Abstract
In this study, measures of the quality and availability of social supports were found to moderate risk for depression associated with a history of maltreatment and the presence of the short (s) allele of the serotonin transporter gene promoter polymorphism (5-HTTLPR). The present investigation (i) replicates research in adults showing that 5-HTTLPR variation moderates the development of depression after stress, (ii) extends the finding to children, and (iii) demonstrates the ability of social supports to further moderate risk for depression. Maltreated children with the s/s genotype and no positive supports had the highest depression ratings, scores that were twice as high as the non-maltreated comparison children with the same genotype. However, the presence of positive supports reduced risk associated with maltreatment and the s/s genotype, such that maltreated children with this profile had only minimal increases in their depression scores. These findings are consistent with emerging preclinical and clinical data suggesting that the negative sequelae associated with early stress are not inevitable. Risk for negative outcomes may be modified by both genetic and environmental factors, with the quality and availability of social supports among the most important environmental factors in promoting resiliency in maltreated children, even in the presence of a genotype expected to confer vulnerability for psychiatric disorder.
Keywords: child maltreatment, gene-by-environment interaction
Child abuse is a pervasive societal problem, with nearly 1 million substantiated reports of child maltreatment each year (1), many reported cases of actual abuse that are not verified (2), and countless other cases that never come to the attention of authorities (3). Although not all abused children develop difficulties, many experience a chronic course of psychopathology, with depression as one of the most common psychiatric sequelae reported in maltreated children (4, 5).
Preclinical studies suggest that stress early in life can promote long-term changes in multiple neurochemical systems (6, 7). Specifically, exposure to prenatal and/or postnatal stress is associated with increased basal and stress-induced responsiveness of the hypothalamic pituitary adrenal axis, increased central corticotropin-releasing hormone and norepinephrine drive, decreased γ-aminobutyric acid/benzodiazepine functioning, multiple alterations in the serotonergic system, and reduction in hippocampal volume, a brain structure vulnerable to the neurotoxic effects of stress-induced elevations in circulating glucocorticoids (e.g., cortisol) and amino acids (e.g., glutamate).
Because many of the biological alterations associated with early stress in preclinical studies have been reported in adults with depression, it has been hypothesized that the neurobiological changes associated with adverse early experiences may confer a vulnerability for the development of depression (6, 8). Consistent with preclinical studies, adults with depression have been reported to have multiple alterations of the hypothalamic pituitary adrenal axis (9), increased cerebrospinal fluid corticotropin-releasing hormone (10), elevated cerebrospinal fluid norepinephrine concentration (11), decreased cortical γ-aminobutyric acid measured in vivo using proton magnetic resonance spectroscopy (12), and multiple indicators of altered serotonin (5-HT) functioning (13), including reduced serotonin transporter (5-HTT) availability as evidenced by reduced [123I] β-CIT spect binding (14). Reduction in hippocampal volume has also been reported in depressed adults in numerous investigations (15).
There are emerging preclinical and clinical data to suggest that the long-term changes associated with early stress are not inevitable and can be modified by genetic factors (16, 17). In studies with non-human primates, polymorphic variation in the gene encoding the serotonin transporter protein (SLC6A4) has been found to moderate the effects of early stress. The serotonin transporter is a protein critical to the regulation of serotonin function in the brain, because serotonin's action in the synapse is terminated by its reuptake. SLC6A4 has a well studied functional variable number of tandem repeats (VNTR) polymorphism in the promoter region; the variant site is commonly known as 5-HTTLPR. There are two common functionally different alleles at the 5-HTTLPR site, the short (s) allele and the long (l) allele. The s allele of 5-HTTLPR contains an attenuated promoter segment and is associated with reduced transcription and functional capacity of the serotonin transporter relative to the l allele (18, 19). In non-human primates, the 5-HTTLPR genotype makes little difference in predicting phenotypes of infants reared under optimal conditions (e.g., parent-reared). However, for infants reared under more stressful conditions (e.g., peer-reared), compared with those homozygous for the l allele, infants with the s allele show increased emotional distress, elevated hypothalamic pituitary adrenal axis response to stress, and reduced basal serotonergic functioning (20–22). There appears to be a gene by environment interaction between experiences of stress and the serotonin transporter gene, with the s allele of the 5-HTTLPR associated with adverse outcomes, but only in primates subjected to early stress. Another SLC6A4 variant, in intron 2, has been shown to have the potential to affect transcriptional regulation during development (23), and 5-HTTLPR is in linkage disequilibrium with this intron 2 variant (24).
Results of a large-scale epidemiological investigation of adults conducted by Caspi et al. (25) are consistent with the findings in primates and likewise suggest a gene-by-environment interaction involving the 5-HTTLPR. In the study, the s allele of the 5-HTTLPR was associated with the development of depression, but only in adults with histories of child maltreatment or recent stressful life events. In the absence of these experiences, the s allele was not associated with an increased risk for psychopathology.
