Abstract
α-Amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptors mediate excitatory synaptic transmission and are dynamically regulated during synaptic plasticity in the CNS. The membrane trafficking of AMPA receptors to synapses is critical for the regulation of the efficacy of excitatory synaptic transmission. Direct imaging of AMPA receptors in various cell compartments is important to dissecting the regulation of distinct steps in receptor membrane trafficking. In this study, we have developed an approach for the imaging of receptor trafficking with subunits tagged with a 13-aa α-bungarotoxin (BTX)-binding site (BBS). The small polypeptide neurotoxin BTX has been used for decades to study the nicotinic acetylcholine receptor. Similar high-affinity ligands are rarely available for most receptors. Engineering the BBS tag into receptor subunits allowed the high-affinity binding of fluorescent, radioactive, and biotinylated BTX to the tagged receptor subunits. By using this approach, the total receptor expression, surface expression, internalization, and insertion of receptors into the plasma membrane could be visualized and quantified in fixed or live cells including cultured neurons. The BBS tag is a flexible approach for labeling membrane proteins and studying their dynamic trafficking.
Keywords: GFP, glutamate receptor, live imaging, synapses, tag
Ionotropic glutamate receptors mediate rapid excitatory transmission in the CNS and play critical role in synaptic transmission and plasticity, in neuronal development, and in several neurological and psychiatric disorders (1–4). The ionotropic glutamate receptors include the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA), kainate, and N-methyl-d-aspartate (NMDA) subtypes (1, 2). AMPA receptors mediate the majority of excitatory synaptic transmission in the CNS and are heterotetramers of four different subunits (GluR1–GluR4) (1, 2). The GluR1–GluR4 subunits have a large N-terminal extracellular domain, three transmembrane domains, a reentrant membrane loop that forms the central ion channel, and an intracellular C-terminal domain (1, 2). The regulation of AMPA-receptor membrane trafficking to synapses is critical for the dynamic modulation of excitatory synaptic transmission and is required for several forms of synaptic plasticity in the brain (3–5).
Recent studies on AMPA-receptor membrane trafficking have used common cell biological techniques. GFP fusion proteins of AMPA-receptor subunits have been used to observe trafficking and targeting of AMPA receptors (6–8). In addition, labeling of live cells with antibodies against extracellular epitopes or tags has been used to visualize the surface expression and internalization of receptors (9–12). Moreover, surface biotinylation techniques have been used to biochemically measure the relative levels of surface and internalized receptors (13, 14). Although these approaches have been used successfully to study AMPA-receptor trafficking and are widely used in many systems, there are several drawbacks to these techniques. Antibodies are large divalent proteins and therefore can promote clustering or capping of surface antigens, which may have significant effects on membrane trafficking. Cellsurface biotinylation experiments covalently modify all reactive free amines on the cell surface, which may affect membrane protein and cell function. Moreover, these techniques can only measure the relative levels of protein expression and cannot quantify absolute expression levels.
In this study, we have developed an approach for imaging receptor trafficking by tagging receptors with a 13-aa α-bungarotoxin (BTX)-binding site (BBS). The small polypeptide neurotoxin BTX is a high-affinity ligand for the nicotinic acetylcholine receptor that has been used for 30 years to study the structure, function, and synaptic trafficking of the receptor (15, 16). Similar high-affinity ligands are not available for most receptors and thus limit the ease of receptor characterization. We took advantage of the fact that a small 13-aa sequence can bind BTX with high affinity (17) and placed this sequence in the extracellular domain of AMPA-receptor subunits. These tagged receptors bind BTX, and using fluorescent and biotinylated BTX, we were able to specifically detect surface receptors and image internalization and insertion of receptors in live cells. Moreover, by using radioactive BTX, the levels of surface and total receptor expression could be quantitatively analyzed. Biotinylated BTX could also be used to biochemically detect and isolate the tagged receptors. The BBS tag is a flexible technique for labeling membrane proteins and is a potentially powerful technique for studying receptor and membrane trafficking in general.
Materials
BTX-Binding-Site Fusion Protein. The complementary DNA fragments of 13-aa BBS were synthesized with the nucleotide sequences CTAGCTGGAGATACTACGAGAGCTCCCTGGAGCCCTACCCTGACA (sense) and CTAGTGTCAGGGTAGGGCTCCAGGGAGCTCTCGTAGTATCTCCAT (antisense). The two fragments were phosphorylated and annealed to each other. The fragment was subcloned into the SpeI site introduced after the fourth amino acid residue of the mature AMPA-receptor subunit GluR2 (just after the signal peptide). The BBS-GluR2 fragment was then subcloned into the pcDNA3.1 vector (Clontech) (BBS-GluR2 construct). Enhanced GFP (Clontech) was also subcloned into the SpeI site for the GFP-GluR2 construct and then subcloned into the pcDNA3.1 vector. The GFP fragment was excised from a GFP-GluR2 construct and inserted into the C termini of the BBS fragment in the BBS-GluR2 construct (BBS-GFP-GluR2 construct). The BBS or BBS-GFP was also engineered into MluI site introduced in the N-terminal of GluR1 subcloned in pRK5 vector (BBS-GluR1 and BBS-GFP-GluR1 constructs, respectively).
