Abstract
Peptido-mimetic inhibitor of apoptosis protein (IAP) antagonists (Smac mimetics (SMs)) can kill tumour cells by depleting endogenous IAPs and thereby inducing tumour necrosis factor (TNF) production. We found that interferon-γ (IFNγ) synergises with SMs to kill cancer cells independently of TNF− and other cell death receptor signalling pathways. Surprisingly, CRISPR/Cas9 HT29 cells doubly deficient for caspase-8 and the necroptotic pathway mediators RIPK3 or MLKL were still sensitive to IFNγ/SM-induced killing. Triple CRISPR/Cas9-knockout HT29 cells lacking caspase-10 in addition to caspase-8 and RIPK3 or MLKL were resistant to IFNγ/SM killing. Caspase-8 and RIPK1 deficiency was, however, sufficient to protect cells from IFNγ/SM-induced cell death, implying a role for RIPK1 in the activation of caspase-10. These data show that RIPK1 and caspase-10 mediate cell death in HT29 cells when caspase-8-mediated apoptosis and necroptosis are blocked and help to clarify how SMs operate as chemotherapeutic agents.
Inhibitor of apoptosis proteins (IAPs) were first identified in baculoviruses where they prevent host cell apoptosis.1 Mammalian IAPs can be antagonised by endogenous proteins such as Smac/DIABLO and HtrA2/Omi,2, 3 and the finding that cell-permeable peptides containing the four N-terminal residues of Smac-sensitised tumour cells to apoptosis4 hastened the development of synthetic Smac mimetics (SMs) that proved similarly efficacious.5, 6, 7, 8
cIAPs play a central role in regulating tumour necrosis factor receptor 1 (TNFR1) signalling. Stimulation of TNFR1 by TNF promotes the formation of a membrane-bound intracellular signalling complex, Complex I, that can contain TRADD,9, 10 RIPK1,10, 11 TRAF2,12 cIAP1/2 and LUBAC.13 Several components of this complex become ubiquitylated by cIAPs and LUBAC.2, 13, 14 This ubiquitylation provides a platform for the recruitment of IKK- and TAK1-containing complexes, ultimately resulting in the activation of NF-κB and MAP kinases and the transcription of prosurvival proteins and proinflammatory cytokines.15 SM-induced cIAP degradation prevents ubiquitylation and formation of this ubiquitin platform. The failure to correctly form Complex I leads to the activation of caspase-8 in a secondary cytoplasmic complex (complex II) that contains TRADD, FADD and RIPK110 and apoptosis.
The caspase-8 homodimer and the caspase-8/cFLIPL heterodimer that may also be present in complex II cleave RIPK1 and thereby prevent an alternative cell death pathway, called necroptosis.16, 17, 18, 19 When caspase-8 is inhibited, or absent, necroptosis occurs following activation and autophosphorylation of RIPK1 and RIPK3. Active RIPK3 phosphorylates the pseudokinase MLKL, leading to its oligomerisation and MLKL-mediated membrane permeabilisation.20
Similar to SMs, TWEAK, a TNF superfamily ligand, can synergise with TNF to kill tumour cells,21, 22, 23, 24 and cells that are sensitive to TWEAK-induced death are also sensitive to SMs.24 Earlier reports demonstrated that TWEAK not only synergises with cell death ligands such as TNF, TRAIL and Fas but also with interferon-γ (IFNγ) to kill cancer cells.25, 26 Classical IFNγ receptor signalling, which involves the SOCS1-inhibitable JAK/STAT pathway,27 differs significantly from typical cell death receptor pathways. It has however been implicated in causing apoptosis,28, 29 and this has been attributed to, among other things, IFNγ-induced upregulation of proapoptotic proteins such as Puma, FasL, TRAIL27, 30, 31 and caspase-8.32
We hypothesised that, similar to TWEAK, SMs would synergise with IFNγ to induce cell death. We found that IFNγ/SM-induced death in primary mouse dermal fibroblasts (MDFs) occurred via RIPK3- and caspase-8-dependent apoptosis. However, human cell lines, and in particular human colorectal adenocarcinoma HT29 cells, behaved differently to MDFs. IFNγ/SM-induced killing of HT29 cells was not prevented by caspase inhibition. Furthermore, CASP8−/−RIPK3−/− or CASP8−/−MLKL−/− HT29 cells remained sensitive to IFNγ/SM treatment. Surprisingly, however, combined loss of caspase-8 and RIPK1 largely protected HT29 cells from IFNγ/SM, indicating that this treatment induced a novel type of RIPK1-dependent cell death. We observed that caspase-10 was significantly upregulated following IFNγ treatment, and HT29 cells deficient for caspase-10, caspase-8 and either MLKL or RIPK3 were resistant to IFNγ/SM. This suggests that the human-specific caspase-10 may have an important role in IFNγ-induced death.
Results
IFNγ and SMs act synergistically to kill cancer cells
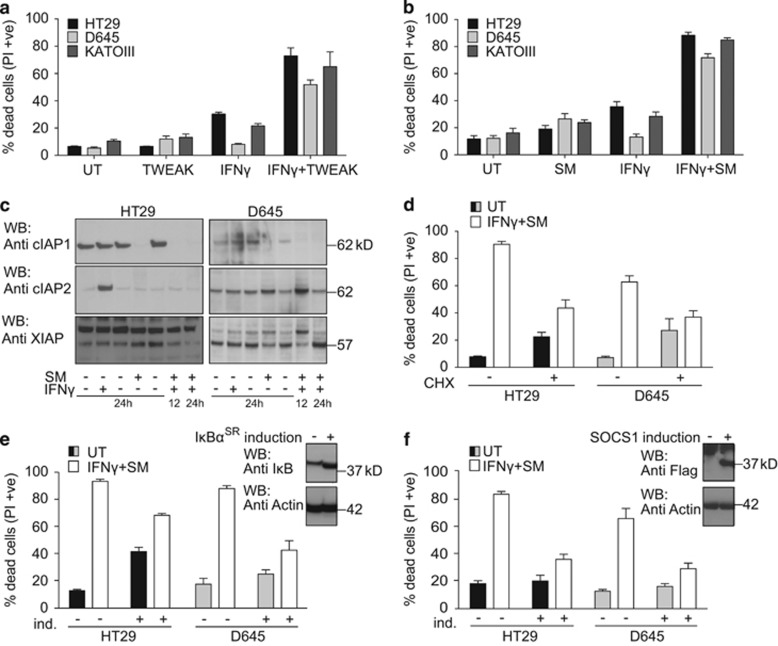
Consistent with earlier reports, we observed that IFNγ synergises with TWEAK to kill HT29, D645 and KATOIII cells26 (Figure 1a). TWEAK and SMs cause similar cellular responses;24, 33, 34 therefore, we tested whether SM and IFNγ synergised to kill IFNγ/TWEAK-sensitive cells, which they did (Figure 1b). Several other cell types were also sensitive to the combination of IFNγ/SM (Supplementary Figure S1A). To test whether loss of a specific IAP was responsible for IFNγ/SM-induced cell death, we used primary MDFs and keratinocytes deficient for either XIAP, cIAP1 or cIAP2, and found that loss of individual IAPs did not sensitise cells to IFNγ death (Supplementary Figures S1B and C). This suggests that pan-IAP inhibition is required for cell death induction.