In addition to genetic factors, there are also emerging preclinical and clinical data to suggest that the long-term neurobiological and behavioral changes associated with early stress can be modified by the availability of positive supports and optimal subsequent care-giving experiences (26). Several investigators using mother–infant separation paradigms in rodents, one of the most frequently used paradigms to examine the effects of early stress, have noted that separation resulted in subtle disruptions in the quality of maternal–pup interaction. By providing the mother with a foster litter during the period of infant separation, the investigators were able to prevent the deterioration in maternal care behaviors and subsequently prevent most of the long-term neurobiological changes associated with early separation (27). These findings are consistent with the results of studies examining the effects of prenatal stress. In these studies, adoption with optimal parenting has also been found to reverse the hypothalamic pituitary adrenal axis alterations typically observed in association with these early stress paradigms (28). Clinical studies of individuals with a history of abuse also suggest that the availability of a caring and stable parent or alternate guardian is one of the most important factors that distinguish abused individuals with good developmental outcomes from those with more deleterious outcomes (29).
Given emerging preclinical and clinical findings demonstrating the importance of both genetic and environmental (e.g., care giving and social supports) factors in determining the consequences of early stress, we examined the moderating role of the 5-HTTLPR and social supports on the development of depression in maltreated children. The objectives of this investigation were (i) to replicate and extend to children the findings in adults that suggest a moderating role of the 5-HTTLPR in the development of depression and (ii) to examine the potential additional modifying impact of social supports on the development of depression in maltreated children. It was hypothesized that both genotype and social supports would predict depression in maltreated children and that maltreated children with the s allele and unavailable or negative social supports would have the most severe depressive symptomatology.
Methods
Sample. Participants included 101 children: 57 were removed from their parents' care by the State of Connecticut Department of Children and Families (DCF) within the past 6 months because of allegations of abuse and/or neglect, and 44 were community controls (CC) with no history of maltreatment or exposure to intrafamilial violence. Participants were drawn from a larger study examining (i) the effects of trauma on children over time and (ii) the efficacy of the SAFE Homes intervention, a DCF program in which children who have been removed from their parents' care are placed temporarily in state-run facilities rather than immediate foster care to facilitate assessment and treatment planning.
The 101 children were from 67 families with one to four siblings and half-siblings in each family. As detailed in the data analyses section, statistical approaches were used to control for familial correlations between subjects resulting from the inclusion of siblings in the sample. Children ranged in age from 5 to 15 years, with a mean age of 10.0 ± 2.3 years. Twenty-one percent of the children were European-American, 25% were Hispanic, 32% were African-American, and 22% were biracial, and the sample was approximately evenly divided by sex (46% male). The DCF and CC groups did not differ in terms of age (t =–0.75, df = 99, P = 0.45), sex (χ2 = 0.68, df = 1, P = 0.41), or ethnic composition (χ2 = 3.34, df = 3, P = 0.34).
Inclusion Criteria for CC Children. Eligible CC families met the following criteria: (i) reported annual household income of $25,000 or below, and (ii) as reported by birth mothers and verified by the DCF computerized record system, no contact with protective services and no history of abuse, neglect, and/or exposure to domestic violence. CCs were recruited through newspaper ads and targeted mailings, and prospective subjects were screened for study inclusion by telephone.
Inclusion Criteria for Maltreated Children. Children recruited for the DCF group met the following criteria: (i) removal from parental care because of allegations of abuse or neglect, and (ii) 96-hour temporary custody of the children awarded to DCF by the courts. Eligible DCF families were informed about the study by their caseworkers. Interested parents signed a form consenting for research staff to contact them about the study.
The Yale University Human Investigations Committee and the DCF Institutional Review Board approved the present investigation. The children's legal guardians provided written consent, and all children provided written assent for study participation. In cases where the children's legal guardian was not their birth parent, the children's caseworker signed for them to participate in the study, and written assent for participation was also obtained from the birth parents if they were available.
Maltreatment History. Multiple informants and data sources (e.g., caseworkers, parents, children, and DCF case records) were used to obtain a best estimate of a child's maltreatment history. Data from the various sources were reviewed and synthesized to summarize the severity of children's maltreatment experiences using the operationalized criteria and coding system delineated in ref. 30. Severity of five maltreatment subtypes (i.e., physical abuse, sexual abuse, neglect, emotional abuse, and exposure to domestic violence) were rated, with the scales showing good inter-rater reliability (mean intraclass correlation across categories of maltreatment = 0.91; range = 0.84–0.97). In the present cohort, >80% of the DCF sample experienced two or more types of maltreatment. Sixty-three percent of the sample had a history of physical abuse; 19% had a history of sexual abuse; 81% had a history of neglect; 79% had a history of emotional maltreatment, and 60% had a history of exposure to domestic violence.
