Abstract
Background
Combatting over-weight or obesity can lead to large fluctuations in an individual’s body weight, often referred to as weight cycling or “yo-yo” dieting. Current evidence regarding the potentially damaging effects of these changes is conflicting.
Methods
Here, we assess the metabolic effects of weight cycling in a murine model, comprising three dietary switches to normal or high fat diets at 6 week intervals; male C57BL/6 mice were fed either a control (C) or high fat (F) diet for 6 weeks (n=140/group). C and F groups were then either maintained on their initial diet (CC and FF respectively) or switched to a high fat (CF) or control (FC) diet (n=35/group). For the final 6 week interval, CC and CF groups were returned to the control diet (CCC and CFC groups) while FC and FF groups were placed on a high fat diet (FCF and FFF) (n=28/group).
Results
For the majority of metabolic outcomes changes aligned with dietary switches; however assessment of neuropeptides and receptors involved in appetite regulation and reward signalling pathways reveal variable patterns of expression. Furthermore, we demonstrate that multiple cycling events leads to a significant increase in internal fat deposition, even when compared to animals maintained on a high fat diet (Internal Fat: FCF: 7.4 ± 0.2g vs. FFF: 5.6 ± 0.2g; p<0.01).
Conclusions
Increased internal adipose tissue is strongly linked to the development of metabolic syndrome associated conditions such as type 2 diabetes, cardiovascular disease and hypertension. While further work will be required to elucidate the mechanisms underlying the neuronal control of energy homeostasis, these studies provide a causative link between weight cycling and adverse health.
Introduction
Effective treatments for obesity and its related morbidities are hindered by both post-surgical complications following bariatric surgery, and side-effects associated with approved anti-obesity drugs 1. Reduced calorie intake and increased energy expenditure via exercise represent the least invasive means of therapy. However, these lifestyle changes are often unsuccessful in the long-term 2, which can lead to multiple, unsuccessful attempts to lose weight, resulting in weight cycling, or “yoyo dieting”.
Although undesirable, as it represents a failure to maintain weight loss, there are conflicting data on whether weight cycling episodes induce a metabolically adverse outcome. Several studies indicate no association between weight-cycling and body composition, metabolic profile, energy expenditure or cardiovascular risk 3–7. In contrast, additional reports have linked weight cycling to future weight gain, altered fat distribution and increased cardiovascular and metabolic risk, 8–12.
These inconsistencies are mirrored in animal studies; with several papers reporting increased fat mass and internal adiposity, insulin resistance and hypertension in weight cycling models 13–15, while others find no association 16, 17. In both human and preclinical studies a number of potential confounders are likely to contribute to this disparity; age, gender, prior changes in bodyweight as well as the timing and physiological background onto which experimental protocols are imposed are all likely to influence long-term outcomes. Here, we comprehensively assess the effects of a weight cycling paradigm in a murine model, comprising three dietary switches of a normal or high fat diet, at 6 weeks intervals.
Materials and Methods
Animals and Treatment
All experiments were performed in compliance with Animals (Scientific Procedure) Act 1986 under the project licence 70/6656 and 70/7502. C57BL/6 males mice (aged 8-10 weeks) (Harlan UK) were acclimatised for a week in ventilated cages under a 12h:12h light cycle (lights on at 7am) at 21-23°C with ad libitum access water. Animals were fed high fat (F) (60% by kcal intake; TD.06414) and control (C) diets (TD.08806, 10% by kcal intake) (Harlan, USA).
Weight cycling study
C57BL/6 male mice were housed in groups of 4 and fed combinations of high fat and control diets. The study ran for a total of 18 weeks, with animals assessed at three time points following dietary manipulation at 6 and 12 weeks (Figure 1). In total 10 dietary groups were generated;
Figure 1. Study protocol.
Weight cycling protocol in mice fed combinations of high fat (F) or control (C) diets for a total of 18 weeks. Dietary changes were introduced at 6 and 12 weeks of feeding.
Time Point 1: After 6 weeks of feeding, no diet swaps; 2 groups: C, F
Male C57BL/6 mice were fed either a control diet (C) or high fat (F) diet for 6 weeks (n=140/group)
Time Point 2: After 12 weeks of feeding, 1 diet swap; 4 groups: CC, CF, FC, FF
After 6 weeks of feeding, C and F groups were either maintained their initial diet (CC and FF respectively) or switched to a high fat (CF) or control (FC) diet for the subsequent 6 weeks (n=35/group).
Time Point 3: After 18 weeks of feeding, 2 diet swaps; 4 groups: CCC, CFC, FCF, FFF
After 12 weeks of feeding, CC and CF groups were returned to the control diet (CCC and CFC groups) while FC and FF groups were placed back on a high fat diet (FCF and FFF) for an additional 6 weeks (n=28/group)
Body weight, food and calorie Intake
Body weight and food intake were monitored weekly. Calorie intake was measured in grams per cage of 4 mice (kcal) calculated from dietary composition
Magnetic resonance Imaging
Subsets of mice from each of the 10 dietary groups underwent whole body MRI and 1H MRS, and localised 1H liver and muscle MRS after 4 weeks, 10 weeks and 16 weeks of their assigned diet regime (n=10-12/group). Adiposity was assessed using whole body MRI and 1H MRS, and localised 1H liver.
Whole body MRI
After an overnight fast (16h), mice were anaesthetised with a 3% oxygen/isofluorane Body temperature and respiration rate were maintained during the subsequent scan. Mice were placed in a whole body birdcage coil and scanned in a 4.7T Unity Inova MR scanner (Varian Inc, USA). 50 consecutive transverse MR images of 2mm thickness covering the whole body were collected using a T1-weighted spin echo sequence (TR: 2.2s, TE: 20ms, FOV: 45mm x 45mm, Matrix: 256 x 192, 2 Averages). Images were converted into a stack using ImageJ (Rasband) and segmentation analysis was performed using SliceOmaticTM (Tomovision®) to provide volumes and mass of internal and subcutaneous adipose tissue deposits. Analysis was performed by a trained analyst blinded to the animal treatment.
Whole Body and Localised 1H MRS
Whole body 1H MRS was performed using a single pulse sequence (SPULS) with the following parameters: TR 10s, pulse angle 45°, 4 averages and spectral width 20,000Hz. Localised 1H liver MRS was performed using a Point Resolved Spectroscopy (PRESS) sequence with a voxel 2x2x2mm3 placed using the images generated with whole body MRI with the following parameters: TR 10s, TE 9ms, averages 64 and spectral width 20,000Hz.
Glucose Tolerance Test
An intraperitoneal glucose tolerance test (IPGTT) was performed on subsets of mice after 5 weeks, 11 weeks and 17 weeks on their assigned diet regime (n=10/12 group). Mice were fasted overnight for 16-18 hours prior to the IPGTT. Fasting glucose was determined with a commercially available glucose metre before injecting a 2g/kg D-glucose bolus i.p. Blood glucose concentration was determined 15, 30, 60 and 120 minutes following injection. Insulin sensitivity was assessed from fasting concentrations of glucose and insulin using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) 18.
Metabolic Hormone Assay
A MILLIPLEX MAP Mouse Metabolic Hormone Magnetic Bead Panel was used to assess metabolic hormone levels in the plasma (Millipore, USA). The assay was performed according to the manufacturer’s protocol using undiluted plasma. The plate was run on a MAGPIX system with xPonent software (Luminex Corp, US).