Figure 1.
SMs and IFNγ act synergistically to kill cancer cells. (a and b) HT29, D645, KATOIII cells were treated as indicated with 30 ng/ml of human recombinant IFNγ and 100 ng/ml of TWEAK (a) or 500 nM SM (b) or not further treated (UT) for 48 h. The same concentrations were used throughout the paper. Cell death was quantified by measuring propidium iodide (PI)-permeable (PI-positive) cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (c) Western blot of HT29 and D645 cells treated with IFNγ and SM for 12 and 24 h as indicated. Degradation of IAPs was determined by immunoblotting for cIAP1, cIAP2 and XIAP. (d) HT29 and D645 cells were treated with 10 μg/ml cycloheximide 1 h before stimulation with IFNγ and SM (white bars) or no stimulation (UT) (black/grey bars) for 48 h. Cell death was analysed as in (a). Data are plotted as mean±S.E.M. (n≥3). (e) HT29 and D645 cells were infected with an inducible IκBαSR lentiviral construct. After pre-treatment with 100 nM of 4-OHT for 24 h to induce IκBαSR or no treatment, cells were treated with IFNγ/SM or not treated (UT) for a further 48 h. Cell death was analysed as in (a). Data are plotted as mean±S.E.M. (n≥3). (Top panel) Western blots showing induction of IκBSR. (f) HT29 and D645 cells were infected with an inducible SOCS1 lentiviral construct. Cells were treated as described in (e). Data are plotted as mean±S.E.M. (n≥6). (Top panel) Western blots showing induction of SOCS1
IFNγ might synergise with SMs by enhancing SM-induced cIAP degradation. However, on the contrary, IFNγ treatment strongly increased the expression of cIAP2 in HT29s (Figure 1c). IFNγ transcriptionally upregulates multiple genes via JAK-STAT and SMs activate NF-κB.33, 34 We therefore tested whether transcription and protein synthesis are required for IFNγ/SM-induced killing. We inhibited protein synthesis with cycloheximide, and despite the fact that cycloheximide is toxic to cells (Figure 1d), this treatment inhibited IFNγ/SM-induced death in HT29 and D645 cells (Figure 1d). We inhibited NF-κB activation with an inducible IκBα super-repressor (IκBSR)33 and this reduced IFNγ/SM-induced death in HT29 and D645 cells (Figure 1e). Similarly, overexpression of the JAK-STAT inhibitor SOCS1 protected HT29 and D645 cells from IFNγ/SM-induced cell death (Figure 1f). These results demonstrated that transcription downstream of both NF-κB and JAK-STAT is required for IFNγ/SM-induced death.
Death receptors are not essential for IFNγ/SM-induced killing
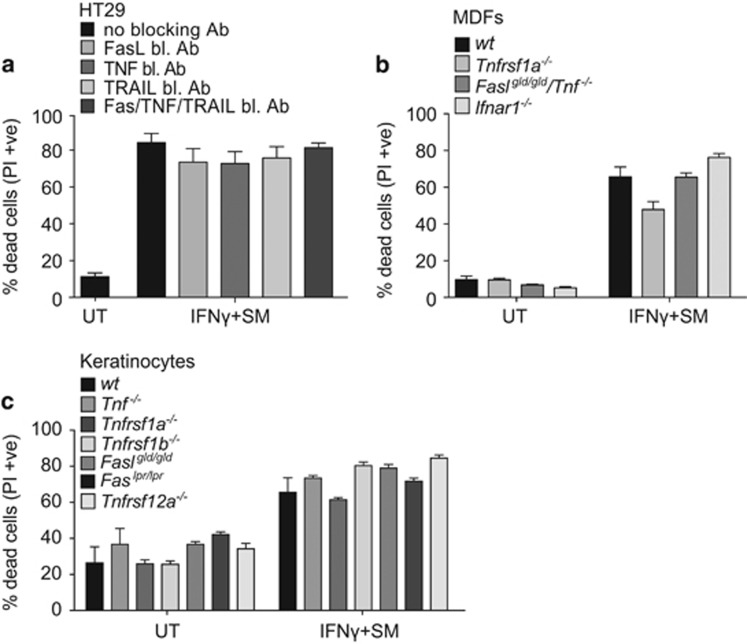
IAP antagonists cause cell death in some cells by promoting autocrine production of TNF and simultaneously sensitising them to the cytotoxic activity of TNF.6, 7, 33, 34 IFNγ can also induce Fas and TRAIL31, 35, 36 and both these ligands can synergise with SMs to kill cells.5, 37 To investigate a potential role for autocrine FasL, TNF or TRAIL in IFNγ/SM-induced killing, we preincubated cells with blocking antibodies. These antibodies blocked cell death induced by high doses of recombinant FasL- and TRAIL- as well as TNF/SM-induced cell death (Supplementary Figure S2). However, IFNγ/SM-induced cell death could not be blocked by single or combined treatment with neutralising FasL, TNF or TRAIL antibodies (Figure 2a).
Figure 2.
IFNγ/SM killing occurs independent of other cell death receptor signalling. (a) Following a 30 min pre-treatment with 10 μg/ml of blocking antibodies against FasL, TNF or TRAIL, HT29 cells were treated with SM and IFNγ for a further 48 h or cells were not treated (UT). Cell death was analysed by measuring PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n=4). (b and c) wild-type, Tnfrsf1a−/−, Faslgld/gld/Tnf-−/−, Ifnar−/− MDFs (b) and wild-type, Tnfrsf1a−/−, Tnfrsf1b−/−, Faslgld/gld, Faslpr/lpr, Tnfrsf12a−/− keratinocytes (c) were treated with 500 nM SM and 30 ng/ml recombinant mouse IFNγ or left untreated (UT) as indicated for 48 h. Cell death was analysed by measuring PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3), except Faslgld/gld (n=2)
We also analysed primary MDFs and keratinocytes, isolated from mutant and knockout mouse strains that were deficient for cell death ligands and receptors. Faslgld/gldTnf-−/− and Ifnar−/− MDFs, and Tnf−/−, Tnfrsf1a−/− (Tnfr1),Tnfrsf1b−/− (Tnfr2), Faslgld/gld, Faslpr/lpr and Tnfrsf12a−/− (Fn14) keratinocytes all showed comparable sensitivity to IFNγ/SM-induced death compared with their wild-type counterparts, whereas Tnfrsf1a−/− MDFs showed modest protection (Figures 2b and c). This indicates that IFNγ/SM-induced cell death is largely or entirely independent of recognised extrinsic death pathways in HT29s, MDFs and keratinocytes.