Depression Severity. The Mood and Feelings Questionnaire (MFQ) was used to assess children's depression symptomatology. The MFQ is a 33-item self-report measure that assesses depression in children, with each item rated on a 0- to 2-point scale (31). It has excellent psychometric properties and has been used extensively in clinical and epidemiological research. The measure was individually administered. Research assistants read the MFQ items to the children and used pictorial scoring aids to facilitate administration with the younger children.
Psychiatric Diagnoses. As previously described (32), a number of standardized parent- and child-report questionnaires, as well as the semistructured diagnostic interview the Schedule for Affective Disorders and Schizophrenia for School Aged Children (33), were used to generate child psychiatric diagnoses. The maltreated children were more likely to meet criteria for major depression, dysthymia, or minor depression than the CCs (any depressive disorder: maltreated, 22.8%; CCs, 4.5%; χ2 = 6.55, df = 1, and P < 0.01), although few children met full diagnostic criteria for major depression (major depressive disorder: maltreated, 7.0%; CCs, 0.0%; χ2 = 3.22, df = 1, and P < 0.08). Given that children are not yet through the period of risk for the development of major depression and that so few children in the comparison group met diagnostic criteria for any depressive disorder, the dimensional severity measure derived from the Mood and Feelings Questionnaire was used in subsequent analyses examining predictors of depression in the children.
Social Supports. The Arizona Social Support Interview Schedule (ASSIS) was used to assess children's social supports. The ASSIS (34) is an interview that was originally developed for adults and was revised for use with school-aged children (5). During the interview, children are asked to name people they (i) talk to about personal things; (ii) count on to buy the things they need; (iii) share good news with; (iv) get together with to have fun; and (v) go to if they need advice. Children are also asked to name people who make them angry or upset (negative relationships). Children provide information on the nature of their relationship with the people named (i.e., parent, relative, other adult, or friend) and the frequency with which they see each support, with frequency of contact rated on a 1- to 5-point scale (1, daily; 2, almost daily; 3, monthly; 4, semiannually; 5, annually). In prior research, scores on the ASSIS distinguished depressed abused children from non-depressed abused children and were found to correlate significantly with measures of basal cortisol secretion (5). Summary social support measures used in the current investigation include (i) number of positive support categories listed for the child's top support and (ii) frequency with which the child reported seeing his or her top support.
Genotyping. Serotonin transporter. We studied the well known functional polymorphism in the 5′ flanking regulatory (promoter) region of the gene (SLC6A4) coding for the serotonin transporter protein. This polymorphism (5-HTTLPR) has two common alleles that have been designated as long (16 repeats) and short (14 repeats), according to their relative size. A rarer extra-long (20 repeats) allele has also been characterized (24). Ancestry informative markers. Thirty-four additional markers were genotyped to provide information useful for clustering the sample into population groups (35).
Saliva was collected for DNA extraction and analyses. To collect the specimens, 10 ml of Original Mint Scope Mouthwash (Procter & Gamble) was dispensed into a 50-ml tube. Children were instructed to swish the Scope in their mouths for 45 seconds and then spit back into the 50-ml tube. Specimens were refrigerated within 2 hours of sample collection, and DNA was extracted by using PUREGENE (Gentra Systems) kits and protocol. The SLC6A4 promoter VNTR polymorphism was genotyped by agarose gel size fractionation as described in ref. 24. To assure the accuracy of genotyping, 100% of the specimens were reanalyzed, and no discrepancies in genotype assignment were identified. Alleles were designated according to their relative size: short (14 repeats), long (16 repeats), or extra-long (20 repeats). The rare extra-long allele was observed only in two subjects, and in characterizing the genotype of these two subjects, the extra-long allele was classified as long. These children were not outliers on any variables.
Ancestral Proportion Scores. Although the maltreated and comparison groups were comparable in terms of racial composition, to prevent spurious associations that can result from variation in allele frequency and prevalence of trait by population (24), ancestry proportion scores were generated and included as a covariate in all analyses. Subjects' ancestries were estimated by using a set of unlinked genetic markers by Bayesian cluster analysis, using the procedures and structure software developed by Pritchard and colleagues (36–38). structure implements Bayesian cluster modeling that can recognize cryptic population genetic patterns without prior information of population origins. We used this method to generate ancestral proportion scores by running structure multiple times such that each run included only one child from each family. Data were submitted to structure using 34 markers with models specified as “admixture” and “allele frequencies correlated” and 500,000 burn-in and 500,000 Markov chain Monte Carlo iterations (39). The best clustering solution occurred when there were two clusters assumed (based on posterior probability). The markers were the set of short tandem repeats described above, plus SLC6A4 itself (also known to distinguish between populations) (24). We have previously demonstrated that a closely related marker set is sufficient to distinguish ancestry of American populations accurately (35). The average missing rate of genotypes for the 34 markers was 6.2% for the full data set of 101 subjects.
