Abstract
Combinatorial antibody libraries have the potential to display the entire immunological record of an individual, allowing one to detect and recover any antibody ever made, irrespective of whether it is currently being produced. We have termed this the “fossil record” of an individual's antibody response. To determine whether cancer patients have ever made antibodies with disease-fighting potential, we screened combinatorial antibody libraries from cancer patients for immunoglobulins that can identify metastatic tumor cells. This strategy yielded human antibodies specific for the activated conformation of the adhesion receptor integrin αvβ3 that is associated with a metastatic phenotype. In a remarkable example of convergent evolution, two of these antibodies were shown to contain the Arg-Gly-Asp integrin recognition motif of the natural ligand within the third complementarity-determining region of the heavy chain. These antibodies interfered with lung colonization by human breast cancer cells in a mouse model and inhibited existing metastatic disease. Our data imply that, at least at some time, these antibodies were part of a patient's surveillance system against metastatic cells, targeting the activated conformer of integrin αvβ3 and disrupting its functions. The ligand-mimetic nature of these antibodies, combined with specificity for a single receptor, is unique in the integrin–ligand repertoire. The convergent evolution of critical sequences in antibodies and other ligands that bind to the same target means that the immune response has sufficient power to find a best chemical solution for the optimization of binding energy, even though antibodies evolve in real time, as compared with billions of years for the natural ligand.
Keywords: convergent evolution, human antibodies, integrin αvβ3
Combinatorial antibody libraries allow the cloning of the entire immunological repertoire of an individual (1, 2). One advantage of this method is that, in principle, it allows the resurrection of any antibody that an individual has ever made, irrespective of whether the antibody is currently being produced. We have referred to this feature of combinatorial antibody libraries as the “fossil record” of an individual's immunological history (3).
In cancer, the successful establishment of a tumor in the host does not necessarily mean that there was a failure to produce antitumor antibodies. Tumor establishment also could be due to the fact that the growth rate of the malignant cells outstripped the ability of the immune response to deal with the tumor mass or that the target antigen has been modulated. Over the years, these ideas have been generally termed “immune surveillance”, but there has been no simple way to test this concept. Insofar as we can now clone the fossil record, we can study whether patients with cancer have made antibodies with specificities unique to cancer cells and whether such antibodies can influence the outcome of the host–tumor encounter.
To test these ideas, we took advantage of studies in our laboratory that have demonstrated that the activated form of integrin αvβ3 presents itself as a functional target associated with the metastatic phenotype in breast cancer cells (4, 5). When expressed in a constitutively activated functional form, αvβ3 may play a role in metastasis of breast cancer as well as prostate cancer, melanoma, and neuroblastic tumors (4, 6–11). To determine whether combinatorial antibody libraries from cancer patients contained immunoglobulins that can recognize αvβ3 specifically in its activated conformation, we designed a subtractive panning strategy on poorly vs. strongly metastatic variants of a human breast cancer cell line. This strategy yielded several human antibodies against the activated conformation of integrin αvβ3. Remarkably, two of these antibodies were shown to contain the Arg-Gly-Asp (RGD) integrin recognition motif of the natural ligand within the third complementarity-determining region of the heavy chain (CDR-H3). These antibodies not only interfered with lung colonization by human breast cancer cells in a mouse model but also inhibited existing metastatic disease. These data imply that the immune repertoire of at least some cancer patients contains antibodies that can specifically recognize the activated conformer of integrin αvβ3, disrupt the functions of the receptor, and inhibit breast cancer metastasis.
Methods
Single-Chain Fv (scFv) Combinatorial Antibody Libraries. A scFv antibody library was previously generated from the total RNA of peripheral blood lymphocytes from 20 cancer patients, 5 of whom had breast cancer. From this library, phage-displaying scFvs on gene III were rescued as detailed in ref. 12.
Subtractive Panning of Antibody Libraries. Phage-displaying antibodies reactive with the activated form of integrin αvβ3 were isolated by five rounds of subtractive panning on metastatic vs. nonmetastatic variants of MDA-MB-435 human breast cancer cells. The library (≈5 × 1012 colony-forming units) was subtracted on 3 × 107 MDA-MB-435 parent combination cells, made up from a pool of 20 clones that contained integrin αvβ3 in its nonactivated functional form, as judged by the fact that the receptor failed to support fibrinogen-directed migration and tumor cell arrest during blood flow. These cells also failed to colonize the lungs of immune-deficient mice. For selection on metastatic cells, the library was panned on 1 × 107 MDA-MB-435 cells isolated from lung metastases after mammary fat pad injection and two rounds of selection in the mouse. These cells express activated αvβ3.
scFv Purification. scFv gene fragments were subcloned into pET-Flag (derived from pET-15b, Novagen) and transformed into Escherichia coli B834(DE3). Expression was induced with 0.5 mM isopropyl β-d-thiogalactoside, and FLAG-tagged scFv fragments were purified on anti-Flag mAb M2 affinity agarose (Sigma) as described in refs. 12 and 13. Monomeric scFv was purified on Sephacryl-100 (Amersham Pharmacia) by FPLC.
