Abstract
Hepatocyte growth factor (HGF) is composed of an α-chain and a β-chain, and these chains contain four kringle domains and a serine protease-like structure, respectively. Activation of the HGF–Met pathway evokes dynamic biological responses that support morphogenesis (e.g., epithelial tubulogenesis), regeneration, and the survival of cells and tissues. Characterizations of conditional Met knockout mice have indicated that the HGF–Met pathway plays important roles in regeneration, protection, and homeostasis in various cells and tissues, which includes hepatocytes, renal tubular cells, and neurons. Preclinical studies designed to address the therapeutic significance of HGF have been performed on injury/disease models, including acute tissue injury, chronic fibrosis, and cardiovascular and neurodegenerative diseases. The promotion of cell growth, survival, migration, and morphogenesis that is associated with extracellular matrix proteolysis are the biological activities that underlie the therapeutic actions of HGF. Recombinant HGF protein and the expression vectors for HGF are biological drug candidates for the treatment of patients with diseases and injuries that are associated with impaired tissue function. The intravenous/systemic administration of recombinant HGF protein has been well tolerated in phase I/II clinical trials. The phase-I and phase-I/II clinical trials of the intrathecal administration of HGF protein for the treatment of patients with amyotrophic lateral sclerosis and spinal cord injury, respectively, are ongoing.
Keywords: amyotrophic lateral sclerosis, clinical trial, HGF, Met, spinal cord injury
1. Background of Hepatocyte Growth Factor (HGF)–Met Pathway Leading to Drug Discovery
HGF was molecularly cloned as a growth factor for hepatocytes [1,2]. The scatter factor, originally identified as a fibroblast-derived cell motility factor for epithelial cells [3], was shown to be an identical molecule to HGF [4]. The receptor for HGF was identified as a c-met protooncogene product of transmembrane receptor tyrosine kinase in 1991 [5,6]. These early findings implicated biological and pathophysiological roles for HGF in epithelial wound healing, tissue regeneration, tumorigenesis, and cancer invasion.
HGF is a glycosylated protein composed of an α-chain and a β-chain linked by one disulfide bridge (Figure 1A) [1,2]. HGF is biosynthesized as a prepro-form, including a signal sequence and both α- and β-chains. After cleavage of a signal peptide of the first 31 amino acids and extracellular secretion, a single-chain HGF is further cleaved between Arg494 and Val495 by serine proteases such as HGF-activator, matriptase, and hepsin [7,8]. Mature HGF is composed of 697/692 amino acids.
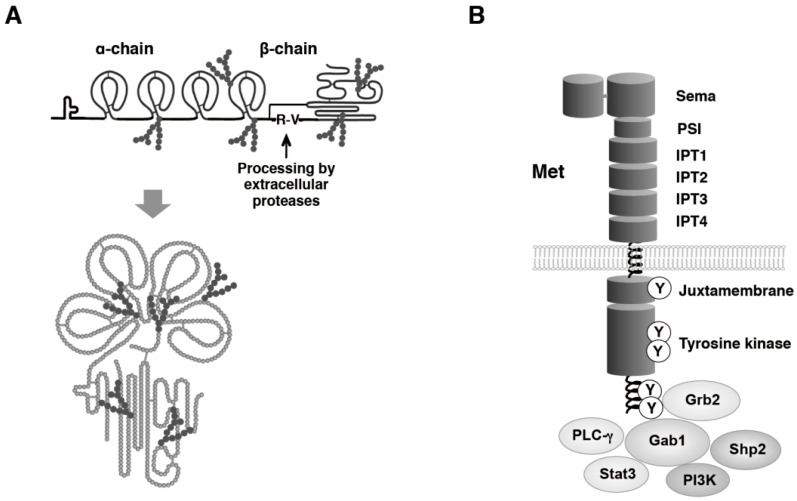
Figure 1.
Schematic structures of HGF (A) and Met (B). A single-chain HGF is cleaved between Arg494 and Val495 by serine proteases and HGF is modified by glycosylation. Domain structure of Met and typical signaling molecules are described.
The Met receptor is composed of structural domains that include the extracellular Sema (the domain found in semaphorin receptors), the PSI (the domain found in plexins, semaphorins and integrins), the IPT (the domain found in immunoglobulins, plexins, and transcription factors), the transmembrane, the intracellular juxtamembrane, and the tyrosine kinase domains (Figure 1B) [9]. The Sema domain serves as a key element for ligand binding [10], while the involvement of IPT-3 and IPT-4 in the binding to HGF was demonstrated by another approach [11].
Binding of HGF to the Met results in the phosphorylation of multiple tyrosine residues within the cytoplasmic region. The phosphorylation of Tyr1234 and Tyr1235 within the tyrosine kinase domain positively regulates the catalytic activity of tyrosine kinase, and the subsequent phosphorylation of C-terminal tyrosine residues Tyr1349 and Tyr1356 recruits intracellular signaling molecules, including growth factor receptor-bound protein 2 (Grb2), GRB2-associated-binding protein 1 (Gab1), phosphoinositide 3-kinase (PI3K), SH2 containing protein tyrosine phosphatase (Shp2), phospholipase Cγ1 (PLCγ1), and signal transducer and activator of transcription-3 (Stat3). Typical biological activities evoked by Met activation include promotion of mitogenesis and migration, suppression of cell death, and induction of epithelial morphogenesis. Among signaling molecules, Gab1 plays a critical role in HGF–Met pathway-dependent biological responses [12,13]. Gab1 is a scaffolding adaptor containing the Met-binding site, by which a direct and robust interaction between Gab1 and Met results in prolonged Gab1 phosphorylation in response to HGF. Association of Gab1 to Met is responsible for unique biological activities of HGF, including epithelial tubulogenesis. Gab1−/− and Met−/− mice were characterized by impairment in migration of myogenic precursor cells and formation of functional placenta.
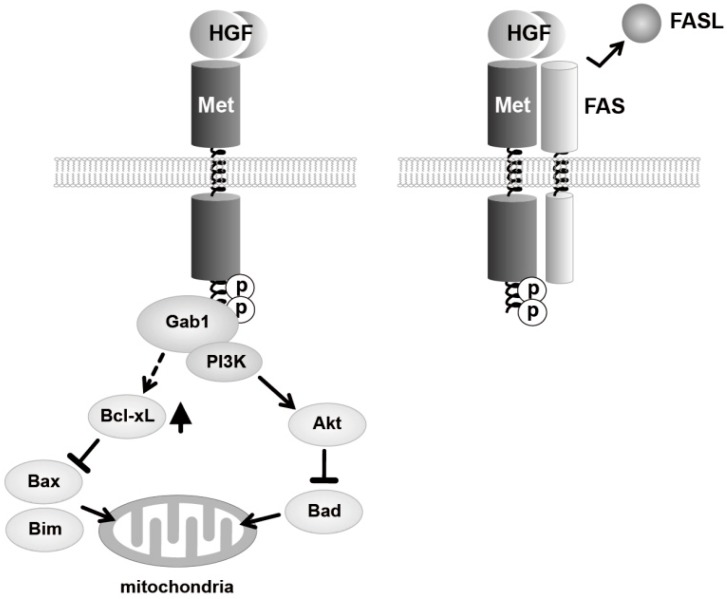
Promotion of cell survival (i.e., suppression of cell death) is a key biological action of HGF–Met pathway in development, regeneration, and therapeutics. Activation of Met induces phosphorylation of PI3K and Akt, and Akt-induced phosphorylation of Bad inhibits pro-apoptotic activity of Bad (Figure 2). In parallel, HGF increases expression anti-apoptotic Bcl-xL, by which pro-apoptotic activity of Bax and Bim is inhibited [14,15]. Fas and Fas-ligand play a marked role in induction of apoptosis. Another anti-apoptotic mechanism involves extracellular interaction between the extracellular domain of Met and the death receptor FAS [16]. Binding of HGF to Met prevents functional association between FAS and FAS-ligand, therefore inhibiting the self-aggregation of FAS, a key event required for induction of FAS-mediated apoptosis.
Figure 2.
Signaling mechanisms responsible for promotion of cell survival mediated by HGF–Met pathway.
The cytoplasmic juxtamembrane domain negatively regulates Met-dependent signal transduction. Cbl ubiquitin ligase binds phosphorylated Y1003, and this Cbl binding results in Met ubiquitination and degradation [17]. Phosphorylation of Ser985 in the juxtamembrane domain also regulates Met activation. Ser985 is phosphorylated by protein kinase-C, and when Ser985 is phosphorylated, HGF-dependent Met tyrosine phosphorylation is suppressed [18].
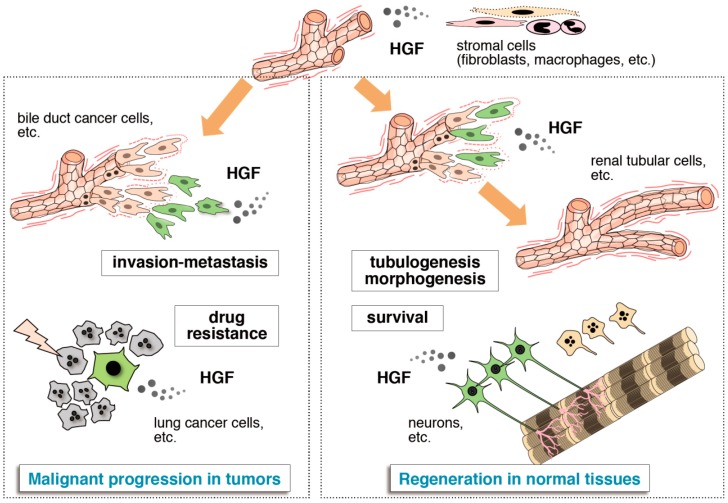
In most cases in the relationship between growth factors and their receptors, a single growth factor activates multiple receptors that have structural similarities, while a single receptor has multiple ligands with structural similarities. By contrast, the sole receptor for HGF is Met, while the sole ligand for Met is HGF; the relationship is a “one-to-one relationship”. This unique biochemical characteristic in the HGF–Met pathway promotes drug development by targeting HGF–Met through either the activation or the inhibition of the HGF–Met pathway. HGF has biological activities involved in dynamic tissue remodeling during embryogenesis and tissue regeneration. Breakdown of the extracellular matrix scaffold and the concomitant cellular migration, mitogenesis, and morphogenesis that is driven by the HGF–Met pathway all make way for the construction and reconstruction of tissues. HGF suppresses cell death and promotes the survival of cells, and this action participates in the protection of cells and tissues against injuries and pathology. However, these biological actions that are driven by the HGF–Met pathway all play a role in the acquisition of the malignant characteristics in tumor cells—invasion, metastasis, and drug resistance in the tumor microenvironment (Figure 3).
