Abstract
Background:
Oral tongue squamous cell carcinoma (OTSCC) metastasises early, especially to regional lymph nodes. There is an ongoing debate on which early stage (T1-T2N0) patients should be treated with elective neck dissection. We need prognosticators for early stage tongue cancer.
Methods:
Mice immunisation with human mesenchymal stromal cells resulted in production of antibodies against tenascin-C (TNC) and fibronectin (FN), which were used to stain 178 (98 early stage), oral tongue squamous cell carcinoma samples. Tenascin-C and FN expression in the stroma (negative, moderate or abundant) and tumour cells (negative or positive) were assessed. Similar staining was obtained using corresponding commercial antibodies.
Results:
Expression of TNC and FN in the stroma, but not in the tumour cells, proved to be excellent prognosticators both in all stages and in early stage cases. Among early stages, when stromal TNC was negative, the 5-year survival rate was 88%. Correspondingly, when FN was negative, no cancer deaths were observed. Five-year survival rates for abundant expression of TNC and FN were 43% and 25%, respectively.
Conclusions:
Stromal TNC and, especially, FN expressions differentiate patients into low- and high-risk groups. Surgery alone of early stage primary tumours might be adequate when stromal FN is negative. Aggressive treatments should be considered when both TNC and FN are abundant.
Keywords: tongue cancer, prognosis, mesenchymal stromal cell, tenascin-C, fibronectin, stroma, immunohistochemistry, tumour microenvironment
Globally an estimated 405 000 new cases of oral cancer are diagnosed annually, of which about 50% are located in the oral/mobile tongue. Oral tongue squamous cell carcinoma (OTSCC) is an aggressive subset of the oral squamous cell carcinomas (OSCCs) with the highest invasion and metastasis properties (Bello et al, 2011; Ganly et al, 2012; Kim and Cha, 2012). Lymph node metastases are the single most important prognostic factor of OSCC. However, in early stage cancers, lymph node metastases are often occult without preoperative signs (Bello et al, 2010). The prognosis can be improved by performing elective neck dissection for all early stage OTSCC patients (D'Cruz et al, 2015), but most of the patients do not benefit from elective neck dissection, from which surgery-related morbidities are substantial (Kelner et al, 2015). Owing to the lack of prognostic markers, the mortality of early stage (cT1-T2N0) OTSCC is still comparatively high (Ganly et al, 2012), as patients with aggressive OTSCC are not recognised. Thus the development of reliable methods to detect aggressive early stage tumours is essential in order to provide treatment with the most efficient modalities.
The role of the tumour microenvironment (TME) in cancer development has become abundantly clear. The fibroblasts and extracellular matrix (ECM) components are crucial for the proliferation and invasion of cancer cells through dynamic interactions between cancer cells and ECM molecules (Metwaly et al, 2012). As mesenchymal stem cells, among other TME components, are known to enhance the invasion of OTSCC cells (Sobral et al, 2011; Salo et al, 2013), we used intact human mesenchymal stromal cells (hMSCs) as an immunising agent to produce specific antibodies in mice. The aim of this study was to characterise novel monoclonal mouse antibodies produced against hMSCs, and to evaluate the prognostic value of two of the target antigens – tenascin-C (TNC) and fibronectin (FN) – in OTSCCs according to the reporting recommendations for tumour marker prognostic studies (REMARK) criteria.
Materials and methods
New anti-TNC and anti-FN antibodies
Mice (C57BL/6) were immunised using cultured live hMSCs as single-cell suspensions in PBS (i.p). The hMSCs were obtained from bone marrow (BM) of volunteer donors (two 16-year-old donors with arthritis) following written consent approved by the Ethical Committee of the Northern Ostrobothnia Hospital District, Oulu, Finland. Bone marrow hMSC isolation and culture followed the protocol described by Leskelä et al (2003). The presence of both MSC-positive (CD73, CD90 and CD105) and MSC-negative (CD14, CD19, CD34, CD45 and HLA-DR) cell surface markers of the cultured cells were assayed by flow cytometry, and the differentiation potential of isolated cells was assessed for adipogenic and osteogenic differentiation capability (data not shown). The preparation of the live cells and the booster immunisation was performed twice. On the basis of the antibody titres, mice were chosen and their spleens prepared for fusion. Positive hybridomas were subcloned several times using standard limiting dilution procedures.
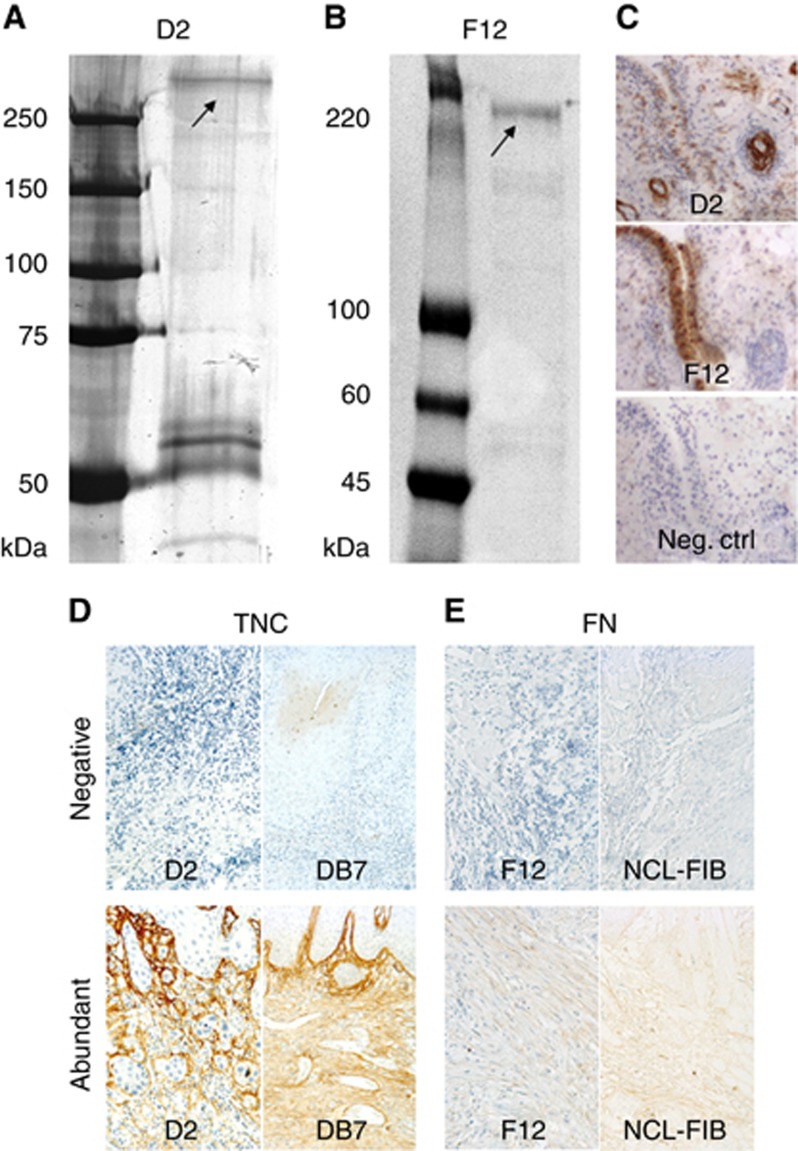
Media from hybridoma lines were screened against several cell types by cyto-ELISA (Grunow et al, 1994), flow cytometry and western blot. The monoclonal antibodies produced by the hybridoma lines were isolated using protein G. The target antigens were isolated from cultured hMSC cell homogenate by immunoprecipitation with the purified antibody covalently coupled to the surface of Dynabeads (M270, Invitrogen, Carlsbad, CA, USA) or using Protein G resin (Sigma-Aldrich, Ayshire, UK). The immunoisolated target molecules were separated using SDS-7.5% PAGE. The bands from protein gels (Figure 1A and B) were identified using peptide fingerprinting (trypsin cleavage and MS Maldi TOF analysis of proteolytic fragments) and Mascot Database search (Matrix Science, London, UK).