Flow Cytometry. scFv clones were analyzed by flow cytometry for binding to integrin αvβ3 on human tumor cells and their ability to distinguish between the activated and nonactivated form of the receptor. The clones were tested on cells that express αvβ3 or lack this receptor but express αv or β3 in combination with other integrin subunits: M21 melanoma cells (αvβ3, no other β3 integrin), M21-L (no αv integrins), M21-LIIb (αIIbβ3, no αv integrins) (6), and UCLA-P3 lung adenocarcinoma (αv integrins but no αvβ3) (14). All of these cells express a variety of other integrins, including β1 integrins. For our study, 2 × 105 tumor cells were blocked with goat serum and incubated either with scFv phage (2–5 × 1010), followed by mouse anti-M13 mAb and goat FITC-anti-mouse or with purified scFv (15 μg/ml), followed by mouse anti-FLAG mAb M2 and goat FITC-antimouse. Binding/washing buffer was Tris-buffered saline with or without 1 mM Ca2+, 1 mM Mg2+, or 0.2 mM Mn2+.
Sequence Analysis of scFvs. Nucleic acid sequencing of selected clones was carried out on a 373-A DNA sequencer (Applied Biosystems). All sequences were searched in the Kabat database (www.nci.nlm.nih.gov) to compare them with previously sequenced VH and VL chains and in the international ImMuno-GeneTics database (www.dnaplot.de/index.html) to propose correlations of scFvs with potential germ-line gene sequences and assess V-segment usage. The gcg wisconsin package (Accelrys, San Diego) was used for alignments.
Cell Adhesion and Migration. Tumor cell adhesion under stationary conditions and haptotactic tumor cell migration in transwell chambers were detailed earlier in refs. 5 and 15.
Tumor Cell Arrest During Blood Flow. Breast cancer cell arrest during blood flow and interaction with platelets was measured as described in refs. 4 and 16. Briefly, tumor cells were suspended in human blood anticoagulated with 50 nM d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (PPACK) and perfused over a collagen I matrix at a venous wall shear rate of 50 s–1, 4 dynes/cm2. Adhesive events and cell interactions were visualized and recorded by using fluorescence video microscopy and quantified by image acquisition at 30 predefined positions, followed by computerized image analysis. Tumor cells were identified by their unique red fluorescence at 543/590 nm. To test inhibition, tumor cells were incubated with 3 μM scFv for 5 min at 37°C and mixed into blood, and scFv was added to a 3 μM final concentration and perfused immediately.
Antibody Internalization. Breast cancer cells grown in chamber slide wells were incubated with 20 μg/ml FITC-labeled scFv in serum-free Eagle's minimal essential medium for 3 h either at 4°C or 37°C, washed 10 times, fixed and permeabilized with 95% ethanol, stained with propidium iodide, mounted in antifade solution, and analyzed with a laser scanning confocal microscope (model 2100, Bio-Rad).
Apoptosis Assay. BMS human breast cancer cells derived from a patient blood sample were seeded into 96-well ultra-low-attachment plates (3 × 104 cells per well in medium with 10% FBS) in the presence or absence of 3.7 μM scFv or 14.36 μM camptothecin as a positive control and cultured for 20 h at 37°C and 5% CO2, and apoptosis was measured by quantifying cytoplasmic histone-associated DNA fragments (Cell Death Detection ELISA, Roche).
Prevention and Treatment of Breast Cancer Metastasis. For in vivo use, scFv preparations were further purified on Detoxy-Gel resin (Pierce). Remaining traces of endotoxin ranged from 0.001 to 0.07 endotoxin units/mg scFv (LAL test, BioWhittaker). To analyze the effects of patient-derived scFvs on target-organ colonization by circulating metastatic breast cancer cells, 1 × 105 BMS human breast cancer cells were injected i.v. into 6- to 8-week-old female C.B17/lcTac-Prkdc scid mice (Taconic Farms) together with 50 μg of scFv Bc-12 or Bc-15 (n = 8–10 mice per group). At an average blood volume of 2 ml per mouse, this amount provides an initial scFv concentration of 1 μM. The clearance time for scFv antibody fragments in the circulation is <1 h (17). Tumor cells may remain in the circulation for several days. Therefore, scFv antibody injections (50-μg bolus doses, i.v.) were repeated on days 2–4. Antibody-treated and control mice appeared to be healthy during the experiment. After 32 days, the mice were euthanized, dissected, and analyzed by gross examination. No obvious abnormalities were observed. The lungs were fixed in Bouin's solution, and metastatic foci were counted at the lung surface under a dissecting microscope. For detailed analysis of metastatic burden, the lungs of each mouse were embedded in paraffin, sectioned, stained with hematoxylin/eosin, and examined histologically for evidence of metastatic colonies within the lung tissue. Per lung, six sets of three consecutive sections, separated by 140 μm, were collected. The sections were randomized and coded, and the total number of metastatic foci was counted.