Figure 3.
Two-pronged roles of HGF in tissue regeneration and cancer tissues. HGF is mainly expressed in stromal cells. Cells responding to HGF are conceptually shown in green. Dynamic morphogenesis (e.g., blanching tubulogenesis in renal tubular cells) and promotion of cell survival (e.g., for neurons) mediated by the HGF–Met pathway play roles in tissue regeneration after tissue injury (right part). Dynamic cell movement and survival promoted by Met activation participate in invasion-metastasis and resistance to anticancer drugs in cancer tissues (left part).
2. Tissue Regeneration/Protection Deduced from Met Disruption
In conventional knockout mice of the HGF or Met gene in whole body is lethal during the embryonic stage due to an impaired organogenesis of the placenta and liver [19,20]. Moreover, HGF provides spatially defined chemoattractant-like motogenic signals for myogenic precursor cells. The migration of myogenic precursor cells from the dermo-myotome in the somite to the limb buds and diaphragm is impaired in Met−/− mice. With this condition, the skeletal muscles of the limbs and diaphragm are not formed in mutant mice [21].
Definitive roles of the HGF–Met pathway in tissue protection and repair have been demonstrated using a conditional knockout of the Met gene in mice (Table 1). Hepatocytes subjected to selective loss of the functional Met were highly susceptible to cell death even after mild liver injury, indicating that the anti-apoptotic activity of HGF plays a role in the protection of the liver [22]. Liver-specific Met−/− mice showed delayed liver regeneration associated with persistent inflammatory reaction [23]. Activation of Met plays a role in the persistent Erk1/2 activation and the G2/M gene expression program throughout liver regeneration following partial hepatectomy [24]. In addition to the regenerative response in mature hepatocytes, HGF–Met signaling supports the in vitro sphere formation of hepatic stem cells (oval cells) and in vivo hepatic stem cell-mediated regeneration [24]. Met-deficient oval cells were more prone to apoptosis when the cells were exposed to proapoptotic conditions after bile duct ligation. The livers in hepatocyte-specific Met−/− mice were more susceptible to chronic inflammation and fibrotic change compared with control mice [25]. The effects shown by these liver- or hepatocyte-specific Met−/− mice indicate the physiological roles of the HGF–Met pathway in the protection, regeneration, anti-inflammation, and anti-fibrosis of the liver.
Table 1.
Physiological roles of HGF deduced from conditional knockout mice.
| Met−/− Tissue/Cell Types | Characteristics | Ref. |
|---|---|---|
| Liver | ||
| Hepatocytes | Highly susceptible to apoptosis after liver injury | [22] |
| Impairment in recovery from liver necrosis after liver injury | ||
| Impairment in Erk1/2 activation and G2/M transition after liver injury | [23] | |
| Hepatocytes | Steatotic change of the liver in aged mice | [24] |
| Decrease in mitotic hepatocytes after partial hepatectomy | ||
| Delayed regeneration after partial hepatectomy | ||
| Hepatocytes | Promoted liver fibrosis after liver injury | [26] |
| Extensive necrosis and lower proliferation of hepatocytes after bile-duct ligation | [25] | |
| Enhanced susceptibility to liver fibrosis | ||
| Oval cells | Decrease in oval cell viability and more prone to apoptosis | [27] |
| Reduction in oval cell pool | [28] | |
| Impairment in migration and differentiation into hepatocytes | ||
| Kidney | ||
| Tubular cells | No appreciable defect in kidney morphology and function | [29] |
| Aggravated renal injury and inflammation after acute kidney injury | ||
| Podocytes | Neither albuminuria nor overt pathologic lesions | [30] |
| Severe podocyte injury and apoptosis, and albuminuria after toxic injury | ||
| Collecting duct | Increased fibrosis and tubular necrosis after unilateral ureteral obstruction | [31] |
| Reduced capacity in regeneration after release of the obstruction | ||
| Ureteric bud | Double knockout of Met and EGF receptor in ureteric bud | [32] |
| Decrease in branching and a reduction in final glomerular number | ||
| Skin | ||
| Keratinocytes | Lack of keratinocyte migration after skin wound | [33] |
| Severe impairment epidermal wound closure | ||
| Pancreas | ||
| β-Cell | Mild hyperglycemia, and decreased serum insulin levels at 6 months | [34] |
| Loss of acute-phase insulin secretion in response to glucose, and impaired glucose tolerance | ||
| Diminished glucose tolerance and reduced plasma insulin after a glucose challenge | [35] | |
| Normal glucose and β-cell homeostasis | [36] | |
| Susceptible to streptozotocin-induced diabetes | ||
| Nervous System | ||
| Ganglionic eminence | Increased numbers of striatal GABAergic interneurons in the lateral sensorimotor | [37] |
| Areas with distinct behavioral deficits | ||
| Delayed procedural learning | ||
| Cerebral cortex and hippocampus | Larger size in the rostral cortex, caudal hippocampus, dorsal striatum, thalamus, and corpus callosum | [38] |
| Dorsal pallial | Increases proximal and reduces distal apical dendritic branching of neocortical pyramidal neurons in post-pubertal period | [39] |
| Forebrain neurons | Reduced volume of cortical tissue | [40] |
| Increase in spine head volume, but no change in density of spines | ||
| Hyperconnectivity in circuit-specific intracortical neurons | ||
| Heart | ||
| Cardiomyocytes | Normal heart development | [41] |
| Cardiomyocyte hypertrophy and interstitial fibrosis by 6 months | ||
| Systolic cardiac dysfunction by 9 months | ||
| Immune System | ||
| Dendritic cells | Impaired emigration toward draining lymph nodes upon inflammation-induced activation | [42] |
| Impaired contact hypersensitivity reaction to contact allergens | ||
Characterization of conditional Met knockout mice indicates that the HGF–Met pathway plays important roles in regeneration, protection, and homeostasis in various cells and tissues (Table 1). The loss of functional Met in renal tubules caused no appreciable defect in renal function. However, when mice were subjected to renal injury, tubular cell-specific Met−/− mice displayed higher serum creatinine, greater severity in morphologic lesions, and an increase in apoptosis compared with control mice [29]. In podocyte-specific Met−/− mice, no pathology was seen, but when subjected to toxic renal injury of the podocytes, these mice developed podocyte apoptosis and albuminurea that was more severe compared with that of control mice [30]. Collective duct-selective Met dysfunction indicated a trend toward increased interstitial fibrosis, infiltration of the interstitium, and acute tubular necrosis after unilateral obstruction, while there was a reduced regenerative response after the release of obstruction [31].
Disruption of the Met gene in epidermal keratinocytes demonstrated an indispensable role for the HGF–Met pathway in skin wound healing [28]. Because the migration of keratinocytes post-wounding was almost completely impaired in Met−/− keratinocytes, re-epithelialization was severely suppressed. Wound closure occurred exclusively in a few keratinocytes that had escaped recombination, which indicated that the skin wounding process had selected and amplified residual cells that expressed a functional Met. Those results indicated a definitive role for the HGF–Met pathway in skin wound healing. In mice with Met-deficient dendritic cells, Met-deficient dendritic cells failed to reach skin-draining lymph nodes upon activation while exhibiting an activated phenotype, and the contact hypersensitivity reactions in response to contact allergens was greatly impaired [42]. HGF–Met signaling in cutaneous dendritic cells may play a critical role in the maintenance of normal immune function.
Conditional knockout mice with selective disruption of Met in pancreatic β-cells displayed significantly diminished glucose tolerance and reduced plasma insulin after a glucose challenge [35]. In vitro glucose-stimulated insulin secretion in the islets from β-cell-Met−/− mice was decreased by ~50% compared with control islets. These changes in β-cell function in the conditional Met knockout mice were not accompanied by changes in total β-cell mass, islet morphology, or β-cell proliferation [35]. Another group using β-cell-Met−/− mice displayed mild hyperglycemia and a complete loss of acute-phase insulin secretion in response to glucose [34]. Therefore, HGF–Met signaling in the β-cell is not essential for β-cell growth, but it is essential for normal glucose-dependent insulin secretion and glucose homeostasis.
3. Neurotrophic Function and Involvement in Neuronal Disorder/Symptoms
HGF also functions as a novel neurotrophic factor for a variety of neurons, including the hippocampal, cerebral cortical, midbrain dopaminergic, motor, sensory, sympathetic, parasympathetic, and cerebellar granule neurons in culture [43]. Knock-out/-in studies and stereotaxic injections of anti-HGF antibody into the striatum have revealed the critical role of HGF in the nervous system [43]. HGF plays important roles in motor (including muscles), sensory, sympathetic, parasympathetic, and cortical neuronal development. In addition to the neuronal development, intrastriatal injections of anti-HGF IgG has revealed the involvement of HGF in the proliferation of oligodendrocyte progenitor cells and their differentiation into oligodendrocytes [44].
Approaches to address the susceptibility genes in neuronal disorders have revealed a relationship between Met transcriptional regulation and autism. The genetic association of a common C allele in the promoter region of the Met gene was found in 204 families with autism [45]. The allelic association for this Met variant was confirmed in a replication sample of 539 families with autism and in a combined sample. These researchers also found that Met protein levels were significantly decreased in autism spectrum disorder cases, compared with control subjects. Their subsequent study supported the association of the Met promoter variant rs1858830 C allele with autism spectrum disorder, implying a promoter mutation in autism spectrum disorder [46]. New mutations were noted in autism, in both single locus and haplotype approaches, with a single nucleotide polymorphism in intron 1 and with one intronic haplotype, in 325 multiplex International Molecular Genetics of Autism Consortium families [47].