Figure 1.
Characterisation of the new matrix antibodies. Coomassie-stained SDS-7.5% PAGE of the target antigens from the cultured hMSC lysate immunoprecipitated using the new anti-tenascin-C monoclonal antibody D2 (A) or anti-fibronectin monoclonal antibody F12 (B). Molecular weight markers are shown on the left of the gels: Bio-Rad Dual Colour (A) and Sigma Colorburst (B). The identified target antigen is marked with arrows. Bands migrating at ∼60 kDa region are mouse antibody fragments detached from the resin (A). The immunostaining of the synovia tissue sections with anti-tenascin-C D2, anti-fibronectin F12 and idiotype IgG1 as negative control (C). New antibodies were compared with commercial ones by staining representative samples from ‘negative' and ‘abundant' groups using both D2 and commercial DB7 for TNC (D), and both F12 and commercial NCL-FIB for FN (E).
Purified antibodies were assayed for immunofluorescence using the Dylight 550 conjugated anti-mouse secondary antibody and DAPI. The formalin fixed, paraffin-embedded synovial tissues were also assayed with the new antibodies (Figure 1C). The negative control was stained with the idiotype antibody (unrelated mouse IgG1). For western blots of various cell types and tissues, the samples were incubated with the new matrix antibodies at a concentration of 1 μg ml−1 for 2 h and, after washing, the bound antibodies were detected with peroxidase conjugated anti-mouse antibody (Sigma-Aldrich). Target antigen for antibodies from two clones (A8 and D2) was identified as human TNC and target antigen for antibodies from three clones (F12, C1 and B6) was identified as human FN. From these antibodies, the ones from clones D2 for TNC and F12 for FN were chosen to be used in this study.
Patients and biological material
This retrospective multi-centre study included 178 OTSCC patients treated in University Hospitals of Oulu (OUH, n=107) and Kuopio (KUH, n=21) in Finland from 1979 to 2009, and UOPECCAN Cancer Hospital (n=27) and Oncology Center of Cascavel (CEONC, n=23) in Brazil from 1998 to 2008. Of these 178 patients, 98 had been diagnosed with clinical stage I (cT1N0M0) or II (cT2N0M0) OTSCC. The inclusion criteria were sufficient clinical data and histological samples to perform the analysis. All patients had undergone primary resection of the tumour. Detailed patient demographic data of the early stage patients are shown in Table 1, and demographic data of the pooled group of all stage patients are in Supplementary Table 1. This study was approved by the Ethical Committee of the Northern Ostrobothnia Hospital District, Finland and the Finnish National Supervisory Authority for Welfare and Health. In Brazil, this study was approved by the Ethics Committee in Research of the Piracicaba Dental School, University of Campinas (approval number 100/2012).
Table 1. Patient data of stage I and II OTSCC patients.
| N (%) | All patients (n=98) | OUH (n=59) | KUH (n=14) | UOPECCAN (n=8) | CEONC (n=17) |
|---|---|---|---|---|---|
|
Patient data, stages I–II | |||||
| Gender | |||||
| Male | 53 (54) | 24 (41) | 9 (64) | 6 (75) | 14 (82) |
| Female | 45 (46) | 35 (59) | 5 (36) | 2 (25) | 3 (18) |
| Age at diagnosis | |||||
| ⩽55 | 26 (26) | 13 (22) | 3 (21) | 3 (38) | 7 (41) |
| 56–69 | 33 (34) | 15 (25) | 7 (50) | 4 (50) | 7 (41) |
| ⩾70 | 39 (40) | 31 (53) | 4 (29) | 1 (12) | 3 (18) |
| Range | 32–99 | 32–99 | 33–80 | 45–77 | 40–80 |
| Mean | 64.18 | 67.17 | 62.29 | 56.50 | 59 |
| Median | 64 | 70 | 63.5 | 57 | 59 |
| Follow-up time | |||||
| Range | 1–298 | 1–298 | 3–175 | 6–127 | 1–139 |
| Mean | 73.65 | 80.75 | 72.71 | 61.38 | 55.59 |
| Median | 57 | 64 | 60 | 47.5 | 51 |
| Cause of death | |||||
| Alive | 49 (50) | 31 (53) | 6 (43) | 5 (62) | 7 (41) |
| Cancer | 22 (22) | 8 (14) | 3 (21) | 3 (38) | 8 (47) |
| Other | 27 (28) | 20 (33) | 5 (36) | – | 2 (12) |
| Grade | |||||
| 1 | 28 (28) | 24 (41) | 4 (29) | – | – |
| 2 | 37 (38) | 32 (54) | 5 (36) | – | – |
| 3 | 6 (6) | 3 (5) | 3 (21) | – | – |
| Missing | 27 (28) | – | 2 (14) | 8 (100) | 17 (100) |
| Neck lymph nodes | |||||
| Positive | 2 (2) | 2 (3) | – | – | – |
| Negative | 96 (98) | 57 (97) | 14 (100) | 8 (100) | 17 (100) |
| Adjuvant therapy | |||||
| No | 67 (69) | 47 (80) | 7 (50) | 3 (37) | 10 (59) |
| Radiotherapy | 22 (22) | 9 (15) | 7 (50) | 2 (26) | 4 (23) |
| Radio- and chemotherapy | 7 (7) | 1 (2) | – | 3 (37) | 3 (18) |
| Missing | 2 (2) | 2 (3) | – | – | – |
| Recurrence | |||||
| Yes | 39 (40) | 17 (29) | 8 (57) | 5 (62) | 9 (53) |
| No | 59 (60) | 42 (71) | 6 (43) | 3 (38) | 8 (47) |
Abbreviations: CEONC=Oncology Center of Cascavel; KUH=Kuopio University Hospital; OTSCC=oral tongue squamous cell carcinoma; OUH=Oulu University Hospital.