IFNγ/SM triggers RIPK3-dependent, caspase-8-mediated apoptosis in MDFs
Stimulation of cell death receptors such as Fas or TNFR1 in combination with SMs can trigger recruitment of caspase-8 to the RIPK1-containing complex II, resulting in caspase-8-mediated apoptosis. However, caspase-8 can also be activated independently of death receptors, for example, by Toll-like receptors or the Ripoptosome, and RIPK3 can be activated by PKR.29, 38 We therefore generated MDFs lacking DAI, TRIF and PKR, which are known to be upregulated by interferons39 and implicated in cell death.29, 38, 40 Dai−/−, Trif−/− and Pkr−/− MDFs or MDFs expressing a kinase-dead PKR variant (PKR K271R) were, however, similar to MDFs lacking the pyroptotic mediator caspase-1, still sensitive to IFNγ/SM, ruling out a role for these proteins in IFNγ/SM killing in MDFs (Supplementary Figures S3A–C). Consistently, doxycycline-induced expression of human PKR in HT29 cells did not kill cells in combination with SM (Supplementary Figure S3D).
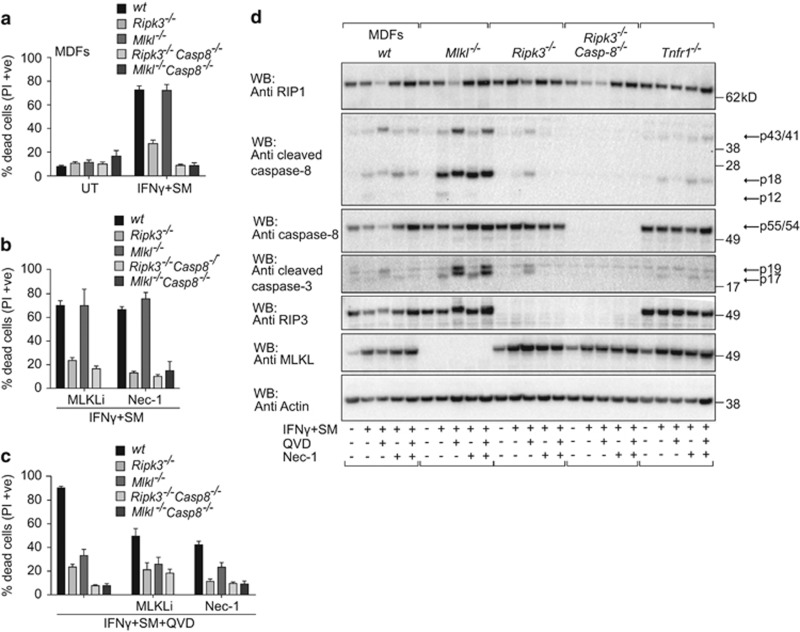
To determine the type of cell death induced by IFNγ/SM, we generated primary MDFs lacking key mediators in the necroptotic and extrinsic apoptotic pathways. Interestingly, in contrast to Mlkl−/− MDFs, Ripk3−/− MDFs were largely protected from IFNγ/SM-induced killing (Figure 3a), suggesting a necroptosis-independent role for RIPK3 that is not wholly unprecedented.41, 42 Both Ripk3−/−Casp8−/− and Mlkl−/−Casp8−/− MDFs were completely resistant to IFNγ/SM-induced cell death, suggesting that IFNγ/SM treatment causes a caspase-8-dependent apoptosis (Figure 3a). Inhibition of necroptosis using the MLKL inhibitor, compound 1,43 and the RIPK1 inhibitor, Nec-1 (necrostatin-1),44 had no impact on the sensitivity to IFNγ/SM killing (Figure 3b), but provided some protection when combined with the caspase inhibitor, QVD (Figure 3c). QVD alone did not stop IFNγ/SM killing because by preventing apoptosis it triggered necroptosis (Figure 3c). We consistently detected TNF-dependent reduction of RIPK1 levels in MDFs upon IFNγ/SM plus QVD treatment, indicating that the absence of IAPs and inhibition of caspases destabilises RIPK1 (Figure 3d). Overall, these data revealed that in MDFs, IFNγ/SM treatment primarily activates RIPK3, which is followed by caspase-8 activation. This is further supported by the reduction of caspase-8 processing in Ripk3−/− MDFs compared with wild-type MDFs detected by western blotting (Figure 3d). If, however, apoptosis is inhibited, then necroptosis occurs. Hence, both caspase-8-mediated apoptosis and necroptosis must be blocked to protect MDFs from IFNγ/SM-induced cell death.
Figure 3.
Synergistic cell death induced by IFNγ/SM occurs via RIPK3-dependent- and caspase-8 mediated apoptosis in murine fibroblasts. (a) Wild-type, Ripk3−/−, Mlkl−/−, Ripk3−/−Casp8−/− and Mlkl−/−Casp8−/− MDFs were treated with IFNγ/SM or not treated (UT) for 48 h. Cell death was measured by detecting PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (b and c) MDFs similar to that in (a) were treated with 1 μM Compound 1 (MLKL inhibitor), 50 μM Nec-1 and IFNγ/SM without (b) or with (c) 10 μM QVD (Q-VD-OPh) or not treated (UT) for 48 h. The same concentrations were used throughout the paper. Cell death was measured by detecting PI-permeable cells using flow cytometry. Data are represented as mean±S.E.M. (n≥3). (d) Wild-type, Mlkl−/−, Ripk3−/−, Ripk3−/−Casp8−/− and Tnfr1−/− MDFs were treated with IFNγ/SM, QVD and Nec-1 for 24 h similar to that in (b) and (c). Total cell lysates were analysed by immunoblotting (n=2). Arrows indicate full-length and processing products of caspases
IFNγ/SM triggers necroptosis in HT29 cells when caspases are inhibited
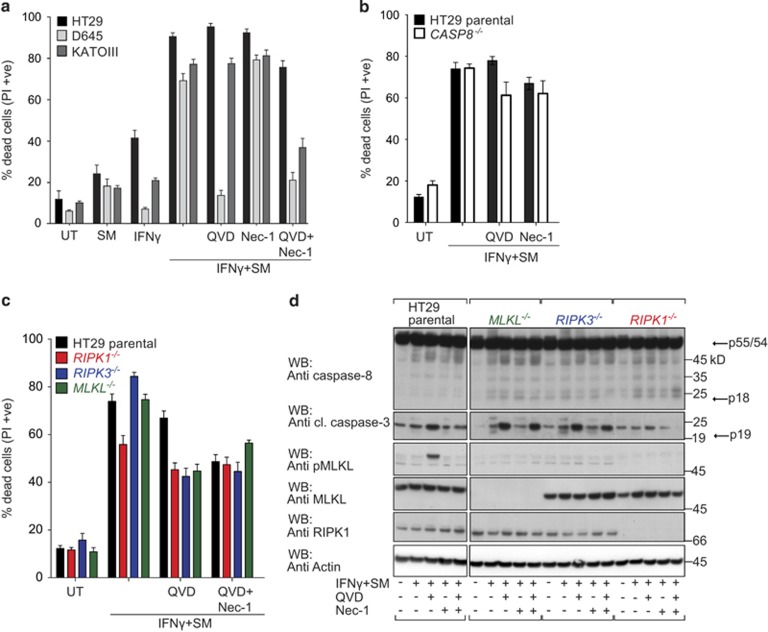
In contrast to MDFs, QVD protected D645 cells from IFNγ/SM-induced death (Figure 4a). Similar to MDFs, however, HT29 and KATOIII cells still underwent cell death in the presence of caspase inhibitors (Figure 4a). To assess whether this caspase-independent cell death was necroptosis, we inhibited RIPK1 using Nec-1.44 Nec-1 alone did not prevent cell death, while the combination of QVD and Nec-1 provided protection in KATOIII cells, but had little impact on death in HT29 cells (Figure 4a). These results suggested that HT29 cells exhibited a different type of cell death in response to IFNγ/SM, such as autophagy. However, when we knocked down the autophagy mediator ATG5 (autophagy protein 5) or treated these cells with autophagy inhibitors (Supplementary Figures S4A and B), we failed to detect any effect on IFNγ/SM killing.