Tissue Analysis
White adipose tissue was collected (subcutaneous, epididymal, retroperitoneal and mesenteric), along with brown adipose tissue, liver, pancreas, kidneys, heart and gastrocnemius muscle. Tissue samples were frozen on dry ice then stored at 80°C. Brains were sliced coronally on an ice cold brain matrix into 3 sections (forebrain, midbrain and hindbrain) and stored in RNAlater (Ambion) overnight at 4°C before being stored at 20° C. A section of the left lobe of the liver was also collected in RNAlater and stored overnight at 4°C before being stored at 20°C.
Gene Expression Analysis
RNA and DNA Extraction
Brain RNA was extracted using a combination of Trizol treatment and an AllPrep DNA/RNA micro kit (Bettscheider et al., 2011). Brains preserved in RNAlater were sectioned coronally at 500μm on an ice cold brain matrix using double edged razor blades. Specific regions of interest were dissected from 2 slices using a mouse brain atlas as a reference (Figure 2.4.9.1.1, Franklin & Paxinos, 2007). Approximately 4μg of tissue was placed in a 2ml eppendorf with 350μl of RLT Buffer Plus (Qiagen, USA), 1% 2-Mercaptoethanol and 0.5% Reagent DX (Qiagen). The tissue was lysed and disrupted using by placing a 5mm steel bead (Qiagen) in the eppendorf and placing in a TissueLyser II (Qiagen) for 4 minutes at 20Hz. The lysate was removed and added to an AllPrep DNA column and centrifuged at 13,500rpm for 3 minutes at 4°C. The columns were then placed in new collection tubes on ice for DNA elution after RNA extraction was completed (as DNA is more stable than RNA). The flow through was added to a new 1.5ml eppendorf, along with 100μl of Trizol reagent (Ambion, USA) and incubated at room temperature for 5 minutes. 20μl of chloroform was added and the tube was vigorously shaken by hand for 15 seconds, before being left a further 2 minutes at room temperature. The sample was then centrifuged at 13,500rpm for 15 minutes at 4°C, after which the aqueous phase (containing RNA) was removed and added to a new 1.5ml eppendorf. 70% ethanol was added to the sample to precipitate the RNA, then added to an RNeasy MinElute spin column (Qiagen). The columns were centrifuged at 13,500rpm for 3 minutes at 4°C and flow through discarded. The spin column membrane underwent a series of washes as described in the manufacturer’s protocol, along with oncolumn DNAse digestion using RNasefree DNase (Qiagen). RNA was eluted using 12μl of THE RNA storage solution (Ambion) and stored at 80°C until further use. AllPrep DNA columns also underwent a series of washes as described in the manufacturer’s protocol and eluted twice using 30μl of EB buffer preheated to 70°C, then stored at 80°C.
cDNA Synthesis
The amount of RNA is each sample was quantified using a NanoDrop (Thermo Scientific, USA). 1μg of liver RNA and 500ng of brain RNA was used to synthesise cDNA as described in the Thermoscript RT-PCR Systems protocol (Invitrogen, UK). RNA was added to a mix of oligo (dT)20 primer, 10mM deoxyribonucleotide phosphate Mix (dNTP), DEPC treated water. The samples were incubated at 65°C for 5 minutes and immediately placed on ice. The samples were then incubated with 5x cDNA synthesis buffer, 0.1M Dithriothreitol (DTT), DEPC treated water and Thermoscript Reverse Transcriptase. cDNA synthesis was completed by incubating the samples at 50°C for 60 minutes and terminating the reaction at 85°C for 5 minutes, after which the samples were stored at 20°C until qPCR analysis.
Quantitative RT-PCR
A master mix of Platinum SYBR Green qPCR superMix-UDG, primer, ROX reference dye, DEPC treated water (Invitrogen) and cDNA template were prepared for qRT-PCR analysis. Each sample was added in triplicate to a MicroAmp Fast 96-well reaction plate (Applied Biosystems, USA), including a 1:5 serial dilution of the cDNA templates used for each primer. Expression of all genes undergoing investigation were normalised to a control gene (GAPDH) (Table 2.4.10.0.1, 2.4.10.0.2). The plate was sealed with a PCR specific seal (Invitrogen) then placed in a preheated (Applied Biosystems 7500 Fast RealTime PCR System) at a cycling program of 50°C for 2 minutes, 94°C for 2 minutes, 40 cycles of 95°C for 15 seconds, 65°C for 30 seconds, then a dissociation step of 95°C for 15 seconds, 60°C for 60 seconds and 95°C for 15 seconds. Gene expression analysis was performed using ΔΔCT values as described previously (Bookout & Mangelsdorf 2003, Bookout et al., 2006).
Indirect calorimetry and acute exercise
Indirect calorimetry were carried out at 18 weeks on a separate group of mice (n=8/group). Ad libitum fed rats were then monitored for a baseline period of 24 hours. During CLAMS monitoring, metabolic parameters (VO2 and VCO2) were measured by indirect calorimetry. Exhaust air from each chamber was sampled at 30 minute intervals for a period of 1 minute. Sample air was sequentially passed through O2 and CO2 sensors (Columbus Instruments) for determination of O2 and CO2 content. In order to compare animals of differing sizes, the O2 consumption and CO2 production values were normalised with respect to body weight and O2 consumption was corrected to metabolic body size (W0.75) (9). RER was calculated by dividing VCO2 by VO2. Ambulatory activity of each individually housed animal was measured simultaneously using the optical beam technique (Opto M3, Columbus Instruments). Consecutive photo-beam breaks were scored as an ambulatory movement. Activity counts in x and z axes were recorded every minute for 24 hours and were used to determine horizontal (XAMB) and vertical (ZTOT) movement respectively. Food intake was measured every minute.
Statistics
All data is presented as mean ± standard error of mean (SEM) unless specified. The normality of the data was tested using a Kolmogorov-Smirnov test. Two-way ANOVA with Bonferroni correction was used to analyse continuous data, including body weight, food intake and CLAMS measurements. Oneway ANOVA with Bonferroni correction was performed between multiple groups (GraphPad Software, USA).
Results
The effects of weight cycling on body weight, food intake, calorie consumption and feeding efficiency
C57BL/6 male mice were fed combinations of high fat and control diets for 18 weeks, with dietary changes at 6 and 12 weeks (Figure 1). In total, 10 dietary groups were generated; for the initial 6 weeks mice were fed either a control diet (C) or high fat (F) diet (n=140/group). During the subsequent 6 weeks C and F groups were either maintained on their initial diet (CC and FF respectively), or switched to a high fat (CF) or control (FC) diet (n=35/group). For the final 6 weeks of the study, CC and CF groups were returned to the control diet (CCC and CFC groups), while FC and FF groups were placed back on a high fat diet (FCF and FFF) (n=28/group).