Data as combination from all the hospitals, as well as separately from each hospital.
Immunohistochemistry
TNC and FN
After characterisation, our novel TNC antibody D2 was qualitatively compared with the well-tested commercial monoclonal TNC antibody DB7 (order #610003, Biohit, Helsinki, Finland), and our FN antibody F12 was compared with the commercial monoclonal antibody NCL-FIB (NCL-FIB, Leica Biosystems, Newcastle, UK; Figure 1D and E). Representative OTSCC samples from negative and abundant groups were stained with both D2 and DB7, as well as F12 and NCL-FIB. Paraffin-embedded resected tumours were obtained and prepared for immunohistochemistry. In essence, 5 μm thick slices were mounted on microscope slides and dewaxed in xylene. For epitope retrieval, the sections were then heated in a microwave oven in Tris-EDTA solution (pH 9) for 10 min and allowed to cool at room temperature (RT) for 20 min. Endogenous peroxidase activity was blocked using peroxidase-blocking solution (DAKO, Glostrup, Denmark) for 10 min. The sections were then incubated with primary antibodies at a dilution of 1 : 500 for 60 min at RT. For visualisation, horseradish peroxidase (HRP)-conjugated antibodies (DAKO) were introduced to sections for 10 min, after which DAB chromogen was used for 5 min, and the sections were then counterstained using Mayer's haematoxylin.
α-Smooth muscle actin
A group of 60 randomly selected OTSCC samples were stained for α-smooth muscle actin (α-SMA). The sections were incubated with primary antibody (Dako monoclonal mouse anti-human, 1 : 500, clone 1A4, M0851) for 30 min in RT.
Analysis of TNC and FN in the stroma and tumour cells
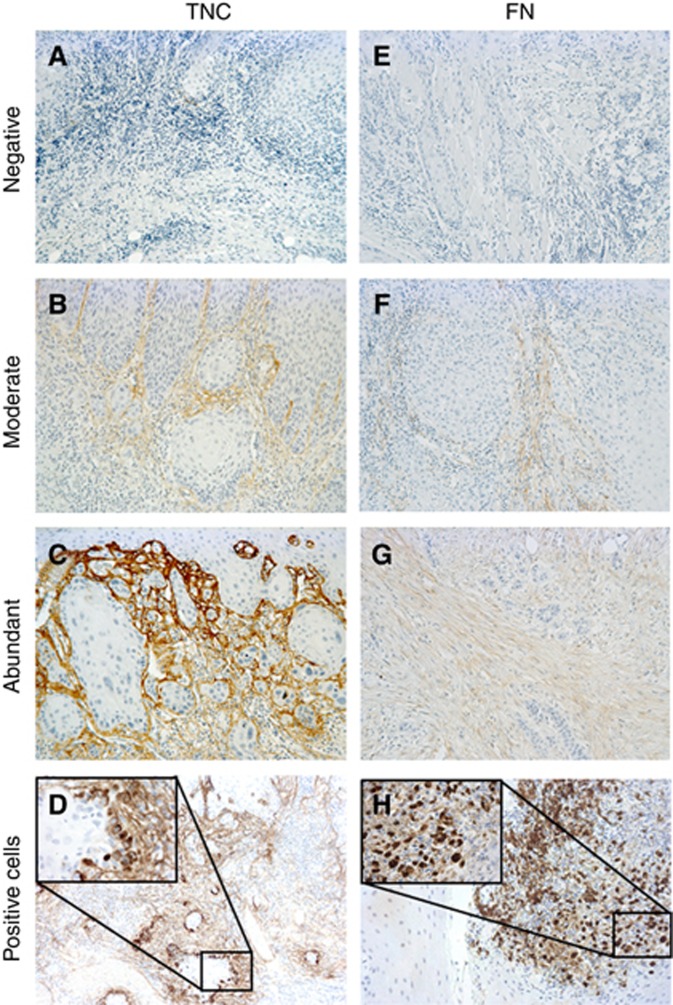
Stromal TNC and FN stain was scored as previously described for α-SMA (Bello et al, 2011), using a scale from 0 to 4 as follows: 0=no extracellular staining; 1=staining of individual confined spots; 2=staining not confined to specific spots, more diffuse; 3=staining throughout the invasive front, distinguishable border between the stained ECM and carcinoma cells; 4=staining throughout the invasive front, no distinguishable border between the stained ECM and carcinoma cells. For statistical analysis the grade 0 was labelled as ‘negative' (Figure 2A and E), grades 1–3 were merged into the ‘moderate' group (Figure 2B and F) and grade 4 was labelled as ‘abundant' (Figure 2C and G). If the same tumour had areas of different staining intensity, the tumour was scored according to the most severe grade.
Figure 2.
Scoring of stromal and intracellular TNC and FN.Stromal TNC (A–C) and FN (E–G) were scored from 0 to 4 and grade 0 was labelled ‘negative' (A and E), grades 1–3 were combined and labelled ‘moderate' (B and F) and grade 4 was labelled ‘abundant' (C and G). Intracellular TNC (D) and FN (H) were scored from 0 to 2. Grade 0 was labelled ‘negative' and 1–2 were combined and labelled ‘positive' (D and H).
Expression in the carcinoma cells was scored as follows: 0=no cells with intracellular staining; 1=individual cells with intracellular staining and 2=groups of cells with intracellular staining. For statistical analysis, groups 1 and 2 were merged into a ‘positive' group (Figure 2D and H). Scoring was performed by two of the authors (ES or RM and JHK) independently and unaware of the clinical outcome at the time of the analysis. Discrepancies in the scoring were settled by an experienced oral pathologist (TS). Most of the discrepancies were related to scores 1–3; scores which were combined to the same group for statistical analyses. There were a total of 44 cases that were scored differently by the assessors due to stromal TNC in the pooled data of all the stages of disease. Of these cases, 17 would have been included in different groups. Furthermore, in 9 cases one of the assessors scored the sample as negative and the other one as ‘not-negative'. The respective numbers of cases for stromal FN scoring were 37, 16 and 8. Among early stage cases, the numbers were 25, 8 and 5 for TNC and 21, 8 and 6 for FN.
Statistical analysis
Survival curves were created using the Kaplan–Meier method with disease-specific death as the end point, and death by other cause and end of follow-up as censoring events. Differences between the groups were analysed by a log-rank test.
The prognosis of the patients related to stromal and intracellular TNC, and FN were evaluated by univariate analysis using the Cox proportional hazard model. Differences between the groups were evaluated using a χ2-test.
Multivariate analysis was conducted using the Cox proportional hazard model. In multivariate analysis the results were adjusted for age, gender, adjuvant therapy and hospital, as well as stage among the pooled group including all the stages. As there were no end points (cancer deaths) in the FN-negative group among patients with stage I–II OTSCC, no hazard ratios (HRs) could be calculated in reference to this group. Hence, HRs for stromal FN in the stage I–II patient group were calculated comparing ‘abundant' with ‘moderate'.