Human Embryonic Kidney (HEK) 293 Cells and Primary Cultured Neurons and Transfection. HEK 293 cells were grown and transfected by using Lipofectamine 2000 (Invitrogen) according to standard methods. Primary cortical and hippocampal cultured neurons were prepared from embryonic day 18 rat brains as described in ref. 18. Coverslips (18 and 25 mm) coated with 1 mg/ml poly(l-lysine) were placed in a 60-mm dish and six-well plates, respectively. The cortical and hippocampal neurons were plated at a density of 6 × 106 per dish or 4 × 106 per well and 4 × 105 per dish, respectively. The neurons were then transfected by using the calcium phosphate method at 14 days in vitro for cortical neurons and 7–14 days in vitro for hippocampal neurons, as described previously (30).
Western Blotting. The transfected cells were harvested with lysis buffer (50 mM Tris·HCl/100 mM NaCl/2 mM EDTA/1% Triton X-100/50 mM Na fluoride/10 mM Na pyrophosphate/1 mM Na orthovanadate, pH 7.5) and mixed with SDS sample buffer, and the sample were separated by SDS/PAGE. The gels were transferred to Immobilon-P membranes (Millipore), Western blotted with anti-GluR2-C (1:1,000) and anti-GFP (1:2,000) antibodies, and detected by using an enhanced chemiluminescence detection kit (PerkinElmer) according to standard methods. To detect BBS-tagged receptors with BTX, Immobilon membranes were incubated in TBST (50 mM Tris·HCl/500 mM NaCl/0.1% Tween 20/1% skim milk, pH 7.4) containing biotin-conjugated BTX (1 μg/ml, Molecular Probes), washed, and incubated with horseradish peroxidase (HRP)-conjugated streptavidin (0.1 μg/ml, Molecular Probes). BTX was detected with an enhanced chemiluminescence detection kit.
Immunostaining of Transfected Cells. Transfected cells were fixed with 4% paraformaldehyde on ice for 10 min in the absence of detergent, incubated with anti-GFP antibody (1:8,000, Molecular Probes) or anti-GluR2-N antibody (1:500), washed, and incubated with Cy3-conjugated secondary antibody.
BTX Binding in HEK 293 Cells. Living cells transfected with BBS-tagged constructs were labeled with 10 μg/ml rhodamine-conjugated BTX (Molecular Probes) in prechilled MEM including 10 mM Hepes (pH 7.4), 1 mM CaCl2, and 0.5 mM MgCl2 at 10°C for 1 h or 17°C for 20 min, washed with the buffer on ice to eliminate free BTX, and fixed. The cells were then observed by using epifluorescence microscopy (Axiovert 200, Zeiss), and the images were collected by using a charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan) with axiovision (Zeiss) analysis software. For the BTX dissociation assay, we incubated the cells with rhodamine-conjugated BTX at 17°C for 20 min, washed them, and incubated them in MEM at 17°C for 6 h. To observe the internalization of receptors, the cells were incubated with 1 μg/ml rhodamine-conjugated BTX at 37°C for various times. To observe receptor insertion into the plasma membrane, the cells were preincubated with 10 μg/ml unlabeled BTX (Molecular Probes) at 17°C for 15 min, and the cells were then washed and incubated with 1 μg/ml rhodamine-conjugated BTX at 37°C. After fixation, the images were observed in 1-μm sections by using confocal microscopy (LSM 510, Zeiss). The time-lapse images of the internalized receptors in living cells were observed at 1-min intervals in 1-μm sections by using confocal microscopy. The images were analyzed with metamorph (Universal Imaging, West Chester, PA) analysis software.
BTX Binding in Primary Cultured Neurons. Because neurons can express low levels of the α7 nicotinic acetylcholine receptor that bind BTX (19), the transfected neurons were preincubated with the nicotinic receptor antagonist tubocurarine (20) to block binding of BTX to the endogenous nicotinic receptors before incubation with 1 μg/ml rhodamine-conjugated BTX to label the BBS-tagged receptors. To observe the insertion of receptors, the transfected neurons were also incubated with 10 μg/ml unlabeled BTX (Molecular Probes) at 17°C for 15 min to block preexisting surface receptors and then briefly washed before incubating with rhodamine-conjugated BTX. To observe internalization of receptors, FITC-conjugated transferrin (Molecular Probes) was coincubated with rhodamine-conjugated BTX at 37°C for 10 min.
