Abstract
Background
Polycystic ovary syndrome (PCOS) is a common gynecological disease characterized by chronic oligoanovulation, clinical/biochemical hyperandrogenism, polycystic ovaries, and insulin resistance. Accumulating evidence has shown that PCOS-related ovarian dysfunction is the main cause of anovulatory infertility. Clomiphene citrate (CC) is the first-line therapy for PCOS patients; however, approximately 15–40% PCOS patients are resistant to CC treatment. It has been demonstrated that PCOS is a chronic pro-inflammatory state, as some pro-inflammatory cytokines were elevated in the peripheral circulation of PCOS patients, but whether altered inflammatory cytokines expression in PCOS patients is associated with blunted response to CC remains unknown.
Material/Methods
We recruited 44 CC-resistant PCOS patients, along with 55 age and body mass index (BMI)-matched CC-sensitive PCOS patients. Ovulation was induced by administrating 50–100 mg/day CC on days 5 to 9 of each menstrual cycle. The cytokine profiles were detected by cytokine antibody microarrays and further validated by ELISAs.
Results
CC-resistant patients had higher levels of high-sensitivity C-reactive protein (hsCRP) than the CC-sensitive individuals. A growth factor, angiopoietin-2, was significantly reduced [1.64 (0.93–1.95) vs. 1.08 (0.85–1.34), p<0.05], while a chemokine CXCL-16 was significantly increased (9.10±2.35 vs. 10.41±2.82, p<0.05) in CC-resistant patients compared to the CC-sensitive subjects. CXCL-16 was positively correlated with hsCRP (r=0.33, p<0.01). Logistic regression analysis showed that angiopoietin-2 and CXCL-16 are associated with CC resistance.
Conclusions
Circulating cytokines are disturbed in CC-resistant PCOS patients. Altered angiopoietin-2 and CXCL-16 levels might compromise the responsiveness of the ovary to CC through up-regulating angiogenesis and inflammation.
MeSH Keywords: Cytokines, Inflammation, Polycystic Ovary Syndrome
Background
Polycystic ovary syndrome (PCOS) is a heterogeneous disease characterized by chronic oligoanovulation, clinical/biochemical hyperandrogenism, polycystic ovaries, and insulin resistance [1]. The PCOS-related ovarian dysfunction has emerged as the main cause of anovulatory infertility [2]. Although several approaches are available to induce ovulation in anovulatory PCOS women [3], clomiphene citrate (CC) is still considered the first-line therapy, with lower risk of adverse effects and lower cost [4]. However, evidence has demonstrated that approximately 15–40% of PCOS patients do not respond to CC treatment [5,6], which is termed CC resistance. It has been reported that PCOS women with obesity, hyperandrogenism, and insulin resistance are less likely to respond to CC, suggesting these factors could be prominent causes of CC resistance [7–9]. Given the multifaceted pathogenesis of PCOS and the great heterogeneity in the studied populations, the aforementioned factors cannot fully explain the origin of CC resistance. On the other hand, current strategies of augmenting therapy with insulin-sensitizing agents such as metformin are not proving to be sufficient [10,11].
Recently, a growing body of evidence has demonstrated that PCOS is a low-grade pro-inflammatory state. Some of the pro-inflammatory cytokines were found to be elevated in the peripheral circulation of PCOS women [12–14]. Cytokines have been shown to be directly involved in maintaining the delicate balance of the hypothalamo-pituitary-ovarian axis and in the maintenance of normal ovarian and menstrual cycles [15]. We thus hypothesized that the dysregulation of cytokines, a main feature of chronic inflammation in PCOS, may be also associated with the blunted ovarian response to CC therapy. The aim of this cross-sectional study was to identify the circulating cytokines that are expressed differently in CC-resistant and CC-sensitive PCOS women.
Material and Methods
Patients
At the Department of Reproduction Health and Infertility of the First Affiliated Hospital of Chongqing Medical University, between February 2013 and December 2014, we recruited 44 anovulatory PCOS women determined to no have response to ovulation induction by CC treatment and assigned them to the CC-resistant group. We recruited 55 age- and body mass index (BMI)-matched anovulatory PCOS women who had ovulated after CC treatment and assigned them to the CC-sensitive group as control. All the patients recruited met the following inclusion criteria:
Age 20–35 years;
Diagnosis of PCOS (with at least both oligo-amenorrhea and polycystic ovaries on ultrasonography) based on the 2003 Rotterdam consensus [16].