Correlations between stromal TNC, FN and α-SMA were calculated using a Spearman's correlation test and 2-tailed significance was applied. Kappa values for inter-rater agreement in TNC and FN scoring was performed by cross tabulation and statistical significance confirmed using a χ2-test. Cross tabulation was used for the analysis of association between clinical parameters and stromal TNC and FN staining.
In all the analyses P-values ⩽0.05 were considered statistically significant. Statistical analyses were performed using either OriginPro 2015 (OriginLab, Northampton, MA, USA) or SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA: IBM Corp.).
Results
Immunohistochemical validation of tenascin-C and fibronectin antibodies
The immunisation of mice with live BM hMSCs resulted in a series of antibodies (anti-CD73, chondroitin sulphate proteoglycan, FN, TNC, type VI collagen and CD13). However, after testing these in paraffin-embedded OTSCC tissue sections, the FN and TNC gave the most specific and strongest staining signals. Two of the clones were characterised as clones of anti-TNC and three as anti-FN, respectively. One clone of each (D2 for TNC and F12 for FN) was selected to be used in immunohistochemistry. When we compared our novel antibodies with commercially available antibodies, the staining patterns were similar in all of the tested samples (Figure 1D and E) and met the same scoring criteria. However, less unspecific staining was detected in the samples stained with D2 in comparison with DB7 (Figure 1D). The number of cases in each staining group from each of the hospitals is indicated in Table 2 for early stages and in Supplementary Table 2 for all stages. In early stage samples, the concordance for TNC and FN staining was high (kappa 0.827 for TNC and kappa 0.849 for FN, Supplementary Table 2).
Table 2. Results of scoring of stromal and intracellular TNC, and FN of stage I and II OTSCC patients.
| N (%) | All patients (n=98) | OUH (n=59) | KUH (n=14) | UOPECCAN (n=8) | CEONC (n=17) |
|---|---|---|---|---|---|
|
Frequencies of TNC and FN scores in different hospitals, stages I–II | |||||
| Stromal TNC | |||||
| Negative | 26 (27) | 20 (34) | – | 2 (25) | 4 (23) |
| Moderate | 65 (66) | 35 (59) | 11 (79) | 6 (75) | 13 (77) |
| Abundant | 7 (7) | 4 (7) | 3 (21) | – | – |
| Cellular TNC | |||||
| Negative | 60 (61) | 32 (54) | 12 (86) | 4 (50) | 12 (71) |
| Positive | 38 (39) | 27 (46) | 2 (14) | 4 (50) | 5 (29) |
| Stromal FN | |||||
| Negative | 35 (36) | 27 (46) | 3 (21) | 1 (13) | 4 (24) |
| Moderate | 55 (56) | 28 (47) | 10 (72) | 6 (74) | 11 (64) |
| Abundant | 8 (8) | 4 (7) | 1 (7) | 1 (13) | 2 (12) |
| Cellular FN | |||||
| Negative | 63 (64) | 47 (80) | 7 (50) | 4 (50) | 5 (29) |
| Positive | 35 (36) | 12 (20) | 7 (50) | 4 (50) | 12 (71) |
Abbreviations: CEONC=Oncology Center of Cascavel; FN=fibronectin; KUH=Kuopio University Hospital; OTSCC=oral tongue squamous cell carcinoma; OUH=Oulu University Hospital; TNC=tenascin-C.
Amounts of various scoring groups shown in total and for each individual hospital separately.
Stromal TNC predicts outcome in OTSCC
The expression patterns of TNC were variable when comparing the stromal fibroblast-like cell and cancer cell components of the tumours. Stromal TNC expression was not associated with any of the clinical and demographic parameters (gender, age, hospital, stage, neck lymph nodes and adjuvant therapy, Supplementary Tables 3 and 4). A total of 65 cancer deaths occurred among combined group of all the stages including 178 patients during the whole follow-up period.
In the pooled group (all stages of disease), abundant stromal TNC served as a prognosticator in univariate analysis when compared with the negative group, with a HR and 95% confidence intervals (CI) of 3.21 (1.48–6.96; Supplementary Table 5). Five-year cumulative survival rates were 70%, 70% and 20% for the negative, moderate and abundant groups, respectively (Supplementary Figure 1A and E). The numbers of cancer deaths/total number of patients in a given group during the whole follow-up time in the negative, moderate and abundant groups were 14 out of 43, 39 out of 119 and 12 out of 16, respectively. When compared with the stromal TNC-negative group, the age, gender, hospital, adjuvant therapy and stage-adjusted HR and 95% CI in the multivariate analysis for abundant group was 2.48 (1.04–5.90). Tenascin-C expression in the tumour cells did not have statistically significant prognostic value (Supplementary Figure 1C and Supplementary Table 5).
Stromal and intracellular FN predict outcome in OTSCC
The stromal expression of FN in fibroblasts and the fibrillary network was associated with hospital, stage, lymph node metastasis and treatment modalities in the combined group of all stages (Supplementary Table 3). Among stages I–II, the stromal expression of FN was associated only with adjuvant therapy (Supplementary Table 4).
In the pooled group (all stages of disease), stromal FN was an excellent prognosticator (Supplementary Figure 1B and Supplementary Table 5) with 5-year cumulative survival rates being 89%, 62% and 22% for the negative, moderate and abundant groups, respectively (Supplementary Figure 1E). The number of cancer deaths/total number of patients during the whole follow-up time in the negative, moderate and abundant groups was 5 out of 51, 43 out of 105 and 17 out of 22, respectively. In this pooled group, intracellular FN expression also predicted a poor prognosis (Supplementary Figure 1D and Supplementary Table 5) with a 5-year cumulative survival rate of 75% for the negative group and 54% for the positive group (Supplementary Figure 1E). In the univariate analysis including all stages, HRs for moderate and abundant stromal FN were 5.45 (2.16–13.77) and 13.58 (4.97–37.11), respectively (Supplementary Table 5). The HR for FN-positive cancer cells was 2.06 (1.25–3.38). In multivariate analysis the respective HRs were 5.33 (2.07–13.73) and 9.64 (3.34–27.83), and 2.08 (1.22–3.56) for FN-positive cancer cells (Supplementary Table 5).
Subgroup analysis of TNC and FN stainings
As staining patterns of both TNC and FN were strongly associated with patient outcome, we did a subgroup analysis in early stage OSTCC patients. Among early stage patients, there were a total of 22 events for 98 patients during the whole follow-up period.