The molecular mechanism by which mutations found in the Met gene become causative for characteristics seen in autism and autism spectrum disorder should be further addressed, but the neuro-developmental role of the HGF–Met pathway may be somewhat altered by these mutations in the Met gene. The HGF–Met pathway contributes to the development of the cerebral cortex [48,49] and the cerebellum [50], both of which exhibit developmental disruptions in autism [51,52]. Hypomorphic Met–HGF signaling in the cerebral cortex results in abnormal interneuron migration from the ganglionic eminence and reduced interneurons in the frontal and parietal regions of the cortex [48,49]. Hypomorphic HGF–Met signaling in the cerebellum causes a decrease in the proliferation of granule cells and a concomitant reduction in the size of the cerebellum, particularly in the vermis [50]. Both of these neuropathologic abnormalities are consistent with those observed in the brains of individuals with autism [51,52]. These results show why the Met gene is pursued as an autism candidate gene.
A comparison of 21 single nucleotide polymorphisms in the Met gene of 173 Caucasian patients with schizophrenia status and 137 controls revealed an association between genetic variation in the Met gene and schizophrenia [53]. Genetic variations of the Met are associated with general cognitive ability. These findings implicated the HGF–Met pathway in psychiatric status and diseases.
Three mutations in the HGF gene were found in patients with nonsyndromic hearing loss, at the autosomal-recessive NSHI locus DFNB39 [54]. Two of the mutations occurred in a region contained in the 3'UTR of an alternate splice form of the HGF gene that had not been previously discovered. The third mutation, a third-position nucleotide change predicted to make a synonymous amino acid substitution, altered splicing by affecting the relative strengths of the spliced forms of the HGF gene. The conditional deletion of exon 5 of the HGF gene in the cochlea, and apparently no other phenotype, resulted in a profound, nonprogressive hearing loss associated with extensive morphometric pathology [54]. Conversely, the ubiquitous overexpression of HGF in a transgenic mouse resulted in progressive hearing loss associated with a degeneration of the outer hair cells; therefore, the dysregulation of HGF might be involved in nonsyndromic hearing loss.
4. HGF as a Biological Drug Candidate
Collectively, tissue-specific disruption of the functional Met in mice indicated that HGF plays a promoting role in the regeneration, protection, and homeostasis of tissues, and an inhibitory role in the progression of chronic inflammation and fibrosis. Thus, enhancement of Met-mediated signaling and biological responses is likely to become therapeutic for the treatment of different types of diseases. In parallel studies addressing the roles of the HGF–Met pathway in genetically modified mice, therapeutic approaches have been pursued in different disease models (Table 2) [55].
Table 2.
Therapeutic approaches with recombinant HGF protein in various disease models.
| Tissues and Disease/Injury Models | References | |
|---|---|---|
| Liver | ||
| Acute hepatitis | [58,59,60,61,62,63] | |
| Chorestasis | [64] | |
| Fulminant hepatitis | [65,66] | |
| Liver fibrosis/cirrhosis | [67,68,69,70,71] | |
| Liver cirrhosis + surgery | [72] | |
| Alcoholic steatohepatitis | [73] | |
| Gastrointestinal | ||
| Ulcerative colitis | [74,75] | |
| Gastric ulcer | [76] | |
| Gastric injury | [77] | |
| Kidney | ||
| Acute kidney injury | [78,79,80,81,82,83] | |
| Acute renal inflammation | [84] | |
| Septic acute renal failure | [85] | |
| Diabetic nephropathy | [86] | |
| Chronic kidney disease | [87,88,89,90] | |
| Glomerulonephritis | [91] | |
| Chronic allograft nephropathy | [92] | |
| Cardiovascular | ||
| Critical limb ischemia | [93,94,95] | |
| Neointimal hyperplasia | [96] | |
| Coronary artery disease | [97] | |
| Myocardial infarction | [98,99] | |
| Cardiac allograft vasculopathy | [100] | |
| Dilated cardiomyopathy | [101] | |
| Respiratory | ||
| Acute lung injury | [102] | |
| Ischemia-reperfusion | [103] | |
| Lung fibrosis | [104,105,106] | |
| Pulmonary emphysema | [107] | |
| Left peumonectomy | [108] | |
| Allergic airway inflammation | [109] | |
| Vocal fold scarring | [110,111] | |
| Skin | ||
| Wounding | [112,113] | |
| Nervous System(s) | ||
| Cerebral ischemia | [114,115,116,117,118] | |
| Peripheral nerve injury | [119] | |
| Amyotrophic lateral sclerosis | [120] | |
| Hydrocephalus | [121] | |
| Retinal injury | [122] | |
| Photoreceotr degeneration | [123,124] | |
| Difficulty in hearing | [125] | |
| Musclosleletal | ||
| Articular cartilage injury | [126] | |
| Skeletal muscle injury | [127] | |
| Rheumatoid arthritis | [128] | |
| Ligament injury | [129] | |
The prevention of cell death against various types of stress and injury explains the protective action of HGF, and this seems to be associated with a subsequent suppression of inflammation. HGF regulates the function of dendritic cells and a subset of regulatory T cells [56,57]. The biological actions of HGF on immune cells are likely to underlie the mechanisms by which HGF exerts its therapeutic effect on diseases associated with allergies, inflammation, and fibrosis, at least in part. The promotion of cell proliferation, migration, and 3-D morphogenesis (e.g., branching tubulogenesis in epithelial cells and endothelial cells) driven by the HGF–Met pathway seems to explain the re-organization of tissues from injuries. Chronic tissue injury and inflammation has been associated with the onset of fibrosis. It should be emphasized that there has been no effective medicine for the treatment of chronic fibrotic diseases, whereas HGF-treatment has been effective in reducing fibrosis and improving tissue function in disease models, including liver cirrhosis, chronic kidney disease, dilated cardiomyopathy, lung fibrosis, and vocal fold scarring.
The administration of HGF exerts neuroprotective effects in the animal models of cerebrovascular diseases, spinal cord injury, and in neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) and neuroimmune diseases by promoting neuronal cell survival and the functioning of glial, vascular, and immune cells. Transgenic expression of HGF in neurons has exhibited beneficial roles in the animal models of Alzheimer’s disease and polyglutamine diseases [43,130]. Experimental allergic encephalomyelitis that is induced either by immunization with myelin oligodendrocyte glycoprotein peptide or by the adoptive transfer of T cells, which mimics multiple sclerosis, was inhibited in a selective overexpression of HGF by neurons in the central nervous system in mice [57]. HGF has mediated mesenchymal stem cell-induced recovery in multiple sclerosis models [131]. Furthermore, systemic HGF treatment has ameliorated experimental allergic encephalomyelitis through the development of tolerogenic dendritic cells [132]. These findings have demonstrated the roles of HGF in the animal models of multiple sclerosis via functioning on not only neural cells but also mesenchymal stem cells and immune cells.
5. Clinical Study and Drug Development
5.1. Chronic Leg Ulcer
Chronic leg ulcer treatment in elderly patients is a significant healthcare problem. These leg wounds result from different causes, such as diabetes and/or inappropriate circulation, and can be very difficult to heal. Between 1.5 and 3.0/1000 people have active leg ulcers. At present, there has been no satisfactory treatment for many of these patients. The methods available are often time-consuming, difficult, and costly. Involvement of the HGF–Met pathway in the pathophysiological roles of HGF might be considered by evaluating the Met activation status in tissues with pathology. In clinical studies in patients with skin ulcer, the phosphorylation of Met was most prominent in keratinocytes and dermal cells in normally healing wounds; however, tyrosine phosphorylation of Met has been barely detectable in non-healing wounds, suggesting reduced Met activation [133].
The disruption of functional Met in epidermal keratinocytes has indicated that Met-deficient keratinocytes were unable to contribute to the re-epithelialization of skin wounds in mice models [28]. Therefore, activation of the HGF–Met pathway is essential for a fundamental regenerative process during skin wound healing and may not be substituted by other bioactive molecules for the generation of hyperproliferative epithelium in skin. In a full-thickness cutaneous excision model in diabetic mice, topical administration of recombinant HGF protein promoted angiogenesis, extracellular matrix remodeling, re-epithelialization, and wound closure [112,113].
The first clinical study using recombinant human HGF protein was done to investigate the physiological and therapeutic effects of HGF on chronic leg ulcers. HGF in gel form was locally applied to chronic leg ulcers in 11 patients [134]. This pilot study observed that excellent (84%–100% area reduction) or partial healing (58%–59%) was seen in eight out of 11 patients. Significant microcirculatory perfusion was statistically correlated to ulcer area reduction in the treated ulcers. This study suggested that topical application of HGF protein might have facilitated the healing of chronic leg ulcers, possibly by improving the microcirculation. Because no control group was included in this pilot study, proper control studies must be performed for further clinical evaluation.
5.2. Critical Limb Ischemia
Critical limb ischemia, the most severe form of peripheral arterial disease due to atherosclerosis, is a common clinical problem that has no effective medical therapy. The standard therapy for critical limb ischemia remains to be lower-extremity revascularization, either through open bypass surgery or by endovascular techniques, or lower-extremity amputation when revascularization is not an option. At present, there is a need for less invasive therapies to improve limb perfusion in patients with critical limb ischemia.