Statistical Analyses. Before performing any analyses, data were examined for entry accuracy, and the distributions of all outcome measures were tested for normalcy. Children's depression scores were skewed and therefore were normalized with a square root transformation. In predicting children's depression scores, generalized estimating equations (GEE) were used to model the effects of risk and protective factors while handling familial correlations between subjects resulting from the inclusion of siblings in the sample. GEE is a generalization of generalized linear model approaches that takes into account within-group correlations. Separate models were used to examine the effect of both social support indices. Age, sex, and ancestral proportion scores were entered in all of the models as covariates and were retained in the models regardless of their significance given the relevance of these potential confounding variables in interpreting the study results. The main effects of 5-HTTLPR genotype (s/s, l/s, and l/l), group (maltreatment and CC), and the social support indices were explored, together with all possible two- and three-way interactions. Within-group analyses were attempted to examine the impact of different maltreatment experiences on child outcome, but, given power limitations, none of these analyses were informative.
Results
Allele Frequencies. There were no differences in 5-HTTLPR allele frequency between the two groups (χ2 = 0.37, df = 2, and P = 0.83). Forty-two percent of the sample was homozygous for the l allele, 41% were heterozygous (s/l), and 17% were homozygous for the s allele. Consistent with prior investigations (40), African-American children were more likely to have the l/l genotype than were Caucasian or Hispanic children (χ2 = 20.1, df = 6, and P < 0.003).
Social Supports. The 5-HTTLPR genotype did not predict children's scores on any of the social support measures (for all comparisons, the P value was not significant). However, group status did relate significantly to all of the social support indices. Although mothers were the most frequently listed top support among children in the two groups, CC children were more likely to list their mothers as their primary support than were the maltreated children (maltreated, 67%; CC, 86%; χ2 = 5.2, df = 1, and P < 0.03). In addition, maltreated children were more likely to list alternative parental figures (e.g., stepfather, foster mother), grandparents, or other relatives as their primary support than CCs (maltreated, 28.1%; CC, 6.8%; χ2 = 7.3, df = 2, and P < 0.03), and, not surprisingly, the quality of the maltreated children's relationship with their biological mothers and top-rated support was lower than that of CCs (P < 0.05, both comparisons). Maltreated children were also less likely to see their primary supports on a regular basis. Although 91% of the CCs reported seeing their primary supports on a daily basis, only 32% of the maltreated children saw their primary supports this frequently. In addition, all but one child within the CC group reported seeing their primary support at least several times a week. In contrast, within the maltreated cohort 18% of the children reported seeing their primary support on only a monthly basis, and 20% reported seeing their primary support semiannually or less often. Such infrequent contact typically occurred if a parent was incarcerated or if the parent was drug addicted and her where-abouts were unknown to protective services. Irregular visitation with birth parents, siblings, and other supports is often a significant problem for children in out-of-home care.
Predicting Depression Symptoms. In the first GEE analysis, none of the covariates (e.g., age, sex, ancestral proportion scores) were significant predictors of children's depression scores. After controlling for these covariates, genotype, group, and the child's relationship with his or her primary support were all significant predictors of children's depression scores. There was also a significant two-way interaction between genotype and group, and a three-way interaction among genotype, group, and the social support measure. The results of this analysis are depicted in Table 1.
Table 1. Results of generalized estimating equation analysis examining the effect of maltreatment, the 5-HTTLPR genotype, and the quality of the child's relationship with his or her primary support (WALD type 3 statistic).
| Source | df | χ2 test | Significance |
|---|---|---|---|
| Age | 1 | 0.35 | ns |
| Sex | 1 | 0.01 | ns |
| Ancestral proportion score | 1 | 0.82 | ns |
| Genotype | 2 | 12.3 | 0.002 |
| Maltreatment group | 1 | 4.2 | 0.04 |
| Social support: quality primary relationship | 3 | 11.2 | 0.01 |
| Genotype × maltreatment | 2 | 10.0 | 0.007 |
| Genotype × social support | 6 | 2.9 | ns |
| Genotype × maltreatment × social support | 7 | 59.9 | 0.0001 |
ns, not significant.