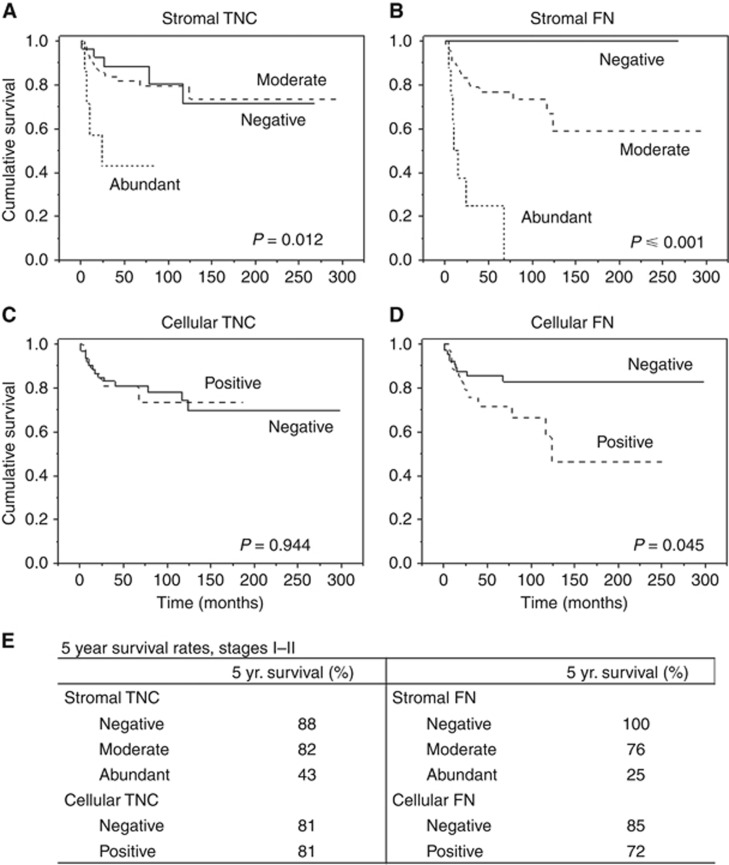
There was no difference in outcome observed between stromal TNC-negative and moderate groups (Figure 3A and E) or between cellular TNC-negative and positive groups (Figure 3C and E) among early stage patients. However, the group with abundant stromal TNC differed significantly from those with negative or moderate staining with a 5-year survival of 43% (in comparison with 88% for negative and 82% for the moderate groups). The HRs were 5.18 (1.29–18.61) and 6.54 (1.20–35.75) in uni- and multivariate analyses in comparison with the negative group, respectively (Table 3). The number of cancer deaths during the whole follow-up time in the negative, moderate and abundant groups was 5 out of 26, 13 out of 65 and 4 out of 7, respectively. Cancer cell TNC expression had no statistically significant prognostic value (Figure 3C and E and Table 3).
Figure 3.
Survival of stage I and II OTSCC patients.Kaplan–Meier curves representing the cumulative survival of early stage OTSCC patients divided by stromal TNC (A) and FN (B) staining, as well as cellular TNC (C) and FN (D). In curves A–D the survivals are shown throughout the entire follow-up time. Five-year survivals are shown separately (E). Log-rank test was used to test statistical significance.
Table 3. Cox regression uni- and multivariate analyses of stage I and II OTSCC patients.
| HR | 95% CI for HR | P-value | |
|---|---|---|---|
|
Univariate analysis, stages I–II | |||
| Stromal TNC | 0.025 | ||
| Negative | 1 | ||
| Moderate | 1.09 | 0.39–3.07 | NS |
| Abundant | 5.18 | 1.29–18.61 | 0.019 |
| Cancer cell TNC | NS | ||
| Negative | 1 | ||
| Positive | 1.03 | 0.43–2.48 | NS |
| Stromal FNa | <0.001 | ||
| Moderate | 1 | ||
| Abundant | 6.10 | 2.36–15.75 | <0.001 |
| Cancer cell FN | NS | ||
| Negative | 1 | ||
| Positive | 2.30 | 0.99–5.33 | NS |
|
Multivariate analysis, stages I–II | |||
| Stromal TNC | 0.012 | ||
| Negative | 1 | ||
| Moderate | 0.76 | NS | |
| Abundant | 6.54 | 0.23–2.48 | 0.030 |
| Cancer cell TNC | NS | ||
| Negative | 1 | ||
| Positive | 1.61 | 0.54–4.78 | NS |
| Stromal FNa | 0.004 | ||
| Moderate | 1 | ||
| Abundant | 5.91 | 1.75–19.94 | 0.004 |
| Cancer cell FN | NS | ||
| Negative | 1 | ||
| Positive | 1.56 | 0.61–4.04 | NS |
Abbreviations: CI=confidence interval; FN=fibronectin; HR=hazard ratio; OTSCC=Oncology Center of Cascavel; TNC=tenascin-C.
Multivariate analysis adjusted for age, gender and hospital.
HR and their 95% CI, as well as P-values. Analyses were performed separately for stromal and cellular TNC and FN. In multivariate analysis the HRs were adjusted for age, gender and hospital. The HR for stromal FN in multivariate analysis was calculated by comparing abundant to moderate due to the lack of end points (cancer deaths) in the FN-negative group. Statistically significant values are bolded.
HR could not be calculated comparing with ‘Negative' group due to lack of end points in that group.
Surprisingly, no cancer deaths of early stage patients were observed when stromal FN was negative. Thus, all of the cancer-specific deaths occurred in the moderately or abundantly stained groups. The numbers of cancer deaths during the whole follow-up time in the negative, moderate and abundant groups were 0 out of 35, 15 out of 55 and 7 out of 8, respectively. The 5-year cumulative survival rates were 100%, 76% and 25% for the negative, moderate and abundant groups, respectively (Figure 3B and E). As there were no disease-specific deaths in the stromal FN-negative group, the HR for the abundant group was calculated by comparing it with the moderate group. This resulted in HRs of 6.10 (2.36–15.75) and 5.91 (1.75–19.94) in uni- and multivariate analyses, respectively (Table 3).
Intracellular FN proved to be a significant prognostic factor in early stage cases in Kaplan–Meier analysis, but not in Cox regression uni- or multivariate analysis with cumulative 5-year survival rates of 85% and 72% for negative and positive groups, respectively (Figure 3D and E; Table 3).
Correlations of stromal TNC, FN and α-SMA
Carcinoma-associated fibroblasts (CAFs), which are identified by their intracellular α-SMA, are known predictors for poor prognosis in various malignancies (de Vlieghere et al, 2015), including OTSCC (Kellermann et al, 2007; Li et al, 2015; Bagordagis et al, 2016). However, CAFs are not prognostic indicators at early stage OTSCC (Almangush et al, 2014; Kelner et al, 2015), only in patient material of pooled stages, and at late stages when the variation in prognostic markers does not affect the choice of treatment so drastically, as more effective modalities are utilised in all cases. In this study we re-evaluated the prognostic significance of stromal α-SMA using a pilot group of 60 (with 17 cancer deaths) random samples from our extensive patient material. Indeed, in concordance to previous work of Almangush et al (2014) and Kelner et al (2015), in our pilot group the stromal α-SMA was found not to be predictive of patient outcome in early stage cases (Supplementary Figure 2). In addition, among early stage cases only a weak correlation was found between stromal α-SMA and TNC (0.395; 0.344 in all stages) or FN (0.441; 0.402 in all stages, Supplementary Table 2), the two proteins shown in this study to have prognostic value in early stage OTSCC. Furthermore, a correlation was found in early stage cases between stromal TNC and FN with a correlation coefficient of 0.612 (0.617 in all stages, Supplementary Table 2).