To treat mice with established metastatic disease, 5 × 105 DsRed2-tagged MDA-MB-435 cells expressing constitutively activated αvβ3D723R were injected i.v. On days 7, 9, 11, 14, 16, and 18 after tumor cell inoculation, the mice received i.v. injections of either 40 μg of scFv Bc-15 or scFv Mut-15 as control. The mice were euthanized on day 19, and lung metastases were counted under a fluorescence microscope. The care and use of the animals complied with National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Results and Discussion
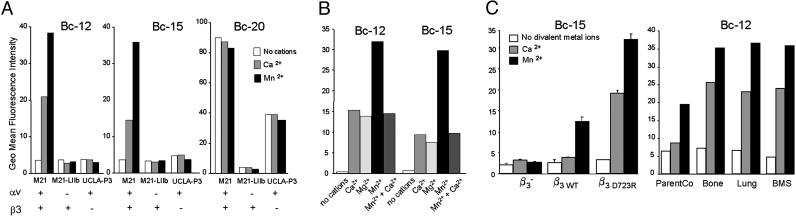
Cancer Patients Have Antibodies That Can Recognize Metastatic Breast Cancer Cells Based on Their Expression of Activated Integrin αvβ3. To generate therapeutic reagents that react specifically with metastatic breast cancer cells, we exploited the metastasis-related expression of the activated conformer of integrin αvβ3 that we identified earlier (4, 5). A combinatorial library of single-chain antibody fragments (scFv), derived from the blood lymphocyte cDNA of patients with cancer (12), allowed us to test the hypothesis that the immune repertoire contains antibodies that recognize tumor cell integrin αvβ3 and distinguish between the activated and nonactivated functional forms of the receptor. After subtractive panning on metastatic vs. nonmetastatic variants of a human breast cancer cell model, we identified two scFv clones, Bc-12 and Bc-15, which reacted only with cells expressing the αvβ3 heterodimer. Bc-12 and Bc-15 failed to bind tumor cells that lack αv integrins but did react with the transfectants of these cells in which αvβ3 expression had been restored (6), confirming the reactivity of these antibodies with αvβ3. A third scFv clone, Bc-20, reacted with αv-positive cells, regardless of the associated β-integrin subunit (Fig. 1A).
Fig. 1.
Cancer patient-derived scFvs Bc-12 and Bc-15 recognize cancer cells based on their expression of activated integrin αvβ3. Shown are flow cytometric analyses with or without 1 mM Ca2+, 1 mM Mg2+, or 0.2 mM Mn2+.(A) scFvs Bc-12 and Bc-15 are specific for αvβ3 and require cations for binding to human tumor cells. M21 melanoma, αvβ3 plus other αv integrins; M21-LIIb transfected melanoma, αIIbβ3, no αv integrins; UCLA-P3 lung adenocarcinoma, αv integrins, no β3 integrin. (B) Effect of cations and their combination on scFv-binding to M21 melanoma cells. (C) Requirement of integrin αvβ3 activation for Bc-12 and Bc-15 binding to human breast cancer cells: MDA-MB-435 (β3–) cells lack αvβ3 or express αvβ3 either in a nonactivated (β3WT and ParentCo) or activated functional form (β3D723R, Bone, and Lung), and BMS cells that were isolated from breast cancer patient blood. Several independent experiments with Bc-12 and Bc-15 on each of these cell types gave similar results.
Importantly, the recognition of αvβ3 by Bc-12 and Bc-15 showed a dependence on cations and receptor activation similar to that of natural ligands (Fig. 1 A and B). Binding was measurable in the presence of physiological Ca2+ levels and greatly enhanced when the cells were treated with Mn2+, a metal ion that can activate integrins (18–20). The enhancing effect of Mn2+ was reduced by Ca2+, consistent with the model of a specific Ca2+ integrin-binding site that interferes with ligand recognition when occupied (21) (Fig. 1B). This indicates that scFvs Bc-12 and Bc-15 have binding requirements reminiscent of natural integrin ligands, such as fibrinogen, vitronectin, and fibronectin (20, 22, 23).
To confirm that scFvs Bc-12 and Bc-15 recognize αvβ3 selectively in its activated form in a physiological setting, we tested binding of these antibodies to in vitro-generated and in vivo-selected variants of the MDA-MB-435 breast cancer cell model (4). Bc-12 and Bc-15 failed to bind β3-negative MDA-MB-435 cells (β3–) and a β3 wild-type-expressing derivative of these cells (β3WT), which requires Mn2+ for activation (Fig. 1C). In contrast, Bc-12 and Bc-15 were able to bind a breast cancer cell variant expressing constitutively activated mutant αvβ3D723R and human breast cancer cells from lung and bone metastases and patient blood samples. This binding did not require exogenous stimulation with Mn2+. These results indicate that the patient-derived scFvs Bc-12 and Bc-15 recognize αvβ3 and require its presentation in an activated state.