Fasting blood glucose levels <7.0 mmol/L and a 2-h glucose levels <11.1 mmol/L following a 75-g oral glucose tolerance test (OGTT);
Follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH) levels within normal ranges;
No intake of the hormone medications (including oral contraceptives) within the past 2 months or of medicines that affect insulin sensitivity (e.g., metformin or thiazolidinediones) within the past 3 months;
No male-related infertility or tubal causes of infertility.
Each subject of the present study provided signed consent. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Ovulation induction
All participants were induced to ovulate by giving 50 mg/day CC on day 5 to 9 of a spontaneous or a progestin-induced menstrual cycle. Ovulation was observed by vaginal B-ultrasound (GE Voluson E8, Zipf, Austria) on days 11 to 14 of the menstrual cycle, and progesterone level was measured on day 21 of the menstrual cycle. If there was no dominant follicle (size <10 mm in diameter) observed and the progesterone level was <5 ng/ml after 1 cycle of CC treatment, 100 mg/day CC was used on the same days of subsequent cycles. The maximum dose of CC used in our hospital was 100 mg/day due to the nature of the Asian population [9,17]. The duration of follow-up for all patients was at least 3 treatment cycles. The CC-resistant group was defined as those who failed to ovulate in response to CC treatment after 3 cycles. The patients who ovulated after at least 1 of 3 cycles of CC treatment were defined as CC-sensitive.
Clinical and Biochemical measurements
BMI was calculated as the ratio between weight in kilograms and the square of height in meters (kg/m2). BMI ≥28 kg/m2 was referred to as obesity [18]. A blood sample was collected from each patient on day 3 of the first menstrual cycle (before the initiation of CC treatment), following overnight fasting. An OGTT was performed after overnight fasting on all patients. Plasma glucose was measured by the Glucose Oxidase method (Mindray, Shenzhen, China). Fasting plasma insulin, total testosterone, FSH, and TSH were determined by chemiluminescence (Beckman, CA, USA). The plasma levels of high-sensitivity C-reactive protein (hsCRP) were measured by immunoturbidimetry on the autoanalyzer (HITACHI 7180, Ichige, Japan). Insulin resistance (IR) was estimated by Homeostasis Model Assessment of IR (HOMA-IR), which was calculated using the formula FPG (mmol/L)× fasting insulin (μIU/ml)/22.5. Patients with HOMA-IR >2.0 were considered as having IR [17].
Cytokine antibody microarray
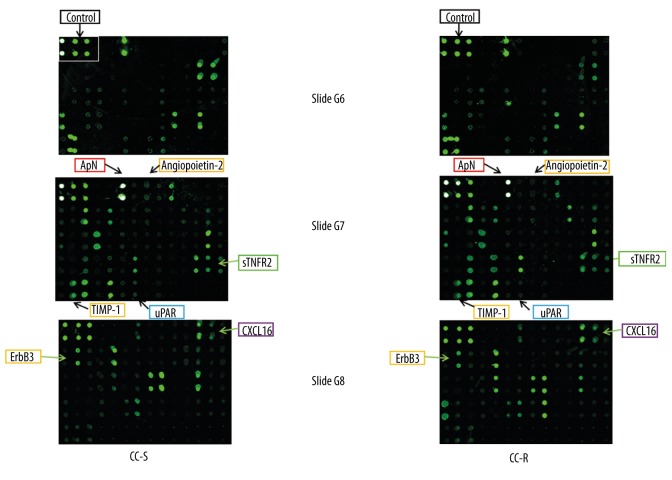
Screening for circulating cytokines secreted by CC-sensitive or CC-resistant patients was performed by hybridizing plasma with antibody-coated glass slides according to the protocol supplied by the manufacturer [RayBio Human Cytokine Antibody Array G Series 2000, a kit combining glass slides of Array G6, G7, and G8 and allowing the simultaneous detection of 174 cytokines (cat. no. AAH-CYT-2000), for details please refer to http://www.raybiotech.com/manual/Antibody%20Array/AAH-CYT-2000.pdf (RayBiotech, Inc, Norcross GA, USA)]. Spots fluorescent signal intensities on slides were quantified by using a laser scanner [GenePix 4000B (Molecular Devices, LLC, CA, USA)]. Signals were normalized to internal positive controls present on each slide (Figure 1), and then expressed as intensity units in each group. The experiments on both groups were always performed simultaneously.
