Abstract
Background: Despite recent advances in cancer immunotherapy and the development of various assays for T cell assessment, a lack of universal standards within immune monitoring remains. The objective of this study was to evaluate the enzyme-linked immunosorbent spot (ELISpot) assay in comparison with major histocompatibility complex-tetramer analysis in the context of dendritic cell (DC)-based cancer immunotherapy. Methods: The ELISpot assay was performed on peripheral blood mononuclear cells to assess reproducibility, daily precision, and linearity using HLA-A*24:02-restricted Cytomegalovirus peptide. Wilms’ tumor 1 (WT1) antigen-specific cytotoxic T cells were then evaluated by both the ELISpot assay and WT1 tetramer analysis in peripheral blood from 46 cancer patients who received DC vaccinations pulsed with human leukocyte antigen (HLA)-A*24:02-restricted modified WT1 peptides. Results: The ELISpot assay was proven to have reproducibility (coefficient of variation (CV) ranged from 7.4% to 16.3%), daily precision (CV ranged from 5.0% to 17.3%), and linearity (r = 0.96–0.98). WT1-specific immune responses were detected by the ELISpot assay in 34 out of 46 patients (73.9%) post-vaccination. A Spearman’s rank-correlation coefficient of 0.82 between the ELISpot assay and WT1 tetramer analysis was obtained. Conclusion: This is the first report of a comparison of an ELISpot assay and tetramer analysis in the context of dendritic cell (DC)-based cancer immunotherapy. The ELISpot assay has reproducibility, linearity, and excellent correlation with the WT1 tetramer analysis. These findings suggest that the validated ELISpot assay is useful to monitor the acquired immunity by DC vaccination targeting WT1.
Keywords: enzyme-linked immunosorbent spot assay, tetramer analysis, dendritic cells, antigen-specific cytotoxic T cells, Wilms’ tumor 1
1. Introduction
Over the last two decades, cancer immunotherapy by dendritic cell (DC) vaccination has become viable and a number of clinical trials have been conducted [1,2,3,4]. DCs, antigen-presenting cells of the mammalian immune system, can induce antigen specific cytotoxic T lymphocytes (CTLs) through interactions of the major histocompatibility complexes (MHCs) [1].
As Wilms’ tumor 1 (WT1) molecules are expressed in various types of solid tumors, DC vaccination targeting this molecule for cancer patients has priority as an immunotherapy [2,3,4,5,6,7].
Notably, the monitoring of immune response has been explored to detect cancer associated antigen-specific cytotoxic T lymphocytes (CTLs) exhibiting antitumor effects. Various types of methods are used for assessing the variables of immunity, such as MHC tetramer analysis, intracellular cytokine assay, and interferon gamma (IFN-γ) real time polymerase chain reaction (RT-PCR) [8,9,10].
The enzyme-linked immunosorbent spot (ELISpot) assay is an additional method to analyze functional release of IFN-γ from CTLs upon exposure to cancer-associated antigens.
In our facilities, the tetramer analysis has been routinely performed to evaluate the immunological effect of DC vaccination. Although this method could detect frequencies of epitope-specific CTLs, it does not necessarily reflect the function of CTLs. Therefore, we evaluated WT1 antigen-specific CTLs (WT1-CTLs) using the IFN-γ ELISpot assay in comparison with the MHC tetramer analysis as a validation method of DC-based cancer immunotherapy. This is the first study to compare these assays in the context of DC vaccination therapy.
2. Materials and Methods
2.1. Study Design
The primary objective of the current study was to validate the ELISpot assay as a tool for detection of CTLs. The secondary objective of the current study was to evaluate WT1-CTLs by the ELISpot assay in comparison with the tetramer analysis in patients who have received DC-based cancer immunotherapy.
2.2. Patient Population
Forty-six HLA-A*24:02 positive patients with carcinomas, including 11 lung, 6 breast, 5 stomach, 14 colorectal, and 10 pancreas cancer patients were enrolled for WT1 peptide (CYTWNQMNL, residue 235–243) (NeoMPS Inc., San Diego, CA, USA) -pulsed dendritic cell therapy after informed consent. The DC vaccination study was conducted at the Shinshu University Hospital and was approved by the Ethics Committee of the Shinshu University School of Medicine (approval number 1199, 2 December 2008; 2704, 8 April 2014).