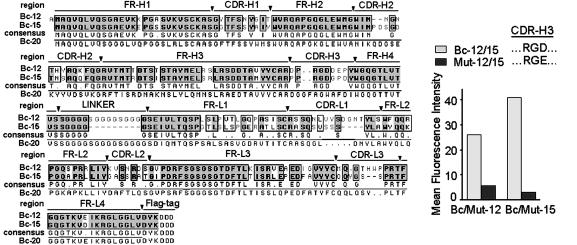
Patient-Derived scFv Antibodies Against Activated αvβ3 Are Mimetics of the Natural Ligand. The findings, that patient-derived scFv antibodies Bc-12 and Bc-15 require divalent metal cations for binding to αvβ3-expressing tumor cells and that the receptor had to be present in an activated functional form, indicate that Bc-12 and Bc-15 could resemble natural ligands of αvβ3. To analyze similarities between the scFv antibodies and natural ligands, the DNA sequences of Bc-12, Bc-15, and Bc-20 were determined and translated (Fig. 2). The protein sequence showed that the CDR-H3 in Bc-12 and Bc-15 contain an RGD ligand-recognition motif. This motif, common to natural αvβ3 ligands, is absent in Bc-20, the activation-independent anti-αv scFv (Fig. 2). In this regard, the patient-derived antibodies Bc-12 and Bc-15 resemble an in vitro engineered derivative of a murine antibody that was experimentally designed to distinguish between activated and nonactivated αv integrins (24). In contrast to that reagent, the antibodies identified here are fully human in nature and are specific for αvβ3. Further, their presence in cancer patient leukocyte expression libraries indicates that the activated conformer of tumor cell integrin αvβ3 can be distinctly recognized by the patient's immune system.
Fig. 2.
Translation of scFv DNA sequence analyses. Consensus sequence of Bc-12 and Bc-15 (specific for activated αvβ3), compared with Bc-20 (specific for αv). (Inset) Flow cytometric analysis with BMS human breast cancer cells indicates loss of binding in mutants of Bc-12 and Bc-15 containing RGE instead of RGD in CDR-H3 (Mut-12 and Mut-15). The Mut-15 signal is equivalent to negative control.
To examine the contribution of the RGD motif in CDR-H3 of scFv antibodies Bc-12 and Bc-15 in the context of their specificity for activated integrin αvβ3, this sequence was mutated to RGE by site-directed mutagenesis. The D-to-E exchange within the RGD motif is known to reduce or abolish ligand recognition by αvβ3 (25). Binding of the mutated scFvs to human breast cancer cells was strongly reduced in Mut-12, the RGE version of Bc-12, and abolished in Mut-15, the RGE version of Bc-15 (Fig. 2). This finding indicates that RGD binding critically determines antibody–antigen recognition. However, the antibodies did not react with the αvβ3-related platelet integrin αIIbβ3 or with other αv integrins or α5β1 (Fig. 1), which are known RGD-binding receptors (26). This finding implies that a synergistic binding region may exist, with contributions from both the antibody tertiary structure and the RGD sequence to the observed selective recognition of the activated conformation of αvβ3.
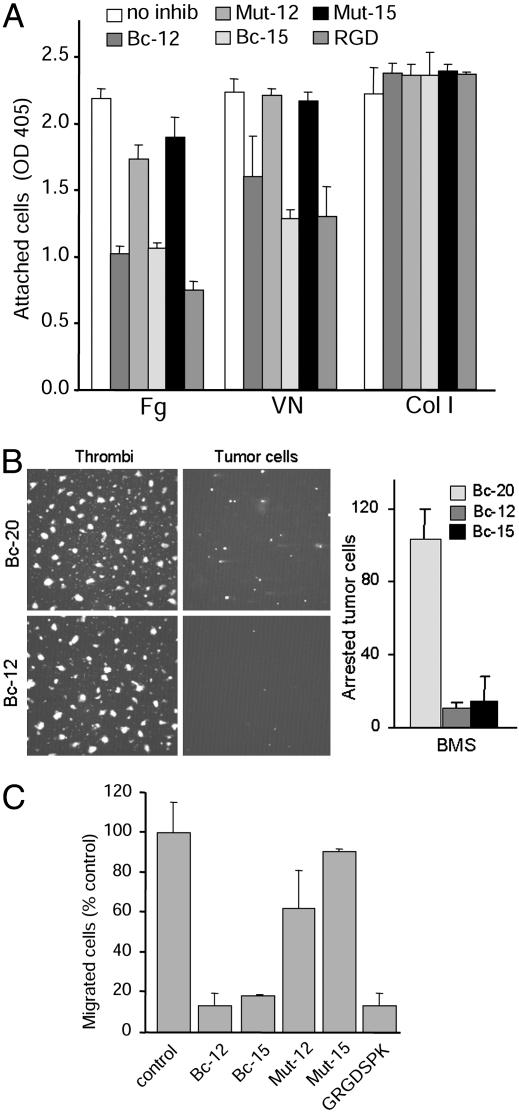
Ligand-Mimetic scFv Antibodies Inhibit αvβ3-Mediated Adhesive Tumor Cell Functions and Induce Apoptosis. Because the patient-derived scFv antibodies Bc-12 and Bc-15 apparently mimic natural ligands of integrin αvβ3, we hypothesized that these antibodies might interfere with αvβ3-mediated ligand-binding and adhesive tumor cell functions that likely contribute to the metastatic process. Treatment of human breast cancer cells isolated from a patient blood sample (5) showed that these antibodies efficiently inhibit stationary tumor cell adhesion, block cell attachment under blood flow conditions as found in the venous circulation, and interfere with matrix-directed migration. The degree to which the scFvs affected these adhesive tumor cell functions depended on the contribution of αvβ3 (Fig. 3). The results indicate that these RGD-containing human antibodies selectively block αvβ3-mediated breast cancer cell functions, which may promote metastatic dissemination. However, as shown by the adhesion and blood perfusion studies, they do not interfere with the properties of other adhesion receptors, such as those that mediate platelet adhesive functions (Fig. 3B). The RGE-containing mutant versions of Bc-12 and Bc-15 and the RGD-lacking anti-αv antibody Bc-20 had no effect on cell adhesion.