Figure 4.
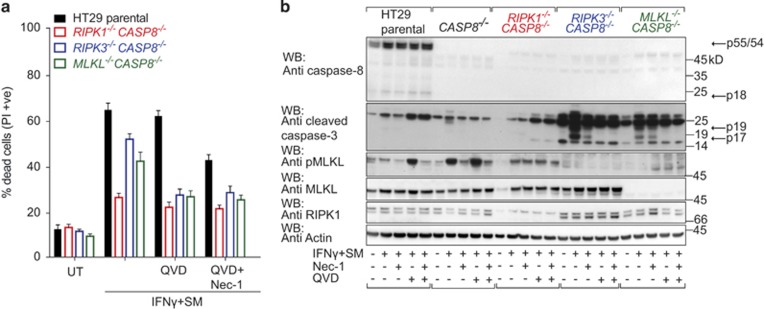
Inhibitors of apoptosis and necroptosis are unable to completely protect HT29 cells from IFNγ/SM-induced killing. (a) HT29, D645 and KATOIII cells were treated with IFNγ/SM, QVD and Nec-1 or not treated (UT) as indicated for 48 h. Cell death was measured by detecting PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (b, and c) Wild-type (black bar) and CRISPR/Cas9 CASP8−/− (white bar; b) and RIPK1−/− (red bar), RIPK3−/− (blue bar), MLKL−/− (green bar; c) HT29 cells were treated with IFNγ/SM, QVD and Nec-1 or left untreated (UT) as indicated for 48 h. Cell death was meaured by detecting PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (d) Immunoblot of wild-type, CRISPR/Cas9 MLKL−/−, RIPK3−/− and RIPK1−/− HT29 cells treated with IFNγ/SM, QVD and Nec-1 or not treated for 24 h. Arrows indicate full-length and processing products of caspases
While inhibitors can provide insights, genetic experiments generally have fewer caveats. We therefore generated single-cell HT29 clones deficient for apoptotic (CASP8−/−) or necroptotic (RIPK1−/−, RIPK3−/− or MLKL−/−) cell death pathways using CRISPR/Cas945, 46 and validated them using next-generation sequencing (Supplementary Figure S5). Similar to their wild-type counterparts, CASP8−/− HT29 cells were sensitive to IFNγ/SM treatment (Figure 4b). In other systems, this would be explained by the fact that loss of caspase-8 leads to the induction of necroptosis; however, Nec-1 had no impact on IFNγ/SM-induced death of CASP8−/− cells. Furthermore, RIPK1−/− HT29 cells were only marginally protected from IFNγ/SM killing (Figure 4c and Supplementary Figure S7), even when QVD was added. These results show that RIPK1 contributes to, but is not required for, cell death.
Consistent with the idea that IFNγ/SM induces necroptosis if caspase-8 is inhibited, combined IFNγ/SM/QVD treatment of wild-type cells resulted in phosphorylation of MLKL, which was absent in RIPK1−/− and RIPK3−/− cell lines (Figure 4d). However, RIPK3−/− and MLKL−/− HT29 cells, that cannot die by necroptosis, were still sensitive to IFNγ/SM-induced death (Figure 4c) and while blocking caspase-8 dependent apoptosis of these necroptosis deficient cells reduced killing, the end result was still a substantial amount of cell death. Similarly, the MLKL inhibitor (compound 1) failed to prevent IFNγ/SM-induced death whether in the presence or absence of QVD (Supplementary Figure S7A). These results show that IFNγ/SM can activate caspase-8 and apoptosis which, if inhibited, results in the activation of MLKL and necroptosis but that, inexplicably, cell death is not entirely dependent on either of these pathways.
To explore this conundrum further, we generated HT29 cells doubly deficient for caspase-8 and either RIPK1, RIPK3 or MLKL and tested for their sensitivity to IFNγ/SM treatment (Figure 5). Intriguingly, RIPK1−/−CASP8−/− cells were almost completely protected from IFNγ/SM-induced cell death; however, RIPK3−/−CASP8−/− and MLKL−/−CASP8−/− cells remained sensitive (Figure 5a). The residual amount of cell death in these lines could now be almost completely prevented by QVD, strongly suggesting that cells are dying via caspase-8-independent apoptosis. Furthermore, we detected substantial amounts of cleaved caspase-3 in RIPK3−/−CASP8−/− and MLKL−/−CASP8−/− HT29 cells following IFNγ/SM treatment, which was reduced by QVD (Figure 5b). Taken together, these data suggest that a Nec-1-independent RIPK1 activity is required for a caspase-dependent IFNγ/SM-induced cell death.
Figure 5.
HT29 cells deficient for caspase-8-mediated apoptosis and necroptosis remain sensitive to IFNγ/SM-induced killing. (a) Wild-type and CRISPR/Cas9 RIPK1−/−CASP8−/− (red frame), RIPK3−/−CASP8−/− (blue frame) and MLKL−/−CASP8−/− (green frame) HT29 cells were treated with IFNγ/SM, QVD and Nec-1 or left untreated (UT) as indicated for 48 h. Cell death was analysed by measuring PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (b) Wild-type and CRISPR/Cas9 CASP8−/−, RIPK1−/−CASP8−/−, RIPK3−/−CASP8−/−, MLKL−/−CASP8−/− HT29 cells were treated with IFNγ/SM, QVD and Nec-1 or not treated for 24 h before SDS lysis, separation on SDS-PAGE and immunoblotting. Arrows indicate full-length and processing products of caspases
Caspase-10 mediates cell death in the absence of caspase-8 and necroptosis
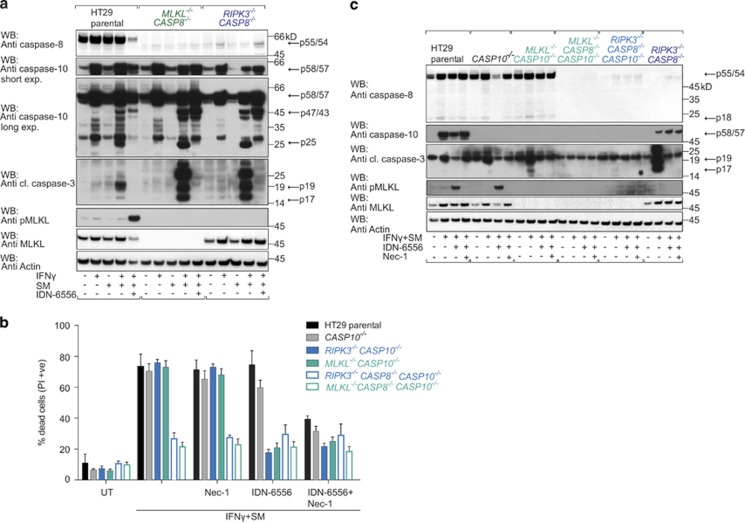
Despite some conflicting data, it appears that caspase-10 can act as an initiator caspase in extrinsic apoptosis pathways.10, 47, 48 To evaluate a role for caspase-10 in IFNγ/SM-induced cell death, we immunoblotted for caspase-10 and observed markedly increased levels when HT29 cells were treated with IFNγ for 24 h (Figure 6a). HT29 cells treated with IFNγ/SM also upregulated caspase-10, and various cleaved forms were detected (Figure 6a). Addition of the pancaspase inhibitor IDN-6556, a more potent caspase inhibitor than QVD,49 prevented the formation of the smallest processed product of caspase-10 (p25), which served as the clearest signature of caspase-10 activation (Figure 6a). As expected, IFNγ/SM/IDN-6556 treatment also induced MLKL phosphorylation (Figure 6a). RIPK3−/−CASP8−/− and MLKL−/−CASP8−/− cells treated with IFNγ/SM activated caspase-10 similarly to their wild-type counterpart, while cleaved caspase-3 levels were significantly increased (Figure 6a), and both caspase-10 and caspase-3 processing were blocked by IDN-6556. Thus, in the absence of caspase-8 and necroptosis effectors, caspase-10 is strongly activated and caspase-3 is an excellent substrate for it.