2.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Peripheral blood samples were obtained from patients using BD Vacutainer™ Blood Collection Tubes (Sodium Heparin/Polyester Gel Samples) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), who received WT1 peptide pulsed DCs therapy before and after seven sessions of intradermal injections of DC vaccines every two weeks.
PBMCs were collected according to manufacturer’s instructions and cryopreserved using TC protector (DS pharma biomedical, Osaka, Japan) under a temperature condition of −80 to −180 °C.
2.4. Interferon (IFN)-γ ELISpot Assay
To assess the functional WT1-CTLs in PBMCs, IFN-γ producing cells were examined using the pre-coated Human IFN-γ ELISpotPLUS Kit (HRP) (Mabtech, Nacka Strand, Sweden), according to manufacturer’s instructions. In brief, cryopreserved cells were thawed and the live cells were counted. Next, 1 × 106 live cells/well were resuspended in AIM medium (Gibco, Gaithersburg, MD, USA) with 10% fetal bovine serum (BioWest, Nuaillé, France) in the presence of 10 μM of WT1 peptide or 10 μM of CMVpp65 peptide (QYDPVAALF, residue 341–349) (MBL, Medical & Biological Laboratories Co, Ltd, Nagoya, Japan). As a negative control, 10 μM HLA-A*24:02 human immunodeficiency virus (HIV) env (RYLRDQQLL, residue 584–592) (MBL, Nagoya, Japan) was used.
In some experiments, the ELISpot assay was performed using CD8+ T cells isolated from patient PBMCs after vaccination. The CD8+ T cells were isolated using microbeads conjugated to CD8 monoclonal antibodies (mAb) (Miltenyi Biotec, San Diego, CA, USA) and then cultured (3 × 105 cells/well) as mentioned above in the presence of CD8− cells pulsed with WT1 peptide as stimulator cells.
After 18–20 h of incubation at 37 °C and 5% CO2, the spots formed by IFN-γ-secreting cells were counted by an automated ELISpot reader (Autoimmun Diagnostika, Strassberg, Germany). Peptide specific spots were considered after deduction of spots of the negative control peptide from that of the WT1 peptide or the CMVpp65 peptide. Results were determined as the mean number of peptide specific spots per 1 × 106 PBMCs from duplicated wells. The presence of WT1-CTLs was defined according to the following criteria: (1) at least 15 WT1-specific spots per 1 × 106 PBMCs and (2) at least 1.5-fold more presence of WT1-specific spots than negative control peptide spots [11,12,13].
2.5. Tetramer Analysis
PBMCs were stained with phycoerythrin (PE)-conjugated WT1-modified peptide/HLA-A*24:02 tetramer (MBL, Nagoya, Japan) or PE-conjugated HIV envelope/HLA-A*24:02 tetramer (MBL, Nagoya, Japan) as a negative control, along with allophycocyanin-conjugated anti-human CD3 mAb (Biolegend, San Diego, CA, USA) and fluorescein isothiocyanate-conjugated anti-human CD8 mAb (Beckman Coulter, Miami, FL, USA). PBMCs were then analyzed by flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA). WT1-tetramer-positive CTLs were defined according to the following criteria: (1) comprising at least 0.02% in the CD3+CD8+ subset of 50,000–100,000 lymphocytes, and (2) forming a clustered and not diffused population.
Chudley and Britten et al. reported that the rate of detection in tetramer analysis was much lower with 10,000 CD8+ T cells than with 100,000 [14,15,16]. However, evaluation of 100,000 CD8+ T cells was very difficult because of the limited number of PBMCs available from most cancer patients. Therefore, cases with less than 10,000 available CD3+CD8+ cells were excluded.