Figure 1.
Cytokine antibody microarray slides used to detect cytokines secreted by CC-sensitive (CC-S) and CC-resistant (CC-R) women with PCOS. A set of 3 human cytokine antibody microarray slides, which tests up to 174 cytokines, was used and probed with fasting plasma. This figure illustrates the representative microarrays of CC-S and CC-R patients. Highlighted cytokines are differentially secreted between the 2 groups.
ELISA
The differentially secreted cytokines between the 2 groups as identified by cytokine antibody microarrays (p<0.05) were further validated by ELISA. The assessment of circulating angiopoietin-2, urokinase receptor (uPAR), tissue inhibitor of metalloproteinase-1 (TIMP-1), soluble tumor necrosis factor receptor-2 (sTNF-R2), adiponectin, CXCL-16, and human epidermal growth factor receptor 3 (ErbB3/Her3) were performed by using ELISA kits from RayBiotech according to the manufacturer’s instructions.
Presentation of the results and statistical analysis
Statistical analysis was performed using SAS 9.13 (SAS Institute, Cary, NC). Variables are presented as means ±SD or median (interquartile ranges) or number (percentage). Means of continuous variables were compared using the unpaired t test or the Wilcoxon 2-sample test. The percentage differences between groups were compared using χ2 tests. Univariate logistic regression analysis was performed to evaluate the associations with response to CC. The differences between groups were considered statistically significant at p<0.05. For logistic regression analysis, the statistical significance was set as p<0.1 and the 95% Wald confidence intervals (CI) did not include 1.0.
Results
Characteristics of subjects
As shown in Table 1, the 2 groups of participants were well matched in terms of age and BMI. There were no significant differences between the 2 groups in terms of hormonal and metabolic parameters, as well the proportions of obesity, hyperandrogenism, and insulin resistance. Compared to the CC-sensitive patients, the CC-resistant patients displayed higher levels of hsCRP, a pro-inflammatory marker.
Table 1.
Clinical and biochemical characteristics of CC-sensitive (CC-S) and CC-resistant (CC-R) women with PCOS.
| CC-S (n=55) | CC-R (n=44) | P value | Normal laboratory value | |
|---|---|---|---|---|
| Age (year) | 26.35±3.13 | 26.82±3.47 | 0.48 | |
| BMI (kg/m2) | 23.02±3.72 | 23.30±3.23 | 0.70 | |
| Subjects with BMI ≥28, n (%) | 6 (10.91) | 2 (4.55) | 0.25 | |
| Total testosterone (ng/ml) | 0.58±0.15 | 0.59±0.17 | 0.87 | <0.75 |
| Subjects with testosterone ≥0.75, n (%) | 4 (7.27) | 7 (15.91) | 0.17 | |
| FSH (mIU/ml) | 6.40±1.82 | 6.10±1.52 | 0.43 | 3.80–8.70 (follicular phase) |
| TSH (μIU/ml) | 2.58 (1.63–3.13) | 2.62 (1.69–3.22) | 0.89 | 0.35–3.50 |
| hsCRP (mg/L) | 1.17 (0.22–1.53) | 1.80 (0.50–2.38) | 0.048 | 0.00–3.00 |
| OGTT | ||||
| Fasting glucose (mmol/L) | 5.24±0.37 | 5.24±0.89 | 0.99 | 3.90–6.00 |
| 30-min glucose (mmol/L) | 9.18±1.46 | 9.06±2.23 | 0.75 | |
| 60-min glucose (mmol/L) | 8.82±2.38 | 9.15±2.93 | 0.55 | |
| 120-min glucose (mmol/L) | 7.31±1.99 | 7.89±2.74 | 0.24 | |
| Fasting insulin (μIU/ml) | 8.38 (4.34–11.66) | 9.05 (5.07–12.49) | 0.57 | 1.90–23.00 |
| 30-min insulin (μIU/ml) | 76.82 (36.54–91.74) | 76.23 (42.05–91.64) | 0.79 | |
| 60-min insulin (μIU/ml) | 78.95 (44.00–99.05) | 92.70 (50.52–118.08) | 0.19 | |
| 120-min insulin (μIU/ml) | 69.75 (38.66–89.10) | 77.04 (38.05–97.35) | 0.63 | |
| HOMA-IR | 1.95 (0.98–2.67) | 2.19 (1.13–2.95) | 0.67 | |
| Subjects with HOMA-IR >2.0, n (%) | 20 (36.36) | 17 (38.64) | 0.82 |
Data are means ±SD or median (interquartile ranges) or number (percentage) for indicated number of subjects in each group. P values for comparisons between two groups are based on Unpaired t test or Wilcoxon two-sample test or χ2 test. BMI – body mass index; FSH – follicle-stimulating hormone; TSH – thyroid-stimulating hormone; hsCRP – high-sensitivity C-reactive protein; OGTT – oral glucose tolerance test; HOMA-IR – homeostasis model assessment of insulin resistance.