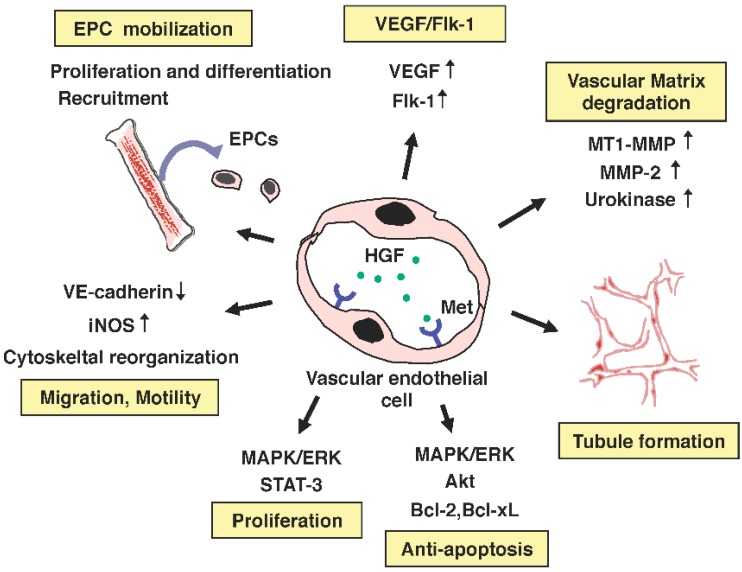
Met receptor is expressed in different types of vascular endothelial cells, and HGF stimulates the migration and motility of different types of endothelial cells [135,136,137]. Further studies have indicated that HGF supports angiogenesis through multiple mechanisms, targeting not only endothelial cells but also endothelial progenitor cells (Figure 4). In preclinical animal models for limb ischemia, intramuscular administration of either recombinant HGF protein or expression plasmid for HGF has facilitated collateral new vessel formation, improved blood flow, and reduced muscle atrophy [93,94]. Thus, HGF is a powerful angiogenic growth factor that is applicable for therapeutic purposes.
Figure 4.
Biological responses leading to angiogenesis driven by the HGF–Met pathway.
The first clinical study of HGF gene therapy by naked expression plasmid was done to investigate its safety for the treatment of patients with arteriosclerosis obliterans, or Berger’s disease [138]. Subsequently, a multicenter, randomized, double-blind, and placebo-controlled clinical trial was performed for the treatment of patients with critical limb ischemia to evaluate the efficacy and safety of HGF gene therapy using naked plasmid [139,140]. This HGF gene therapy was proven safe and effective for critical limb ischemia. Phase-II and phase-III clinical trials of HGF gene therapy for the treatment of peripheral arterial disease have been completed in the USA and in Japan, respectively.
5.3. Hepatitis and Acute Kidney Injury
Conditional Met disruption in hepatocytes indicated they were highly susceptible to apoptosis even in mild injury, and the regenerative response in the liver was retarded when mice were subjected to liver injury. In preclinical models for fulminant hepatitis, systemic administration of recombinant HGF suppressed the onset of fulminant hepatitis [141]. In a similar manner, conditional Met disruption in mature renal cells indicated mice had no appreciable defect in renal function, whereas they were susceptible to severe renal dysfunction and pathology when subjected to renal injury. In preclinical animal models for acute kidney injury, systemic administration of recombinant HGF suppressed tubular cell apoptosis and renal dysfunction, and promoted regenerative cell proliferation [141]. HGF protein may be applicable to the treatment of patients with acute hepatitis or acute kidney injury.
The phase-I clinical trial of the systemic administration of recombinant HGF protein has been completed in both Japan and the USA. In phase I/II clinical trials, intravenous administration of HGF protein was well tolerated by patients with fulminant hepatitis [142].
5.4. Amyotrophic Lateral Sclerosis (ALS)
ALS is a fatal neurodegenerative disease characterized by progressive loss of motor neurons and degeneration of motor axons. Approximately 5%–10% of patients have familial ALS, and of those ~15%–25% carry a mutation(s) in the gene encoding Cu2+/Zn2+ superoxide dismutase (SOD1). Neurons with transgenic expression of mutant SOD1 develop the typical deficits found in both familial and sporadic ALS. Because motoneuronal degeneration is thought to be a primary event in disease progression, treatment approaches have focused on promoting the survival, or at least preventing the death, of motor neurons. In addition to motor neurons, since a reduction in the astrocyte-specific glutamate transporter has been found in ALS patients, astrocytes also seem to be potential targets for ALS therapy.
As described, HGF exerts potent neurotrophic action to promote the survival of a variety of neurons, including motor neurons. The first implication that HGF might be a therapeutic agent for the treatment of patients with ALS was obtained by transgenic over-expression of HGF in the nervous system in mouse model for ALS with mutant SOD1 [143,144]. Neuronal overexpression of HGF has attenuated motor neuron death and axonal degeneration and prolonged the life span of ALS model mice. HGF expression retained the levels of the astrocyte-specific glutamate transporter in reactive astrocytes. Thus, HGF seems to alleviate the symptoms of ALS by direct neurotrophic activities on motor neurons and indirect activities on astrocytes, by reducing glutamatergic neurotoxicity.
Preclinical study to address the therapeutic action of HGF was done using rat model of ALS with mutant SOD1 [120]. In that study, recombinant HGF protein was administered locally into the medullary cavity. The continuous intrathecal administration of HGF attenuated motor neuron degeneration and prolonged the duration of the disease by 63%, even after administration from the onset of paralysis. The phase-I clinical trial for the intrathecal administration of recombinant HGF protein for the treatment of patients with ALS is ongoing at Tohoku University in Japan.
5.5. Spinal Cord Injury
Spinal cord injury (SCI) is followed by secondary degeneration, which is characterized by progressive tissue necrosis. Many therapeutic interventions using neurotrophic factors or pharmacological agents have focused on secondary degeneration after SCI to reduce damaged areas and promote axonal regeneration and functional recovery. The therapeutic action of recombinant HGF protein was tested in a primate (common marmoset) model of contusive cervical SCI [145]. Intrathecal HGF administration preserved the intact spinal cord parenchyma, corticospinal fibers, and myelinated areas, thereby promoting functional recovery. HGF-treatment did not give rise to an abnormal outgrowth of calcitonin gene-related peptide positive fibers compared with that seen in the control group, indicating that this treatment neither induced nor exacerbated allodynia. The phase-I/II clinical trial for the intrathecal administration of recombinant human HGF protein for the treatment of patients with SCI was initiated at Keio University in Japan.
6. Small-Molecule HGF-Inducers and Therapeutic Approaches
The gene expression of HGF is regulated by growth factors, cytokines, and prostaglandins. Among these, prostaglandin receptors are G-protein-coupled receptors from which different effectors and signaling pathways are evoked. EP2 and EP4 receptors for prostaglandin E1/E2 (PGE1/2) and prostacyclin receptor (also known as PGI2 receptor or IP receptor) for PGI2 activate adenylate cyclase upon ligand binding, thereby increasing intracellular cAMP levels. Prostaglandins and their analogs that activate EP2, EP4, and IP receptors induce transcriptional activation of HGF [146]. On the other hand, transgenic mice that overexpressed COX-2 in hepatocytes were resistant to liver injury [147]. Mice with disrupted cyclooxygenase-2 (COX-2) genes, which is a key gene for the production of prostaglandins, developed much more severe liver damage than wild-type mice in a hepatic injury model [148]. The results of these studies have implicated prostaglandins and prostaglandin analogs as being capable of activating EP2, EP4, or IP receptors, which may exert therapeutic action for pathology and injuries by enhancing endogenous HGF expression.
ONO-1301 was developed as a new type of PGI2/IP receptor agonist lacking the typical prostanoid structures [149]. PGI2 and its analogs are not stable in vivo, whereas ONO-1301 is chemically and biologically more stable than prostacyclin and its analogs because of the absence of prostanoid structures. The therapeutic actions of ONO-1301 and the involvement of HGF have been demonstrated in injury and pathology models in different tissues. In mice models of hepatotoxin-induced liver injury, the administration of ONO-1301 increased the hepatic expression of HGF and suppressed the hepatocyte death and the onset of liver injury [150]. However, the actions of ONO-1301 to suppress hepatocyte apoptosis and to expand necrotic areas were cancelled via the neutralization of endogenous HGF. These results indicate that ONO-1301 increases the expression of HGF and that the hepato-protective action of ONO-1301 against liver injury may be largely attributable to HGF.
ONO-1301 enhanced angiogenesis in the subcutaneous sponge discs of mice, whereas it was mostly inhibited via the neutralization of HGF [151]. In an obstructive nephropathy model, a single injection of sustained-release ONO-1301 suppressed fibrogenic gene expression and increased renal HGF expression, and this was associated with a suppression of interstitial fibrosis of the kidney [152]. These therapeutic effects of ONO-1301 were significantly cancelled via the neutralization of HGF. In a rat model of myosin-induced experimental autoimmune myocarditis (the heart transits from an acute inflammatory phase to a chronic dilated cardiomyopathy phase in this model), the oral administration of ONO-1301 increased capillary density in the myocardium and circulating endothelial progenitor cells, and improved hemodynamic functions, and these beneficial effects of ONO-1301 were partially abrogated via the neutralization of HGF [153]. In an experimental chronic asthma model, the subcutaneous administration of ONO-1301 increased pulmonary HGF expression, suppressed airway hyperresponsiveness, and improved airway remodeling/fibrotic change, while neutralization of HGF significantly abrogated the effects of ONO-1301 [154]. In a model of pulmonary arterial hypertension, the oral administration of ONO-1301 significantly attenuated the increases in right ventricular systolic pressure and the increases in the medial wall thickness of pulmonary arterioles [155]. The neutralization of HGF together with ONO-1301 cancelled the improvement in survival rates that had been achieved by ONO-1301 alone, which suggested the involvement of HGF in the therapeutic action of ONO-1301.
These studies using ONO-1301 PGI2/IP receptor agonist demonstrated the therapeutic approach of using a small molecule capable of inducing HGF, as a regenerative medicine. The use of such HGF-inducible small molecules seems to have an advantage in terms of versatility as a medical drug, including oral administration and a combination of drug delivery techniques.
7. Conclusions
Based on the close involvement of HGF and its receptor Met—not only in tumor development, invasion, and metastasis but also in resistance to anticancer therapies—drug discovery targeting the HGF–Met pathway has become a hot target in anticancer drug development [156,157,158,159,160]. On the other hand, based on studies using cell/tissue-specific disruption of functional Met and preclinical disease models in experimental animals, recombinant HGF proteins and HGF genes have become biological drug candidates for the treatment of patients with diseases marked by impaired tissue function [43,141]. Thus, both selective and appropriate inhibition and activation of the HGF–Met pathway are therapeutic approaches that are based on a scientific basis.
Growth factors and cytokines have profound biological and physiological activities, and several growth factors and cytokines have been used as medical drugs: insulin-like growth factor-1, granulocyte-colony stimulating factor, erythropoietin, interferon-γ, and fibroblast growth factor-2. These cytokines and growth factors exert a characteristic pharmaceutical efficacy, and therefore cannot be replaced by other small molecules and chemical compounds.