2.6. Statistical Analyses
The statistical analysis was conducted using the R Software package, Version 3.0.2 (R foundation for Statistical Computing, Vienna, Austria). Pearson’s correlation coefficient was used to assess linearity. The correlation between results of the ELISpot assay and that of the Tetramer analysis was analyzed using the Spearman’s rank-correlation coefficient test. To determine the responses of WT1-CTLs between pre- and post-vaccination, a Wilcoxon signed rank test was applied. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Reproducibility of the ELISpot Assay
First, experiments were conducted to assess quantitative reproducibility of the ELISpot assay in three CMV-responder patients.
To evaluate the repeatability of the ELISpot assay, each 15 wells of 1 × 106 PBMCs stimulated with CMVpp65 peptide were examined. As shown in Table 1a, the CV ranged from 7.4% to 16.3%. Next, to evaluate the daily precision, each four wells of 1 × 106 PBMCs with CMVpp65 peptide were analyzed on three different days. As shown in Table 1b, CV ranged from 5.0% to 17.3%.
Table 1.
Precision of the ELISpot assay. The ELISpot assay was performed using peripheral blood mononuclear cells (PBMCs) from three cytomegalovirus (CMV)-responder patients. The number of spots per well are shown. (a) To evaluate the repeatability of the ELISpot assay, 1 × 106 PBMCs were examined with CMVpp65 in each of 15 wells. (b) To evaluate the daily precision, 1 × 106 PBMCs with CMVpp65 peptides were suspended in each of four wells and analyzed over three times on different days.
| (a) Assay Reproducibility | |||
|---|---|---|---|
| Sample | A | B | C |
| Mean | 73.5 | 15.0 | 22.2 |
| Median | 73.0 | 14.0 | 24.0 |
| SD | 5.4 | 3.1 | 3.6 |
| CV (%) | 7.4 | 11.8 | 16.3 |
| (b) Daily Precision | |||
| Sample | A | B | C |
| Mean | 77.4 | 24.6 | 20.1 |
| Median | 77.5 | 24.3 | 20.5 |
| SD | 3.9 | 4.3 | 3.1 |
| CV (%) | 5.0 | 17.3 | 15.7 |
3.2. Dilution Linearity
To evaluate the dilution linearity, ELISpot assay was performed using PBMCs from three CMV-responder patients. Experiments were performed in serial cell dilution (1.25 × 105, 2.5 × 105, 5.0 × 105, and 10.0 × 105 cells/well) with CMVpp65 peptide. As shown in Figure 1, all three results showed high linearity (Pearson’s correlation coefficient r = 0.96–0.98).
Figure 1.
The linearity of ELISpot assay in sample dilution experiments. ELISpot assay was performed using peripheral blood mononuclear cells from three cytomegalovirus-responder patients with serial cell dilution (1.25 × 105, 2.5 × 105, 5.0 × 105, 10.0 × 105 cells/well) and CMVpp65 peptide. The mean of the CMV specific spots in duplicated wells was indicated in the graph. Pearson’s correlation coefficient (r) was 0.96–0.98.
3.3. Detection of WT1-Specific Immune Response by ELISpot Assay
As shown in Figure 2, WT1 specific responses analyzed by ELISpot assay were detected in 34 out of 46 cancer patients (73.9%) after seven pulsed DCs vaccinations of WT1 peptide. A Wilcoxon signed-rank test showed a statistically significant increase in WT1-specific T cell response from the pre- to post-vaccination (p < 0.05).
Figure 2.
Assessment of Wilms’ tumor 1 (WT1)-specific immune response by ELISpot Assay. WT1-specific immune responses were analyzed both pre- and post-vaccination by ELISpot assay. Subjects were 46 patients who received WT1 peptide-pulsed dendritic cell therapy. Positive patients ●; Negative patients ○; WT1-specific cell responses were detected in 34 out of 46 patients (73.9%) after vaccination. Wilcoxon signed-rank test was p < 0.05. A representative positive case is shown in Figure 3.