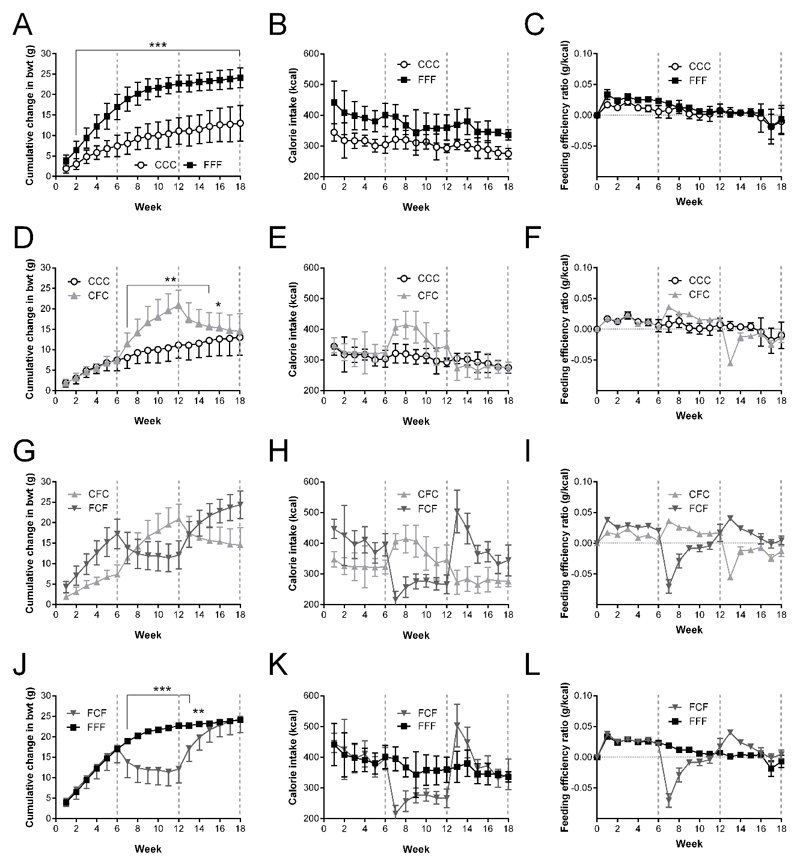
Cumulative changes in body weight, caloric intake and feeding efficiency are shown in Figure 2. Regardless of the specific weight cycling sequence, a group’s body weight consistently reflects the preceding dietary composition, with significant differences between high fat and control groups typically manifesting after the first week. Changes in caloric intake and feeding efficiency align with dietary switches, with difference in caloric intake between high fat and normal chow groups reduced with each successive cycle (Figure 1). Differences in food intake and caloric intake normalised to body weight also aligned with dietary switches (Supplementary Figure 1). There is a sharp increase in caloric intake in FCF animals upon return to a high fat diet at 12 weeks (Figure 2).
Figure 2. Changes in body weight, caloric intake and feeding efficiency in weight cycled animals.
Parameters recorded in mice placed on combinations of high fat (F) or normal fat (C) diet for recorded at 6 (C, F), 12 (CC, CF, FC, FF) and 18 weeks (CCC, CFC, FCF, FFF). CCC vs. FFF: (A) Cumulative change in body weight (Cuml. Bwt (g)), (B) Caloric intake (CI (kcal)), (C) Feeding efficiency (FE (g/kcal)); CCC vs. CFC: (D) Cuml. Bwt, (E) CI, (F) FE; CFC vs FCF: (G) Cuml. Bwt, (H) CI, (I) FE; FCF vs FFF: (J) Cuml. Bwt, (K) CI, (L) FE. Data presented as mean ± sem; n=28/group; statistical analysis performed using Two-Way ANOVA with Bonferroni Correction;* = p<0.05; ** = p<0.01; *** = p<0.001; dotted lines indicate at dietary switch points at 6 and 12 weeks. ○: CCC; ▴: CFC ; ▾: FCF; ■: FFF
The effects of weight cycling on body fat and lipid deposition as measured by MRI and MRS
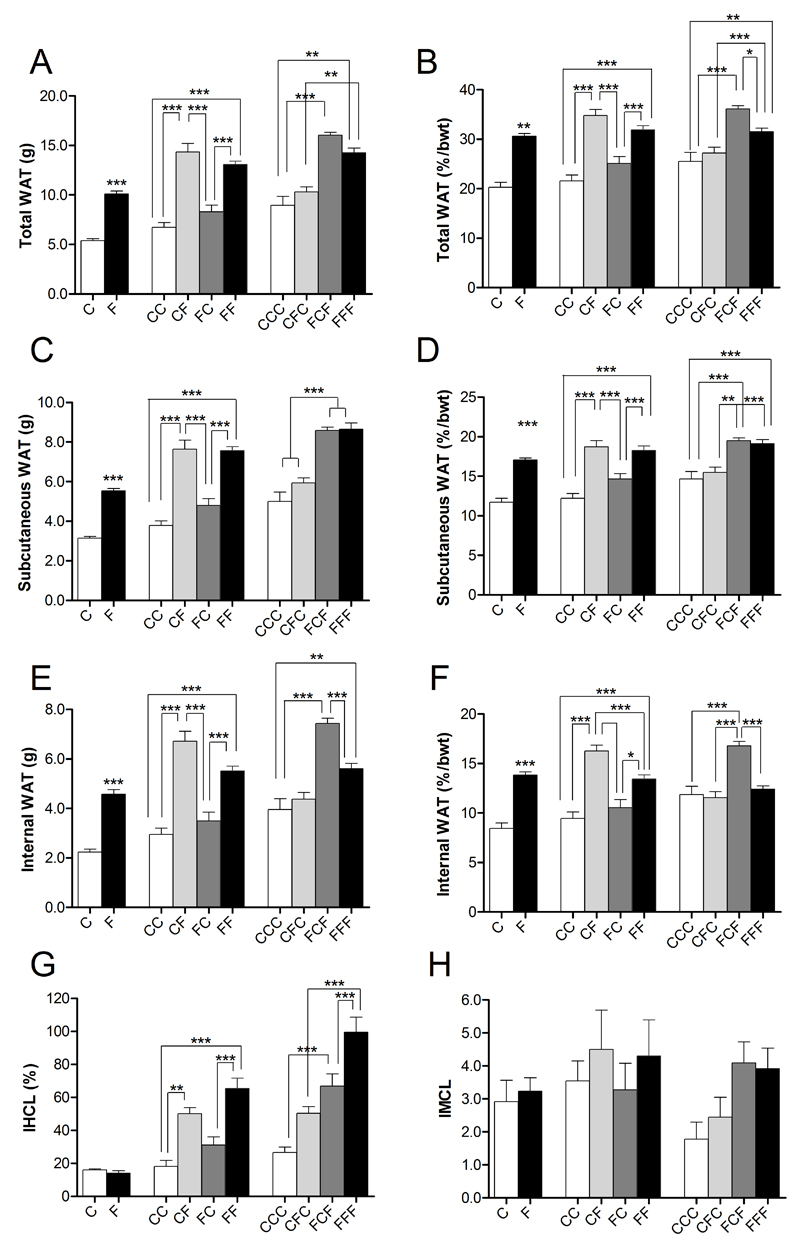
Whole body magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) techniques provide an accurate means of quantifying internal fat depots strongly associated with metabolic derangement 19, 20. Measurements were recorded at 4, 10 and 16 weeks of the diet swap. Differences in total, subcutaneous and internal white adipose tissue (WAT) before and after normalising for body weight are shown in Figure 3. At 4 weeks, animals on a high fat diet had significantly higher percentage of total (Figure 3A), subcutaneous (Figure 3C) and internal fat (Figure 3E) than controls (p<0.001). Similarly, at 10 and 16 weeks, high fat feeding led to significantly increased total, subcutaneous and internal WAT in FF mice compared to CC controls (p<0.001). These changes persisted after normalisation of WAT to body weight (Figures 3B, 3D and 3F). FCF animals demonstrated a trend towards increased total (Figure 3B) and internal WAT (Figure 3E) compared to FFF mice which emerged as significant following normalisation to body weight (p<0.05). Fat depot weights measured following dissection at 6, 12 and 18 weeks reflect comparable patterns in dietary groups to MRI data, including the increased internal adiposity in FCF compared to FFF animals (Supplementary Figure 3).