The characterization of mice with cell/tissue-selective disruption of the Met gene particularly revealed the irreplaceable role of the HGF–Met pathway in survival, regeneration, and homeostasis of cells/tissues, which thereby provides a rationale for the therapeutic use of HGF. An appropriate activation of the HGF–Met pathway is performed by different approaches, such as recombinant HGF proteins, HGF genes (expression vector for HGF genes), and small-molecule HGF inducers. These approaches have different advantages and characteristics for medical use. Because clinical development of the HGF gene and recombinant HGF protein as a regeneration-based drug candidate has progressed and is ongoing, proof-of-concept is expected to be established after further clinical trials.
Acknowledgments
The studies from the authors’ laboratories were supported by Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Science, Sports, and Technology of Japan (No. 24300329 to Kunio Matsumoto), Grant-in-Aid for Research on rare and intractable diseases, the Research Committee on Establishment of Novel Treatments for Amyotrophic Lateral Sclerosis, from the Ministry of Health, Labour and Welfare of Japan to Hiroshi Funakoshi, and a research grant for young investigators by Hokurik Bank to Katsuya Sakai.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 2.Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K., et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1989;163:967–973. doi: 10.1016/0006-291X(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 3.Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 4.Weidner K.M., Behrens J., van der Kerckhove J., Birchmeier W. Scatter factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottaro D.P., Rubin J.S., Faletto D.L., Chan A.-M.I., Kmiecik T.E., vande Woude G.F., Aaronson S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 6.Naldini L., Vigna E., Narcimham R.P., Gaudino G., Zarnegar R., Michalopoulos G.K., Comoglio P.M. Hepatocyte growth factor stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 7.Kataoka H., Miyata S., Uchinokura S., Itoh H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003;22:223–236. doi: 10.1023/A:1023051500010. [DOI] [PubMed] [Google Scholar]
- 8.Parr C., Jiang W.G. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int. J. Cancer. 2006;119:1176–1183. doi: 10.1002/ijc.21881. [DOI] [PubMed] [Google Scholar]
- 9.Park M., Dean M., Kaul K., Braun M.J., Gonda M.A., vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gherardi E., Sandin S., Petoukhov M.V., Finch J., Youles M.E., Ofverstedt L.G., Miguel R.N., Blundell T.L., vande Woude G.F., Skoglund U., et al. Structural basis of hepatocyte growth factor/scatter factor and MET signaling. Proc. Natl. Acad. Sci. USA. 2006;103:4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basilico C., Arnesano A., Galluzzo M., Comoglio P.M., Michieli P. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J. Biol. Chem. 2008;283:21267–21277. doi: 10.1074/jbc.M800727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidner K.M., di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 13.Sachs M., Brohmann H., Zechner D., Müller T., Hülsken J., Walther I., Schaeper U., Birchmeier C., Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am. J. Physiol. 1999;277:F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Ocana A., Takane K.K., Reddy V.T., Lopez-Talavera J.C., Vasavada R.C., Stewart A.F. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces β-cell death. J. Biol. Chem. 2003;278:343–351. doi: 10.1074/jbc.M207848200. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., DeFrances M.C., Dai Y., Pediaditakis P., Johnson C., Bell A., Michalopoulos G.K., Zarnegar R. A mechanism of cell survival: Sequestration of Fas by the HGF receptor Met. Mol. Cell. 2002;9:411–421. doi: 10.1016/S1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 17.Peschard P., Fournier T.M., Lamorte L., Naujokas M.A., Band H., Langdon W.Y., Park M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell. 2001;8:995–1004. doi: 10.1016/S1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 18.Hashigasako A., Machide M., Nakamura T., Matsumoto K., Nakamura T. Bi-directional regulation of Ser-985 phosphorylation of c-met via protein kinase-C and protein phosphatase 2A involves c-Met activation and cellular responsiveness to hepatocyte growth factor. J. Biol. Chem. 2004;279:26445–26452. doi: 10.1074/jbc.M314254200. [DOI] [PubMed] [Google Scholar]
- 19.Uehara Y., Minowa O., Mori C., Shiota K., Kuno J., Noda T., Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E., Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 21.Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 22.Huh C.G., Factor V.M., Sanchez A., Uchida K., Conner E.A., Thorgeirsson S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowiak M., Garratt A.N., Wustefeld T., Strehle M., Trautwein C., Birchmeier C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Factor V.M., Seo D., Ishikawa T., Kaposi-Novak P., Marquardt J.U., Andersen J.B., Conner E.A., Thorgeirsson S.S. Loss of c-Met disrupts gene expression program required for G2/M progression during liver regeneration in mice. PLoS One. 2010;5:e12739. doi: 10.1371/journal.pone.0012739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giebeler A., Boekschoten M.V., Klein C., Borowiak M., Birchmeier C., Gassler N., Wasmuth H.E., Müller M., Trautwein C., Streetz K.L. c-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in mice. Gastroenterology. 2009;137:297–308. doi: 10.1053/j.gastro.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 26.Marquardt J.U., Seo D., Gómez-Quiroz L.E., Uchida K., Gillen M.C., Kitade M., Kaposi-Novak P., Conner E.A., Factor V.M., Thorgeirsson S.S. Loss of c-Met accelerates development of liver fibrosis in response to CCl4 exposure through deregulation of multiple molecular pathways. Biochim. Biophys. Acta. 2012;1822:942–951. doi: 10.1016/j.bbadis.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Castillo G., Factor V.M., Fernández M., Alvarez-Barrientos A., Fabregat I., Thorgeirsson S.S., Sánchez A. Deletion of the Met tyrosine kinase in liver progenitor oval cells increases sensitivity to apoptosis in vitro. Am. J. Pathol. 2008;172:1238–1247. doi: 10.2353/ajpath.2008.070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa T., Factor V.M., Marquardt J.U., Raggi C., Seo D., Kitade M., Conner E.A., Thorgeirsson S.S. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D., Tan R.J., Lin L., Zhou L., Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai C., Saleem M.A., Holzman L.B., Mathieson P., Liu Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010;77:962–973. doi: 10.1038/ki.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma H., Saenko M., Opuko A., Togawa A., Soda K., Marlier A., Moeckel G.W., Cantley L.G., Ishibe S. Deletion of the Met receptor in the collecting duct decreases renal repair following ureteral obstruction. Kidney Int. 2009;76:868–876. doi: 10.1038/ki.2009.304. [DOI] [PubMed] [Google Scholar]
- 32.Ishibe S., Karihaloo A., Ma H., Zhang J., Marlier A., Mitobe M., Togawa A., Schmitt R., Czyczk J., Kashgarian M., et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337–345. doi: 10.1242/dev.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chmielowiec J., Borowiak M., Morkel M., Stradal T., Munz B., Werner S., Wehland J., Birchmeier C., Birchmeier W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai C., Huh C.G., Thorgeirsson S.S., Liu Y. β-Cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am. J. Pathol. 2005;167:429–436. doi: 10.1016/S0002-9440(10)62987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roccisana J., Reddy V., Vasavada R.C., Gonzalez-Pertusa J.A., Magnuson M.A., Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in β-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of β-cell mass. Diabetes. 2005;54:2090–2102. doi: 10.2337/diabetes.54.7.2090. [DOI] [PubMed] [Google Scholar]
- 36.Mellado-Gil J., Rosa T.C., Demirci C., Gonzalez-Pertusa J.A., Velazquez-Garcia S., Ernst S., Valle S., Vasavada R.C., Stewart A.F., Alonso L.C., et al. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic β-cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525–536. doi: 10.2337/db09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins G.J., Shahrokh M., Powell E.M. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience. 2011;176:199–209. doi: 10.1016/j.neuroscience.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith J.M., Xu J., Powell E.M. Age dependent forebrain structural changes in mice deficient in the autism associated gene Met tyrosine kinase. Neuroimage Clin. 2012;1:66–74. doi: 10.1016/j.nicl.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judson M.C., Eagleson K.L., Wang L., Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J. Comp. Neurol. 2010;518:4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu S., Anderson C.T., Levitt P., Shepherd G.M. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J. Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arechederra M., Carmona R., González-Nuñez M., Gutiérrez-Uzquiza A., Bragado P., Cruz-González I., Cano E., Guerrero C., Sánchez A., López-Novoa J.M., et al. Met signaling in cardiomyocytes is required for normal cardiac function in adult mice. Biochim. Biophys. Acta. 2013;1832:2204–2215. doi: 10.1016/j.bbadis.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Baek J.H., Birchmeier C., Zenke M., Hieronymus T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J. Immunol. 2012;189:1699–1707. doi: 10.4049/jimmunol.1200729. [DOI] [PubMed] [Google Scholar]