Cytokine secretion in CC-sensitive and CC-resistant PCOS patients
Fasting plasma from CC-sensitive and CC-resistant PCOS patients were subjected to cytokine antibody microarray for profiling 174 human cytokines (Figure 1). The secretory profile of plasma demonstrated that 7 cytokines – angiopoietin-2, uPAR, TIMP-1, sTNF-R2, adiponectin, CXCL-16, and ErbB3 – were differentially secreted between the 2 groups (Table 2, p<0.05).
Table 2.
Screening secretion of cytokines of CC-sensitive (CC-S) and CC-resistant (CC-R) women with PCOS.
| Cytokines | Fluorescence intensity | P-value | |
|---|---|---|---|
| CC-S (n=4) | CC-R (n=4) | ||
| Angiopoietin-2 | 299.03±109.37 | 160.35±12.56 | 0.05 |
| uPAR | 501.69±27.76 | 372.13±93.69 | 0.04 |
| TIMP-1 | 2846.80±349.61 | 2143.04±295.11 | 0.02 |
| sTNFR2 | 2214.21±203.90 | 1696.79±254.46 | 0.02 |
| Acrp30 | 68648.05±1721.00 | 65635.77±1280.93 | 0.03 |
| CXCL-16 | 1107.60±130.55 | 1549.45±194.84 | 0.01 |
| ErbB3/Her3 | 3459.52±329.81 | 2693.26±266.28 | 0.01 |
Fasting plasma of each subject was incubated with the sets of cytokine antibody microarrays like those shown in Figure 1. Cytokine levels were measured by laser scanner (GenePix 4000B Microarray Scanner), normalized to internal positive controls and expressed as fluorescence intensity. Only the cytokines, which differed between the two groups of subjects, are shown in the table. Seven cytokines were differentially secreted between CC-S and CC-R women. Values are presented as means ±SD for 4 subjects per group. uPAR – urokinase receptor; TIMP-1 – tissue inhibitor of metalloproteinase-1; sTNF-R2 – soluble tumor necrosis factor receptor-2; CXCL-16 – chemokine (C-X-C motif) ligand 16; ErbB3/Her3 – human epidermal growth factor receptor 3.
These cytokines were further validated in 55 CC-sensitive and 44 CC-resistant individuals by ELISA. The ELISA results confirmed that 2 of 7 candidate cytokines were altered in CC-resistant individuals. Compared to the CC-sensitive group, the secretion of angiopoietin-2 was significantly reduced in the CC-resistant group [1.64 (0.93–1.95) vs. 1.08 (0.85–1.34), p<0.05], while CXCL-16 was over-secreted by the CC-resistant group (9.10±2.35 vs. 10.41±2.82, p<0.05).
Correlation analysis
Pearson correlation analysis showed that CXCL-16 was positively correlated with hsCRP (r=0.33, p<0.01). Univariate logistic regression analysis further showed that decreased angiopoietin-2 levels and increased CXCL-16 levels were significantly associated with the presence of CC resistance (Table 3).
Table 3.