Figure 3. The effects of weight cycling on body fat and lipid deposition as measured by MRI and MRS.
Changes in white adipose tissue (WAT) measured by MRI and ectopic fat deposition measured by MRS, in mice placed on combinations of high fat (F) or normal fat (C) measured at 4 (C, F), 10 (CC, CF, FC, FF) and 16 weeks (CCC, CFC, FCF, FFF). (A) Total WAT; (B) Total WAT normalised to body weight; (C) Subcutaneous WAT; (D) Subcutaneous WAT normalised to body weight; (E) Internal WAT; (F) Internal WAT normalised to body weight; (G) Intrahepatocellular Lipid (IHCL); (H) Intramyocellular Lipid (IMCL). Data presented as mean ± sem; n=10/group; statistical analysis performed using an ANOVA with Bonferroni post-hoc test; * = p<0.05, ** = p<0.01; *** = p<0.001
Dietary changes in intrahepatocellular lipid (IHCL) and intramyocellular lipid (IMCL) as measured by localised 1H MRS are shown in Figure 3G and Figure 3H respectively. By 10 weeks, significant increases in IHCL emerge in the groups placed on a high fat diet compared to controls (10 week IHCL: CC: 18.0 ± 3.8 vs. CF: 50.1 ± 3.9; FF: 65.4 ± 6.2, p<0.01 for all, Figure 3G). After 16 weeks of feeding, FFF mice had a significantly higher IHCL content compared to CCC mice (16 week IHCL: CCC: 26.6 ± 3.3; FFF: 102.9 ± 9.1, p<0.001). Of the weight cycled groups, only FCF mice showed a significantly higher IHCL content compared to CCC animals (16 week IHCL: CFC: 50.3 ± 4.0; FCF: 60.9 ± 6.0, CCC vs FCF, p<0.05, Figure 2G). A trend towards increased IMCL content was observed in HF fed mice at 10 and 16 weeks but no significant differences were observed (Figure 3H).
The effects of weight cycling on metabolic markers
Table 1 shows the concentrations of circulating metabolic markers recorded at 6, 12 and 18 weeks. Significantly elevated fasting levels of glucose and insulin were observed in all high fat dietary groups compared to controls, with the exception of CC vs. CF animals for glucose. The differences in fasting glucose and insulin were reflected in the composite HOMA-IR measure of insulin resistance (Table 1).
Table 1. The effects of weight cycling on metabolic markers.
Concentrations of circulating metabolic markers in blood (glucose) and plasma (all others) recorded at 6 (C, F), 12 (CC, CF, FC, FF) and 18 weeks (CCC, CFC, FCF, FFF). Fasting glucose (mmol/L), glucose (mmol/L), insulin (ng/ml), C-Peptide (ng/ml), glucagon (pg/ml), GIP (pg/ml), leptin (ng/ml), ghrelin (pg/ml), resistin (ng/ml), IL-6 (pg/ml), ß-ketone (mmol/L), IL-6 (pg/ml), MCP-1 (pg/ml), TNF-α (pg/ml); n=10-12/group; statistical analysis performed using One-way ANOVA with Bonferroni correction; All measurements performed in fed animals except fasting glucose; ○ = p<0.05, ○○ = p<0.01; ○○○ = p<0.001 versus control diet (C, CC or CCC) ; ▾ = p<0.05, ▾▾ = p<0.01; ▾▾▾ = p<0.001 versus FC or FCF diet; ■ = p<0.05, ■■ = p<0.01; ■■■ = p<0.001 versus FF or FFF diet.
| 6 weeks | 12 weeks | 18 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | F | CC | CF | FC | FF | CCC | CFC | FCF | FFF | |
| Glucose (Fast) | 5.1 ± 1.4 | 9.0 ± 1.7○○○ | 8.4 ± 2.8 | 10.8 ± 2.6▾▾▾ | 6.8 ± 2.2 | 8.2 ± 2.6 | 6.3 ± 1.2 | 6.7 ± 2.2▾▾;■ | 9.7 ± 1.2○○○ | 9.7 ± 1.6○○ |
| Glucose (Fed) | 12.5 ± 2.2 | 13.9 ± 2.5 | 10.0 ± 2.6 | 10.5 ± 1.8 | 10.8 ± 2.7 | 12.2 ± 2.1 | 8.8 ± 2.2 | 9.1 ± 1.2 | 10.5 ± 1.8 | 9.1 ± 2.3 |
| Insulin | 1.0 ± 0.6 | 2.9 ± 1.8○○○ | 0.6 ± 0.3 | 3.9 ± 2.6○○; ▾▾ | 0.9 ± 0.8 | 4.5 ± 2.8○○; ▾▾ | 1.1 ± 0.9 | 1.4 ± 0.8▾;■■■ | 2.7 ± 0.92○○; | 3.9 ± 0.65○○○ |
| C-peptide | 2.1 ± 0.8 | 4.0 ± 1.1○○○ | 1.6 ± 0.3 | 3.9 ± 2.1○; ▾▾ | 1.4 ± 1.0 | 4.6 ± 1.8○○○; ▾▾▾ | 2.2 ± 0.9 | 2.7 ± 1.3▾▾; ■■ | 5.1 ± 2.2○○○ | 5.3 ± 0.4○○○ |
| Glucagon | 51.5 ± 34.5 | 51.9 ± 18.6 | 32 ± 49 | 67.1 ± 72.5 | 52 ± 54.9 | 43 ± 45.3 | 21.7 ± 9.3 | 41.4 ± 31.5 | 72.2 ± 70.9 | 48.8 ± 49.1 |
| GIP | 128 ± 86 | 339 ± 184○○ | 97 ± 42 | 450 ± 203○○; ■ | 183 ± 224 | 220 ± 125 | 184 ± 146 | 194 ± 201 | 258 ± 132 | 313 ± 180 |
| Leptin | 9.0 ± 6.9 | 36.1 ± 10.4○○○ | 5.5 ± 3.5 | 29.7 ± 14.4 | 7.3 ± 5.4 | 32.2 ± 18.6 | 9.5 ± 7.0 | 12.9 ± 8.4▾ | 25.9 ± 11.4○○ | 23.8 ± 7.7○○○ |
| Ghrelin | 268 ± 141 | 220 ± 91 | 321 ± 174 | 194 ± 99 | 263 ± 128 | 182 ± 58○ | 263 ± 153 | 194 ± 64■ | 163 ± 92 | 127 ± 58○○ |
| Resistin | 16.2 ± 5.7 | 33.8 ± 10.3○○○ | 12.0 ± 3.9 | 27.9 ± 8.8 | 10.4 ± 3.4 | 20.3 ± 8.5 | 9.9 ± 4.4 | 12.8 ± 6.2▾▾ | 23.4 ± 10.3○○○ | 21.0 ± 12.1○ |
| IL-6 | 47.6 ± 53.4 | 24.8 ± 36.9 | 16 ± 17 | 10.8 ± 11.1 | 18 ± 13.8 | 18.5 ± 17.4 | 14.6 ± 10.9 | 13.5 ± 9.9 | 19.7 ± 5.2 | 15.7 ± 5.9 |
| ß-ketone | 0.29 ± 0.06 | 0.35 ± 0.06 | 0.2 ± 0.1 | 0.33 ± 0.06 | 0.26 ± 0.02 | 0.36 ± 0.05 | 0.26 ± 0.04 | 0.26 ± 0.03 | 0.30 ± 0.03 | 0.29 ± 0.04 |
| IL-6 | 35.7 ± 8.0 | 24.8 ± 10.7 | 17.9 ± 6.5 | 12.5 ± 3.7 | 18.0 ± 3.7 | 17.7 ± 4.9 | 14.6 ± 3.9 | 13.5 ± 3.5 | 19.7 ± 1.8 | 15.8 ± 2.7 |
| MCP-1 | 43.4 ± 5.8 | 43.7 ± 4.7 | 34 ± 11 | 37.0 ± 5.3 | 22.8 ± 4.0 | 51.2 ± 11.4 | 59.9 ± 5.8 | 43.2 ± 8.0 | 66.6 ± 1.7 | 52.9 ± 7.3 |
| TNF-α | 8.8 ± 1.7 | 8.4 ± 2.1 | 8.1 ± 2.7 | 22.9 ± 4.7 | 7.5 ± 3.2 | 21.1 ± 6.5 | 10.3 ± 3.2 | 34.0 ± 9.1 | 12.7 ± 1.5 | 11.3 ± 4.8 |
No differences were observed in fed glucose levels between dietary groups at any stage of the study. Both insulin (fed) and c-peptide were significantly higher in all groups placed on a high fat diet for the 6 weeks prior to measurement. Plasma GP was increased in F vs. C and CC vs. CF groups. Significant differences in ghrelin manifested at 12 weeks (CC vs. FF, p<0.05) and became more pronounced at 18 weeks (CCC vs FFF, p<0.01; CFC vs. FFF, p<0.05). Increased plasma concentrations of leptin and resistin were observed in high fat groups compared to controls. No significant differences were found between dietary groups in the plasma levels of ß-ketone, IL-6, THF-α or MCP-1.