- 43.Funakoshi F., Nakamura T. Hepatocyte growth factor (HGF): Neurotrophic functions and therapeutic implications for neuronal injury/diseases. Curr. Signal. Transduct. Ther. 2011;6:156–167. doi: 10.2174/157436211795659982. [DOI] [Google Scholar]
- 44.Ohya W., Funakoshi H., Kurosawa T., Nakamura T. Hepatocyte growth factor (HGF) promotes oligodendrocyte progenitor cell proliferation and inhibits its differentiation during postnatal development in the rat. Brain Res. 2007;1147:51–65. doi: 10.1016/j.brainres.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 45.Campbell D.B., Sutcliffe J.S., Ebert P.J., Militerni R., Bravaccio C., Trillo S., Elia M., Schneider C., Melmed R., Sacco R., et al. A genetic variant that disrupts MET transcription is associated with autism. Proc. Natl. Acad. Sci. USA. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell D.B., Li C., Sutcliffe J.S., Persico A.M., Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa I., Clark T.G., Toma C., Kobayashi K., Choma M., Holt R., Sykes N.H., Lamb J.A., Bailey A.J., Battaglia A., et al. MET and autism susceptibility: Family and case-control studies. Eur. J. Hum. Genet. 2009;17:749–758. doi: 10.1038/ejhg.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell E.M., Mars W.M., Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/S0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 49.Powell E.M., Campbell D.B., Stanwood G.D., Davis C., Noebels J.L., Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ieraci A., Forni P.E., Ponzetto C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proc. Natl. Acad. Sci. USA. 2002;99:15200–15205. doi: 10.1073/pnas.222362099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmen S.J., van Engeland H., Hof P.R., Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 52.Courchesne E., Redcay E., Kennedy D.P. The autistic brain: Birth through adulthood. Curr. Opin. Neurol. 2004;17:489–496. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- 53.Burdick K.E., DeRosse P., Kane J.M., Lencz T., Malhotra A.K. Association of genetic variation in the MET proto-oncogene with schizophrenia and general cognitive ability. Am. J. Psychiatr. 2010;167:436–443. doi: 10.1176/appi.ajp.2009.09050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz J.M., Khan S.N., Ahmed Z.M., Riazuddin S., Waryah A.M., Chhatre D., Starost M.F., Ploplis B., Buckley S., Velásquez D., et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am. J. Hum. Genet. 2009;85:25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura T., Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. B. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutella S., Bonanno G., Procoli A., Mariotti A., de Ritis D.G., Curti A., Danese S., Pessina G., Pandolfi S., Natoni F., et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12l°w/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 57.Benkhoucha M., Santiago-Raber M.L., Schneiter G., Chofflon M., Funakoshi H., Nakamura T., Lalive P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishiki Y., Ohnishi H., Muto Y., Matsumoto K., Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and for potent antihepatitis effect in vivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- 59.Roos F., Terrell T.G., Godowski P.J., Chamow S.M., Schwall R.H. Reduction of α-naphthylisothiocyanate-induced hepatotoxicity by recombinant human hepatocyte growth factor. Endocrinology. 1992;131:2540–2544. doi: 10.1210/endo.131.6.1446596. [DOI] [PubMed] [Google Scholar]
- 60.Masunaga H., Fujise N., Shiota A., Ogawa H., Sato Y., Imai E., Yasuda H., Higashio K. Preventive effects of the deleted form of hepatocyte growth factor against various liver injuries. Eur. J. Pharmacol. 1998;342:267–279. doi: 10.1016/S0014-2999(97)01485-4. [DOI] [PubMed] [Google Scholar]
- 61.Sakakura Y., Kaibori M., Oda M., Okumura T., Kwon A.H., Kamiyama Y. Recombinant human hepatocyte growth factor protects the liver against hepatic ischemia and reperfusion injury in rats. J. Surg. Res. 2000;92:261–266. doi: 10.1006/jsre.2000.5913. [DOI] [PubMed] [Google Scholar]
- 62.Oe S., Hiros T., Fujii H., Yasuchika K., Nishio T., Iimuro Y., Morimoto T., Nagao M., Yamaoka Y. Continuous intravenous infusion of deleted form of hepatocyte growth factor attenuates hepatic ischemia-reperfusion injury in rats. J. Hepatol. 2001;34:832–839. doi: 10.1016/S0168-8278(01)00030-7. [DOI] [PubMed] [Google Scholar]
- 63.Yoshikawa A., Kaido T., Seto S., Yamaoka S., Sato M., Ishii T., Imamura M. Hepatocyte growth factor promotes liver regeneration with prompt improvement of hyperbilirubinemia in hepatectomized cholestatic rats. J. Surg. Res. 1998;78:54–59. doi: 10.1006/jsre.1998.5350. [DOI] [PubMed] [Google Scholar]
- 64.Li Z., Mizuno S., Nakamura T. Antinecrotic and antiapoptotic effects of hepatocyte growth factor on cholestatic hepatitis in a mouse model of bile-obstructive diseases. Am. J. Physiol. 2007;292:G639–G646. doi: 10.1152/ajpcell.00204.2006. [DOI] [PubMed] [Google Scholar]
- 65.Kosai K., Matsumoto K., Nagata S., Tsujimoto Y., Nakamura T. Hepatocyte growth factor abrogates Fas-induced lethal liver apoptosis in mice. Biochem. Biophys. Res. Commun. 1998;244:683–690. doi: 10.1006/bbrc.1998.8293. [DOI] [PubMed] [Google Scholar]
- 66.Kosai K., Matsumoto K., Nakamura T. Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. Hepatology. 1999;30:151–159. doi: 10.1002/hep.510300102. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda Y., Matsumoto K., Ichida T., Nakamura T. Hepatocyte growth factor prevents liver cirrhosis caused by dimethylnitrosamine in rats. J. Biochem. 1995;118:643–649. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 68.Matsuda Y., Matsumoto K., Yamada A., Ichida T., Asakura H., Komoriya K., Nishiyama E., Nakamura T. Preventive and therapeutic effects in rat of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology. 1997;26:81–89. doi: 10.1002/hep.510260111. [DOI] [PubMed] [Google Scholar]
- 69.Oe S., Fukunaka Y., Hirose T., Yamaoka Y., Tabata Y. A trial on regeneration therapy of rat liver cirrhosis by controlled release of hepatocyte growth factor. J. Control. Release. 2003;88:193–200. doi: 10.1016/S0168-3659(02)00463-7. [DOI] [PubMed] [Google Scholar]
- 70.Kim W.H., Matsumoto K., Bessho K., Nakamura T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am. J. Pathol. 2005;166:1017–1028. doi: 10.1016/S0002-9440(10)62323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusumoto K., Ido A., Moriuchi A., Katsura T., Kim I., Takahama Y., Numata M., Kodama M., Hasuike S., Nagata K., Uto H., Inui K., Tsubouchi H. Repeated intravenous injection of recombinant human hepatocyte growth factor ameliorates liver cirrhosis but causes albuminuria in rats. Int. J. Mol. Med. 2006;17:503–509. [PubMed] [Google Scholar]
- 72.Kaido T., Yoshikawa A., Seto S., Yamaoka S., Sato M., Ishii T., Imamura M. Portal branch ligation with a continuous hepatocyte growth factor supply makes extensive hepatectomy possible in cirrhotic rats. Hepatology. 1998;28:756–760. doi: 10.1002/hep.510280323. [DOI] [PubMed] [Google Scholar]
- 73.Tahara M., Matsumoto K., Nukiwa T., Nakamura T. Hepatocyte growth factor leads to recovery from alcohol-induced fatty liver in rats. J. Clin. Investig. 1999;103:313–320. doi: 10.1172/JCI4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tahara Y., Ido A., Yamamoto S., Miyata Y., Uto H., Hori T., Hayashi K., Tsubouchi H. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J. Pharmacol. Exp. Ther. 2003;307:146–151. doi: 10.1124/jpet.103.054106. [DOI] [PubMed] [Google Scholar]
- 75.Numata M., Ido A., Moriuchi A., Kim I., Tahara Y., Yamamoto S., Hasuike S., Nagata K., Miyata Y., Uto H., Tsubouchi H. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm. Bowel Dis. 2005;11:551–558. doi: 10.1097/01.MIB.0000164192.71381.5c. [DOI] [PubMed] [Google Scholar]
- 76.Schmassmann A., Stettler C., Poulsom R., Tarasova N., Hirschi C., Flogerzi B., Matsumoto K., Nakamura T., Halter F. Roles of hepatocyte growth factor and receptor c-Met during gastric ulcer healing in rats. Gastroenterology. 1997;113:1858–1872. doi: 10.1016/S0016-5085(97)70005-2. [DOI] [PubMed] [Google Scholar]
- 77.Nakahira R., Mizuno S., Yoshimine T., Nakamura T. The loss of local HGF, an endogenous gastrotrophic factor, leads to mucosal injuries in the stomach of mice. Biochem. Biophys. Res. Commun. 2006;341:897–903. doi: 10.1016/j.bbrc.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 78.Kawaida K., Matsumoto K., Shimazu H., Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerate renal regeneration in mice. Proc. Natl. Acad. Sci. USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller S.B., Martin D.R., Kissane J., Hammerman M.R. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am. J. Physiol. 1994;266:F129–F134. doi: 10.1152/ajprenal.1994.266.1.F129. [DOI] [PubMed] [Google Scholar]
- 80.Amaike H., Matsumoto K., Oka T., Nakamura T. Preventive effect of hepatocyte growth factor on acute side effects of cyclosporin A in mice. Cytokine. 1996;8:387–394. doi: 10.1006/cyto.1996.0053. [DOI] [PubMed] [Google Scholar]
- 81.Yamasaki N., Nagano T., Mori-Kudo I., Tsuchida A., Kawamura T., Seki H., Taiji M., Noguchi H. Hepatocyte growth factor protects functional and histological disorders of HgCl2-induced acute renal failure mice. Nephron. 2002;90:195–205. doi: 10.1159/000049042. [DOI] [PubMed] [Google Scholar]
- 82.Nagano T., Mori-Kudo I., Tsuchida A., Kawamura T., Taiji M., Noguchi H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron. 2002;91:730–738. doi: 10.1159/000065037. [DOI] [PubMed] [Google Scholar]