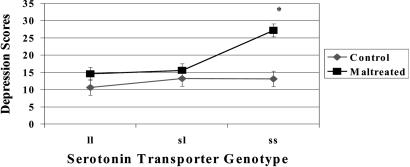
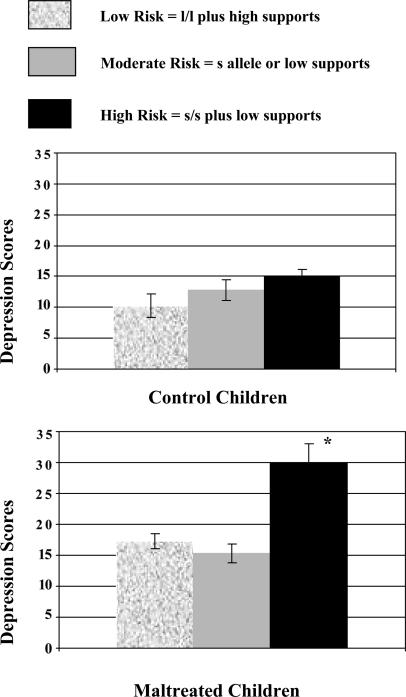
Figs. 1,2,3 depict the primary results of this analysis. Fig. 1 depicts the main effects of maltreatment, genotype, and social supports in predicting children's depression scores. As expected, maltreated children had significantly elevated depression scores compared with CCs (maltreated, 17.0 ± 10.3; CC, 12.0 ± 8.3). Elevated depression scores were also associated with the s/s genotype (s/s, 20.6 ± 12.4; s/l, 14.7 ± 9.7; l/l, 12.7 ± 7.7) and having low social supports (low social supports, 17.0 ± 11.3; high social supports, 13.4 ± 8.4). Fig. 2 shows the interaction between maltreatment experiences and the 5-HTTLPR genotype. The children in the CC group had relatively low depression scores, regardless of their genotype. Within the maltreated group, the children with l/l or l/s genotype had only slight elevations in their depression scores compared with CCs, but the children with s/s, the most vulnerable genotype, had depression scores that were twice as high as the depression scores of the CC children with the same genotype (maltreatment plus s/s genotype, 27.2 ± 13.0; CC plus s/s genotype, 13.1 ± 6.4) and almost twice as high as the depression scores of the maltreated children with the other genotypes. Fig. 3 depicts the results of the three-way interaction. The depression scores of the maltreated children with the s/s genotype and low supports were two times higher than the depression scores of CC children with the same genotype and social support profile (high-risk maltreatment, 30.0 ± 12.3; high-risk CCs, 15.0 ± 6.4). Although the presence of these two risk factors was associated with markedly elevated depression scores within the maltreated cohort, maltreated children with only one of these risk factors had only modest increases in their depression scores compared with CCs.
Fig. 1.
Predictors of depression. Group status (maltreatment vs. CC), 5-HTTLPR genotype (l/l vs. l/s vs. s/s), and social supports (high vs. low) were all significant predictors of children's depression scores in the GEE analysis (P < 0.05, all main effects).
Fig. 2.
Gene–maltreatment interaction in predicting depression in children. The interaction between the 5-HTTLPR genotype and maltreatment history was significant (P < 0.01). The s/s genotype conferred a significant vulnerability for depression, but only in the maltreated children.
Fig. 3.
Depression scores of high-, moderate, and low-risk children in the maltreatment and CC groups. There was a significant three-way interaction among maltreatment, genotype, and social supports in predicting children's depression scores (P < 0.0001). Maltreated children with the s/s genotype and low social supports had markedly elevated depression scores, ratings that were approximately twice as high as those of CCs with the same genotype and social support profile (high-risk CC, 15.0 ± 8.3; high-risk maltreated, 30.0 ±12.3). However, although the combination of the s/s genotype and low social supports was associated with very high depression scores in the maltreated cohort, maltreated children with only one of these risk factors had only modest increases in their depression scores compared with CCs (moderate-risk CC, 12.8 ± 8.8; moderate-risk maltreated, 15.3 ± 9.7).
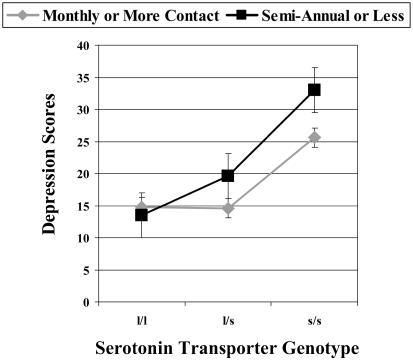
A similar pattern of findings emerged when frequency of contact between the child and his or her primary support was examined in the GEE analysis (see Table 2). In this analysis, age was a significant covariate, with less frequent contact with the primary support more deleterious for younger children. As noted previously, only 32% of the maltreated children saw their primary support on a daily basis, 18% reported seeing their primary support on a monthly basis, and 20% reported seeing their primary support semiannually or less often. As depicted in Fig. 4, the amount of contact maltreated children had with their primary support interacted significantly with genotype in predicting children's depression scores. Monthly or more frequent contact with children's primary support was associated with decreased depression scores. Although there was no increase in depression associated with less frequent contact for children with the l/l genotype, maltreated children with at least one s allele that had semiannual or less frequent contact with their primary support had a 33% increase in depression scores compared with maltreated children with the same genotypes that had more regular contact with their primary support. The depression scores of maltreated children with the s/s genotype and semiannual contact with their primary support were the highest and were also ≈33% higher than those of maltreated children with the s/s genotype that had more regular contact with their supports and slightly more than twice as high as those of maltreated children with the l/l genotype.