BTX Binding Assay with Radioactive BTX. For cell-surface binding assay, HEK 293 cells transfected with BBS-GFP-GluR2 or GFP-GluR2 constructs were incubated with 5 nM 125I-BTX (Amersham Biosciences) in MEM including 10 mM Hepes (pH 7.4), 1 mM CaCl2, and 0.5 mM MgCl2 with or without 5 μM unlabeled BTX at room temperature for 30 min. The cells were washed, harvested with lysis buffer, and centrifuged at 14,000 × g at 4°C for 10 min. The extract was spotted on DEAE paper (DE81, Fisher Scientific) and immediately counted in a gamma counter. The BTX binding assay in vitro was performed as described (21, 22) with some modifications. The cells were harvested with the lysis buffer the resulting extracts were incubated with 34 nM 125I-BTX with or without unlabeled BTX at room temperature for various time points. The fractions were spotted on DEAE papers, washed with the buffer, and counted on DEAE papers in a gamma counter. The dissociation constant (Kd) was quantified from binding experiments in which extracts were incubated with various concentrations of 125I-BTX using Scatchard analysis (23).
Affinity Isolation with Biotinylated BTX. HEK 293 cells transfected with BBS-GFP-GluR2 or GFP-GluR2 constructs were incubated with 25 μg/ml biotinylated BTX in PBS (containing 1 mM CaCl2 and 0.5 mM MgCl2) at 17°C for 20 min. The cells were washed with PBS, harvested with lysis buffer, and centrifuged at 15,000 rpm at 4°C for 10 min. The detergent extracts were incubated with NeutrAvidin-agarose (Pierce) at 4°C overnight. The agarose was washed, and the bound receptors were then eluted with SDS sample buffer and analyzed by SDS/PAGE and Western blotting with anti-GluR2-C (1:2,000) antibody.
Results
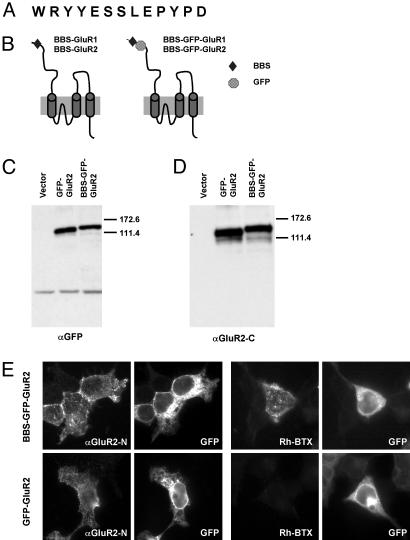
Expression of BBS-Tagged Receptor Subunits. To analyze the expression and membrane trafficking of AMPA receptors, we engineered a 13-aa minimal BBS (Fig. 1A) derived from the nicotinic acetylcholine receptor (17) into the N-terminal region of the GluR1 and GluR2 subunits of the AMPA-type glutamate receptors. Constructs were generated with (BBS-GFP-GluR1 and BBS-GFP-GluR2) and without (BBS-GluR1 and BBS-GluR2) N-terminal GFP-tags (Fig. 1B). Similar results were obtained for GluR1 and GluR2 constructs, but only the results for GluR2 are shown here. The GluR2 constructs were transfected into HEK 293 cells and detected by using anti-GluR2-C and anti-GFP antibodies in Western blots to confirm expression of the tagged proteins (BBS-GFP-GluR2) (Fig. 1 C and D). The expression of the BBS-tagged receptor on the cell surface was detected immunocytochemically by using anti-GluR2-N (BBS-GFP-GluR2) (Fig. 1E) and anti-GFP (data not shown) antibodies in nonpermeabilized transfected HEK 293 cells demonstrating that the tagged receptors were efficiently expressed on the cell surface. Interestingly, the BBS-tagged receptors expressed on the cell surface could be detected in live cells by incubating cells with 10 μg/ml rhodamine-conjugated BTX at 10°C for 1 h (Fig. 1E). In contrast, no labeling of the GFP-GluR2 transfected cells was observed with rhodamine-conjugated BTX (Fig. 1E).
Fig. 1.
Expression of BBS-tagged receptor subunits in HEK 293 cells. (A) Amino acid sequence of the BBS. (B) Design of the BBS-tagged receptor subunits. BBS and GFP were inserted into the N-terminal extracellular region of the receptor subunits. (C and D) Western blotting of BBS-tagged subunits in HEK 293 cells. Both the GFP-GluR2 and BBS-GFP-GluR2 constructs were detected with anti-GFP (C) and anti-GluR2-C (D) antibodies. (E) Surface labeling of cells expressing BBS-GFP-GluR2 or GFP-GluR2 with anti-GluR2-N antibody (αGluR2N) or rhodamine-BTX (Rh-BTX). GFP, GFP signal detected in the cells. Both BBS-GFP-GluR2 and GFP-GluR2 constructs were expressed on the cell surface, whereas only the BBS-GFP-GluR2 construct was labeled with rhodamine-BTX on the cell surface.