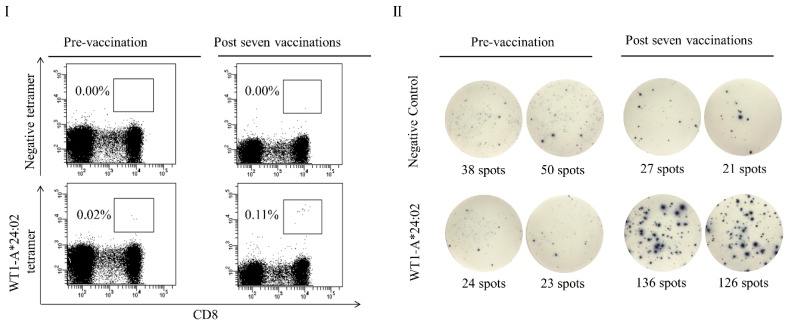
Figure 3.
WT1 peptide-specific responses post-DC vaccination in a representative case. (I) The frequencies of CD8+ and Tetramer+ cells in the CD3+ population are shown. Numbers indicate the percentages of tetramer-positive cells within the CD8+ population. (II) ELISpot assays of PBMCs. WT1-specific IFN-γ secretion by PBMCs increased after vaccination.
3.4. IFN-γ Producing Cells in the PBMCs
To identify WT1 peptide-specific IFN-γ-producing cells among the CD8+ T cells, the ELISpot assay was performed using CD8+ T cells isolated from PBMCs of vaccinated patients. The CD8+ T cells (1 × 105 cells/well) were cultured in the presence of CD8− PBMCs pulsed with the WT1 peptide (2 × 105 cells/well) at 37 °C for 30 min as stimulator cells.
As shown in Figure 4, WT1-specific spots were detected only in the wells containing CD8+ cells in these cases, suggesting that WT1-specific IFN-γ-producing PBMCs were primarily CD8+.
Figure 4.
ELISpot assay results of two representative cases of CD8+ T cells isolated from PBMCs. The CD8+ cells (1 × 105 cells/well) were cultured in the presence of CD8− PBMCs pulsed with the WT1 peptide (2 × 105 cells/well) as stimulator cells. Black and gray bars indicate the number of IFN-γ-producing cells per well stimulated by CD8− cells pulsed with the WT1 peptide and with negative control peptide, respectively.
3.5. Correlation between ELISpot Assay and Tetramer Analysis
The ELISpot assay was compared with the tetramer analysis in 46 patients who were treated with WT1 peptide pulsed DC therapy. As shown in Figure 5(I), a significant positive correlation between the number of WT1 specific spots from 1 × 106 PBMCs and the percentages of WT1 Tetramer+ cells in PBMCs was observed. The Spearman’s rank-correlation coefficient was 0.78. Figure 5(II) also showed a significant positive correlation between the number of WT1 specific spots from 1 × 106 PBMCs and the percentages of WT1 Tetramer+ cells in CD3+CD8+ cells. The Spearman’s rank-correlation coefficient was 0.82, indicating a positive relationship between the ELISpot assay and the tetramer analysis. Percentage tetramers were rounded off to the second decimal point, resulting in very similar values between graph (I) and (II) for samples with low tetramer positive cells.
Figure 5.
Correlation between the ELISpot assay and tetramer analysis. Subjects were 46 patients who were received WT1 peptide-pulsed DC therapy. Each tetramer data was rounded off to the second decimal place. The horizontal axis indicates the percentages of WT1 tetramer positive cells within PBMCs (I) or CD3+CD8+ population (II). The vertical axis indicates the number of WT1-specific IFN-γ secreting cells in 1 × 106 PBMCs. Cut off line of each analysis was shown.
As shown in Table 2, WT1-specific immune responses were detected by both ELISpot and tetramer analyses in 33 out of 46 post-vaccination patients. Positive responses were detected in one patient by only ELISpot assay, and seven patients by only tetramer analysis. Accordingly, total positive results were detected in 41 out of 46 (89.1%) patients by either analysis.
Table 2.
Cross tabulation of the findings by ELISpot and tetramer (CD3+CD8+) analyses from 46 cancer patients. The number of patients in each category is shown. WT1-specific immune responses were detected in 41 (89.1%) out of 46 patients post-vaccination by ELISpot and/or tetramer analysis.
| - | ELISpot | |||
|---|---|---|---|---|
| + | − | Total | ||
| Tetramer | + | 33 | 7 | 40 |
| − | 1 | 5 | 6 | |
| Total | 34 | 12 | 46 | |
4. Discussion
The evaluation of T-cell responses is very important to determine the effectiveness of vaccination-induced immune reaction. The ELISpot assay is considered as a unique method that allows the quantification of the actual cytokine secretory activity of individual cells among various approaches to evaluate T cell response [17,18].