The effects of weight cycling on indirect calorimetry as measured by CLAMS metabolic cages
CLAMS metabolic cage measurements recorded at 18 weeks are shown in Supplementary Figure 4. CCC and CFC mice demonstrated significantly greater O2 consumption, VO2 production and RER during both light and dark phases compared to FCF and FFF mice (p<0.05 for all). A significant increase in heat production was observed in FFF compared to CCC mice in the dark phase (p<0.05). Vertical beam breaks (ZTOT) indicative of movement and rearing were significantly greater in CCC and CFC compared to FCF and FFF groups in the dark phase (p<0.001 for all).
The effects of weight cycling on neuronal mRNA expression
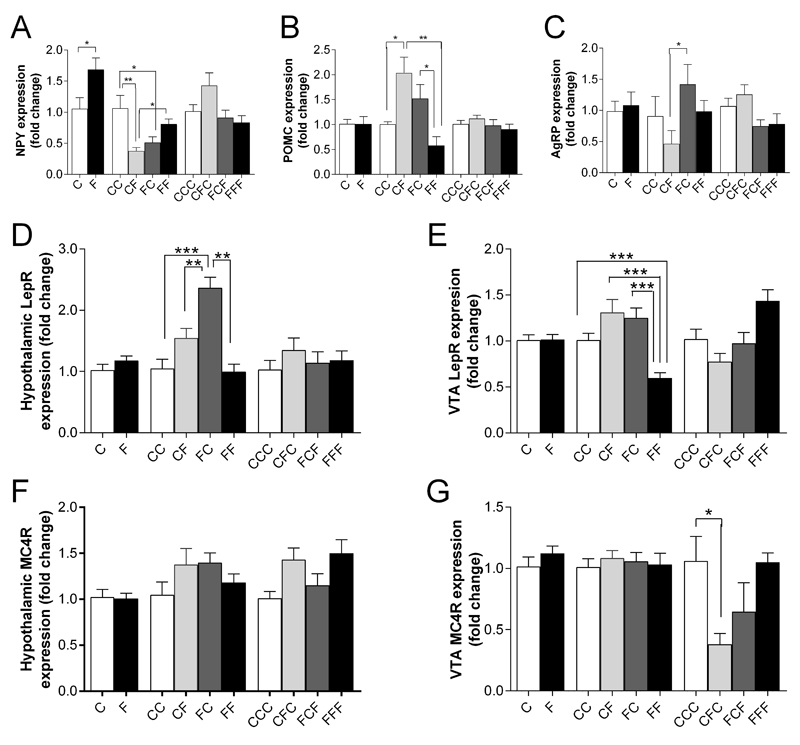
RT-qPCR was used to assess changes in mRNA expression in homeostatic and hedonic regions. Figure 4 shows the hypothalamic expression of the neuropeptides NPY (Figure 4A), Agouti-related peptide (AGRP) (Figure 4B) and proopiomelanocortin (POMC) (Figure 4C). After 6 weeks of HF feeding, F mice showed a significant increase in the expression of NPY compared to control animals, with no differences in POMC or AgRP expression. At 12 weeks, CF demonstrated a significant decrease in NPY mRNA expression and increased POMC mRNA expression compared to CC and FF mice. After 18 weeks of feeding, no differences in hypothalamic NPY, POMC or AgRP mRNA expression were observed between any dietary groups.
Figure 4. The effects of weight cycling on hypothalamic neuropeptide expression and receptor expression in the hypothalamus and ventral tegmental region (VTA).
RT-qPCR was used to assess hypothalamic neuropeptide and neuropeptide receptor expression in fold change (arbitrary units) at 6 (C, F), 12 (CC, CF, FC, FF) and 18 weeks (CCC, CFC, FCF, FFF). (A) NPY; (B) AgRP; (C) POMC; (D) LepR: Hypothalamus; (E) LepR: VTA; (F) MC4R: Hypothalamus; (G) MC4R: VTA. Data presented as mean ± sem; n=5-8/group; statistical analysis performed using an ANOVA with Bonferroni post-hoc test; * = p<0.05, ** = p<0.01, *** = p<0.001
No significant differences between C and F groups were observed in either LepR or MC4R expression in the hypothalamus (Figure 4D) or ventral tegmental area (VTA) (Figure 4E). At 12 weeks, FC mice showed a significant increase in hypothalamic LepR mRNA expression in compared to CC (p<0.001), CF (p<0.01) and FF (p<0.001) mice (Figure 4D). In the VTA, LepR expression was significantly lower in FF mice compared to all other groups (p<0.001 for all, (Figure 4E)). No significant changes in MC4R expression in either the hypothalamus or VTA were observed between groups at 6 and 12 weeks. At 18 weeks, expression of MC4R in the VTA was reduced in CFC compared to CCC animals in the VTA (p<0.05, Figure 4G).
Assessment of dopaminergic gene expression in the hypothalamus, VTA, NAc and PFC are shown in Figure 5. TH expression in the hypothalamus was significantly increased in CF mice compared to CC mice (p<0.05) at 12 weeks (Figure 5B). CF mice also showed a significant increase in D2R (p<0.05) and COMT (p>0.01) mRNA expression compared to FF animals (Figure 5B). In the VTA, mice fed on a high fat diet demonstrated a significant increase in D1R, D2R and DAT mRNA expression at 6 weeks (all p<0.05, Figure 5D). DAT mRNA expression was also significantly increased in CF mice compared to CC controls at 12 weeks (p<0.05, Figure 5E). Both CF and FC mice showed a significant increase in VTA DAT mRNA expression compared to FF animals (Figure 5E). At 18 weeks, TH, D2R and DAT mRNA expression were all significantly higher in FFF mice compared to both CCC and FCF groups (p<0.05 for all, Figure 5F). FFF mice also had significantly higher DAT mRNA expression compared to CFC animals (Figure 5F). CFC mice showed a significant decrease in D1R mRNA and an increase in CORT mRNA expression compared to CCC mice (p<0.05 for both, Figure 5F).