The dissociation of BTX from the BBS-tagged receptors was examined by incubating cells with rhodamine-conjugated BTX at 17°C for 20 min to minimize internalization of the receptor, washing the cells to remove free BTX, and examining the dissociation of the rhodamine-BTX labeling at various time points at 17°C (Fig. 2 A and B). BTX binding to surface receptors was very stable under these conditions, and no detectable loss of binding was seen even after 6 h of incubation (Fig. 2 A and B).
Fig. 2.
Detection of internalization and insertion of BBS-tagged GluR2 in HEK 293 cells. (A) Cells transfected with BBS-GFP-GluR2 were incubated with rhodamine-BTX at 17°C for 30 min, washed to remove free rhodamine-BTX, incubated for several hours at 17°C (to inhibit internalization), and imaged at the time points indicated (0–6 h). (B) Quantification of the surface signals in the cells. BTX binding to the BBS-tagged GluR2 was stable for 6 h(n = 4). (C) Observation of internalization of BBS-GFP-GluR2 in the transfected HEK 293 cells. Cells transfected with BBS-GFP-GluR2 were incubated with rhodamine-BTX at 17°C for 30 min, washed to remove free rhodamine-BTX, incubated at 37°C to allow internalization of the receptor, and imaged at the indicated times (0–30 min) with confocal microscopy. Internalized BBS-GFP-GluR2 vesicles were seen in the intracellular region of the cells after incubation at 37°C. (D) Observation of plasma membrane insertion of BBS-GFP-GluR2 in transfected HEK 293 cells. Cells transfected with BBS-GFP-GluR2 were preincubated with unlabeled BTX at 17°C and incubated with rhodamine-BTX at 37°C for the indicated times to image the insertion of new receptors into the plasma membrane. Rapid insertion of the receptors into the plasma membrane within 10 min could be visualized with this method.
Internalization of AMPA Receptors in HEK 293 Cells. To examine internalization of the receptors using the BBS tag, living cells were incubated with rhodamine-conjugated BTX at 17°C for 20 min to allow BTX binding while limiting internalization. The cells were then washed to eliminate free rhodamine-BTX and incubated at 37°C for various times. The internalization of the BTX-labeled GluR2 from the plasma membrane could be visualized after fixation (Fig. 2C) or in living cells by using time-lapse imaging (data not shown). Vesicles labeled with BTX were observed near the cell membrane immediately after incubating the cells at 37°C for 10 min (Fig. 2C) and rapidly accumulated in intracellular perinuclear regions after 20–30 min (Fig. 2C). In addition, the signal at the plasma membrane decreased and the size of labeled intracellular vesicles increased after 30 min (Fig. 2C).
Insertion of AMPA Receptors to Cell Surface in HEK 293. The BBS tag was then tested to see whether we could specifically detect insertion of new receptors into the plasma membrane in HEK 293 cells. The receptors present on the cell surface were first incubated with unlabeled BTX in living cells at 17°C for 15 min to block all preexisting surface receptors, and the cells were then washed and incubated with rhodamine-BTX at 37°C for the indicated times (Fig. 2D). The cells were observed after eliminating the free-BTX and fixation. No surface labeling occurred immediately after addition of the rhodamine-BTX, indicating that the preexisting receptors were efficiently blocked with unlabeled BTX (Fig. 2D, 0 m). However, specific BTX labeling appeared on the cell surface rapidly after 10 min incubation (Fig. 2D). Upon longer incubation, the newly inserted receptors were observed in internalized vesicles, indicating the cycling of the AMPA receptors back into the cell from the cell surface (Fig. 2D). The level of labeling of new receptors increased rapidly, and the new receptors were then rapidly internalized, demonstrating the cycling of receptors into and out of the plasma membrane.