Fig. 3.
scFvs Bc-12 and Bc-15 inhibit αvβ3-mediated adhesive breast cancer cell functions. (A) Adhesion of BMS breast cancer cells to fibrinogen (Fg), vitronectin (VN), or type I collagen (Col I) with or without 3 μM Bc-12, Bc-15, or their RGE mutants Mut-12 and Mut-15, or 200 μM linear Gly-Arg-Gly-Asp-Ser-Pro-Lys (GRGDSPK) peptide. (B) Effect of scFvs on breast cancer cell arrest during blood flow. BMS breast cancer cells were labeled with hydroethidine, suspended in human blood (anticoagulated with 50 nM PPACK), and perfused over a collagen I matrix at a venous wall shear rate of 50 s–1. As shown earlier (4), breast cancer cells arrest by αvβ3-mediated binding to platelet thrombi. This arrest was quantified by image acquisition at 30 predefined positions during blood flow. (Left) Representative images of platelet thrombi (green fluorescence) and tumor cells (red fluorescence) at identical x,y positions. (Right) Number of arrested tumor cells in the presence of 3 μM nonfunction-blocking anti-αv scFv Bc-20 or function-blocking anti-αvβ3 scFvs Bc-12 or Bc-15. (C) Effect of scFvs on haptotactic BMS cell migration toward a fibrinogen substrate in transwell chambers with or without 2 μM Bc-12, Bc-15, or their RGE mutants Mut-12 and Mut-15, compared with 200 μM GRGDSPK peptide.
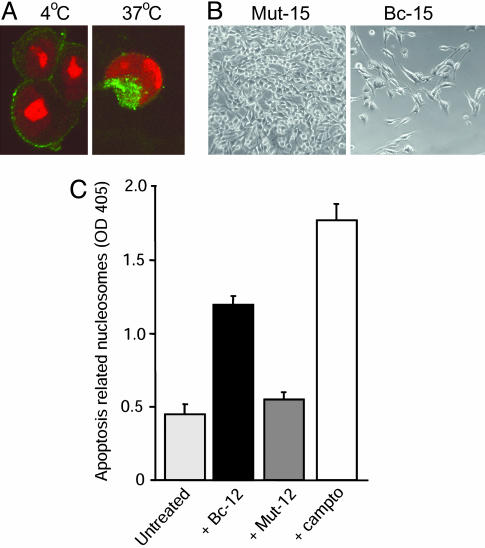
As mimetics of the natural ligand, scFvs Bc-12 and Bc-15 may impact not only breast cancer cell adhesive functions but also cell survival. It has been demonstrated that disruption of ligand-binding to αvβ3 can stimulate apoptosis (27). In addition, internalized RGD-containing compounds may directly induce cell death by activating the proapoptotic enzyme caspase-3 through an alternative pathway (28). We showed that scFvs Bc-12 and Bc-15 were efficiently bound and readily internalized by adherent human breast cancer cells at permissive temperatures (Fig. 4A). The growth of several human breast cancer cell lines, isolated from patient blood samples, was retarded in the presence of the RGD-containing scFvs Bc-12 and Bc-15, whereas their RGE-mutant versions, Mut-12 and Mut-15, had no effect (Fig. 4B). Furthermore, when breast cancer cells were deprived of an adhesive matrix, as occurs in the circulation, exposure to scFv Bc-12 resulted in apoptotic cell death (Fig. 4C). Thus, patient-derived, ligand-mimetic antibodies can disrupt specific adhesive breast cancer cell functions mediated by activated integrin αvβ3 and affect the viability and growth behavior of tumor cells bearing this receptor.
Fig. 4.
Patient-derived ligand-mimetic scFvs against activated αvβ3 are internalized and affect breast cancer cell survival. (A) scFv Bc-15 is internalized by breast cancer cells isolated from patient blood: confocal images of BMS cells incubated for 4 h with FITC-Bc-15 at 4°C (binding) vs. 37°C (allowing internalization). (B) Phase-contrast images of BMS cell cultures 4 days after seeding the cells in the presence of 2 μM RGD-expressing scFv Bc-15 vs. its RGE-mutant scFv Mut-15. (C) Effect of scFv Bc-12 on the viability of matrix-deprived BMS breast cancer cells in ultra-low-adhesion plates in the presence or absence of 3.7 μM scFv or 14.36 μM camptothecin as apoptosis-inducing control. Measurement of apoptosis was based on cytoplasmic histone-associated DNA fragments after 20 h.