Univariate logistic regression analysis of hsCRP and the two cytokines in 99 patients for the associations with CC-resistance.
| OR (95% CI) | P value | |
|---|---|---|
| hsCRP | 1.28 (0.94–1.75) | 0.12 |
| Angiopoietin-2 | 0.29 (0.12–0.70) | <0.01 |
| CXCL16 | 1.23 (1.03–1.46) | 0.02 |
Discussion
Some studies have suggested that serum androgen levels, BMI, blood glucose, and insulin resistance are associated with CC resistance. Other researchers have reported that FSH receptor polymorphism at position 680 and basal FSH levels predict the ovarian response to CC [19]. However, in the present study, the CC-sensitive and CC-resistant groups had similar BMI, and showed no significant differences in blood glucose, insulin levels, total testosterone, or FSH levels before CC treatment. However, the CC-resistant group displayed a higher pro-inflammatory state, as shown by an increase in hsCRP levels. This indicates that inflammation might be associated with the blunted CC response. Therefore, we then focused on investigating the correlation between circulating cytokines and CC resistance without the interference of obvious hormonal and metabolic confounding factors.
The regulation of cytokines in the ovary has been described as promoting processes of follicular growth, steroidogenesis, recruitment, and activation of leukocytes necessary for ovulation and tissue remodelling during ovulation, luteinization, and luteolysis [20]. Ovulation is regarded as an inflammatory event that is followed by anti-inflammatory reactions; these processes need the coordination of both pro-inflammatory and anti-inflammatory cytokines [21]. Moreover, cytokines are involved in both the inhibition and stimulation of follicular responsiveness to gonadotrophins [22]. However, the involvement of cytokines in the ovulatory response to CC medication has not been thoroughly investigated. Serum levels of IL-6 have been reported to be decreased in anovulatory PCOS women resistant to CC when compared to ovulatory women, suggesting that low serum IL-6 could be a marker of CC resistance [23]. Another study found that circulating levels of leptin, an adipokine, were significantly higher in CC non-responders and could be used to predict CC resistance [8]. Using cytokine antibody microarray technology, we investigated 174 human cytokines, including the pro-inflammatory factors, growth factors, chemokines, anti-inflammatory factors, extracellular matrix factors, and adipokines. By subsequently performing ELISA, we ultimately identified 2 cytokines that had different circulating levels between the CC-sensitive and CC-resistant patients. Angiopoietin-2, a growth factor, is decreased in CC-resistant women, and is also reversely correlated with the presence of CC resistance as determined by logistic regression analysis. The angiopoietins, including angiopoietin-1 and angiopoietin-2, are key regulators of angiogenesis. It is known that angiopoietin-1 is necessary for the recruitment of peri-vascular cells that lead to the maturation and stabilization of newly developed capillaries, while angiopoietin-2 acts as a natural antagonist of angiopoietin-1, resulting in loosening of the supporting cell matrix and destabilization of existing vessels [24]. In the physiological condition, angiopoietin-2 protein level in the ovary increases during follicular enlargement and decreases during maturation of follicles, suggesting that angiopoietin-2 may be critical for follicular development [25,26]. In contrast, in the pathological PCOS condition, angiopoietin-2 level was decreased in the ovaries of PCOS rats compared to the normal controls, resulting in higher ovarian vascularity and vessel stabilization [27]. The compromised angiopoietin-2 expression in CC-resistant PCOS women suggest that defects of follicular development and ovulation may result from excessive ovary angiogenesis.
We also found that CXCL-16 was increased in CC-resistant women and was positively correlated with CC resistance. Although the function of CXCL-16 in the ovaries or hypothalamo-pituitary is still poorly documented, it is known that CXCL-16 plays an important role in initiating and shaping immune-inflammatory responses by attracting inflammatory mononuclear cells [28]. Consistently, we have found a significant positive correlation between CXCL-16 and hsCRP, indicating that CXCL-16-mediated pro-inflammation might also be involved in regulating CC sensitivity in PCOS patients.
Conclusions
Circulating cytokines are disturbed in CC-resistant PCOS patients. Altered angiopoietin-2 and CXCL-16 levels might impair the responsiveness of the ovaries to CC through up-regulating angiogenesis and inflammation.