Figure 6.
Caspase-10 mediates IFNγ/SM cell death in the absence of caspase-8 and necroptosis. (a) Immunoblot of wild-type and CRISPR/Cas9 MLKL−/−CASP8−/−, RIPK3−/−CASP8−/− HT29 cells treated with IFNγ/SM and IDN-6556 (10 μM) and lysed in SDS lysis buffer. Immunostaining was performed for the indicated proteins. (b) Wild-type (black bar) and CRISPR/Cas9 CASP10−/− (grey bar), RIPK3−/−CASP10−/− (light blue bar), MLKL−/−CASP10−/− (light green bar), RIPK3−/−CASP8−/−CASP10−/− (light blue frame), MLKL−/−CASP8−/−CASP10−/− (light green frame) HT29 cells were treated with IFNγ/SM, IDN-6556 and Nec-1 or not treated (UT) for 48 h before measuring dead cells by detecting PI-permeable cells using flow cytometry. Data are plotted as mean±S.E.M. (n≥3). (c) Wild-type and CRISPR/Cas9 CASP10−/−, MLKL−/−CASP10−/−, MLKL−/−CASP8−/−CASP10−/−, RIPK3−/−CASP8−/−CASP10−/−, RIPK3−/−CASP8−/− HT29 cells were treated as indicated for 24 h, lysed in SDS lysis buffer and immunoblotted. Arrows indicate full-length and processing products of caspases
Caspase-10 upregulation and activation upon IFNγ or IFNγ/SM stimulation was not restricted to HT29s because we also observed it in melanoma, glioblastoma, monocytic and other colon cancer cell lines (Supplementary Figures S8A–G). To determine the contribution of caspase-10 to IFNγ/SM-induced cell death, we generated HT29 single-cell clones lacking caspase-10 (Supplementary Figure S5). CASP10−/− cells were as sensitive as the parental HT29 cells when treated with IFNγ/SM (Figure 6b). Similarly, double deficiency of RIPK3 and caspase-10 or MLKL and caspase-10 in HT29 cells had no effect on the extent of cell death induced by IFNγ/SM, but these cells were now well protected from cell death when IDN-6556 was added (Figure 6b). Most importantly, RIPK3−/−CASP8−/−CASP10−/− and MLKL−/−CASP8−/−CASP10−/− HT29 cells were largely resistant to IFNγ/SM-induced cell death (Figure 6b). Consistent with this, we failed to detect an IFNγ/SM-induced increase in cleaved caspase-3 in triple-knockout cells (Figure 6c). Collectively, these data demonstrate that IFNγ/SM primarily triggers extrinsic apoptosis through activation of caspase-8 and caspase-10. If caspase-8-mediated apoptosis is prevented, then necroptosis occurs. HT29 cells unable to undergo caspase-8 mediated apoptosis and classic necroptosis can, however, still die via caspase-10.
Caspase-10 requires RIPK1 to induce cell death and cleaved caspase-10 is detected in a caspase-8-containing complex in HT29 cells
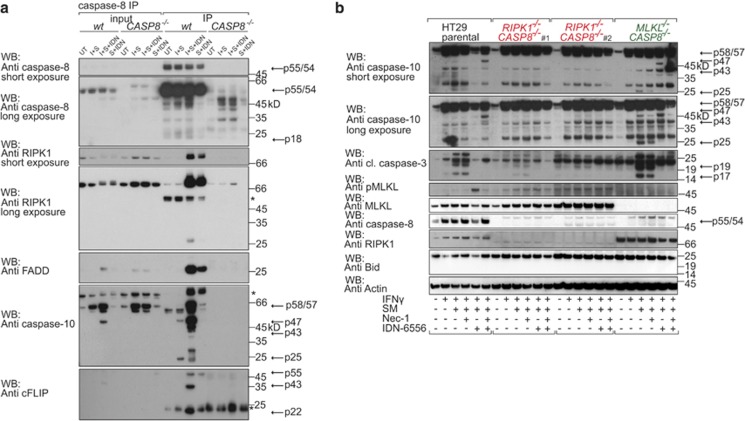
Caspase-10 has been shown to be recruited to the Ripoptosome complex upon TNF stimulation.10, 50 To examine whether a similar complex forms upon IFNγ/SM treatment, we immunoprecipitated caspase-8 (Figure 7a and Supplementary Figure S7B). Because caspase inhibitors stabilise the Ripoptosome,37, 50, 51 we immunoprecipitated caspase-8 in HT29 cells in the presence and absence of IDN-6556 (Figure 7a). At 24 h after IFNγ/SM treatment, when the amount of cell death was still low, we observed recruitment of RIPK1, FLIP and FADD to caspase-8 when IDN-6556 was also added (Figure 7a). A similar pattern of binding was also observed in cells treated with IDN-6556 and SM alone. However, binding of cleaved caspase-10 and caspase-8 was only detected in IFNγ/SM and IFNγ/SM- plus IDN-6556 treated cells (Figure 7a). Overall, this shows that cleaved and therefore presumably active caspase-10 is a component of a caspase-8-, FADD-, RIPK1-containing complex upon IFNγ/SM treatment, which provides further evidence for a role of caspase-10 in IFNγ/SM-induced cell death.
Figure 7.
