Abstract
Background:
Severe chronic hypoxia is associated with tumour necrosis. In patients with muscle invasive bladder cancer (MIBC), necrosis is prognostic for survival following surgery or radiotherapy and predicts benefit from hypoxia modification of radiotherapy. Adding mitomycin C (MMC) and 5-fluorouracil (5-FU) chemotherapy to radiotherapy improved locoregional control (LRC) compared to radiotherapy alone in the BC2001 trial. We hypothesised that tumour necrosis would not predict benefit for the addition of MMC and 5-FU to radiotherapy, but would be prognostic.
Methods:
Diagnostic tumour samples were available from 230 BC2001 patients. Tumour necrosis was scored on whole-tissue sections as absent or present, and its predictive and prognostic significance explored using Cox proportional hazards models. Survival estimates were obtained by Kaplan–Meier methods.
Results:
Tumour necrosis was present in 88/230 (38%) samples. Two-year LRC estimates were 71% (95% CI 61–79%) for the MMC/5-FU chemoradiotherapy group and 49% (95% CI 38–59%) for the radiotherapy alone group. When analysed by tumour necrosis status, the adjusted hazard ratios (HR) for MMC/5-FU vs. no chemotherapy were 0.46 (95% CI: 0.12–0.99; P=0.05, necrosis present) and 0.55 (95% CI: 0.31–0.98; P=0.04, necrosis absent). Multivariable analysis of prognosis for LRC by the presence vs. absence of necrosis yielded a HR=0.89 (95% CI 0.55–1.44, P=0.65). There was no significant association for necrosis as a predictive or prognostic factor with respect to overall survival.
Conclusions:
Tumour necrosis was neither predictive nor prognostic, and therefore MMC/5-FU is an appropriate radiotherapy-sensitising treatment in MIBC independent of necrosis status.
Keywords: necrosis, hypoxia, muscle invasive bladder cancer, radiotherapy, chemotherapy, randomised controlled trial, BC2001
Patients with muscle invasive bladder cancer (MIBC) undergo a radical cystectomy to remove the whole bladder or organ preserving tri-modality treatment. The most common tri-modality treatment involves a transurethral resection of the bladder (TURBT) to remove gross tumour from the bladder wall followed by radiotherapy with concurrent chemotherapy with or without prior neoadjuvant chemotherapy. Combining radiation with chemotherapy gives comparable rates of disease-specific and overall survival (OS) to cystectomy (approximately 30–50% at 5 years; Hoskin et al, 2010; Choudhury et al, 2011; James et al, 2012; Booth et al, 2014). Two large phase III randomised control trials from the UK showed the benefit of giving either chemotherapy (BC2001) or hypoxia-modifying therapy (BCON) with radiation. The BC2001 study showed that the addition of mitomycin C (MMC) and 5-fluorouracil (5-FU) to radiotherapy improved locoregional control; at two years LRC estimates were 67% (95% CI: 59 to 74) in the chemoradiotherapy group and 54% (95% CI: 46–62) in the radiotherapy group (HR 0.68, 95% CI, 0.48–0.96; P=0.03). The BCON study showed adding carbogen and nicotinamide (CON) to radiotherapy increased 3-year OS rates by 13% (Hoskin et al, 2010). With increasing options for treating the disease, there is a need to predict which patients are likely to benefit from tri-modality treatment and to which radiation modifying combination. Although there are a number of accepted prognostic factors for MIBC such as stage, grade and performance status of the patient, there are as yet no predictive factors, which can be used to select patients who are likely to benefit from the different treatment regimens.
Necrosis can be seen histopathologically using haematoxylin and eosin (H&E) staining and is likely to result from periods of low oxygen tension resulting in cell death. Thus, necrosis may be a surrogate for extreme hypoxia within tumours. Hypoxia is an adverse prognostic factor in MIBC (Hoskin et al, 2003; Palit et al, 2005; Ord et al, 2007; Hunter et al, 2014) and necrosis was associated with a poor outcome in three independent studies (Ord et al, 2007; Eustace et al, 2013; Soave et al, 2015). The presence of frank necrosis was also shown to correlate with the expression of hypoxia-associated markers and to predict benefit from hypoxia modification in patients enroled in the BCON trial (Eustace et al, 2013).