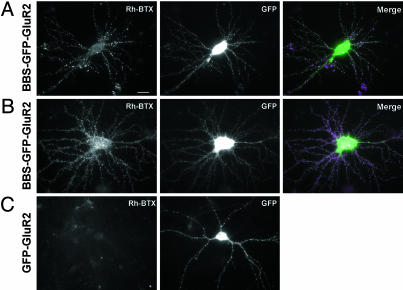
Detection of BBS-Tagged Receptors in Neurons. To visualize receptor trafficking in neurons, we transfected the BBS-tagged receptor constructs into primary cortical and hippocampal cultured neurons. As with the HEK 293 cells, we observed surface expression of the receptor subunit when using an anti-GFP antibody in cortical neurons transfected with the BBS-GFP-GluR2 and GFP-GluR2 constructs (data not shown). Incubation of the transfected neurons with rhodamine-BTX labeled punctate signals on the surface of spines and dendrites in the transfected neurons (Fig. 3 A and B). The surface BTX signals overlapped with clusters of GFP signals in the spines and dendrites (Fig. 3 A and B). Control neurons or neurons transfected with the GFP-GluR2 constructs lacking the BBS tag had a very low background of rhodamine-BTX binding (Fig. 3C). Interestingly there appeared to be a low level of specific labeling of small puncta in dendrites in some untransfected and transfected neurons. This background labeling is most likely due to the low expression of the α7 nicotinic acetylcholine receptor, which binds BTX (19). To eliminate this low background, we took advantage of the fact that binding of BTX to the α7 nicotinic acetylcholine receptor is sensitive to the nicotinic antagonist tubocurarine, whereas the BTX binding to the BBS tag should not be sensitive to tubocurarine (20). Preincubation of the neurons with tubocurarine completely eliminated this background binding but did not block detection of the BBS-tagged receptors (data not shown), allowing us to specifically examine the trafficking of the BBS-tagged receptors in neurons.
Fig. 3.
Detection of surface-expressed BBS-tagged GluR2 with rhodamine-BTX in cultured cortical and hippocampal neurons. (A) Cortical cultured neurons transfected with BBS-GFP-GluR2 were incubated with rhodamine-BTX at 17°C for 15 min, washed, and fixed. The rhodamine-BTX (Rh-BTX) labeled surface receptors, whereas the GFP signal showed the total expression of the BBS-GFP-GluR2 construct. (Scale bar: 10 μm.) (B) BBS-GFP-GluR2 was labeled with rhodamine-BTX on the surface of hippocampal neurons in culture. (C) GFP-GluR2 transfected neurons had a low background level of rhodamine-BTX binding.
Imaging Insertion and Internalization of Surface Receptors in Neurons. To measure receptor insertion into the surface plasma membrane, we first blocked the α7 nicotinic acetylcholine receptors with tubocurarine and masked the surface BBS-GFP-GluR2 with unlabeled BTX by incubating the cells with BTX at 17°C for 15 min. The free unlabeled BTX was removed and rhodamine-BTX was added in the presence tubocurarine and incubated at 17°C for 15 min (Fig. 4 A and B). Under these conditions, the unlabeled BTX completely blocked preexisting surface receptors in the neuron (Fig. 4A). However, if the neurons were incubated with rhodamine-BTX at 37°C for 5 min, newly inserted receptors could be seen as clusters in the dendrites and spines (Fig. 4B). Interestingly, the level of BTX labeling of newly inserted receptors after a 5-min incubation at 37°C approached the BTX labeling of transfected cells in the absence of blocking of preexisting surface receptors, suggesting that receptor insertion is very rapid (Fig. 4C).
Fig. 4.
Insertion and internalization of BBS-GFP-GluR2 in cortical neurons. Insertion of BBS-GFP-GluR2 into the surface membrane in the transfected neurons was observed by preincubating neurons with unlabeled BTX, followed by labeling with rhodamine-BTX (A and B). Neurons were preincubated for 15 min with unlabeled BTX, washed, incubated for 0 min (A) or 5 min (B) at 37°C, and labeled with rhodamine-BTX at for 15 min at 17°C. (Scale bar: 10 μm.) Magnified images of dendritic regions are shown in Insets. The insertion of new receptors could be seen on the cell surface in B. (C) Neurons were transfected with BBS-GFP-GluR2 and then labeled with rhodamine-BTX for 15 min at 17°C to label all cell-surface receptors. (D) Internalization of the BBS-GFP-GluR2 construct with transferrin in cortical neurons. The transfected neurons were observed after simultaneous labeling with rhodamine-BTX and FITC-transferrin. The BBS-GFP-GluR2 was partially colocalized with transferrin (arrows).
To observe the endocytosis of rhodamine-BTX-labeled receptors in living neurons, neurons were incubated with rhodamine-BTX and FITC-transferrin at 17°C to limit internalization; the cells were then briefly washed to remove the free rhodamine-BTX and FITC-transferrin and incubated at 37°C. Under these conditions, the internalization of the receptor could be visualized as indicated by the colocalization of the rhodamine-BTX-labeled receptors with the internalized FITC-conjugated transferrin, a marker of internalized vesicles and early endosomes (Fig. 4D) (24).