IFNγ/SM-induced cell death mediated by caspase-10 is dependent on RIPK1. (a) Immunoprecipitation of caspase-8 from HT29 cells left untreated or stimulated with IFNγ (I)/SM alone or in combination with IDN-6556 for 24 h. To control for a Ripoptosome formation upon SM plus IDN-6556, we treated cells with SM plus IDN-6556 alone. To control for specific binding to caspase-8, we stimulated CRISPR/Cas9 CASP8−/− HT29 cells similar to wild-type HT29. Immunoprecipitates and lysates were separated on SDS-PAGE and immunoblotted. Asteriks indicate unspecific bands. (b) Immunoblot of wild-type and CRISPR/Cas9 RIPK1−/−CASP8−/− and MLKL−/−CASP8−/− HT29 cells treated with IFNγ/SM, IDN-6556 and Nec-1 as indicated for 24 h and lysed in SDS lysis buffer. Arrows indicate full-length and processing products of caspases
RIPK1−/−CASP8−/− cells were largely protected from IFNγ/SM-induced killing in contrast to RIPK3−/−CASP8−/− and MLKL−/−CASP8−/− cells (Figure 5a). Therefore, we analysed caspase-10 activation in cells where RIPK1 and caspase-8 were absent and detected reduced caspase-10 processing compared with the parental HT29 cells or cells deficient for MLKL and caspase-8 (Figure 7b). The smallest caspase-10 product was not detected when RIPK1 and caspase-8 were absent (Figure 7b). Reduced activation of caspase-10 coincided with reduced caspase-3 processing when RIPK1−/−CASP8−/− cells were stimulated with IFNγ/SM (Figure 7b). This suggests that independent of its function in necroptosis, RIPK1 is required for full caspase-10 activation in the context of IFNγ/SM stimulation.
Discussion
TNF binding to its receptor TNFR1 induces recruitment of a number of components including cIAPs and RIPK1.15 Subsequent ubiquitylation of RIPK1 by cIAPs and LUBAC provides a binding platform for kinases such as TAK1, leading to NF-κB and MAPK signalling and upregulation of prosurvival proteins. Degradation of cIAPs induced by SMs stops efficient formation of the TNFR1 signalling complex and prevents upregulation of prosurvival proteins. RIPK1, TRADD, FADD, caspase-8 and, in human cells, the less studied caspase-10 form a cytoplasmic complex (complex II), which can lead to caspase activation and apoptosis.10, 50 Active caspase-8 not only induces apoptosis but also inhibits necroptosis, predominantly as a heterodimer with cFLIP, by cleaving RIPK1. Therefore, blocking caspase-8 not only prevents apoptosis but also unleashes the brake on RIPK1, allowing necroptosis to occur.19, 52, 53 cIAPs also inhibit activation of noncanonical NF-κB and SMs can thereby cause production of autocrine TNF.6, 7, 33, 34 Thus, in some cells, SMs can induce cell death by simultaneously up regulating TNF production and sensitising those same cells to TNF-induced cell death.
The TNF superfamily ligand TWEAK, which, upon binding to its receptor Fn14, promotes depletion of cIAPs in a manner analogous to SMs, can also induce TNF in a subset of cells and sensitise them to TNF killing.24 IFNγ also has an apoptotic activity in some cell types,54 and the pivotal role of IFNγ in inhibiting tumour cell growth has recently been highlighted by new studies showing that tumours resistant to checkpoint therapy acquire mutations in the IFNγ signalling pathway.55, 56 We were intrigued by two old reports showing that IFNγ and TWEAK synergise to kill tumour cell lines.25, 26 We confirmed these original observations and found that SMs can also synergise with IFNγ to kill cells. IFNγ can transcriptionally upregulate target genes and this was essential for IFNγ/SM killing because this death could be blocked by SOCS1 overexpression. We suspected that IFNγ/SM-induced TNF caused cell death; however, blocking TNF had no effect on IFNγ/SM killing. IFNγ can also induce FasL and TRAIL54 and these can synergise with SMs to kill cells.4, 37 However, blocking TNF, Fas and TRAIL did not prevent IFNγ/SM-induced cell death.
Although PKR has been claimed to have a role in IFNγ-induced cell death,29 MDFs deficient in PKR or other targets of IFNγ signalling such as DAI or TRIF were as sensitive to IFNγ/SM treatment as wild-type cells. IFNγ did induce the expression of MLKL in MDFs and HT29 cells as previously reported for MEFs.29 While MLKL upregulation might prime cells for necroptosis, we did not observe IFNγ-induced necroptosis unless caspases were inhibited. IFNγ has also been shown to upregulate caspase-8.32, 57 Although we did not observe an increase in caspase-8 levels in MDFs, IFNγ/SM-induced cell death was caspase-8-dependent. Furthermore, RIPK3 was required upstream of caspase-8. Interestingly, Mlkl−/− MDFs showed increased levels of cleaved caspase-8 levels compared with other genotypes tested. Potential explanations of this phenomenon are either that MLKL somehow directly inhibits caspase-8 or that the absence of MLKL increases availability of RIPK3, resulting in a more potent activation of caspase-8.
IFNγ/SM-induced killing in HT29 cells was more complex. To determine the role of caspase-8 in IFNγ/SM-induced killing in HT29 cells, we generated CRISPR/Cas9 HT29 cells deficient for caspase-8. Three out of five CASP8−/− HT29 cell clones were still sensitive to IFNγ/SM stimulation, whereas the other two cell lines were largely protected (Supplementary Figures S6A and B). This makes it difficult to be sure which is the 'correct' phenotype. We believe that the sensitive phenotype is the relevant phenotype because first IFNγ/SM-induced cell death of HT29 cells could not be blocked by Q-VD-OPh (QVD) and second even combined loss of RIPK3 or MLKL and caspase-8 did not prevent IFNγ/SM-induced death. Because we only ever observed a sensitive phenotype in CASP8−/−RIPK3−/− and CASP8−/−MLKL−/− HT29 cells, we hypothesise that the resistant CASP8−/− clones acquired additional changes that allowed them to overcome an unstable state caused by caspase-8 deficiency.
IFNγ/SM treatment of CASP8−/−RIPK3−/− or CASP8−/−MLKL−/− cells induced substantial amounts of cleaved caspase-3. The human-specific caspase-10 can activate caspase-3,58, 59 suggesting that it might be involved in IFNγ/SM killing. Supporting this hypothesis, we found that caspase-10 was strongly induced by IFNγ in HT29 and several other cell lines. Furthermore, IFNγ/SM treatment induced processing of caspase-10. However, loss of caspase-10 alone did not reduce IFNγ/SM-induced death, and neither did it promote necroptosis. Nevertheless, CASP8−/−CASP10−/−RIPK3−/− or CASP8−/−CASP10−/−MLKL−/− triply deficient HT29 cells were, finally, almost completely resistant to IFNγ/SM-induced death. Interestingly, in HT29 cells, it appears that RIPK1 is required for full caspase-10 activation because CASP8−/−RIPK1−/− were as resistant to IFNγ/SM-induced death as the triple CASP8−/−CASP10−/−RIPK3−/−-knockout cells. RIPK1 probably provides a platform for caspase-10 activation via FADD.