Table 2. Results of generalized estimating equation analysis examining the effect of maltreatment, the 5-HTTLPR genotype, and frequency of contact with the child's primary support (WALD type 3 statistic).
| Source | df | χ2 test | Significance |
|---|---|---|---|
| Age | 1 | 5.35 | 0.02 |
| Sex | 1 | 2.7 | ns |
| Ancestral proportion score | 1 | 2.2 | ns |
| Genotype | 2 | 55.0 | 0.0001 |
| Maltreatment group | 1 | 4.9 | 0.03 |
| Social support: contact with primary support | 3 | 64.03 | 0.0001 |
| Genotype × maltreatment | 2 | 22.8 | 0.0001 |
| Genotype × social support | 2 | 32.9 | 0.0001 |
| Genotype × maltreatment × social support | 2 | 62.4 | 0.0001 |
ns, not significant.
Fig. 4.
Effect of social support availability on depression scores in maltreated children. The relative availability of maltreated children's social supports also affected their depression scores. Except for maltreated children with the s/s genotype, maltreated children with monthly or more frequent contact with their primary support had relatively low depression scores (which were, on average, only 3 points higher than the mean depression score of the CC group). The depression scores of the maltreated children with the s/s genotype that had relatively regular contact with their primary supports were 67% higher than those of the maltreated children with less vulnerable genotypes who had comparable contact with their supports. However, the maltreated children with the s/s genotype and semiannual or less frequent contact with their primary support had the highest depression scores. Their depression scores were, on average, 9 points higher than those of the maltreated children with the s/s genotype that had monthly or more frequent contact with their supports and 18 points higher than those of the maltreated children with the less vulnerable genotypes that had more regular contact with their supports.
Discussion
To our knowledge, this is the first investigation to examine social support indices together with genetic factors in predicting depression in maltreated children. The findings of this study replicate and extend prior work (25) and suggest that in children, as in adults, the s allele of the 5-HTTLPR gene confers a vulnerability to depression only in individuals with histories of significant stress. In the absence of these experiences, the s allele appears to contribute little to the development of depression. The study also demonstrates that risk for depression associated with the s allele and stressful life events is further moderated by social support quality and availability. Maltreated children with the s/s genotype and an absence of positive supports had depression scores that were approximately twice as high as those of maltreated children with the s/s genotype and positive social supports. This latter group had only modest increases in their depression scores compared with CC children with the same genotype. These results are consistent with emerging preclinical and clinical data suggesting that the negative sequelae associated with early stress are not inevitable. Risk for negative outcomes may be modified by both genetic and environmental factors, with the quality and availability of social supports among the most important environmental factors in promoting resiliency, even in the presence of a genotype expected to predispose to psychiatric disorder.
One significant limitation of the present study is that the depression and social support data were collected contemporaneously. In the future, it will be important to obtain longitudinal assessments and examine the predictive power of measures of social supports in determining severity and persistence of depressive symptomatology over time. A second limitation of the study is the relatively small sample size. Although there was adequate power to detect associations in this study because the sample was enriched for maltreatment experiences and psychopathology, independent replication is warranted.
Nonetheless, the results have profound treatment and social policy implications, because failures within the child protective services system too often result in recurrent episodes of abuse, frequent changes in children's out-of-home placements, and an absence of positive social supports in the lives of maltreated children. For example, longitudinal follow-up studies suggest that, within 5 years of a child's first substantiated report of maltreatment, 35% of all cases will have one or more additional substantiated report of abuse and/or neglect (41). Of children who enter out-of-home care, although 50–75% will return home, 20–40% will reenter care within 1–2 years because of new allegations of abuse (42, 43). Of those remaining in care, many spend the majority of their lives in foster care drift, moving from one home to the next without ever securing a permanent home (44).
Recognizing these problems, in 1997 Congress passed the Adoption and Safe Families Act (45), which mandates permanency be attained in a timely fashion for children who enter out-of-home care. Currently, however, there are few data to guide interventions with maltreated children and their families to assure that this goal is achieved (46). Clinicians treating maltreated children need to focus not only on reducing depressive symptoms but also on working collaboratively with protective services to decrease risk of recurring abuse, use interventions to promote placement stability, and facilitate the development of lasting, positive relationships in the lives of these children.
Closing Remarks
There is converging evidence that maltreated children are at an elevated risk for depression. The present study suggests that this risk for depression is moderated by social supports and polymorphic variation of the VNTR in the promoter region of the serotonin transporter gene (5-HTTLPR). However, failures in the child protective services system further exacerbate maltreated children's risk for depression and increase the likelihood that these problems will become chronic. We believe the life course trajectory of maltreated children can be improved through ongoing research efforts that span from neurobiology to social policy, identifying mechanisms responsible for the etiology of depression and other stress-related psychiatric disorders, and systematically testing interventions to improve the system of care for these children.