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81170585, 81100444, 81370732), Chongqing Science and Technology Commission (No. 2011BB5121), and Chongqing Municipal Health Bureau (No.2011-2-046)
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–49. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Palomba S, Orio F, Jr, Russo T, et al. Is ovulation induction still a therapeutic problem in patients with polycystic ovary syndrome? J Endocrinol Invest. 2004;27:796–805. doi: 10.1007/BF03347527. [DOI] [PubMed] [Google Scholar]
- 4.Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev. 2000;(2):CD000056. doi: 10.1002/14651858.CD000056. [DOI] [PubMed] [Google Scholar]
- 5.Beck JI, Boothroyd C, Proctor M, et al. Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database Syst Rev. 2005;(1):CD002249. doi: 10.1002/14651858.CD002249.pub3. [DOI] [PubMed] [Google Scholar]
- 6.O’Flynn N. Assessment and treatment for people with fertility problems: NICE guideline. Br J Gen Pract. 2014;64:50–51. doi: 10.3399/bjgp14X676609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauser BC, Diedrich K, Devroey P. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14. doi: 10.1093/humupd/dmm034. [DOI] [PubMed] [Google Scholar]
- 8.Imani B, Eijkemans MJ, de Jong FH, et al. Free androgen index and leptin are the most prominent endocrine predictors of ovarian response during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 2000;85:676–82. doi: 10.1210/jcem.85.2.6356. [DOI] [PubMed] [Google Scholar]
- 9.Kurabayashi T, Suzuki M, Fujita K, et al. Prognostic factors for ovulatory response with clomiphene citrate in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2006;126:201–5. doi: 10.1016/j.ejogrb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Moll E, Bossuyt PM, Korevaar JC, et al. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: Randomized double blind clinical trial. BMJ. 2006;332:1485. doi: 10.1136/bmj.38867.631551.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: A randomized, double-blinded placebo-controlled trial. Hum Reprod. 2001;16:1625–31. doi: 10.1093/humrep/16.8.1625. [DOI] [PubMed] [Google Scholar]
- 12.Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97:7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77:300–5. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335:30–41. doi: 10.1016/j.mce.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Tabibzadeh S. Cytokines and the hypothalamic-pituitary-ovarian-endometrial axis. Hum Reprod. 1994;9:947–67. doi: 10.1093/oxfordjournals.humrep.a138621. [DOI] [PubMed] [Google Scholar]
- 16.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Igarashi H, Hara S, et al. Brachial-to-ankle pulse wave velocity as an independent prognostic factor for ovulatory response to clomiphene citrate in women with polycystic ovary syndrome. J Ovarian Res. 2014;7:74. doi: 10.1186/1757-2215-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 19.Overbeek A, Kuijper EA, Hendriks ML, et al. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. 2009;24:2007–13. doi: 10.1093/humrep/dep114. [DOI] [PubMed] [Google Scholar]
- 20.Buscher U, Chen FC, Kentenich H, Schmiady H. Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries; Is ovulation a suppressed inflammatory reaction? Hum Reprod. 1999;14:162–66. doi: 10.1093/humrep/14.1.162. [DOI] [PubMed] [Google Scholar]
- 21.Sarapik A, Velthut A, Haller-Kikkatalo K, et al. Follicular pro-inflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol. 2012;2012:606459. doi: 10.1155/2012/606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gougeon A. [Inhibin, activin, follistatin, and transforming growth factor beta (TGF-beta): Presence in the ovary and possible role in the regulation of folliculogenesis in primates]. Contracept Fertil Sex. 1994;22:571–76. [in French] [PubMed] [Google Scholar]
- 23.Omu AE, Al-Azemi MK, Makhseed M, et al. Differential expression of T-helper cytokines in the peritoneal fluid of women with normal ovarian cycle compared with women with chronic anovulation. Acta Obstet Gynecol Scand. 2003;82:603–9. doi: 10.1034/j.1600-0412.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 24.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–69. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi KG, Acosta TJ, Tetsuka M, et al. Involvement of angiopoietin-tie system in bovine follicular development and atresia: messenger RNA expression in theca interna and effect on steroid secretion. Biol Reprod. 2003;69:2078–84. doi: 10.1095/biolreprod.103.017152. [DOI] [PubMed] [Google Scholar]
- 26.Nishigaki A, Okada H, Tsuzuki T, et al. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011;96:1378–83. doi: 10.1016/j.fertnstert.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Abramovich D, Irusta G, Bas D, et al. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology. 2012;153:3446–56. doi: 10.1210/en.2012-1105. [DOI] [PubMed] [Google Scholar]
- 28.van der Voort R, Verweij V, de Witte TM. An alternatively spliced CXCL16 isoform expressed by dendritic cells is a secreted chemoattractant for CXCR6+ cells. J Leukoc Biol. 2010;87:1029–39. doi: 10.1189/jlb.0709482. [DOI] [PMC free article] [PubMed] [Google Scholar]