In our facilities, the tetramer analysis has been performed to evaluate the efficacy of T cell responses in before and after seven-time application of WT1 peptide-pulsed DC vaccination. However, this test is limited as it detects only the frequency of epitope specific CTLs, all of which may not produce IFN-γ with antigen-specificity. On the other hand, the ELISpot assay can evaluate functional CTLs in response to stimulation by WT1 peptide. Therefore, using the IFN-γ ELISpot assay, we can assess not only the frequencies, but also the function of antigen-specific CTLs.
In the clinical setting, the validated methods should be used for immunological monitoring of the patients’ responses to treatment. Therefore, we assessed the reproducibility and linearity of the ELISpot assay. The results showed that there was reproducibility and daily precision as shown in Table 1. The dilution linearity results also showed a dose dependent response as shown in Figure 1. In the ELISpot assay, various concentrations (from 1 × 104 to 1 × 106 cells per well) and various types of cells have been used with many kinds of objective peptide [11,12,13,14,15]. However, the results of dilution linearity showed that when small numbers of cells were applied in a well, a very low number of antigen-specific T cells in the PBMCs may not be detected as Karlsson et al. reported [19]. Therefore, we decided to plate 1 × 106 PBMCs per well for the ELISpot assay.
Next, we identified WT1-specific IFN-γ-producing populations of PBMCs using the ELISpot assay. As shown in Figure 4, IFN-γ-producing PBMCs in response to WT1 were indeed CD8+. Thus, we used PBMCs instead of bead-separated CD8+ cells in the ELISpot assay, which was easier and is more applicable to clinical settings.
We then assessed the correlation between the ELISpot assay and the tetramer analysis. A significant correlation was observed between WT1-specific spots from 1 × 106 PBMCs and the percentages of WT1 tetramer positive cells in CD8+ cells, as shown in Figure 5(II). We also assessed the correlation between WT1-specific spots from 1 × 106 PBMCs and the percentages of WT1 tetramer positive cells in the PBMCs in Figure 5(I). Although the percentages of WT1 tetramer positive cells in the PBMCs were lower than that of in the CD8+ population, there was a significant correlation between both analyses.
However, as shown in Table 2, some cases showed mismatched results between the ELISpot assay and the tetramer analysis. More specifically, seven cases (three cases of lung cancer and four of pancreatic cancer) were tetramer analysis positive and ELISpot assay negative. Tetramer analysis could detect T cells harboring T cell receptor (TCR) with sufficient affinity to bind to WT1-peptide. On the other hand, the IFN-γ ELISpot assay could detect the cells that secrete IFN-γ stimulated by WT1-peptide. Therefore, despite the WT1-specific T cells being phenotypically detected, they might have no functional release of IFN-γ in these seven cases.
Only one case of colorectal cancer was positively detected by ELISpot assay while not detected by tetramer analysis. In this case, the count of WT1-specific spots by ELISpot assay was 16, which was far smaller than other cases. However, the size of each spot was larger than that of the others. Therefore, a small number of WT1-specific CTLs that can produce a high amount of IFN-γ upon WT1 stimulation may exist. We propose that an important advantage of the ELISpot assay is its ability to detect small numbers of functional CTLs, which are otherwise undetectable via tetramer analysis in clinical assessment of cancer immunotherapy. However, the question remains as to whether these CTLs are sufficiently functional to convey anti-cancer effects in vivo. Thus, further investigation is warranted.
Because DC therapy is usually combined with chemotherapy and/or radiotherapy, a reduction of tumor size and a decrease of tumor markers do not always indicate the effect of DC therapy only [20,21,22]. However, the IFN-γ ELISpot assay and tetramer analysis would provide an indication of the efficacy of the DC therapy by detection of antigen specific CTLs [2,5,23,24]. Again, this is the first report to reveal the usefulness of the ELISpot assay in the context of DC vaccination anti-cancer therapy in comparison with tetramer analysis, which is widely used clinically to assess the effects of therapy.