This study highlights how complex cell death pathways can be and their resilience to disruption, perhaps reflecting the defensive nature of cell death. Intriguingly, immune checkpoint inhibitors appear to require tumour cell intrinsic IFNγ signalling to cure melanomas in patients55, 56 and it was proposed that this was, in part, due to the apoptotic activity of IFNγ. Because IFNγ upregulates caspase-10 in multiple cell lines including human melanoma cell lines, and that this contributes to SM-induced killing our results open up the enticing possibility that SMs could be combined with immune checkpoint inhibitors to increase T-cell killing by synergising with T-cell-secreted IFNγ.
Materials and Methods
Cell culture, transfection, lentiviruses and lentiviral production
MDFs and keratinocytes were generated as per Gerlach et al.14and Etemadi et al.60 and similar to 239Ts cultured in Dulbecco's modified Eagle's medium with the addition of 8% FBS, 1 mM l-glutamine, 100 U/ml penicillin and 100 g/ml streptomycin (purchased from Gibco, Melbourne, VIC, Australia) at 37 °C with 10% CO2 in a humidified incubator. All other cell lines were cultured in HTRPMI, respectively, with additives and conditions like that described above.
The inducible lentiviral system has been described,33 but briefly the inducible transcriptional activator Gal4 ERT2 VP16 (GEV16) was cloned into the lentiviral vector pFU PGK Hygro and infected with pF 5x UAS SV40 Puro vectors encoding for human IκBSR61 and human SOCS1 in HT29 and D645 cells. The cDNA encoding for human PKR was purchased by Addgene (Cambridge, MA, USA) and was cloned into the pFTRE 3G vector, which was generated by Toru Okamoto, and allows doxycycline-inducible expression.
For the generation of the CRISPR/Cas9 cell lines, we used two vectors generated by Marco Herold: the vector pFU Cas9 Cherry, which allows constitutive expression of the Cas9 protein, and the pF GH1t UTG vector, which allows doxycycline-inducible expression of different guide RNA sequences complementary to their target sequence.46
Infected cells were selected with 5 μg/ml of puromycin (for IκBαSR, SOCS1, PKR selection) and/or 10–50 μg/ml of hygromycin (for GEV16 selection) or single cells were sorted for GFP and mCherry (selection of CRISPR/Cas9 cell lines) into 96-well plates. Lentiviral constructs were induced with 10 nM of 4-hydroxy tamoxifen or 20 ng/ml (for pFTRE 3G human PKR vector) and 1 μg/ml (for pF GH1t UTG vector) doxycycline.
To knock down ATG5 in HT29 cells, cells were infected with pLKO.1 encoding for the shRNA against ATG5, which is constitutively expressed.
Reagents
Recombinant mouse and human IFNγ were purchased from R&D Systems (Minneapolis, MN, USA) and Q-VD-OPH was purchased from MP Biomedicals (Seven Hills, NSW, Australia). SM also known as Compound A,33 Nec-1 and the caspase inhibitor IDN-6556 were a gift from TetraLogic (Malvern, PA, USA). 4-Hydroxy-tamoxifen, cycloheximide, propidium iodide, doxycycline, wortmannin, bafilomycin and 3-methyladenine were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Compound 1 (MLKL inhibitor) was a gift from Guillaume Lessene and was generated in-house (WEHI). Fc-TWEAK and Fc-TNF were generated in-house as described. TRAIL ligand was a gift from Prof. Henning Walczak (Imperial College, London, UK) and the Fas ligand was purchased from Peprotech (Rocky Hill, NJ, USA).
Statistical analyses
Error bars represent mean±S.E.M. of specified number of independent and/or biological repeats of cell death assays.
Immunoblotting and co-immunoprecipitation
For co-immunoprecipitation, HT29 cells were lysed in DISC lysis buffer (1% (v/v) Triton X-100, 150 mM NaCl, 20 mM Tris, pH 7.5, 10% (v/v) glycerol, 2 mM EDTA) with complete protease inhibitor cocktail (Roche, Dee Why, NSW, Australia), phosphatase inhibitors (2 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM sodium molybdate, 5 mM β-glycerophosphate, 2 mM sodium pyrophosphate) and 1 mM NEM (N-ethylmaleimide; Sigma, Castle Hill, NSW, Australia). Lysates were incubated overnight with 2 μg caspase-8 antibody (Santa Cruz, Santa Cruz, CA, USA; sc6136) and 20 μl packed Sepharose Protein G beads were incubated overnight with 1% BSA in PBS. The next day, lysates were incubated with beads for 2 h, washed four times in lysis buffer and boiled for 5 min. For expression tests, cells were harvested from tissue culture plates and washed with ice-cold PBS, and then either lysed in DISC lysis buffer on ice for 20 min before the addition of SDS sample loading buffer or lysed directly in SDS lysis buffer (126 mM Tris-HCl, pH 8, 20% (v/v) glycerol, 4% (w/v) SDS, 0.02% (w/v) bromophenol blue, 5% (v/v) 2-mercaptoethanol) boiled and sonicated. Separation occurred on 4–12% NuPAGE Bis-Tris gels (Life Technologies, Scorseby, Vic, Australia) and transferred onto PVDF membranes (Millipore, Bayswater, Vic, Australia). Membranes were blocked in 5% milk and antibodies diluted in 2% BSA in PBST. Antibodies used for immunoblotting were as follows: anti-human FADD (BD Pharmingen, North Ryde, NSW, Australia; 556402), anti-human FLIP (Enzo Life Sciences, Redfern, NSW, Australia; ALX-804-961-0100), anti-full-length mouse caspase-8 (Enzo Life Sciences; ALX-804-448-C100), anti-cleaved mouse caspase-8 (Cell Signalling Technology, Danvers, MA, USA; 8592), anti-cIAP1 and anti-cIAP2 (Alexis Biochemicals, San Diego, CA, USA; ALX-803-341), anti-XIAP (MBL, M044-3), anti-FLAG M2 (Sigma; F-3165), anti-β-actin (Sigma; A-1978), anti-mouse caspase-8 (Cell Signalling Technology; 4927), anti-human caspase-8 (MBL, Woburn, MA, USA; M058-3), anti-caspase-10 (MBL; M059-3), anti-cleaved caspase-3 (Cell Signalling Technology; 9661), anti-human PKR (Santa Cruz; sc6282), anti-RIPK1 (BD Transduction Laboratories, North Ryde, NSW, Australia, 610458), anti-mouse RIPK3 (Axxora, Farmingdale, NY, USA; PSC-2283-c100), anti-human phospho-MLKL (Abcam, Milton, Cambridge, UK; ab187091), anti-total mouse and human MLKL (housemade, 3H1). Antibodies used for neutralisation/blocking assays were as follows: anti-TNF (MAB610), anti-FasL (MAB126) and anti-TRAIL (MAB375) were purchased from R&D Systems (Noble Park, Vic, Australia).