Figure 5. The effects of weight cycling on dopamine-related gene expression in the hypothalamus and reward areas.
Dopamine-related neuropeptide expression as measured by RT-qPCR; in the hypothalamus (hypo) (A-C); ventral tegmental area (VTA) (D-F); nucleus accumbens (NAc) (G-I); prefrontal cortex (PFC) (K-L) at 6 (C, F), 12 (CC, CF, FC, FF) and 18 weeks (CCC, CFC, FCF, FFF). (A) Hypo: 6 weeks, (B) Hypo: 12 weeks, (C) Hypo: 18 weeks, (D) VTA: 6 weeks, (E) VTA: 12 weeks, (F) VTA: 18 weeks, (G) NAc: 6 weeks; (H) NAc: 12 weeks, (I) NAc: 18 weeks; (J) PFC: 6 weeks, (K) PFC: 12 weeks, (L) PFC: 18 weeks. Statistical TH: tyrosine hydroxylase; D1R: dopamine receptor D1A; D2R: dopamine receptor 2; DAT: dopamine active transporter; DARPP: dopamine and cAMP-regulated neuronal phosphoprotein; COMT: Catechol-O-methyl-transferase. Measurements in fold change (arbitrary units). Data presented as mean ± sem; n=5-8/group; statistical analysis performed using an unpaired t-test; * = p<0.05, ** = p<0.01; *** = p<0.001
At 6 weeks, a significant reduction in DAT mRNA expression was recorded in both the NAc (p<0.01, Figure 5G) and PFC (p<0.001, Figure 5J) of F mice compared to controls (p<0.01). At 12 weeks, a significant decrease in D1R mRNA expression was seen in CF and FC groups compared to CC and FF groups, in both the NAc (p<0.05 for all, Figure 5H) and PFC (p<0.05 for all, Figure 5K). DAT expression in FC mice was also significantly increased compared to CC mice in the NAc (p<0.01, Figure 5H) and PCF (p<0.00, Figure 5K). In the PFC, FC DAT mRNA expression was also significantly higher than CF and FF groups (p<0.001 for all, Figure 5K). In the PFC, a significant decrease in D2R mRNA expression was seen in CF compared to FF mice (p<0.05, Figure 5K). D2R mRNA expression was significantly increased in CFC (p<0.01) and FCF (p<0.05) groups compared to CCC controls in the PFC (Figure 5L).
Discussion
A considerable amount of conflicting data exists regarding the long-term effects of weight-cycling in both human and animal studies. Here, we implemented a weight cycling protocol in a murine model, measuring a wide array of markers associated with energy homeostasis; including feeding, body composition, circulating gut hormones, lipid and muscle metabolism, indirect calorimetry and neuronal signalling in the central nervous system (CNS). While the majority of outcomes aligned with changes in diet, the effects of weight cycling events on several key markers indicate the development of an adverse metabolic phenotype.
There is a lack of consensus regarding the effects on weight cycling in available human studies, which vary considerably with regard to design, age of subjects and definition of weight cycling 21. Most of these analyses have been carried out in adults who are already overweight, whereas the majority of animal studies are performed in young animals that are relatively lean. Here, we selected male mice in ‘adolescence/ early adulthood” at 8-10 weeks of age so as to reduce the influence of early life programming effects 22. Male mice are more susceptible to developing metabolic syndrome associated features in diet-induced obesity studies than females, and as such represent a more sensitive physiological background to assess our weight cycling paradigm. A key contributing factor is likely to be their predisposition to deposit greater amounts of fat in visceral areas, as opposed to the potentially protective subcutaneous fat stores utilised by females 23. Furthermore, different phases of the ovarian cycle alternately affect feeding and sensitivity to high fat diets 24, and exclusive use of male mice removes this potential confounder. It should be noted that the prevalence of weight cycling events is greater in women compared to men 25, and additional work will be required to study this paradigm in female mice.
The 6 week cycle duration in our protocol was chosen in order to measure the metabolic flexibility of animals following intervals inducing rapid weight gain or loss. Previous studies implementing a 4 week interval reported weight cycled animals failed to reach the body weight observed in mice maintained on a high fat diet 26, 27. Conversely, a weight cycling study that employed 7 weeks cycle duration revealed extended periods of high fat feeding impinge on subsequent weight loss following a return to a control diet 28. We therefore chose a 6 week interval in order to reduce the confounding effects of differences in body weight between feeding groups. In accord with previous weight-cycling studies, we found changes in body weight, calorie intake and feeding efficiency aligned with dietary switches 26, 29. Furthermore, significant differences between high fat and control groups in these parameters were lost following any subsequent period in which animals were placed on the same diet. Six week dietary intervals therefore appear to provide sufficient time for groups to normalise body weight and feeding. It should be noted that cycled mice had a non-significant trend towards increased body weight after three weight cycles. In addition, the magnitude of difference in calorie intake and feeding efficiency between high fat and normal chow groups reduced with each successive dietary switch following normalisation for body weight. Together these data suggest that multiple cycling may reduce the ability to reach a control body weight, as previously indicated 26.
Increases in internal and ectopic fat deposition are strongly associated with dyslipidaemia, cardiovascular disease and type 2 diabetes 30, 31. Excessive dietary fat intake has been shown to overload the capacity for lipid storage in subcutaneous adipose tissue, leading to an initial increase in internal depots and ectopically, in organs such as the muscle and liver 32, 33. Individual adipose depots also respond differentially to an energy deficit, with a preferential loss of fat from visceral compared to subcutaneous stores 34, 35. Together, these data indicate key differences in depot flexibility following fluctuations in dietary fat intake 36. There have been contradictory reports regarding the effects of weight-cycling on adiposity and body composition; with several studies indicating changes in percentage lean mass and an increase in visceral adiposity 8, 37–39, while others show no effects 5, 6, 40, 41.
In our model we demonstrate body fat deposition is highly responsive to changes in diet, with significant increases in all compartments following transfer to a high fat diet. We found no difference in adipose tissue between FC and CC, or CFC and CCC groups, indicating that 6 weeks of a control diet is sufficient to normalise these depots following a high fat diet cycle. However, both MRI and dissection data corroborate a significantly increased internal adiposity in weight cycled FCF compared to FFF animals, with no differences in subcutaneous stores. These results indicate that weight-cycling alters the response to a high fat diet, leading to preferential fat deposition in internal depots. The changes observed in intrahepatocellular lipid (IHCL) are not suggestive of any additionally negative effects of weight cycling. Rather, IHCL appears to reflect long-term dietary consequences, with differences between groups only manifesting later in the study, and increases directly proportional to the duration of time animals spend on a high fat diet. Similarly, analyses of metabolic markers and indirect calorimetry data indicate groups vary by diet, with no additional effects of weight-cycling.