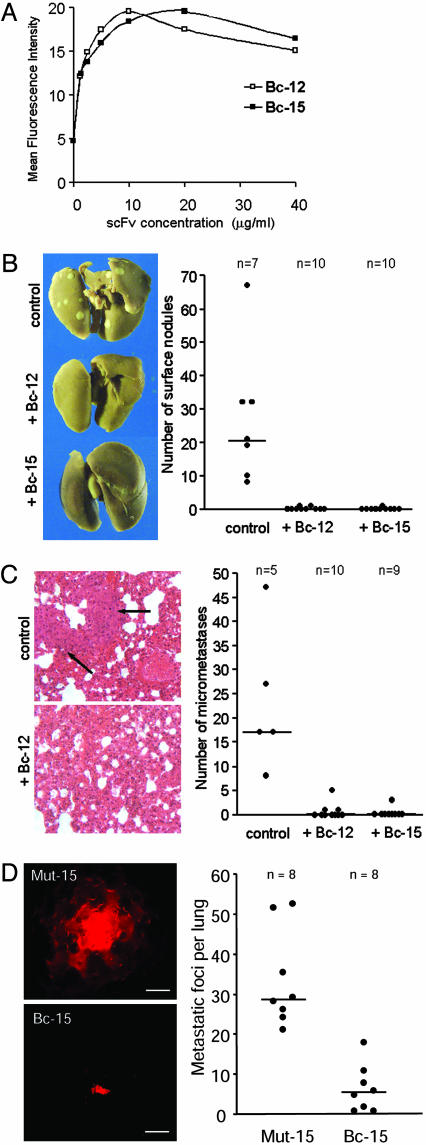
scFv Antibodies Against Activated αvβ3 Prevent Hematogenous Breast Cancer Metastasis and Inhibit Established Metastatic Disease. Having demonstrated that the immune repertoire contains antibodies that specifically recognize the activated, metastasis-supporting form of integrin αvβ3, we next tested whether binding of these antibodies to circulating tumor cells affects metastasis from the blood stream. To be effective in that microenvironment, the antibodies must bind to tumor cell integrin αvβ3 in blood or plasma. Our blood perfusion studies demonstrated that scFvs Bc-12 and Bc-15 can inhibit breast cancer cell arrest during blood flow, indicating that these antibodies recognize and affect tumor cells under conditions as found in the vasculature. To confirm this, BMS breast cancer cells were incubated with Bc-12 or Bc-15 in fresh human plasma anticoagulated with PPACK, which does not affect the cation milieu. The antibodies bound in a saturable manner with half-maximal binding at 40 nM scFv (1 μg/ml) (Fig. 5A). Similar results were obtained with other metastatic breast cancer cell lines. This finding demonstrates that the RGD-containing scFvs Bc-12 and Bc-15 bind to activated tumor cell integrin αvβ3 in the presence of a multitude of RGD-containing plasma proteins, the most abundant of which is fibrinogen at a physiological concentration of 6–12 μM.
Fig. 5.
scFvs Bc-12 and Bc-15 prevent hematogenous breast cancer metastasis and inhibit established metastatic disease. (A) Flow cytometric analysis of scFv binding to BMS breast cancer cells in human plasma anticoagulated with 50 nM PPACK. (B) Effect of Bc-12 and Bc-15 on lung colonization by human breast cancer cells. (Left) Lungs of female severe combined immunodeficient mice 32 days after i.v. injection with 1 × 105 BMS cells. The mice were treated with either 50 μg of Bc-12 or Bc-15 (i.v.) on days 1, 2, 3, and 4. Controls received PBS. (Right) Numbers of lung-surface metastases for each animal. The horizontal line indicates the median number of metastases per group. ScFv-treated mice had significantly fewer metastases (P < 0.001 by the Kruskal–Wallis test). (C) (Left) Histological sections of the above mouse lungs stained with hematoxylin/eosin. Metastases were counted for each lung in six sets of three consecutive sections separated by 140 μm. (Right) Number of metastases counted in sections of individual lungs. The horizontal line indicates the median number of metastases per group. ScFv-treated mice had significantly fewer detectable metastases (P < 0.005 by the Kruskal–Wallis test). (D) Effect of Bc-15 treatment on established breast cancer metastasis in the lungs. Female severe combined immunodeficient mice were injected i.v. with 5 × 105 DsRed2-tagged MDA-MB-435 breast cancer cells expressing constitutively activated integrin αvβ3D723R. Mice were treated on days 7, 9, 11, 14, 16, and 18 by i.v. injections of either scFv Bc-15 or its RGE mutant, Mut-15 (40 μg per dose). Metastatic foci were enumerated by fluorescence microscopy on day 19. (Left) Images of typical tumor foci within the lung tissue in Mut-15-treated (Upper) or Bc-15-treated (Lower) mice. (Scale bar, 50 μm.) (Right) Number of metastatic foci within the lung tissue of each animal. Horizontal line, median number of metastases per group. Bc-15-treated mice had significantly fewer metastases than Mut-15-treated mice (P < 0.001 by the two-sided Mann–Whitney U test).