Time-Lapse Imaging of Receptor Insertion into the Plasma Membrane. One of the major advantages of the BBS tag is that we found we could visualize BTX binding to surface receptor clusters in real time in the presence of free rhodamine-BTX. Although free rhodamine-BTX in the bath produced a high background when visualized with normal epifluorescence, this background was quite low, and a high signal-to-noise ratio was observed by using confocal microscopy, allowing us to measure BTX binding to BBS-tagged receptors in real time even in the presence of free rhodamine-BTX in the bath (Fig. 5A). To measure the rate of receptor insertion into the surface plasma membrane in living neuron, we first blocked the α7 nicotinic acetylcholine receptors with tubocurarine and also masked the surface BBS-GFP-GluR2 with unlabeled BTX at 17°C for 15 min. The free unlabeled BTX was removed, rhodamine-BTX was added in the continued presence of tubocurarine, and time-lapse images were used to observe the insertion of new receptors at the surface at 37°C (Fig. 5). New BBS-tagged receptors rapidly appeared in the dendrites and spines of the neurons after 1 min as punctate clusters in the dendrites. The number and intensity of the signals gradually increased for 3 min and reached a plateau by 5 min (Fig. 5 A and B). The insertion rate of the BBS-GFP-GluR2 could be quantitated by analysis of individual puncta (Fig. 5B).
Fig. 5.
Time-lapse imaging of receptor insertion into the surface plasma membrane in cortical neurons. (A) The insertion of BBS-GFP-GluR2 into the surface membrane in the transfected neurons was observed after preincubating neurons with unlabeled BTX, followed by labeling with rhodamine-BTX. The clusters of receptors newly inserted in the surface could be seen in the dendrites and spines. Arrowheads show individual clusters of BBS-GFP-GluR2. (B) Quantification of signal intensity of individual clusters (n = 19).
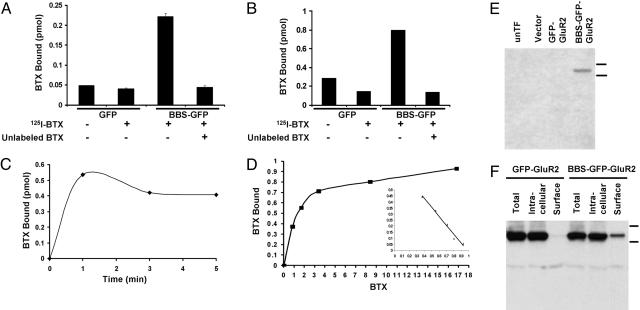
Quantitation of the Expression of BBS-Tagged Receptors by Using 125I-BTX Binding in HEK 293. Another advantage of the BBS tag is that the use of radioactive BTX allowed us to quantitate the absolute number of BTX-binding sites expressed on the cell surface or in total cell extracts. We tested whether BTX binding could be assayed by using standard BTX binding assays developed for the nicotinic acetylcholine receptor (21). Living cells expressing BBS-GFP-GluR2 or GFP-GluR2 were incubated with 125I-BTX at room temperature for 30 min, and the cells were washed with buffer and then harvested and counted in a gamma counter. As shown in Fig. 6A, the BBS-GFP-GluR2 transfected cells specifically bound 125I-BTX. Robust 125I-BTX binding could also be observed with solubilized transfected cells by using a DEAE filter paper assay developed for the nicotinic receptor (Fig. 6B). The assays allowed us to quantitate the absolute level of surface and total expression of the BBS-tagged receptors. The surface binding of 125I-BTX was 0.23 pmol and the total 125I-BTX binding was 1.5 pmol in these experiments in HEK cells. The ratio of the surface BBS-tagged receptors to the total BBS-tagged receptors was 15.3% in HEK cells. The 125I-BTX binding occurred very rapidly and was essentially completed within 1 min at the concentration of 34 nM (Fig. 6C). Binding experiments to determine the affinity of binding indicated that BTX had a quite high affinity for BBS-tagged receptors (Kd = 13.6 nM) (Fig. 6D).
Fig. 6.
BTX binding assay using 125I-BTX in HEK 293 cell. (A) To measure surface expression, the cells expressing BBS-GFP-GluR2 or GFP-GluR2 were incubated with 125I-BTX in the absence or presence of unlabeled BTX at 17°C for 30 min, the cells were washed, and the 125I-BTX bound in the cell surface was measured. Robust 125I-BTX binding was detected in BBS-GFP-GluR2-transfected cells. (B) To measure total expression, detergent extracts of membrane fractions were incubated with 125I-BTX with or without unlabeled BTX for 30 min at room temperature, spotted onto DEAE filters, washed, and counted. (C) The time course of 125I-BTX binding was measured by using detergent extracts of membrane fractions for the indicated times. 125I-BTX binding was completed within 1 min. (D) The detergent extracts of membrane fractions were incubated with various concentrations of 125I-BTX, and the dissociation constant (Kd affinity) was quantified (Kd = 1.36 × 10–8 M) from the resulting Scatchard plot (Inset). (E) The BBS-GFP-GluR2 was specifically detected on Western blot by using biotinylated BTX and streptavidin-HRP. Lanes show untransfected cells (unTF), vector, GFP-GluR2, and BBS-GFP-GluR2 from left to right. (F) Surface BBS-GFP-GluR2, but not GFP-GluR2, was isolated from the transfected cells by using biotinylated BTX and NeutrAvidin beads.