It could be argued that the effects of a single high fat diet interval in the CFC group are reflected in non-significant trends towards increased IHCL, insulin, glucose, glucagon and leptin compared to CCC controls. Furthermore, weight-cycled FCF animals showed trends towards increased leptin, resistin, glucagon and inflammatory markers compared to FFF animals, in accord with their elevated levels of abdominal fat. While these trends did not reach statistical significance, these data serve to inform the timescale of changes in various markers following weight cycling events; with significant differences in adiposity manifesting before changes in more established clinical outcomes.
There is little consensus regarding the effects of weight cycling on hypothalamic neuropeptide expression, with variability in cycle length, feeding state, animal age and dietary constituents likely to be major contributing factors. Following the switch to a high fat diet, the anticipated decrease in orexigenic neuropeptides such as NPY and AgRP often disappear after prolonged exposure 42, 43, while other studies demonstrate a delayed decrease in NPY after 9 weeks of a cafeteria style HF diet 44 or no effect at all 45. Here, we noted an unexpected increase in hypothalamic expression of NPY in HFD compared to control animals after a single cycling event. At 12 weeks the neuropeptide profile is somewhat clearer, with a reduction in NPY, an increase in POMC and a trend towards decreased AgRP in CF compared to CC animals indicating a decrease in appetite in response to maintained weight gain. It would be tempting to suggest that the lack of difference in neuropeptide profiles at 18 weeks in the consequent CCC and CFC groups is indicative of a reduction in sensitivity of the system, induced by weight cycling events. However, no differences in POMC or AGRP were noted at 6 weeks and FC and FF groups do not show clear patterns of expression. Future studies, which incorporate additional time points, fed and fasted state measurements and analyses of specific hypothalamic nuclei will be required to fully resolve the effects of weight cycling on feeding centres within the CNS. Similarly, no clear patterns of expression for MC4R, leptin receptor or dopamine mRNA were seen either in the hypothalamic or ventral tegmental area at any time-point. A more detailed analysis which differentiates between the orexigenic and anorexigenic neurones that leptin and MC4 receptors connect to 46, 47 will be required.
Previous studies which demonstrated a negative correlation between BMI and dopamine receptor availability in the striatum led to the “reward deficit” hypothesis, whereby a deficiency in dopamine signalling leads to overeating and obesity 48, 49, and is supported by much of the subsequent animal model data 50, 51. In contrast, the results presented here do not replicate these findings, with animals placed on a high fat diet for 18 weeks showing an increase in D2R and TH mRNA expression in the VTA, suggestive of increased dopamine signalling. These increases in dopamine signalling in various reward centres were not observed after 12 weeks of HF feeding but closely resembled those observed after 6 weeks of HF feeding. Together these results may suggest differences in dopaminergic gene expression are not the product of a gradual change in one direction, but represent differential expression according to the time spent feeding on a HF diet. More work will be required to determine if this is the result of producing a new “set point” for body weight homeostasis. Ultimately, the lack of a coherent narrative arising from the neuropeptide and dopamine-related gene expression data highlight the need for a more in depth analysis of weight cycling on the neuronal control of energy homeostasis.
While animal models permit unique insight into the impact of dietary fluctuations on a wide array of metabolic outcomes, the precise control over macronutrient intake does not adequately reflect human dietary patterns. Caution must therefore be taken extrapolating the results of animal studies such as ours with the effects of weight cycling in humans; the average lifespan for a male C57BL/6 mouse is 820 ± 20 days, meaning the six week cycling paradigm we employ crudely corresponds to approximately 4 years in a human life-time (based on a 78 year life span in UK males). However, the six week cycle we employ appears to successfully nullify the confounding effects of differences in body weight, and provides a platform from which future studies can be carried out exploring additional weight cycling events and sex comparisons, allowing more meaning comparison with human data.
The question remains as to the underlying mechanism(s) responsible for the increase in internal fat observed in weight cycled animals (FCF), notably compared to those maintained on a high fat diet (FFF). Recent work by Dulloo et al. has proposed the “fat over-shoot” hypothesis whereby following weight loss, body composition is regulated with preferential accumulation of fat mass persisting until complete recovery of lean mass 52. Here, analysis of fat mass and lean mass, individually and as a percentage of body weight revealed no difference between FCF and FFF groups (data not shown), and no difference in thermogenesis as measured by metabolic cage analysis at the end of the study (Supplementary Figure 4). Future analysis of 6 week cycles which impose periods of dieting, food restriction and/or periods of weight recovery will provide a more accurate assessment of the influence of this potential mechanism. Alternatively, caloric intake and feeding efficiency data presented here demonstrate a sharp increase in energy intake in FCF animals upon return to a high fat diet for their final dietary switch. This increase in internal fat may have arisen from the “overflow” of lipid storage into ectopic areas, a hypothesis which posits that an acute increase in energy intake can result in metabolically “exhausted” subcutaneous adipose tissue stores, unable to deal with the influx of fat. This in turn leads to an overflow of triglyceride storage in the liver and internally around the organs 53. Determining how preceding weight cycles influence energy intake and distribution of fat storage will be required to confirm the exact nature of the adverse metabolic phenotype we have observed.
In conclusion, despite the uncertainty regarding the effects of weight cycling and yo-yo dieting, here we demonstrate multiple weight cycling events have an acute effect on fat composition, specifically increasing the amount and proportion of the metabolically adverse internal adipose. These data, in conjunction with our 6 week cycling model, are suggestive of an altered, adverse phenotype arising from rapid fluctuations in body weight.
Supplementary Material
Supplementary information is available at IJO's website
Acknowledgements
We acknowledge the Medical Research Council for funding and support.
Footnotes
Author Contributions
SES conducted research, analysed and interpreted data; ABH, GJSC and MS conducted research, JRCP interpreted data and wrote paper. JDB designed research and had primary responsibility for final content. All authors contributed to the preparation of the final manuscript.