After we found a strong correlation between stromal TNC and FN, we analysed those cases in which abundant staining of both these markers was observed in the stroma. A total of 14 patients showed abundant staining of both stromal TNC and FN, and 5 of these were among early stage OTSCC. Remarkably, among all stages there was only 1 patient alive after follow-up; 12 deaths were due to cancer and 1 due to another cause. There were no survivors at follow-up among the early stage patients; four were cancer deaths and one due to another cause.
Discussion
There is a lack of potential markers to assess the need of elective neck dissection for early stage tongue cancer. In this international multi-centre study, we show that immunohistochemical staining for FN and TNC could potentially be used in clinical decision-making for early stage OTSCC.
The strengths and limitations of our study should be taken into account when interpreting the results. Our study had an international, multi-centre study design, with which we aimed to counteract country-specific biases. The sample size in early stage group is small (n=98), especially in TNC and FN abundant groups, and might limit the statistical power and analyses in this group. This can be seen in the wide CIs in some of the analyses. However, the effects of TNC and FN expression were similar in both early and late-stage cancers, suggesting actual effect for the biomarkers. Finally, we adjusted for several pre-determined confounding factors in our analyses and showed that TNC and FN are associated to survival, also after the adjustment.
Although several OTSCC prognostic molecular markers have been reported (Bello et al, 2010), so far none is in clinical use to pinpoint those individuals who benefit from the primary neck dissection and multimodality treatment (Barnes et al, 2005). In this study, we hypothesised that the expression of two ECM proteins could predict the prognosis of cancers. After mice immunisation with stromal cells and production of related antibodies, we decided to assess the efficacy of TNC and FN as prognostic markers in OTSCC, as the role of these proteins as prognostic indicators in several cancers is well known (Jahkola et al, 1999; Emoto et al, 2001; Ioachim et al, 2002; Brunner et al, 2004; Ioachim et al, 2005; O'Shannessy et al, 2014). Neither of these proteins had been previously studied for their ability to predict prognosis of early stage OTSCC. In this study we showed that the identification of patients in low-risk and high-risk groups could potentially be achieved by assessing the stromal expression of TNC and FN. Stromal TNC and FN were both independent prognostic indicators not only in pooled stages of OTSCC, but most importantly in cases with clinical stages I–II when abundant stromal staining is detected.
Tenascin-C is an ECM glycoprotein with a six-armed quaternary structure. Under physiological conditions, TNC is found abundantly in several embryonic tissues, especially related to the epithelial mesenchymal transition (EMT), and to cell migration pathways. In adult tissues, it is expressed mainly during inflammation, wound healing and cancer progression (Orend and Chiquet-Ehrismann, 2006). Increased TNC expression in the tumour-adjacent ECM has been shown to correlate with a clinically aggressive phenotype or poor prognosis in several cancers such as oesophageal (Yang et al, 2016), breast (Ishihara et al, 1995; Jahkola et al, 1998) colorectal (Emoto et al, 2001; Sis et al, 2004), bladder (Brunner et al, 2004) and Merkel cell carcinomas (Koljonen et al, 2005); glioma (Herold-Mende et al, 2002), malignant pleural mesothelioma (Kaarteenaho-Wiik et al, 2003), glioblastoma multiforme (Leins et al, 2003), malignant melanoma (Kääriäinen et al, 2006) and astrocytoma (Varga et al, 2012). In OSCC, TNC is produced by carcinoma cells (Driemel et al, 2007) or by surrounding mesenchymal fibroblast-like cells (Hindermann et al, 1999; Metwaly et al, 2012). Tenascin-C is present in the stroma of potentially malignant oral lesions and its expression is increased along the degree of dysplasia (Tiitta et al, 1994). Furthermore, TNC expression is high in budding cancer cells and in the surrounding stroma (Tiitta et al, 1994; Hindermann et al, 1999). Tenascin-C has a variety of pro-tumourigenic interactions with cancer cells, CAFs, lymphocytes and tumour-associated macrophages, as well as angiogenesis promoting interactions with endothelial cells. It interacts with cancer cells by reducing adhesion, enhancing proliferation, migration and invasion, and increasing dissemination and homing. Tenascin-C inhibits adhesion of CAFs, and activation, proliferation and cytokine production of tumour infiltrating lymphocytes (Midwood and Orend, 2009). Increased expression of TNC by carcinoma cells correlates with the grade of OSCC (Hindermann et al, 1999). In our study, TNC expression in tumour cells was not a prognosticator in early stage OTSCC, nor was it a prognosticator among all the stages. On the other hand, the results of the present study indicate that stromal TNC was an independent prognosticator among all stages, and more importantly, among early stages. However, TNC predicted poor prognosis only when detected abundantly in the tumour stroma as there was no difference in prognosis between negative and moderate stromal TNC staining. Taken together, we hypothesise that TNC is related to dissemination of cancer cells and aiding metastasis formation leading to poor prognosis.
Fibronectin is a multifunctional glycoprotein organised in a fibrillary network. It is expressed mainly by fibroblasts, and during development and wound healing. Fibronectin regulates a variety of cell activities such as adhesion, migration, growth and differentiation (Mao and Schwarzbauer, 2005; White et al, 2008) and is involved in cancer invasion and metastasis (Stivarou and Patsavoudi, 2015), and stromal FN correlates with poor prognosis or aggressive phenotypes in colorectal cancer (O'Shannessy et al, 2014), urothelial carcinoma (Ioachim et al, 2005) and breast cancer (Ioachim et al, 2002). Fibronectin is one of the markers of EMT, a process essential for dissemination and invasion of carcinoma cells (Chong et al, 2012), as well as one of the markers of angiogenesis in primary tumours and metastases (Rybak et al, 2007). Fibronectin is upregulated in head and neck cancer (Al Moustafa et al, 2002; Ferrari et al, 2009), and the presence and amount of FN in tumour stroma is linked to an increased number of cervical metastases, extracapsular spread and elevated risk for disease-specific mortality in OSCCs (Lyons et al, 2001). Here, in concordance to previous studies, stromal FN was shown to be an excellent prognosticator among all stages of OTSCC and more importantly among early stage cases. The fact that there were no disease-specific deaths in the stromal FN-negative group among stage I–II patients is itself a remarkable demonstration of the specific nature of this marker. Cellular FN, on the other hand, had prognostic value in the pooled group of all stages in univariate analysis, and additionally it was an independent prognosticator in multivariate analysis. Furthermore, cellular FN predicted survival in stages I–II in Kaplan–Meier, but not in Cox regression uni- or multivariate analysis.