- 83.Takada S., Takahara S., Nishimura K., Ichimaru N., Hongsi J., Kokado Y., Matsumiya K., Matsumoto K., Nakamura T., Okuyama A. Effect of hepatocyte growth factor on tacrolimus-induced nephrotoxicity in spontaneously hypertensive rats. Transplant. Intern. 1999;12:27–32. doi: 10.1111/j.1432-2277.1999.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 84.Gong R., Rifai A., Dworkin L.D. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney Int. 2006;69:1166–1174. doi: 10.1038/sj.ki.5000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamimoto M., Mizuno S., Matsumoto K., Nakamura T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem. Biophys. Res. Commun. 2009;380:333–337. doi: 10.1016/j.bbrc.2009.01.080. [DOI] [PubMed] [Google Scholar]
- 86.Mizuno S., Nakamura T. Suppressions of chronic glomerular injuries and TGF-β1 production by HGF in attenuation of murine diabetic nephropathy. Am. J. Physiol. 2004;286:F134–F143. doi: 10.1152/ajprenal.00199.2003. [DOI] [PubMed] [Google Scholar]
- 87.Mizuno S., Kurosawa T., Matsumoto K., Mizuno-Horikawa Y., Okamoto M., Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Investig. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong R., Rifai A., Dworkin L.D. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J. Am. Soc. Nephrol. 2006;17:2464–2473. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- 89.Mizuno S., Matsumoto K., Wen J., Nakamura T. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2001;59:1304–1314. doi: 10.1046/j.1523-1755.2001.0590041304.x. [DOI] [PubMed] [Google Scholar]
- 90.Yang J., Liu Y. Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am. J. Physiol. 2003;284:F349–F357. doi: 10.1152/ajpcell.00066.2002. [DOI] [PubMed] [Google Scholar]
- 91.Bessho K., Mizuno S., Matsumoto K., Nakamura T. Counteractive effects of HGF on PDGF-induced mesangial cell proliferation in a rat model of glomerulonephritis. Am. J. Physiol. 2003;284:F1171–F1180. doi: 10.1152/ajprenal.00326.2002. [DOI] [PubMed] [Google Scholar]
- 92.Azuma H., Takahara S., Matsumoto K., Ichimaru N., Wang J., Moriyama T., Wagga A., Kitamura M., Otsuki Y., Okuyama A., et al. Hepatocyte growth factor prevents development of chronic allograft nephropathy in an experimental rat transplant model. J. Am. Soc. Nephrol. 2001;12:1280–1292. doi: 10.1681/ASN.V1261280. [DOI] [PubMed] [Google Scholar]
- 93.Van Belle E., Witzenbichler B., Chen D., Silver M., Chang L., Schwall R., Isner J.M. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: The case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.CIR.97.4.381. [DOI] [PubMed] [Google Scholar]
- 94.Morishita R., Nakamura S., Hayashi S., Taniyama Y., Moriguchi A., Nagano T., Taiji M., Noguchi H., Takeshita S., Matsumoto K., et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.HYP.33.6.1379. [DOI] [PubMed] [Google Scholar]
- 95.Marui A., Kanematsu A., Yamahara K., Doi K., Kushibiki T., Yamamoto M., Itoh H., Ikeda T., Tabata Y., Komeda M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J. Vasc. Surg. 2005;41:82–90. doi: 10.1016/j.jvs.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 96.Yasuda S., Noguchi T., Gohda M., Arai T., Tsutsui N., Matsuda T., Nonogi H. Single low-dose administration of human recombinant hepatocyte growth factor attenuates intimal hyperplasia in a balloon-injured rabbit iliac artery model. Circulation. 2000;101:2546–2549. doi: 10.1161/01.CIR.101.21.2546. [DOI] [PubMed] [Google Scholar]
- 97.Yamaguchi T., Sawa Y., Miyamoto Y., Takahashi T., Jau C.C., Ahmet I., Nakamura T., Matsuda H. Therapeutic angiogenesis induced by injecting hepatocyte growth factor in ischemic canine hearts. Surg. Today. 2005;35:855–860. doi: 10.1007/s00595-005-3042-3. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura T., Mizuno S., Matsumoto K., Sawa Y., Matsuda H., Nakamura T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Investig. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin H., Yang R., Li W., Ogasawara A.K., Schwall R., Eberhard D.A., Zheng Z., Kahn D., Paoni N.F. Early treatment with hepatocyte growth factor improves cardiac function in experimental heart failure induced by myocardial infarction. J. Pharmacol. Exp. Ther. 2003;304:54–60. doi: 10.1124/jpet.102.041772. [DOI] [PubMed] [Google Scholar]
- 100.Yamaura K., Ito K., Tsukioka K., Wada Y., Makiuchi A., Sakaguchi M., Akashima T., Fujimori M., Sawa Y., Morishita R., et al. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: Induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation. 2004;110:1650–1657. doi: 10.1161/01.CIR.0000143052.45956.71. [DOI] [PubMed] [Google Scholar]
- 101.Nakamura T., Matsumoto K., Mizuno S., Sawa Y., Matsuda H., Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am. J. Physiol. 2005;288:H2131–H2139. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
- 102.Ohmichi H., Matsumoto K., Nakamura T. In vivo mitogenic action of hepatocyte growth factor on lung epithelial cells: Pulmotrophic role in lung regeneration. Am. J. Physiol. 1996;270:L1031–L1039. doi: 10.1152/ajplung.1996.270.6.L1031. [DOI] [PubMed] [Google Scholar]
- 103.Makiuchi A., Yamaura K., Mizuno S., Matsumoto K., Nakamura T., Amano J., Ito K. Hepatocyte growth factor prevents pulmonary ischemia-reperfusion injury in mice. J. Heart Lung Transplant. 2007;26:935–943. doi: 10.1016/j.healun.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Yaekashiwa M., Nakayama S., Ohnuma K., Sakai T., Abe T., Satoh K., Matsumoto K., Nakamura T., Takahashi T., Nukiwa T. Simultaneous or delayed administration of hepatocyte growth factor (HGF) equally repress the fibrotic change in murine lung injury induced by bleomycin: A morphologic study. Am. J. Resp. Crit. Care Med. 1997;156:1937–1944. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- 105.Dohi M., Hasegawa T., Yamamoto K., Marshall B.C. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000;162:2302–2307. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- 106.Mizuno S., Matsumoto K., Li M.Y., Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 107.Ishizawa K., Kubo H., Yamada M., Kobayashi S., Suzuki T., Mizuno S., Nakamura T., Sasaki H. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem. Biophys. Res. Commun. 2004;324:276–280. doi: 10.1016/j.bbrc.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 108.Sakamaki Y., Matsumoto K., Mizuno S., Miyoshi S., Matsuda H., Nakamura T. Hepatocyte growth factor stimulates proliferation of respiratory epithelial cells during postneumonectomy compensatory lung growth in mice. Am. J. Respir. Cell Mol. Biol. 2002;26:525–533. doi: 10.1165/ajrcmb.26.5.4714. [DOI] [PubMed] [Google Scholar]
- 109.Ito W., Kanehiro A., Matsumoto K., Hirano A., Ono K., Maruyama H., Kataoka M., Nakamura T., Gelfand E.W., Tanimoto M. Hepatocyte growth factor attenuates airway hyperresponsiveness, inflammation, and remodeling. Am. J. Respir. Cell Mol. Biol. 2005;32:268–280. doi: 10.1165/rcmb.2004-0058OC. [DOI] [PubMed] [Google Scholar]
- 110.Ohno T., Hirano S., Kanemaru S., Yamashita M., Umeda H., Suehiro A., Tamura Y., Nakamura T., Ito J., Tabata Y. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann. Otol. Rhinol. Laryngol. 2007;116:762–769. doi: 10.1177/000348940711601008. [DOI] [PubMed] [Google Scholar]
- 111.Kishimoto Y., Hirano S., Kitani Y., Suehiro A., Umeda H., Tateya I., Kanemaru S., Tabata Y., Ito J. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope. 2010;120:108–113. doi: 10.1002/lary.21016. [DOI] [PubMed] [Google Scholar]
- 112.Bevan D., Gherardi E., Fan T.P., Edwards D., Warn R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004;203:831–838. doi: 10.1002/path.1578. [DOI] [PubMed] [Google Scholar]
- 113.Yoshida S., Matsumoto K., Tomioka D., Bessho K., Itami S., Yoshikawa K., Nakamura T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors. 2004;22:111–119. doi: 10.1080/08977190410001701005. [DOI] [PubMed] [Google Scholar]
- 114.Miyazawa T., Matsumoto K., Ohmichi H., Yamashima T., Chigasaki H., Nakamura T. Protection of hippocampal neurons from ischemia-induced delayed neuronal death by hepatocyte growth factor: A novel neurotrophic factor. J. Celebral. Blood Flow Metab. 1998;18:345–348. doi: 10.1097/00004647-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 115.Tsuzuki N., Miyazawa T., Matsumoto K., Nakamura T., Shima K. Hepatocyte growth factor reduces the infarct volume after transient focal cerebral ischemia in rats. Neurol. Res. 2001;23:417–424. doi: 10.1179/016164101101198659. [DOI] [PubMed] [Google Scholar]
- 116.Date I., Takagi N., Takagi K., Kago T., Matsumoto K., Nakamura T., Takeo S. Hepatocyte growth factor improved learning and memory dysfunction of microsphere-embolized rats. J. Neurosci. Res. 2004;78:442–453. doi: 10.1002/jnr.20263. [DOI] [PubMed] [Google Scholar]
- 117.Niimura M., Takagi N., Takagi K., Mizutani R., Tanonaka K., Funakoshi H., Matsumoto K., Nakamura T., Takeo S. The protective effect of hepatocyte growth factor against cell death in the hippocampus after transient forebrain ischemia is related to the improvement of apurinic/apyrimidinic endonuclease/redox factor-1 level and inhibition of NADPH oxidase activity. Neurosci. Lett. 2006;407:136–140. doi: 10.1016/j.neulet.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 118.Shang J., Deguchi K., Yamashita T., Ohta Y., Zhang H., Morimoto N., Liu N., Zhang X., Tian F., Matsuura T., et al. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J. Neurosci. Res. 2010;88:2197–2206. doi: 10.1002/jnr.22373. [DOI] [PubMed] [Google Scholar]
- 119.Okura Y., Arimoto H., Tamura N., Matsumoto K., Nakamura T., Yamashita T., Miyazawa T., Matsumoto Y. Analysis of neurotrophic effects of hepatocyte growth factor in the adult hypoglossal nerve axotomy model. Eur. J. Neurosci. 1999;11:4130–4144. doi: 10.1046/j.1460-9568.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 120.Ishigaki A., Aoki M., Nagai M., Warita H., Kato S., Kato M., Nakamura T., Funakoshi H., Itoyama Y. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J. Neuropathol. Exp. Neurol. 2007;66:1037–1044. doi: 10.1097/nen.0b013e318159886b. [DOI] [PubMed] [Google Scholar]