To test directly whether targeting activated integrin αvβ3 with the ligand-mimetic antibodies affects target organ colonization by circulating tumor cells, metastatic human breast cancer cells were injected into the blood stream of immunodeficient mice, and the animals were treated with scFv Bc-12 or Bc-15 for the first 4 days. This caused a nearly complete inhibition of metastatic burden in the lungs. Whereas each of the control mice had tumor foci at the lung surface, measured after 32 days (median, 21; range, 8–68), only two mice in the Bc-12-treated group and one mouse in the Bc-15-treated group had a single visible nodule at the lung surface (Fig. 5B). Histological examination of each lung revealed that scFv treatment had prevented metastatic colonization of the lung tissue. All control animals had metastases of considerable size in their lung tissue (median, 17; range, 8–48 counts per lung) (Fig. 5C Upper Left). In contrast, only 3 of 10 Bc-12-treated mice and 1 of 9 Bc-15-treated mice had detectable metastases, and these were very small (median, 1 for Bc-12 and 0 for Bc-15; range, 0–5 counts per lung for Bc-12 and 0–3 counts per lung for Bc-15) (Fig. 5C). Thus, injections with scFv Bc-12 or Bc-15 interfered with lung colonization by circulating breast cancer cells at a statistically significant level.
Prevention of breast cancer's spreading to distant organs is clinically highly relevant. In many cases, however, micrometastases have already established when a patient is first diagnosed with breast cancer. To test whether our antibodies could interfere with established metastatic disease, immunodeficient mice were injected i.v. with human breast cancer cells expressing constitutively activated integrin αvβ3D723R and metastases were allowed to develop before treatment was initiated. The number of detectable metastases was significantly reduced in mice treated with the RGD-containing scFv Bc-15 (median, 5.5; range, 1–18 counts per lung), compared with mice that had received the RGE-mutant version of this antibody, Mut-15 (median, 28.5; range, 21–52 counts per lung). Residual metastases in mice treated with Bc-15 were very small, in stark contrast to large multicellular foci observed in the lungs of the control animals (Fig. 5D). Thus, targeting the activated conformer of integrin αvβ3 with patient-derived antibodies can indeed interfere with established metastatic breast cancer.
Taken together, our data imply that the immune repertoire of at least some cancer patients contains antibodies that specifically recognize and disrupt the activated form of integrin αvβ3, a functional target present on metastatic breast cancer cells. Such antibodies may offer a treatment for breast cancer metastasis. In that activated αvβ3 also is expressed during angiogenesis, these antibodies may further find a role in the control of other solid tumors.
Finally, it is interesting to speculate why cancer patients make antibodies to a self-antigen, albeit of an activated conformation. Perhaps self/nonself discrimination is context-dependent and we are not as tolerant to self-antigens when they present in the wrong tissue environment, a situation that may occur during metastasis.
Acknowledgments
We thank Drs. E. Beutler, Z. M. Ruggeri, and D. O'Sullivan of The Scripps Research Institute for support and stimulating discussions. This work was supported by National Institutes of Health Grants NCI-CA95458 (to B.F.-H.), NIAID-AI47127 (to K.D.J.), and M01RR00833 (to The Scripps General Clinical Research Center); U.S. Army Breast Cancer Research Program Grant DAMD 17-99-1-9368 (to B.F.-H.); the Stein Endowment Fund; and The Skaggs Institute for Chemical Biology.
Author contributions: B.F.-H., R.A.L., A.L., S.Z., M.R.W., S.A., C.G., S.M., A.S., and K.D.J. designed research, performed research, contributed new reagents/analytic tools, analyzed data, and wrote the paper.
Abbreviations: CDR-H3; third complementarity-determining region of the heavy chain; PPACK, d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone; scFv, single-chain Fv.