The BC2001 study used MMC and 5-FU as radio-sensitising agents. MMC is the prototype bioreductive agent, that is, a pro-drug that is selectively metabolised in hypoxic cells to a cytotoxic compound (McKeown et al, 2007). However, although it has some bioreductive activation and selective toxicity towards hypoxic cells in vitro, it is a weak bioreductive agent in vivo because hypoxia-independent toxicity occurs at a dose level lower than that required for hypoxia-dependent toxicity (McKeown et al, 2007). Therefore, we hypothesised that the presence of necrosis in tumour samples from BC2001 patients would not predict benefit for the addition of MMC and 5-FU to radiotherapy, but would be prognostic. The study reported here aimed to test this hypothesis.
Patients and methods
Patients and samples
A retrospective cohort study was carried out using diagnostic tumour samples from patients enroled in the BC2001 phase III trial (ISRCTN68324339, CRUK/01/004). BC2001 has been described in detail elsewhere (James et al, 2012; Huddart et al, 2013). In brief, 360 patients with MIBC suitable for radical treatment with radiotherapy were randomised to either radiotherapy alone (n=178) or with concurrent MMC (12 mg m−2 on D1) and 5-FU (500 mg m−2 on D1-5 and D16–20, n=182). Patients were treated with one of two radiotherapy regimens in standard use in the UK, either 64 Gy in 32 fractions over 6.5 weeks or 55 Gy in 20 fractions over 4 weeks.
Formalin-fixed and paraffin-embedded (FFPE) tissue samples from the TURBT were available for central histological assessment from 245 patients diagnosed with MIBC between May 2001 and December 2007. Data from 230 were analysed (Figure 1). Approval for the necrosis study was obtained from the North West Greater Manchester East Ethics Committee (09/H1013/24). Informed consent for sample collection and analysis were obtained prospectively from each patient as part of the main trial. REMARK guidelines for reporting tumour marker prognostic studies were followed (McShane et al., 2005).
Figure 1.
The CONSORT diagram showing the flow of patients through the study.
Histopathology
Methods have been described previously in detail (Eustace et al, 2013). In summary, a single 4 μm whole-tissue section from the FFPE block was obtained for H&E staining. As in other standard solid tumour histopathological reporting, tissue-hypoxia-related coagulative necrosis, characterised by loss of tissue architecture, increased eosinophilia and nuclear breakdown, was scored by a consultant urohistopathologist as absent vs present. Supplementary Figure S1 is a selection of histological images illustrating necrosis. The pathologist was blinded to the treatment received by the patient and to the clinical outcome data.
Statistical analyses
Analyses were undertaken for locoregional control (LRC) and OS. LRC was the primary endpoint of the trial and was measured from the time of randomisation to recurrence in pelvic nodes or bladder (muscle invasive or non-muscle invasive), with data censored at the first sign of metastasis (if this occurred ⩾30 days before locoregional failure), a second primary cancer or death. Median follow-up for the 230 patients in this cohort was 71.3 months.
Heterogeneity in the treatment effect according to the tumour necrosis status was first explored graphically by Kaplan–Meier curves comparing treatment by necrosis status, and log-rank tests for each comparison reported. The predictive significance of necrosis was formally addressed by a test for interaction, first in a Cox proportional hazards model including treatment, necrosis status and their interaction (unadjusted analysis), and then including known prognostic factors (in the literature or relevant in the main BC2001 trial (adjusted analysis). These factors were: sex, age, WHO status, stage, tumour size (only for LRC), haemoglobin (only for OS), residual mass after resection and presence of Cis. The prognostic significance of necrosis was similarly explored by Kaplan–Meier curves comparing necrosis status and fitting Cox models with necrosis and treatment, but without the interaction term.