- 121.Tada T., Zhan H., Tanaka Y., Hongo K., Matsumoto K., Nakamura T. Intraventricular administration of hepatocyte growth factor treats mouse communicating hydrocephalus induced by transforming growth factor-b1. Neurobiol. Disease. 2006;21:576–586. doi: 10.1016/j.nbd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Shibuki H., Katai N., Kuroiwa S., Kurokawa T., Arai J., Matsumoto K., Nakamura T., Yoshimura N. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2002;43:528–536. [PubMed] [Google Scholar]
- 123.Ohtaka K., Machida S., Ohzeki T., Tanaka M., Kurosaka D., Masuda T., Ishii T. Protective effect of hepatocyte growth factor against degeneration of the retinal pigment epithelium and photoreceptor in sodium iodate-injected rats. Curr. Eye Res. 2006;31:347–355. doi: 10.1080/02713680600629797. [DOI] [PubMed] [Google Scholar]
- 124.Machida S., Tanaka M., Ishii T., Ohtaka K., Takahashi T., Tazawa Y. Neuroprotective effect of hepatocyte growth factor against photoreceptor degeneration in rats. Investig. Ophthalmol. Vis. Sci. 2004;45:4174–4182. doi: 10.1167/iovs.04-0455. [DOI] [PubMed] [Google Scholar]
- 125.Inaoka T., Nakagawa T., Kikkawa Y.S., Tabata Y., Ono K., Yoshida M., Tsubouchi H., Ido A., Ito J. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2009;129:453–457. doi: 10.1080/00016480902725197. [DOI] [PubMed] [Google Scholar]
- 126.Wakitani S., Imoto T., Kimura T., Ochi T., Matsumoto K., Nakamura T. Hepatocyte growth factor facilitates cartilage repair: full thickness articular cartilage defect studied in rabbit knee. Acta Orthop. Stand. 1997;68:474–480. doi: 10.3109/17453679708996266. [DOI] [PubMed] [Google Scholar]
- 127.Miller K.J., Thaloor D., Matteson S., Pavlath G.K. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. 2000;278:C174–C181. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- 128.Okunishi K., Dohi M., Nakagome K., Tanaka R., Mizuno S., Matsumoto K., Miyazaki J., Nakamura T., Yamamoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 129.Nakase J., Kitaoka K., Matsumoto K., Tomita K. Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy. 2010;26:84–90. doi: 10.1016/j.arthro.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 130.Noma S., Ohya-Shimada W., Kanai M., Ueda K., Nakamura T., Funakoshi H. Overexpression of HGF attenuates the degeneration of Purkinje cells and Bergmann glia in a knockin mouse model of spinocerebellar ataxia type 7. Neurosci. Res. 2012;773:115–121. doi: 10.1016/j.neures.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 131.Bai L., Lennon D.P., Caplan A.I., DeChant A., Hecker L., Kranso J., Zaremba A., Miller R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benkhoucha M., Molnarfi N., Dunand-Sauthier I., Merkler D., Schneiter G., Bruscoli S., Riccardi C., Tabata Y., Funakoshi H., Nakamura T., et al. Hepatocyte growth factor limits autoimmune neuroinflammation via GILZ expression in dendritic cells. J. Immunol. 2014;193:2743–2752. doi: 10.4049/jimmunol.1302338. [DOI] [PubMed] [Google Scholar]
- 133.Buchstein N., Hoffmann D., Smola H., Lang S., Paulsson M., Niemann C., Krieg T., Eming S.A. Alternative proteolytic processing of hepatocyte growth factor during wound repair. Am. J. Pathol. 2009;174:2116–2128. doi: 10.2353/ajpath.2009.080597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nayeri F., Strömberg T., Larsson M., Brudin L., Söderström C., Forsberg P. Hepatocyte growth factor may accelerate healing in chronic leg ulcers: A pilot study. J. Dermatol. Treat. 2002;13:81–86. doi: 10.1080/095466302317584449. [DOI] [PubMed] [Google Scholar]
- 135.Rosen E.M., Jaken S., Carley W., Luckett P.M., Setter E., Bhargava M., Goldberg I.D. Regulation of motility in bovine brain endothelial cells. J. Cell Physiol. 1991;146:325–335. doi: 10.1002/jcp.1041460218. [DOI] [PubMed] [Google Scholar]
- 136.Morimoto A., Okamura K., Hamanaka R., Sato Y., Shima N., Higashio K., Kuwano M. Hepatocyte growth factor modulates migration and proliferation of human microvascular endothelial cells in culture. Biochem. Biophys. Res. Commun. 1991;179:1042–1049. doi: 10.1016/0006-291X(91)91924-2. [DOI] [PubMed] [Google Scholar]
- 137.Bussolino F., di Renzo M.F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morishita R., Aoki M., Hashiya N., Makino H., Yamasaki K., Azuma J., Sawa Y., Matsuda H., Kaneda Y., Ogihara T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 139.Shigematsu H., Yasuda K., Iwai T., Sasajima T., Ishimaru S., Ohashi Y., Yamaguchi T., Ogihara T., Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 140.Shigematsu H., Yasuda K., Sasajima T., Takano T., Miyata T., Ohta T., Tanemoto K., Obitsu Y., Iwai T., Ozaki S., et al. Transfection of human HGF plasmid DNA improves limb salvage in Buerger’s disease patients with critical limb ischemia. Int. Angiol. 2011;30:140–149. [PubMed] [Google Scholar]
- 141.Nakamura T., Sakai K., Nakamura T., Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011;26:188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 142.Ido A., Moriuchi A., Numata M., Murayama T., Teramukai S., Marusawa H., Yamaji N., Setoyama H., Kim I.D., Chiba T., et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: A phase I/II clinical trial, following preclinical studies to ensure safety. J. Transl. Med. 2011;9:55. doi: 10.1186/1479-5876-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun W., Funakoshi H., Nakamura T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 2002;22:6537–6548. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kadoyama K., Funakoshi H., Ohya W., Nakamura T. Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci. Res. 2007;59:446–456. doi: 10.1016/j.neures.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Kitamura K., Fujiyoshi K., Yamane J., Toyota F., Hikishima K., Nomura T., Funakoshi H., Nakamura T., Aoki M., Toyama Y., et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011;6:e27706. doi: 10.1371/journal.pone.0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matsumoto K., Okazaki H., Nakamura T. Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J. Biochem. 1995;117:458–464. doi: 10.1093/jb/117.2.458. [DOI] [PubMed] [Google Scholar]
- 147.Casado M., Mollá B., Roy R., Fernández-Martínez A., Cucarella C., Mayoral R., Boscá L., Martín-Sanz P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 148.Yin H., Cheng L., Langenbach R., Ju C. Prostaglandin I2 and E2 mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45:159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi K., Nagamatsu T., Oka T., Suzuki Y. Modulation of anti-glomerular basement membrane nephritis in rats by ONO-1301, a non-prostanoid prostaglandin I2 mimetic compound with inhibitory activity against thromboxane A2 synthase. Jpn. J. Pharmacol. 1997;73:73–82. doi: 10.1254/jjp.73.73. [DOI] [PubMed] [Google Scholar]
- 150.Xu Q., Nakayama M., Suzuki Y., Sakai K., Nakamura T., Sakai Y., Matsumoto K. Suppression of acute hepatic injury by a synthetic prostacyclin agonist through hepatocyte growth factor expression. Am. J. Physiol. 2012;302:G420–G429. doi: 10.1152/ajpgi.00216.2011. [DOI] [PubMed] [Google Scholar]
- 151.Uchida T., Hazekawa M., Yoshida M., Matsumoto K., Sakai Y. A novel long-acting prostacyclin agonist (ONO-1301) with an angiogenic effect: Promoting synthesis of hepatocyte growth factor and increasing cyclic AMP concentration via IP receptor signaling. J. Pharmacol. Sci. 2013;123:392–401. doi: 10.1254/jphs.13073FP. [DOI] [PubMed] [Google Scholar]
- 152.Nasu T., Kinomura M., Tanabe K., Yamasaki H., Htay S.L., Saito D., Hinamoto N., Watatani H., Ujike H., Suzuki Y., et al. A sustained-release prostacyclin analog ONO-1301 ameliorates tubulointerstitial alterations in a mouse obstructive nephropathy model. Am. J. Physiol. Renal Physiol. 2012;302:F1616–F1629. doi: 10.1152/ajprenal.00538.2011. [DOI] [PubMed] [Google Scholar]
- 153.Hirata Y., Kurobe H., Uematsu E., Yagi S., Soeki T., Yamada H., Fukuda D., Shimabukuro M., Nakayama M., Matsumoto K., et al. Beneficial effect of a synthetic prostacyclin agonist, ONO-1301, in rat autoimmune myocarditis model. Eur. J. Pharmacol. 2013;699:81–87. doi: 10.1016/j.ejphar.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 154.Yamabayashi C., Koya T., Kagamu H., Kawakami H., Kimura Y., Furukawa T., Sakagami T., Hasegawa T., Sakai Y., Matsumoto K., et al. A novel prostacyclin agonist protects to airway hyperresponsiveness and remodeling in mice. Am. J. Respir. Cell Mol. Biol. 2012;47:170–177. doi: 10.1165/rcmb.2011-0350OC. [DOI] [PubMed] [Google Scholar]
- 155.Nakamura A., Nagaya N., Obata H., Sakai K., Sakai Y., Yoshikawa M., Hamada K., Matsumoto K., Kimura H. Oral administration of a novel long-acting prostacyclin agonist with thromboxane synthase inhibitory activity for pulmonary arterial hypertension. Circ. J. 2013;77:2127–2133. doi: 10.1253/circj.CJ-13-0107. [DOI] [PubMed] [Google Scholar]
- 156.Comoglio P.M., Giordano S., Trusolino L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 157.Martin T.A., Jiang W.G. Hepatocyte growth factor and its receptor signalling complex as targets in cancer therapy. Anticancer Agent Med. Chem. 2010;10:2–6. doi: 10.2174/1871520611009010002. [DOI] [PubMed] [Google Scholar]
- 158.Cecchi F., Rabe D.C., Bottaro D.P. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:553–572. doi: 10.1517/14728222.2012.680957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gherardi E., Birchmeier W., Birchmeier C., Vande Woude G.F. Targeting MET in cancer: Rationale and progress. Nat. Rev. Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 160.Giordano S., Columbano A. Met as a therapeutic target in HCC: Facts and hopes. J. Hepatol. 2014;60:442–452. doi: 10.1016/j.jhep.2013.09.009. [DOI] [PubMed] [Google Scholar]