References
- 1.Huse, W. D., Sastry, L., Iverson, S. A., Kang, A. S., Alting-Mees, M., Burton, D. R., Benkovic, S. J. & Lerner, R. A. (1989) Science 246, 1275–1281. [DOI] [PubMed] [Google Scholar]
- 2.McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. (1990) Nature 348, 552–554. [DOI] [PubMed] [Google Scholar]
- 3.Lerner, R. A., Barbas, C. F., III, Kang, A. S. & Burton, D. R. (1991) Proc. Natl. Acad. Sci. USA 88, 9705–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felding-Habermann, B., O'Toole, T. E., Smith, J. W., Fransvea, E., Ruggeri, Z. M., Ginsberg, M. H., Hughes, P. E., Pampori, N., Shattil, S. J., Saven, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolli, M., Fransvea, E., Pilch, J., Saven, A. & Felding-Habermann, B. (2003) Proc. Natl. Acad. Sci. USA 100, 9482–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felding-Habermann, B., Fransvea, E., O'Toole, T. E., Manzuk, L., Faha, B. & Hensler, M. (2002) Clin. Exp. Metastasis 19, 427–436. [DOI] [PubMed] [Google Scholar]
- 7.Gladson, C. L., Hancock, S., Arnold, M. M., Faye-Petersen, O. M., Castleberry, R. P. & Kelly, D. R. (1996) Am. J. Pathol. 148, 1423–1434. [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng, D. Q., Woodard, A. S., Fornaro, M., Tallini, G. & Languino, L. R. (1999) Cancer Res. 59, 1655–1664. [PubMed] [Google Scholar]
- 9.Zheng, D. Q., Woodard, A. S., Tallini, G. & Languino, L. R. (2000) J. Biol. Chem. 275, 24565–24574. [DOI] [PubMed] [Google Scholar]
- 10.Van Belle, P. A., Elenitsas, R., Satyamoorthy, K., Wolfe, J. T., Guerry, D., Schuchter, L., Van Belle, T. J., Albelda, S., Tahin, P., Herlyn, M., et al. (1999) Hum. Pathol. 30, 562–567. [DOI] [PubMed] [Google Scholar]
- 11.Gui, G. P., Wells, C. A., Browne, P. D., Yeomans, P., Jordan, S., Puddefoot, J. R., Vinson, G. P. & Carpenter, R. (1995) Surgery 117, 102–108. [DOI] [PubMed] [Google Scholar]
- 12.Mao, S., Gao, C., Lo, C. H., Wirsching, P., Wong, C. H. & Janda, K. D. (1999) Proc. Natl. Acad. Sci. USA 96, 6953–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, C., Mao, S., Ronca, F., Zhuang, S., Quaranta, V., Wirsching, P. & Janda, K. D. (2003) J. Immunol. Methods 274, 185–197. [DOI] [PubMed] [Google Scholar]
- 14.Wayner, E. A., Orlando, R. A. & Cheresh, D. A. (1991) J. Cell Biol. 113, 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilch, J., Habermann, R. & Felding-Habermann, B. (2002) J. Biol. Chem. 277, 21930–21938. [DOI] [PubMed] [Google Scholar]
- 16.Felding-Habermann, B., Habermann, R., Saldivar, E. & Ruggeri, Z. M. (1996) J. Biol. Chem. 271, 5892–5900. [DOI] [PubMed] [Google Scholar]
- 17.Kortt, A. A., Dolezal, O., Power, B. E. & Hudson, P. J. (2001) Biomol. Eng. 18, 95–108. [DOI] [PubMed] [Google Scholar]
- 18.Xiong, J. P., Stehle, T., Diefenbach, B., Zhang, R., Dunker, R., Scott, D. L., Joachimiak, A., Goodman, S. L. & Arnaout, M. A. (2001) Science 294, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong, J. P., Stehle, T., Zhang, R., Joachimiak, A., Frech, M., Goodman, S. L. & Arnaout, M. A. (2002) Science 296, 151–155. [DOI] [PubMed] [Google Scholar]
- 20.Mould, A. P., Barton, S. J., Askari, J. A., Craig, S. E. & Humphries, M. J. (2003) J. Biol. Chem. 278, 51622–51629. [DOI] [PubMed] [Google Scholar]
- 21.Xiong, J. P., Stehle, T., Goodman, S. L. & Arnaout, M. A. (2003) J. Thromb. Haemost. 1, 1642–1654. [DOI] [PubMed] [Google Scholar]
- 22.Smith, J. W. (2002) Methods Cell Biol. 69, 247–259. [DOI] [PubMed] [Google Scholar]
- 23.Hughes, P. E., Diaz-Gonzalez, F., Leong, L., Wu, C., McDonald, J. A., Shattil, S. J. & Ginsberg, M. H. (1996) J. Biol. Chem. 271, 6571–6574. [DOI] [PubMed] [Google Scholar]
- 24.Pampori, N., Hato, T., Stupack, D. G., Aidoudi, S., Cheresh, D. A., Nemerow, G. R. & Shattil, S. J. (1999) J. Biol. Chem. 274, 21609–21616. [DOI] [PubMed] [Google Scholar]
- 25.Ruoslahti, E. (1996) Annu. Rev. Cell Dev. Biol. 12, 697–715. [DOI] [PubMed] [Google Scholar]
- 26.Ruoslahti, E. (2003) Matrix Biol. 22, 459–465. [DOI] [PubMed] [Google Scholar]
- 27.Stupack, D. G., Puente, X. S., Boutsaboualoy, S., Storgard, C. M. & Cheresh, D. A. (2001) J. Cell Biol. 155, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley, C. D., Pilling, D., Henriquez, N. V., Parsonage, G., Threlfall, K., Scheel-Toellner, D., Simmons, D. L., Akbar, A. N., Lord, J. M. & Salmon, M. (1999) Nature 397, 534–539. [DOI] [PubMed] [Google Scholar]