Death assays
Keratinocytes were treated like in Gerlach et al.,14 and MDFs were left to settle in 24-well tissue plates for 24 h. All other cells were plated in 48-well tissue plates and left to settle for 48 h before treatment with Q-VD-OPH (QVD; 10 μM), IDN-6556 (10 μM), Compound 1 (MLKL inhibitor; 1 μM), GSK872 (RIPK3 inhibitor, 5 μM), Nec-1 (RIPK1 inhibitor; 50 μM) and IFNγ (30 ng/ml)/SM (500 nM) for 48 h. Blocking antibodies for TNF, Fas and TRAIL were used at 10 μg/ml 30 min before cell death induction by IFNγ/SM or TNF (100 ng/ml), Fas (5 μg/ml) or TRAIL (1 μg/ml). Cell death was subsequently measured by propidium iodide (100 ng/ml in PBS) staining and flow cytometry.
Acknowledgments
We thank Toru Okamoto for the pFTRE 3G construct, David Baltimore for lentiviral packaging constructs, Theo Mantamadiotis for the ERT2 construct, Robert Gerl for early work with the GAL4 ERT2 VP16 system, Henning Walczak for the TRAIL ligand, Tracy Putoczki for LIM2837, LIM1215, SW480, LIM2560, LIM2405, SW481 cells, Conor Kearney for MEWO, ME-RM, SK-Mel-28, ME4405, HT-144, Oliver Sieber for SKCO1, HCT116, OLDI, LIM2099 cells, Ben Kile for Ifnar−/− mouse tails, Paul Hertzog for Pkr−/−, PKR K271R and Ifnar−/− tails, Seth Masters for Casp1−/− tails, Andreas Strasser for Casp8−/−/Mlkl−/− tails, Hans-Uwe Simon for the pLKO.1 construct encoding for the shRNA ATG5, James Murphy and Guillaume Lessene for Compound 1 (MLKL inhibitor). Andrew Kueh and Stephen Wilcox for helping with the next-generation sequencing. J Silke is funded by the NHMRC (433013, 541901 and 541902). MC Tanzer is funded by the Victorian International Research Scholarship. Fc-TNFSF ligand DNA was provided by J Tschopp (University of Lausanne, Lausanne, Switzerland). We thank Wendy Cook and David Vaux for their input and guidance in editing this paper.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by C Borner.
J Silke was variously a consultant and member of the Scientific Advisory Board and SM Condon, CA Benetatos, SK Chunduru and M McKinlay were employees of TetraLogic Pharmaceuticals Corporation over some of the course of this work. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312–1316. [DOI] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 2010; 10: 561–574. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Kratina TK, Hawkins CJ, Silke J, Ekert PG, Vaux DL. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ 2007; 14: 348–357. [DOI] [PubMed] [Google Scholar]
- Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 2002; 8: 808–815. [DOI] [PubMed] [Google Scholar]
- Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science 2004; 305: 1471–1474. [DOI] [PubMed] [Google Scholar]
- Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res 2007; 67: 11493–11498. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 2007; 12: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon SM, Mitsuuchi Y, Deng Y, LaPorte MG, Rippin SR, Haimowitz T et al. Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. J Med Chem 2014; 57: 3666–3677. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995; 81: 495–504. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003; 114: 181–190. [DOI] [PubMed] [Google Scholar]
- Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ 2010; 17: 482–487. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996; 84: 299–308. [DOI] [PubMed] [Google Scholar]
- Tokunaga F. Linear ubiquitination-mediated NF-kappaB regulation and its related disorders. J Biochem 2013; 154: 313–323. [DOI] [PubMed] [Google Scholar]
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 2011; 471: 591–596. [DOI] [PubMed] [Google Scholar]
- Silke J. The regulation of TNF signalling: what a tangled web we weave. Curr Opin Immunol 2011; 23: 620–626. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 1998; 188: 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–714. [DOI] [PubMed] [Google Scholar]
- Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J 2011; 433: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471: 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Silke J. Ars Moriendi; the art of dying well—new insights into the molecular pathways of necroptotic cell death. EMBO Rep 2014; 15: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M, Zimmermann G, Gottfried E, Chen CM, Grunwald U, Huang DC et al. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J 1999; 18: 3034–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicovsky A, Henkler F, Salzmann S, Scheurich P, Kneitz C, Wajant H. Tumor necrosis factor receptor-associated factor-1 enhances proinflammatory TNF receptor-2 signaling and modifies TNFR1-TNFR2 cooperation. Oncogene 2009; 28: 1769–1781. [DOI] [PubMed] [Google Scholar]
- Salzmann S, Seher A, Trebing J, Weisenberger D, Rosenthal A, Siegmund D et al. Fibroblast growth factor inducible (Fn14)-specific antibodies concomitantly display signaling pathway-specific agonistic and antagonistic activity. J Biol Chem 2013; 288: 13455–13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol 2008; 182: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 1997; 272: 32401–32410. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Kayagaki N, Yamaguchi N, Okumura K, Yagita H. Involvement of TWEAK in interferon gamma-stimulated monocyte cytotoxicity. J Exp Med 2000; 192: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264: 1415–1421. [DOI] [PubMed] [Google Scholar]
- Beug ST, Tang VA, LaCasse EC, Cheung HH, Beauregard CE, Brun J et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat Biotechnol 2014; 32: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci USA 2013; 110: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Lamphier MS, Tanaka N. IRF-1: the transcription factor linking the interferon response and oncogenesis. Biochim Biophys Acta 1997; 1333: M9–17. [DOI] [PubMed] [Google Scholar]
- Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood 1995; 85: 3183–3190. [PubMed] [Google Scholar]
- Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene 2002; 21: 2295–2308. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681. [DOI] [PubMed] [Google Scholar]
- Ahn EY, Pan G, Vickers SM, McDonald JM. IFN-gamma upregulates apoptosis-related molecules and enhances Fas-mediated apoptosis in human cholangiocarcinoma. Int J Cancer 2002; 100: 445–451. [DOI] [PubMed] [Google Scholar]
- Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res 1998; 58: 2832–2837. [PubMed] [Google Scholar]
- Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol 2009; 187: 1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 2013; 288: 31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Comella N, Tognazzi K, Brown LF, Dvorak HF, Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene 1999; 240: 157–163. [DOI] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012; 11: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014; 343: 1357–1360. [DOI] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 2014; 56: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA 2014; 111: 15072–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013; 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey BJ, Kelly GL, Kueh AJ, Brennan MS, O'Connor L, Milla L et al. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep 2015; 10: 1422–1432. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem 2001; 276: 46639–46646. [DOI] [PubMed] [Google Scholar]
- Lamy L, Ngo VN, Emre NC, Shaffer AL, Yang Y, Tian E et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell 2013; 23: 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med 2016; 8: 339ra69. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011; 43: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencho T, Katiuscia B, Maurice D, Meike B, Claudia L, Fredrik W et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 2011; 43: 432–438. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011; 471: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 2011; 471: 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8: 237–249. [DOI] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 2016; 167: 397–404 e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Munoz-Pinedo C, Lopez-Rivas A. Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res 2000; 60: 5673–5680. [PubMed] [Google Scholar]
- Sprick MR, Rieser E, Stahl H, Grosse-Wilde A, Weigand MA, Walczak H. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J 2002; 21: 4520–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA 2001; 98: 13884–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadi N, Holien JK, Chau D, Dewson G, Murphy JM, Alexander WS et al. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J 2013; 280: 5283–5297. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 1996; 274: 787–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.