In the scientific magazine Science, anti-cancer immunotherapy was chosen as the top scientific breakthrough of 2013 [25]. Immunotherapy will further develop as the fourth routine method of cancer treatment in the future. Within this context, the evaluation of monitoring methods assessing immunotherapy by an established clinical laboratory procedure will become increasingly important.
5. Conclusions
The ELISpot assay has reproducibility, linearity, and excellent correlation with the WT1 tetramer analysis. The validated ELISpot assay is useful to monitor the acquired immunity by DC vaccination targeting WT1.
Acknowledgments
The authors thank Tsukasa Higuchi for assistance in preparation of this manuscript and Enago (www.enago.jp) for the English language review.
Author Contributions
Yumiko Higuchi and Shigetaka Shimodaira conceived and designed the study; Yumiko Higuchi, Terutsugu Koya, and Miki Yuzawa manufactured dendritic cell vaccines; Yumiko Higuchi, Yumiko Mizuno, Naoko Yamaoka, and Kiyoshi Yoshizawa provided immune monitoring data; Koichi Hirabayashi and Takashi Kobayashi treated patients with DC vaccines; Kenji Sano performed pathological diagnosis; Shigetaka Shimodaira took special responsibility for the clinical study. Yumiko Higuchi analyzed immune monitoring data and wrote the article.
Conflicts of Interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Steinman R.M. The dendritic Cell and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Saito S., Yanagisawa R., Yoshikawa K., Higuchi Y., Koya T., Yoshizawa K., Tanaka M., Sakashita K., Kobayashi T., Kurata T., et al. Safety and tolerability of allogeneic dendritic cell vaccination with induction of WT1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: A case report and review of the literature. Cytotherapy. 2015;17:330–335. doi: 10.1016/j.jcyt.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Oka Y., Tsuboi A., Taguchi T., Osaki T., Kyo T., Nakajima H., Elisseeva O.A., Oji Y., Kawakami M., Ikegame K., et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA. 2004;21:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koido S., Homma S., Okamoto M., Takakura K., Mori M., Yoshizaki S., Tsukinaga S., Odahara S., Koyama S., Imazu H., et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin. Cancer Res. 2014;20:4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi Y., Koya T., Yuzawa M., Yamaoka N., Mizuno Y., Yoshizawa K., Takahashi K., Soneda M., Horiuchi K., Hirabayashi K., et al. Detection of specific immune response by dendritic cell-based cancer immunotherapy. J. Jpn. Soc. Transfus. Med. Cell Ther. 2015;61:401–402. doi: 10.3925/jjtc.61.401. [DOI] [Google Scholar]
- 6.Nakatsuka S., Oji Y., Horiuchi T., Kanda T., Kitagawa M., Takeuchi T., Kawano K., Kuwae Y., Yamauchi A., Okumura M., et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 7.Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M., et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clay T.M., Hobeika A.C., Mosca P.J., Lyerly H.K., Morse M.A. Assays for Monitoring Cellular Immune Responses to Active Immunotherapy of Cancer. Clin. Cancer Res. 2001;7:1127–1135. [PubMed] [Google Scholar]
- 9.Xu Y., Theobald V., Sung C., DePalma K., Atwater L., Seiger K., Perricone M.A., Richards S.M. Validation of a HLA-A2 tetramer flow cytometric method, IFNγ real time RT-PCR, and IFNγ ELISPOT for detection of immunologic response to gp100 and MelanA/MART-1 in melanoma patients. J. Transl. Med. 2008;6:61. doi: 10.1186/1479-5876-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keilholz U., Weber J., Finke J.H., Gabrilovich D.I., Kast W.M., Disis ML., Kirkwood J.M., Scheibenbogen C., Schlom J., Maino V.C., et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J. Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Godard B., Gazagne A., Gey A., Baptiste M., Vingert B., Pegaz-Fiornet B., Strompf L., Fridman W.H., Glotz D., Tartour E. Optimization of an elispot assay to detect cytomegalovirus-specific CD8+ T lymphocytes. Hum. Immunol. 