscript, and provided laboratory resources and oversight for the experiments.
All authors have read and approve the final manuscript.
DECLARATION OF INTEREST: Dr. Morilak has served on a Psychopharmacology advisory board for H. Lundbeck A/S within the past three years, and receives research funding from Lundbeck Research USA, Inc. These activities have no relation to any of the work presented in this paper. All other authors have no interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Walter M, Sajonz B, Lucke C, Hagele C, Sterzer P, Adli M, Heinz A, Northoff G. Attentional modulation of emotional stimulus processing in patients with major depression--alterations in prefrontal cortical regions. Neurosci Lett. 2009;463:108–113. doi: 10.1016/j.neulet.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Bing G, Chen S, Zhang Y, Hillman D, Stone EA. Noradrenergic-induced expression of c-fos in rat cortex: neuronal localization. Neurosci Lett. 1992;140:260–264. doi: 10.1016/0304-3940(92)90116-o. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bruno V, Scapagnini U, Canonico PL. Excitatory amino acids and neurotoxicity. Funct Neurol. 1993;8:279–292. [PubMed] [Google Scholar]
- Bubser M, de Brabander JM, Timmerman W, Feenstra MG, Erdtsieck-Ernste EB, Rinkens A, van Uum JF, Westerink BH. Disinhibition of the mediodorsal thalamus induces fos-like immunoreactivity in both pyramidal and GABA-containing neurons in the medial prefrontal cortex of rats, but does not affect prefrontal extracellular GABA levels. Synapse. 1998;30:156–165. doi: 10.1002/(SICI)1098-2396(199810)30:2<156::AID-SYN5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology (Berl) 2011;216:525–535. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G. Reduced Metabotropic Glutamate Receptor 5 Density in Major Depression Determined by [11C]ABP688 PET and Postmortem Study. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–340. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigg J, Tan AM, Finch DM. Glutamatergic hippocampal formation projections to prefrontal cortex in the rat are regulated by GABAergic inhibition and show convergence with glutamatergic projections from the limbic thalamus. Hippocampus. 1994;4:189–198. doi: 10.1002/hipo.450040209. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Castillo-Gomez E, Guirado R, Molto MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013;218:1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, Gerhardt GA. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology (Berl) 2015a doi: 10.1007/s00213-015-3957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Evans L, Patton M, Morilak DA. Chronic unpredictable stress dysregulates glutamate transmission in the rat medial prefrontal cortex: A potential role for noradrenergic modulation. Soc Neurosci Abstr. 2015b;41 online:Program number 254.08. [Google Scholar]
- Jett JD, Morilak DA. Too much of a good thing: blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology. 2013;38:585–595. doi: 10.1038/npp.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Yokofujita J, Oda S, Price JL. Synaptic relationships between axon terminals from the mediodorsal thalamic nucleus and gamma-aminobutyric acidergic cortical cells in the prelimbic cortex of the rat. J Comp Neurol. 2004;477:220–234. doi: 10.1002/cne.20249. [DOI] [PubMed] [Google Scholar]
- Lee YA, Goto Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure--prefrontal cortex interaction. Eur J Neurosci. 2011;34:426–436. doi: 10.1111/j.1460-9568.2011.07750.x. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ. Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast. 2010;2010:139891. doi: 10.1155/2010/139891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Moquin L, Gratton A. Chronic stress alters the dendritic morphology of callosal neurons and the acute glutamate stress response in the rat medial prefrontal cortex. Stress. 2015;18:654–667. doi: 10.3109/10253890.2015.1073256. [DOI] [PubMed] [Google Scholar]
- Lupinsky D, Moquin L, Gratton A. Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J Neurosci. 2010;30:7624–7633. doi: 10.1523/JNEUROSCI.1187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7(Suppl 3):S323–328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joels M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011;59:138–149. doi: 10.1016/j.neuint.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry. 2015;77:445–453. doi: 10.1016/j.biopsych.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. San Diego: Academic Press; 2007. [Google Scholar]
- Pirot S, Jay TM, Glowinski J, Thierry AM. Anatomical and electrophysiological evidence for an excitatory amino acid pathway from the thalamic mediodorsal nucleus to the prefrontal cortex in the rat. Eur J Neurosci. 1994;6:1225–1234. doi: 10.1111/j.1460-9568.1994.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Strijbos PJ. Neuronal protein kinase signaling cascades and excitotoxic cell death. Ann N Y Acad Sci. 2001;939:11–22. doi: 10.1111/j.1749-6632.2001.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- Yuan H, Gao B, Duan L, Jiang S, Cao R, Xiong YF, Rao ZR. Acute hyperosmotic stimulus-induced Fos expression in neurons depends on activation of astrocytes in the supraoptic nucleus of rats. J Neurosci Res. 2010;88:1364–1373. doi: 10.1002/jnr.22297. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zhang T. Synaptic plasticity-related neural oscillations on hippocampus-prefrontal cortex pathway in depression. Neuroscience. 2015;292:170–180. doi: 10.1016/j.neuroscience.2015.01.071. [DOI] [PubMed] [Google Scholar]