Affinity Isolation of the BBS-Tagged Receptors by Using Biotinylated BTX. Biotinylated bungarotoxin is also commercially available, so we wanted to see whether it could be used to detect or isolate BBS-tagged receptors when using streptavidin-coupled HRP or streptavidin affinity resins. The BBS-tagged receptor subunits could be specifically detected on Western blots by using biotinylated BTX and streptavidin-conjugated HRP, confirming BTX binding to the BBS-tagged receptors (Fig. 6E). BTX binding was only observed by using the BBS-GFP-GluR2 construct (Fig. 6E, lane 4) and was not observed by using the GFP-GluR2 construct (Fig. 6E, lane 3). To see whether streptavidin-agarose and biotinylated BTX could isolate BBS-tagged receptors, living cells transfected with BBS-tagged receptors were incubated with biotinylated BTX at 17°C for 20 min. The cells were washed to eliminate the free BTX, solubilized with detergent, and incubated with streptavidin-agarose. The streptavidin-agarose was then washed, eluted with SDS sample buffer, and analyzed by SDS/PAGE and Western blotting. As shown in Fig. 6F, the BBS-tagged receptors expressed on the cell surface could be isolated by this procedure.
Discussion
The dynamic trafficking of membrane receptors has been studied for many decades and has been shown to be important for the cellular absorption of nutrients, such as glucose and iron, and for the regulation of many membrane receptors including G protein-linked, growth factor, and ionotropic receptors (4, 25, 26). The complex regulation of membrane protein insertion, endocytosis, and recycling is now recognized as one of the most common and profound mechanisms to regulate membrane surface events and to play a critical role in most cellular events from cell adhesion and differentiation to synaptic transmission. AMPA receptors mediate excitatory synaptic transmission in the CNS, and it has recently been shown that rapid dynamic trafficking of the receptor is critical for regulating synaptic strength and plasticity processes that underlie higher brain functions such as learning and memory (3–5).
To generate a versatile tool to examine AMPA-receptor trafficking, we developed a method for imaging membrane proteins with a tag based on the BBS of the nicotinic acetylcholine receptor. BTX is a small polypeptide toxin that binds with very high affinity to the nicotinic acetylcholine receptor and has been used for decades to biochemically isolate, quantitate, and image the nicotinic acetylcholine receptor (16). A 13-aa minimal BBS derived from the nicotinic acetylcholine receptor binds BTX with high affinity (17). We used the BBS to tag the N-terminal region of AMPA-receptor subunits and then used it to analyze the expression and membrane trafficking of the tagged receptors. The tagged receptor subunits bind BTX and using rhodamine-labeled BTX we were able to specifically detect surface receptors as well as internalization and insertion of receptors into the plasma membrane. In addition, the BBS-GFP-tagged receptor subunits could be used to image receptor trafficking in live cells including primary cultured neurons. Moreover, using confocal microscopy we could assay the insertion of new receptors into the plasma membrane with rapid kinetics in living neurons.
The BBS-tagged receptors also bound commercially available 125I-BTX, allowing the absolute quantitation of the expression of the tagged proteins on the cell surface and in total solubilized cell extracts. By using biotinylated BTX, the surface receptors could be isolated with streptavidin affinity resin as well as detected on Western blots by using HRP-coupled streptavidin in heterologous cells. The BBS tag, along with the diverse forms of commercially available labeled BTX, makes this method of tagging membrane proteins a very versatile approach that is generally applicable to membrane proteins.
Powerful methods to visualize protein expression have been developed over the last decade to directly visualize recombinant proteins in heterologous expression systems. The introduction of GFP and related fluorescent proteins as reagents has revolutionized cell biology (27, 28). In addition, classical methods such as labeling of cells with epitope tagged receptors, followed by antibody detection, have been an extremely powerful approach to studying the distribution of expressed proteins. However, there are several drawbacks to these methods that limit their application. GFP-fusion protein constructs label the total population of expressed protein in the cell, and although pH-sensitive GFP (29) allows the selective detection of the GFP fusion protein in nonacidic subcellular regions of the cell, the selective labeling of other populations of GFP-tagged receptors is difficult. Epitope tags rely on antibodies for detection and antibodies are large divalent proteins that can dimerize antigens and cause clustering or capping of surface proteins (13). The BBS-tagged system complements these methods and adds to the tools available to cell biologists for studying membrane protein function.
Acknowledgments
We thank L. Ding for preparing primary hippocampal cultured neurons and Drs. R. Sattler and G. Rumbaugh for helpful comments during the preparation of the manuscript. This work was supported by the Howard Hughes Medical Institute (R.L.H.).
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate; BTX, α-bungarotoxin; BBS, BTX-binding site; HEK, human embryonic kidney; HRP, horseradish peroxidase.