2004;65:1307–1318. doi: 10.1016/j.humimm.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Scheibebogen C., Letsch A., Thiel E., Schmittel A., Mailaender V., Baerwolf S., Nagorsen D., Keilholz U. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Khader A., Krause S. Rapid monitoring of immune reconstitution after allogeneic stem cell transplantation—a comparison of different assays for the detection of cytomegalovirus-specific T cells. Eur. J. Haematol. 2013;91:534–545. doi: 10.1111/ejh.12187. [DOI] [PubMed] [Google Scholar]
- 14.Chudley L., McCann K.J., Coleman A., Cazaly A.M., Bidmon N., Britten C.M., van der Burg S.H., Gouttefangeas C., Jandus C., Laske K., et al. Harmonisation of short-term in vitro culture for the expansion of antigen-specific CD8+ T cells with detection by ELISPOT and HLA-multimer staining. Cancer Immunol. Immunother. 2014;63:1199–1211. doi: 10.1007/s00262-014-1593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britten C.M., Gouttefangeas C., Welters M.J., Pawelec G., Koch S., Ottensmeier C., Mander A., Walter S., Paschen A., Müller-Berghaus J., et al. The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunol. Immunother. 2008;57:289–302. doi: 10.1007/s00262-007-0378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britten C.M., Janetzki S., Ben-Porat L., Clay T.M., Kalos M., Maecker H., Odunsi K., Pride M., Old L., Hoos A., et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol. Immunother. 2009;58:1701–1713. doi: 10.1007/s00262-009-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann P.V., Zhang W. Unique strengths of ELISPOT for T cell diagnostics. In: Kalyuzhny A.E., editor. Handbook of ELISPOT: Methods and protocols, Methods in molecular biology. 2nd ed. Volume 792. Humana Press; New York City, NY, USA: 2012. pp. 3–23. [DOI] [PubMed] [Google Scholar]
- 18.Miyahara Y., Murata K., Rodriguez D., Rodriguez JR., Esteban M., Rodrigues M.M., Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-S. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson A.C., Martin J.N., Younger S.R., Bredt B.M., Epling L., Ronquillo R., Varma A., Deeks S.G., McCune J.M., Nixon D.F., et al. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J. Immunol. Methods. 2003;283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Shibamoto Y., Okamoto M., Kobayashi M., Ayakawa S., Iwata H., Sugie C., Mitsuishi Y., Takahashi H. Immune-maximizing (IMAX) therapy for cancer: Combination of dendritic cell vaccine and intensity-modulated radiation. Mol. Clin. Oncol. 2013;1:649–654. doi: 10.3892/mco.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimodaira S., Kobayashi T., Hirabayashi K., Horiuchi K., Koya T., Mizuno Y., Yamaoka N., Yuzawa M., Ishikawa S., Higuchi Y., et al. Induction of Antigen-Specific Cytotoxic T Lymphocytes by Chemoradiotherapy in Patients Receiving Wilms Tumor 1-Targetted Dendritic Cell Vaccinations for Pancreatic Cancer. OMICS J. Radiol. 2015;4 doi: 10.4172/2167-7964.1000196. [DOI] [Google Scholar]
- 22.Johung K., Saif M.W., Chang B.W. Treatment of locally advanced pancreatic cancer: The role of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:508–518. doi: 10.1016/j.ijrobp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Mayanagi S., Kitago M., Sakurai T., Matsuda T., Fujita T., Higuchi H., Taguchi J., Takeuchi H., Itano O., Aiura K., et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015;106:397–406. doi: 10.1111/cas.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai K., Shimodaira S., Maejima S., Udagawa N., Sano K., Higuchi Y., Koya T., Ochiai T., Koide M., Uehara S., et al. Dendritic cell-based immunotherapy targeting Wilms’ Tumor 1 (WT1) in patients with relapsed malignant glioma. J. Neurosurg. 2015;7:1–9. doi: 10.3171/2015.1.JNS141554. [DOI] [PubMed] [Google Scholar]
- 25.Couzin-Frankel J. Breakthrough of the year 2013, Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]