Fibronectin and TNC are co-expressed in several cancers (Hanamura et al, 1997; Yoshida et al, 1997) including OSCC (Ramos et al, 1998) and play a role in the healing by influencing cell adherence and migration especially in epithelial cells (Mackie et al, 1988). It has been suggested that both TNC and FN, as antagonists for each other (TNC being an anti-adhesive and FN an adhesive protein), are essential in cancer invasion (Yoshida et al, 1997). In support of this, Ramos et al (1998) found that TNC matrix production was blocked in vitro when FN antibody was introduced to CAF monolayer culture cultivated on FN-coated glass coverslips. This hypothesis is supported by our results as well, as we observed a strong correlation between stromal TNC and FN in our clinical sample (Supplementary Table 2). Furthermore, additional prognostic value was observed in our cohort among those cases with abundant stromal TNC and FN; among all the stages only one patient survived and no patients among the early stages survived. This implies a strong biological synergy of these proteins in cancer progression.
Our results provide feasible tools for more accurate division of the early stage OTSCC patients into low-risk and high-risk groups. These could be utilised in clinical decision-making in terms of primary neck dissection and multimodality treatments. Furthermore, our results should encourage more basic research on the biology of cancer/TME-interactions, and demonstrate the translational relevance of research on specific proteins. Even though our results provide a basis for using TNC and FN in routine pathology to categorise early stage OTSCC patients into low- and high-risk groups, the cohort was relatively small (n=98 for early stage patients). The small number of patients led to relatively broad CIs in Cox regression analysis, especially in abundant stromal FN and TNC among early stage patients. Although we successfully compared our antibodies with commercial ones, these studies should be replicated using other antibodies for TNC and FN, and preferably with larger patient groups.
In conclusion, based on our multi-centre study we recommend the analysis of stromal TNC and FN as an integral part of diagnosis and treatment planning in early stage OTSCC. When stromal TNC and FN both turn out to be negative, elective neck dissection and multimodality treatments should be avoided, whereas abundant stromal staining of these proteins provides a strong signal to consider more aggressive treatments. If future studies demonstrate that immunohistochemical staining of TNC and FN assessed from biopsies is equally specific in the prognostication to that of surgical specimens, the treatment regimen can be chosen preoperatively.
Acknowledgments
Mrs Sanna Juntunen and Tanja Kuusisto are acknowledged for their excellent technical work on immunohistochemistry. This work was funded by Sigrid Juselius Foundation, Finnish Cancer Foundation, Orion Research Foundation, Paulo Foundation, Ida Montin Foundation, The Finnish Funding Agency for Technology and Innovation, Mary and Georg C Ehrnrooths Foundation, Thelma Mäkikyrö Foundation, Finnish Anti-Tuberculosis Association, Finnish Dental Society Apollonia, Medical Research Centre Oulu and research funds from the Medical Faculty of the University of Oulu and Oulu University Hospital special state support for research. In Brazil, research was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, Brasília, Brazil (473825/2013-9); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, Brasília, Brazil (AUXPE-PVES-570/2013).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Al Moustafa A-E, Alaoui-Jamali MA, Batist G, Hernandez-Perez M, Serruya C, Alpert L, Black MJ, Sladek R, Foulkes WD (2002) Identification of genes associated with head and neck carcinogenesis by cDNA microarray comparison between matched primary normal epithelial and squamous carcinoma cells. Oncogene 21(17): 2634–2640. [DOI] [PubMed] [Google Scholar]
- Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola S, Nieminen O, Soini Y, Kosma V-M, Koivunen P, Grénman R, Leivo I, Salo T (2014) Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck 36(6): 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagordakis E, Sawazaki-Calone I, Macedo CC, Carnielli CM, de Oliveira CE, Rodrigues PC, Rangel AL, Dos Santos JN, Risteli J, Graner E, Salo T, Leme AF, Coletta RD (2016) Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumour Biol 37(7): 9045–9057. [DOI] [PubMed] [Google Scholar]
- Barnes L, Eveson JW, Reichart P, Sidransky D (2005) Pathology and Genetics of Head and Neck Tumours. IARC Press: Lyon. [Google Scholar]
- Bello IO, Soini Y, Salo T (2010) Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol 46(9): 630–635. [DOI] [PubMed] [Google Scholar]
- Bello IO, Vered M, Dayan D, Dobriyan A, Yahalom R, Alanen K, Nieminen P, Kantola S, Läärä E, Salo T (2011) Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol 47(1): 33–38. [DOI] [PubMed] [Google Scholar]
- Brunner A, Mayerl C, Tzankov A, Yerdorfer I, Tschörner I, Rogatsch H, Mikuz G (2004) Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J Clin Pathol 57(9): 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong HC, Tan CK, Huang R-L, Tan NS (2012) Matricellular proteins: a sticky affair with cancers. J Oncol 2012: 351089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A, Kane S, Arya S, Ghosh-Laskar S, Chaturvedi P, Pai P, Nair S, Nair D, Badwe R (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373(6): 521–529. [DOI] [PubMed] [Google Scholar]
- de Vlieghere E, Verset L, Demetter P, Bracke M, de Wever O (2015) Cancer-associated fibroblasts as target and tool in cancer therapeutics and diagnostics. Virchows Arch 467(4): 367–382. [DOI] [PubMed] [Google Scholar]
- Driemel O, Dahse R, Berndt A, Pistner H, Hakim SG, Zardi L, Reichert TE, Kosmehl H (2007) High-molecular tenascin-C as an indicator of atypical cells in oral brush biopsies. Clin Oral Invest 11(1): 93–99. [DOI] [PubMed] [Google Scholar]
- Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y (2001) Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer 92(6): 1419–1426. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Codecà C, Fiore J, Moneghini L, Bosari S, Foa P (2009) Biomolecular markers in cancer of the tongue. J Oncol 2009: 412908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganly I, Patel S, Shah J (2012) Early-stage squamous cell cancer of the oral tongue - clinicopathologic features affecting outcome. Cancer 118(1): 101–111. [DOI] [PubMed] [Google Scholar]
- Grunow R, D'Apuzzo M, Wyss-Coray T, Frutig K, Pichler WJ (1994) A cell surface ELISA for the screening of monoclonal antibodies to antigens on viable cells in suspension. J Immunol Methods 171(1): 93–102. [DOI] [PubMed] [Google Scholar]
- Hanamura N, Yoshida T, Matsumoto E-I, Kawarada Y, Sakakura T (1997) Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer 73(1): 10–15. [DOI] [PubMed] [Google Scholar]
- Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner H-H (2002) Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer 98(3): 362–369. [DOI] [PubMed] [Google Scholar]
- Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, Kosmehl H (1999) Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol 189(4): 475–480. [DOI] [PubMed] [Google Scholar]
- Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ, Pavlidis N (2002) Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer 38(18): 2362–2370. [DOI] [PubMed] [Google Scholar]
- Ioachim E, Michael M, Stavropoulos NE, Kitsiou E, Salmas M, Malamou-Mitsi V (2005) A clinicopathological study of the expression of extracellular matrix components in urothelial carcinoma. BJU Int 95(4): 655–659. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Yoshida T, Tamaki H, Sakakura T (1995) Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res 1(9): 1035–1041. [PubMed] [Google Scholar]
- Jahkola T, Toivonen T, Virtanen I, Von Smitten K, Nordling S, Von Boguslawski K, Haglund C, Nevanlinna H, Blomqvist C (1998) Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 78(11): 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahkola T, Toivonen T, Von Smitten K, Virtanen I, Wasenius V-M, Blomqvist C (1999) Cathepsin-D, urokinase plasminogen activator and type-1 plasminogen activator inhibitor in early breast cancer: an immunohistochemical study of prognostic value and relations to tenascin C and other factors. Br J Cancer 80(1-2): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kääriäinen E, Nummela P, Soikkeli J, Yin M, Lukk M, Jahkola T, Virolainen S, Ora A, Ukkonen E, Saksela O, Hölttä E (2006) Switch to an invasive growth phase in melanoma is associated with tenascin-C, fibronectin, and procollagen-I forming specific channel structures for invasion. J Pathol 210(2): 181–191. [DOI] [PubMed] [Google Scholar]
- Kaarteenaho-Wiik R, Soini Y, Pöllänen R, Pääkkö P, Kinnula VL (2003) Over-expression of tenascin-C in malignant pleural mesothelioma. Histopathology 42(3): 280–291. [DOI] [PubMed] [Google Scholar]
- Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, Nishimoto I, Kowalski LP, Coletta RD (2007) Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology 51(6): 849–853. [DOI] [PubMed] [Google Scholar]
- Kelner N, Rodrigues PC, Bufalino A, Fonseca FP, Santos-Silva AR, Miguel MC, Pinto CA, Leme AF, Graner E, Salo T, Kowalski LP, Coletta RD (2015) Activin A immunoexpression as predictor of occult lymph node metastasis and overall survival in oral tongue squamous cell carcinoma. Head Neck 37(4): 479–486. [DOI] [PubMed] [Google Scholar]
- Kim K-Y, Cha I-H (2012) The identification of significant chromosomal regions correlated with oral tongue cancer progression. J Cancer Res Clin Oncol 138(10): 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koljonen V, Jahkola T, Tukiainen E, Granroth G, Haglund C, Böhling T (2005) Tenascin-C in primary Merkel cell carcinoma. J Clin Pathol 58(3): 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leins A, Riva P, Lindstedt R, Davidoff MS, Mehraein P, Weis S (2003) Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer 98(11): 2430–2439. [DOI] [PubMed] [Google Scholar]
- Leskelä H-V, Risteli J, Niskanen S, Koivunen J, Ivaska KK, Lehenkari P (2003) Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun 311(4): 1008–1013. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang J, Chen S-W, Liu LL, Li L, Gao F, Zhuang S-M, Wang L-P, Li Y, Song M (2015) Cancer-associated fibroblasts provide a suitable microenvironment for tumor development and progression in oral tongue squamous cancer. J Transl Med 13(1): 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AJ, Bateman AC, Spedding A, Primrose JN, Mandel U (2001) Oncofetal fibronectin and oral squamous cell carcinoma. Br J Oral Maxillofac Surg 39(6): 471–477. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Halfter W, Liverani D (1988) Induction of tenascin in healing wounds. J Cell Biol. 107(6): 2757–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE (2005) Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24(6): 389–399. [DOI] [PubMed] [Google Scholar]
- Metwaly H, Maruyama S, Yamazaki M, Tsuneki M, Abé T, Jen KY, Cheng J, Saku T (2012) Parenchymal-stromal switching for extracellular matrix production on invasion of oral squamous cell carcinoma. Hum Pathol 43(11): 1973–1981. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Orend G (2009) The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 3(3-4): 287–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R (2006) Tenascin-C induced signaling in cancer. Cancer Lett 244(2): 143–163. [DOI] [PubMed] [Google Scholar]
- O'Shannessy DJ, Somers EB, Chandrasekaran LK, Nicolaides NC, Bordeaux JB, Gustavson MD (2014) Influence of tumor microenvironment on prognosis in colorectal cancer: tissue architecture-dependent signature of endosialin (TEM-1) and associated proteins. Oncotarget 5(12): 3983–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos DM, Chen B, Regezi J, Zardi L, Pytela R (1998) Tenascin-C matrix assembly in oral squamous cell carcinoma. Int J Cancer 75(5): 680–687. [DOI] [PubMed] [Google Scholar]
- Rybak J, Roesli C, Kaspar M, Villa A, Neri D (2007) The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res 67(22): 10948–10957. [DOI] [PubMed] [Google Scholar]
- Salo S, Bitu C, Merkku K, Nyberg P, Bello IO, Vuoristo J, Sutinen M, Vähänikkilä H, Costea DE, Kauppila J, Lehenkari P, Dayan D, Vered M, Risteli J, Salo T (2013) Human bone marrow mesenchymal stem cells induce collagen production and tongue cancer invasion. PLoS One 8(10): e77692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sis B, Saĉol O, Küpelioĉ A, Sokmen S, Terzi C, Fuzun M, Özer E, Bishop P (2004) Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol Res Pract 200(5): 379–387. [DOI] [PubMed] [Google Scholar]
- Sobral LM, Bufalino A, Lopes MA, Graner E, Salo T, Coletta RD (2011) Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol 47(9): 840–846. [DOI] [PubMed] [Google Scholar]
- Stivarou T, Patsavoudi E (2015) Extracellular molecules involved in cancer cell invasion. Cancers 7(1): 238–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitta O, Happonen RP, Virtanen I, Luomanen M (1994) Distribution of tenascin in oral premalignant lesions and squamous cell carcinoma. J Oral Pathol Med 23(10): 446–450. [DOI] [PubMed] [Google Scholar]
- Varga I, Hutóczki G, Szemcsák CD, Zahuczky G, Tóth J, Adamecz Z, Kenyeres A, Bognár L, Hanzély Z, Klekner A (2012) Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol Oncol Res 18(2): 413–420. [DOI] [PubMed] [Google Scholar]
- White ES, Baralle FE, Muro AF (2008) New insights into form and function of fibronectin splice variants. J Pathol 216(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z-T, Yeo S-Y, Yin Y-X, Lin Z-H, Lee H-M, Xuan Y-H, Cui Y, Kim S-H (2016) Tenascin-C, a prognostic determinant of esophageal squamous cell carcinoma. PLoS One 11(1): e0145807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Matsumoto E-I, Hanamura N, Kalembyi I, Katsuta K, Ishihara A, Sakakura T (1997) Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 182(4): 421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.