References
- 1.Hollmann, M. & Heinemann, S. (1994) Annu. Rev. Neurosci. 17, 31–108. [DOI] [PubMed] [Google Scholar]
- 2.Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. (1999) Pharmacol. Rev. 51, 7–61. [PubMed] [Google Scholar]
- 3.Malinow, R. & Malenka, R. C. (2002) Annu. Rev. Neurosci. 25, 103–126. [DOI] [PubMed] [Google Scholar]
- 4.Song, I. & Huganir, R. L. (2002) Trends Neurosci. 25, 578–588. [DOI] [PubMed] [Google Scholar]
- 5.Bredt, D. S. & Nicoll, R. A. (2003) Neuron 40, 361–379. [DOI] [PubMed] [Google Scholar]
- 6.Shi, S.-H., Hayashi, Y., Petralia, R. S., Zaman, S. H., Wenthold, R. J., Svoboda, K. & Malinow, R. (1999) Science 284, 1811–1816. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi, Y., Shi, S.-H., Esteban, J. A., Piccini, A., Poncer, J.-C. & Malinow, R. (2000) Science 287, 2262–2267. [DOI] [PubMed] [Google Scholar]
- 8.Shi, S.-H., Hayashi, Y., Esteban, J. A. & Malinow, R. (2001) Cell 105, 331–343. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien, R. J., Mammen, A. L., Blackshaw, S., Ehlers, M. D., Rothstein, J. D. & Huganir, R. L. (1997) J. Neurosci. 17, 7339–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beattie, E. C., Carroll, R. C., Yu, X., Morishita, W., Yasuda, H., von Zastrow, M. & Malenka, R. C. (2000) Nat. Neurosci. 3, 1291–1300. [DOI] [PubMed] [Google Scholar]
- 11.Lin, J. W., Ju, W., Foster, K., Lee, S. H., Ahmadian, G., Wyszynski, M., Wang, Y. T. & Sheng, M. (2000) Nat. Neurosci. 3, 1282–1290. [DOI] [PubMed] [Google Scholar]
- 12.Passafaro, M., Piech, V. & Sheng, M. (2001) Nat. Neurosci. 4, 917–926. [DOI] [PubMed] [Google Scholar]
- 13.Mammen, A. L., Huganir, R. L. & O'Brien, R. J. (1997) J. Neurosci. 17, 7351–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung, H. J., Xia, J., Scannevin, R. H., Zhang, X. & Huganir, R. L. (2000) J. Neurosci. 20, 7258–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzartos, S. J. & Changeux, J.-P. (1983) EMBO J. 2, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luetje, C. W., Patrick, J. & Seguela, P. (1990) FASEB J. 4, 2753–2760. [DOI] [PubMed] [Google Scholar]
- 17.Harel, M., Kasher, R., Nicolas, A., Guss, J. M., Balass, M., Fridkin, M., Smit, A. B., Brejc, K., Sixma, T. K., Katchalski-Katzir, E., et al. (2001) Neuron 32, 265–275. [DOI] [PubMed] [Google Scholar]
- 18.Liao, D., Scannevin, R. H. & Huganir, R. L. (2001) J. Neurosci. 21, 6008–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen, J. J., Winzer-Serhan, U. H. & Leslie, F. M. (1997) J. Neurochem. 68, 112–120. [DOI] [PubMed] [Google Scholar]
- 20.Chiara, D. C. & Cohen, J. B. (1997) J. Biol. Chem. 272, 32940–32950. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, J. & Raftery, M. A. (1973) Anal. Biochem. 52, 349–354. [DOI] [PubMed] [Google Scholar]
- 22.Huganir, R. L. & Racker, E. (1982) J. Biol. Chem. 257, 9372–9378. [PubMed] [Google Scholar]
- 23.Scatchard, G. (1949) Ann. N.Y. Acad. Sci. 51, 660–672. [Google Scholar]
- 24.Prekeris, R., Foletti, D. L. & Scheller, R. H. (1999) J. Neurosci. 19, 10324–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson, R. T., Kanzaki, M. & Pessin, J. E. (2004) Endocr. Rev. 25, 177–204. [DOI] [PubMed] [Google Scholar]
- 26.Gaborik, Z. & Hunyady, L. (2004) Trends Endocrinol. Metab. 15, 286–293. [DOI] [PubMed] [Google Scholar]
- 27.Tsien, R. Y. (1998) Annu. Rev. Biochem. 67, 509–544. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. (2002) Nat. Rev. Mol. Cell Biol. 3, 906–918. [DOI] [PubMed] [Google Scholar]
- 29.Miesenbock, G., Angelis, D. A. D. & Rothman, J. E. (1998) Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- 30.Xia, Z., Dudek, H., Miranti, C. K. & Greenberg, M. E. (1996) J. Neurosci. 16, 5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]