Acknowledgments
We thank the children and families who participated in this study; the staff at the DCF, who facilitated the completion of this work; Eileen Billingslea, Daryn David, and Damion Grasso for their help with the collection of the clinical data; and Greg Kay for his work in genotyping the sample. This research was funded by National Institute of Mental Health Grant 1R01MH65519-01 (to J.K.), monies from the state of Connecticut to evaluate the SAFE Homes Initiative (to J.K.), Biological Sciences Training Program Grant MH14276 (to B.-Z.Y.), National Institute on Drug Abuse Grants K24 DA15105 (to J.G.) and KO2AA00261-01 (to J.H.K.), and National Institute on Alcohol Abuse and Alcoholism Grant K05 AA 014906-01 (to J.H.K.). This work was also supported by the National Center for Posttraumatic Stress Disorder of the Department of Veterans Affairs, the Veterans Affairs Depression Research Enhancement Award Program (J.K., J.H.K., and J.G.), and Yale University General Clinical Research Center Grant M01RR06022.
Author contributions: J.K. and J.H.K. designed research; J.K., H.D.-P., S.H., and D.L. performed research; J.K. and B.-Z.Y. analyzed data; J.K. and J.G. wrote the paper; and B.-Z.Y. proposed data analytic strategy and derived ancestral proportion scores.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DCF, Department of Children and Families; CC, community control; l, long; s, short; GEE, generalized estimating equation.
References
- 1.U.S. Department of Health and Human Services (2001) Child Maltreatment 1999: Reports from the States to the National Child Abuse and Neglect Data System (U.S. Dept. of Health and Human Services, Washington, DC).
- 2.Kaufman, J. & Zigler, E. (1996) in Children, Families and Government: Preparing for the Twenty-First Century, eds. Zigler, E., Kagan, S. & Hall, N. (Cambridge Univ. Press, New York), pp. 233–255.
- 3.Wolfner, G. D. & Gelles, R. J. (1993) Child Abuse Neglect 17, 197–212. [DOI] [PubMed] [Google Scholar]
- 4.Famularo, R., Kinscherff, R. & Fenton, T. (1992) J. Am. Acad. Child Adolesc. Psychiatry 31, 863–867. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman, J. (1991) J. Am. Acad. Child Adolesc. Psychiatry 30, 257–265. [DOI] [PubMed] [Google Scholar]
- 6.Heim, C., Owens, M. J., Plotsky, P. M. & Nemeroff, C. B. (1997) Ann. N.Y. Acad. Sci. 821, 194–207. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman, J., Plotsky, P., Nemeroff, C. & Charney, D. (2000) Biol. Psychiatry 48, 778–790. [DOI] [PubMed] [Google Scholar]
- 8.Sapolsky, R. M. (2000) Arch. Gen. Psychiatry 57, 925–935. [DOI] [PubMed] [Google Scholar]
- 9.Schildkraut, J., Green, A. & Mooney, J. (1989) in Comprehensive Textbook of Psychiatry, eds. Kaplan, H. & Sadock, B. (Williams & Wilkins, Baltimore), Vol. 1, pp. 868–879. [Google Scholar]
- 10.Nemeroff, C. B., Bissette, G., Akil, H. & Fink, M. (1991) Br. J. Psychiatry 158, 59–63. [DOI] [PubMed] [Google Scholar]
- 11.Wong, M. L., Kling, M. A., Munson, P. J., Listwak, S., Licinio, J., Prolo, P., Karp, B., McCutcheon, I. E., Geracioti, T. D., Jr., DeBellis, M. D., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanacora, G., Mason, G. F., Rothman, D. L., Behar, K. L., Hyder, F., Petroff, O. A., Berman, R. M., Charney, D. S. & Krystal, J. H. (1999) Arch. Gen. Psychiatry 56, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 13.Arango, V., Underwood, M. D. & Mann, J. J. (2002) Prog. Brain Res. 136, 443–453. [DOI] [PubMed] [Google Scholar]
- 14.Malison, R. T., Price, L. H., Berman, R., van Dyck, C. H., Pelton, G. H., Carpenter, L., Sanacora, G., Owens, M. J., Nemeroff, C. B., Rajeevan, N., et al. (1998) Biol. Psychiatry 44, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 15.Vythilingam, M., Heim, C., Newport, J., Miller, A. H., Anderson, E., Bronen, R., Brummer, M., Staib, L., Vermetten, E., Charney, D. S., et al. (2002) Am. J. Psychiatry 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anisman, H., Zaharia, M. D., Meaney, M. J. & Merali, Z. (1998) Int. J. Dev. Neurosci. 16, 149–164. [DOI] [PubMed] [Google Scholar]