Conflict of Interest
The authors confirm there are no competing financial interest in relation to this work
References
- 1.Adan RA. Mechanisms underlying current and future anti-obesity drugs. Trends in neurosciences. 2013;36(2):133–40. doi: 10.1016/j.tins.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, et al. Long-term weight loss maintenance in the United States. International journal of obesity (2005) 2010;34(11):1644–54. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graci S, Izzo G, Savino S, Cattani L, Lezzi G, Berselli ME, et al. Weight cycling and cardiovascular risk factors in obesity. Int J Obes Relat Metab Disord. 2004;28(1):65–71. doi: 10.1038/sj.ijo.0802537. [DOI] [PubMed] [Google Scholar]
- 4.Field AE, Manson JE, Laird N, Williamson DF, Willett WC, Colditz GA. Weight cycling and the risk of developing type 2 diabetes among adult women in the United States. Obesity research. 2004;12(2):267–74. doi: 10.1038/oby.2004.34. [DOI] [PubMed] [Google Scholar]
- 5.Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, et al. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. Omics : a journal of integrative biology. 2009;13(1):21–35. doi: 10.1089/omi.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason C, Foster-Schubert KE, Imayama I, Xiao L, Kong A, Campbell KL, et al. History of weight cycling does not impede future weight loss or metabolic improvements in postmenopausal women. Metabolism. 2013;62(1):127–36. doi: 10.1016/j.metabol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens VL, Jacobs EJ, Sun J, Patel AV, McCullough ML, Teras LR, et al. Weight cycling and mortality in a large prospective US study. American journal of epidemiology. 2012;175(8):785–92. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 8.Wallner SJ, Luschnigg N, Schnedl WJ, Lahousen T, Sudi K, Crailsheim K, et al. Body fat distribution of overweight females with a history of weight cycling. Int J Obes Relat Metab Disord. 2004;28(9):1143–8. doi: 10.1038/sj.ijo.0802736. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Tamakoshi K, Yatsuya H, Murata C, Wada K, Otsuka R, et al. Long-term body weight fluctuation is associated with metabolic syndrome independent of current body mass index among Japanese men. Circulation journal : official journal of the Japanese Circulation Society. 2005;69(1):13–8. doi: 10.1253/circj.69.13. [DOI] [PubMed] [Google Scholar]
- 10.Montani JP, Viecelli AK, Prevot A, Dulloo AG. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the 'repeated overshoot' theory. International journal of obesity (2005) 2006;30(Suppl 4):S58–66. doi: 10.1038/sj.ijo.0803520. [DOI] [PubMed] [Google Scholar]
- 11.Vergnaud AC, Bertrais S, Oppert JM, Maillard-Teyssier L, Galan P, Hercberg S, et al. Weight fluctuations and risk for metabolic syndrome in an adult cohort. International journal of obesity (2005) 2008;32(2):315–21. doi: 10.1038/sj.ijo.0803739. [DOI] [PubMed] [Google Scholar]
- 12.Savage JS, Birch LL. Patterns of weight control strategies predict differences in women's 4-year weight gain. Obesity (Silver Spring, Md.) 2010;18(3):513–20. doi: 10.1038/oby.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Buison A, Uhley V, Jen KL. Long-term weight cycling in female Wistar rats: effects on metabolism. Obesity research. 1995;3(6):521–30. doi: 10.1002/j.1550-8528.1995.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 14.Ernsberger P, Koletsky RJ, Baskin JS, Collins LA. Consequences of weight cycling in obese spontaneously hypertensive rats. The American journal of physiology. 1996;270(4 Pt 2):R864–72. doi: 10.1152/ajpregu.1996.270.4.R864. [DOI] [PubMed] [Google Scholar]
- 15.Lim K, Murakami E, Lee S, Shimomura Y, Suzuki M. Effects of intermittent food restriction and refeeding on energy efficiency and body fat deposition in sedentary and exercised rats. Journal of nutritional science and vitaminology. 1996;42(5):449–68. doi: 10.3177/jnsv.42.449. [DOI] [PubMed] [Google Scholar]
- 16.Graham B, Chang S, Lin D, Yakubu F, Hill JO. Effect of weight cycling on susceptibility to dietary obesity. The American journal of physiology. 1990;259(6 Pt 2):R1096–102. doi: 10.1152/ajpregu.1990.259.6.R1096. [DOI] [PubMed] [Google Scholar]
- 17.Lauer JB, Reed GW, Hill JO. Effects of weight cycling induced by diet cycling in rats differing in susceptibility to dietary obesity. Obesity research. 1999;7(2):215–22. doi: 10.1002/j.1550-8528.1999.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 20.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta T, Smith DL, Jr, Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev. 2014;15(11):870–81. doi: 10.1111/obr.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Gronert MS, Ozanne SE. Early life programming of obesity. Medycyna wieku rozwojowego. 2013;17(1):7–12. [PubMed] [Google Scholar]
- 23.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99(5):931–40. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 24.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1215–67. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahti-Koski M, Mannisto S, Pietinen P, Vartiainen E. Prevalence of weight cycling and its relation to health indicators in Finland. Obesity research. 2005;13(2):333–41. doi: 10.1038/oby.2005.45. [DOI] [PubMed] [Google Scholar]
- 26.List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ. The effects of weight cycling on lifespan in male C57BL/6J mice. International journal of obesity (2005) 2013;37(8):1088–94. doi: 10.1038/ijo.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism. 1998;47(9):1089–96. doi: 10.1016/s0026-0495(98)90283-9. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS One. 2009;4(4):e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012;7(7):e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perseghin G. Lipids in the wrong place: visceral fat and nonalcoholic steatohepatitis. Diabetes Care. 2011;34(Suppl 2):S367–70. doi: 10.2337/dc11-s249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne CD. Dorothy Hodgkin Lecture 2012: non-alcoholic fatty liver disease, insulin resistance and ectopic fat: a new problem in diabetes management. Diabetic medicine : a journal of the British Diabetic Association. 2012;29(9):1098–107. doi: 10.1111/j.1464-5491.2012.03732.x. [DOI] [PubMed] [Google Scholar]
- 32.Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59(9):2288–96. doi: 10.2337/db10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carobbio S, Rodriguez-Cuenca S, Vidal-Puig A. Origins of metabolic complications in obesity: ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Current opinion in clinical nutrition and metabolic care. 2011;14(6):520–6. doi: 10.1097/MCO.0b013e32834ad966. [DOI] [PubMed] [Google Scholar]
- 34.Palou M, Sanchez J, Priego T, Rodriguez AM, Pico C, Palou A. Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. The Journal of nutritional biochemistry. 2010;21(1):23–33. doi: 10.1016/j.jnutbio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Somers VK, Romero-Corral A, Sert-Kuniyoshi FH, Pusalavidyasagar S, Davison DE, et al. Effects of weight gain and weight loss on regional fat distribution. Am J Clin Nutr. 2012;96(2):229–33. doi: 10.3945/ajcn.111.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. Journal of obesity. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrne NM, Weinsier RL, Hunter GR, Desmond R, Patterson MA, Darnell BE, et al. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: comparison of responses in white and black women. Am J Clin Nutr. 2003;77(6):1368–73. doi: 10.1093/ajcn/77.6.1368. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2010;65(1):78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr. 2011;94(3):767–74. doi: 10.3945/ajcn.110.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitmann BL, Garby L. Composition (lean and fat tissue) of weight changes in adult Danes. Am J Clin Nutr. 2002;75(5):840–7. doi: 10.1093/ajcn/75.5.840. [DOI] [PubMed] [Google Scholar]
- 41.Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. International journal of obesity (2005) 2013;37(10):1371–7. doi: 10.1038/ijo.2013.1. [DOI] [PubMed] [Google Scholar]
- 42.Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. American journal of physiology. Endocrinology and metabolism. 2000;279(4):E838–45. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. American journal of physiology. Endocrinology and metabolism. 2002;282(6):E1352–9. doi: 10.1152/ajpendo.00230.2001. [DOI] [PubMed] [Google Scholar]
- 44.Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. Journal of neurochemistry. 2004;88(4):909–16. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behavioural brain research. 2009;205(1):50–6. doi: 10.1016/j.bbr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Ellacott KL, Halatchev IG, Cone RD. Interactions between gut peptides and the central melanocortin system in the regulation of energy homeostasis. Peptides. 2006;27(2):340–349. doi: 10.1016/j.peptides.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 47.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 48.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 49.Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nature reviews. Endocrinology. 2014;10(9):540–52. doi: 10.1038/nrendo.2014.91. [DOI] [PubMed] [Google Scholar]
- 50.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley interdisciplinary reviews. Systems biology and medicine. 2010;2(5):577–93. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 52.Dulloo AG, Jacquet J, Montani JP, Schutz Y. How dieting makes the lean fatter: from a perspective of body composition autoregulation through adipostats and proteinstats awaiting discovery. Obes Rev. 2015;16(Suppl 1):25–35. doi: 10.1111/obr.12253. [DOI] [PubMed] [Google Scholar]
- 53.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring, Md.) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.