- 17.Dhabhar, F. S., McEwen, B. S. & Spencer, R. L. (1997) Neuroendocrinology 65, 360–368. [DOI] [PubMed] [Google Scholar]
- 18.Lesch, K. P., Gross, J., Franzek, E., Wolozin, B. L., Riederer, P. & Murphy, D. L. (1995) Biol. Psychiatry 37, 215–223. [DOI] [PubMed] [Google Scholar]
- 19.Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Muller, C. R., Hamer, D. H. & Murphy, D. L. (1996) Science 274, 1527–1531. [DOI] [PubMed] [Google Scholar]
- 20.Barr, C. S., Newman, T. K., Becker, M. L., Parker, C. C., Champoux, M., Lesch, K. P., Goldman, D., Suomi, S. J. & Higley, J. D. (2003) Genes Brain Behav. 2, 336–340. [DOI] [PubMed] [Google Scholar]
- 21.Bennett, A. J., Lesch, K. P., Heils, A., Long, J. C., Lorenz, J. G., Shoaf, S. E., Champoux, M., Suomi, S. J., Linnoila, M. V., Higley, J. D., et al. (1998) Am. J. Psychiatry 155, 118–122. [DOI] [PubMed] [Google Scholar]
- 22.Champoux, M., Bennett, A., Shannon, C., Higley, J. D., Lesch, K. P. & Suomi, S. J. (2002) Mol. Psychiatry 7, 1058–1063. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie, A. & Quinn, J. (1999) Proc. Natl. Acad. Sci. USA 96, 15251–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelernter, J., Kranzler, H. & Cubells, J. F. (1997) Hum. Genet. 101, 243–246. [DOI] [PubMed] [Google Scholar]
- 25.Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., McClay, J., Mill, J., Martin, J., Braithwaite, A. & Poulton, R. (2003) Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- 26.Wiedenmayer, C. P., Magarinos, A. M., McEwen, B. S. & Barr, G. A. (2003) Ann. N.Y. Acad. Sci. 1008, 304–307. [DOI] [PubMed] [Google Scholar]
- 27.Huot, R. L., Gonzalez, M. E., Ladd, C. O., Thrivikraman, K. V. & Plotsky, P. M. (2004) Psychoneuroendocrinology 29, 279–289. [DOI] [PubMed] [Google Scholar]
- 28.Barbazanges, A., Vallee, M., Mayo, W., Day, J., Simon, H., Le Moal, M. & Maccari, S. (1996) J. Neurosci. 16, 7783–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman, J. & Henrich, C. (2000) in Handbook of Infant Mental Health, ed. Zeanah, C., Jr. (Guilford, New York), pp. 195–207.
- 30.Kaufman, J., Jones, B., Steiglitz, E., Vitulano, L. & Mannarino, A. (1994) J. Family Violence 9, 227–248. [Google Scholar]
- 31.Costello, E. J. & Angold, A. (1988) J. Am. Acad. Child Adolesc. Psychiatry 27, 726–737. [DOI] [PubMed] [Google Scholar]
- 32.Pine, D. S., McClure, E. B., Hoberman, A. J., Schweder, A., Leibenluft, E., Ernst, M., Charney, D. S. & Kaufman, J., Am. J. Psychiatry, in press.
- 33.Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., Williamson, D. & Ryan, N. (1997) J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- 34.Barrera, M. (1980) Connections 3, 8–13. [Google Scholar]
- 35.Stein, M. B., Schork, N. J. & Gelernter, J. (2004) Biol. Psychiatry 56, 217–224. [DOI] [PubMed] [Google Scholar]
- 36.Falush, D., Stephens, M. & Pritchard, J. K. (2003) Genetics 164, 1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritchard, J. K. & Rosenberg, N. A. (1999) Am. J. Hum. Genet. 65, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritchard, J. K., Stephens, M. & Donnelly, P. (2000) Genetics 155, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, M. W., Lautenberger, J. A., Shin, H. D., Chretien, J. P., Shrestha, S., Gilbert, D. A. & O'Brien, S. J. (2001) Am. J. Hum. Genet. 69, 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelernter, J., Kranzler, H., Coccaro, E. F., Siever, L. J. & New, A. S. (1998) Am. J. Psychiatry 155, 1332–1338. [DOI] [PubMed] [Google Scholar]
- 41.Drake, B., Jonson-Reid, M., Way, I. & Chung, S. (2003) Child Maltreatment 8, 248–260. [DOI] [PubMed] [Google Scholar]
- 42.Barth, R. P. (1994) Child Welfare 73, 625–638. [PubMed] [Google Scholar]
- 43.Barth, R. P. (1996) Future Child 6, 100–110. [PubMed] [Google Scholar]
- 44.Webster, D., Barth, R. P. & Needell, B. (2000) Child Welfare 79, 614–632. [PubMed] [Google Scholar]
- 45.U.S. Dept. of Health and Human Services (1997) Adoption and Safe Families Act of 1997, Public Law 105-89 (105th Congress).
- 46.DeSena, A. D., Murphy, R. A., Douglas-Palumberi, H., Blau, G., Kelly, B., Horwitz, S. M. & Kaufman, J., Child Abuse Neglect, in press. [DOI] [PubMed]