Hazard ratios (HR) and their 95% confidence intervals are reported for all variables in the final models. The association between necrosis status and other prognostic factors was explored by χ2-tests for categorical factors and t-test for continuous. The proportional hazards assumption of the Cox model was tested with the use of Schoenfeld residuals and found to hold for all three survival end points. All statistical analyses were performed using Stata (version 13.1, Stata Corp, College Station, TX, USA).
Results
Tumour samples from 230 of the 360 patients participating in the BC2001 chemoradiotherapy randomisation were available for analysis: 121 out of 230 (52.6%) patients received chemoradiotherapy and 109 out of 230 (47.5%) patients received radiotherapy alone (Figure 1). Baseline characteristics (Table 1) were generally balanced between the two randomisation groups within the main study, but patients in this analysis set undergoing radiotherapy alone tended to have had a higher percentage of incomplete resection (P=0.03) and larger tumours (P=0.07). Necrosis was present in 88 out of 230 (38%) of the tumours, and did not differ between treatment groups (P=0.82). The baseline characteristics of the necrosis cohort broadly represented the patients of the BC2001 trial (Supplementary Table S1) but, in comparison with the 130 patients with no tissue available for analysis, the 230 patient cohort had significantly more T2 disease (P=0.001), higher levels of haemoglobin (P=0.02) and a higher percentage received 64 Gy/32F (P<0.001). Supplementary Figure 2 shows there were no significant differences in LRC (P=0.76) and OS (P=0.16) for patients with or without tissue available.
Table 1. Baseline characteristics.
| Total | Chemoradiotherapy | Radiotherapy | ||
|---|---|---|---|---|
| 230 (100.0%) | 121 (100.0%) | 109 (100.0%) | P-valuea | |
|
Sex | ||||
| Male | 183 (79.6%) | 97 (80.2%) | 86 (78.9%) | 0.81 |
| Female | 47 (20.4%) | 24 (19.8%) | 23 (21.1%) | |
|
Age (years) | ||||
| N | 230 | 121 | 109 | 0.97 |
| Median (Q25–Q75) | 72.1 (65.7–76.4) | 72.1 (66–76.4) | 71.9 (65.7–76.2) | |
| Min–Max | 40.2–87 | 40.2–87 | 49–85.9 | |
|
WHO performance status | ||||
| 0 | 146 (63.5%) | 77 (63.6%) | 69 (63.3%) | 0.33 |
| 1 | 79 (34.3%) | 43 (35.5%) | 36 (33.0%) | |
| 2 | 5 (2.2%) | 1 (0.8%) | 4 (3.7%) | |
|
Pathological stage—primary tumour | ||||
| 1b | 1 (0.4%) | 0 (0.0%) | 1 (0.9%) | 0.72 |
| 2 | 202 (87.8%) | 107 (88.4%) | 95 (87.2%) | |
| 3a | 8 (3.5%) | 3 (2.5%) | 5 (4.6%) | |
| 3b | 10 (4.3%) | 6 (5.0%) | 4 (3.7%) | |
| 4a | 9 (3.9%) | 5 (4.1%) | 4 (3.7%) | |
|
Grade primary tumour | ||||
| 2 | 26 (11.3%) | 11 (9.1%) | 15 (13.8%) | 0.26 |
| 3 | 204 (88.7%) | 110 (90.9%) | 94 (86.2%) | |
|
Multiple tumours | ||||
| Yes | 46 (20.0%) | 20 (16.5%) | 26 (23.9%) | 0.21 |
| No | 183 (79.6%) | 101 (83.5%) | 82 (75.2%) | |
| Unknown | 1 (0.4%) | 0 (0.0%) | 1 (0.9%) | |
|
Extent of tumour resection | ||||
| Not resected/Biopsy | 25 (10.9%) | 18 (14.9%) | 7 (6.4%) | 0.03 |
| Complete resection | 123 (53.5%) | 67 (55.4%) | 56 (51.4%) | |
| Incomplete resection | 77 (33.5%) | 32 (26.4%) | 45 (41.3%) | |
| Resected (extent unknown) | 2 (0.9%) | 1 (0.8%) | 1 (0.9%) | |
| Unknown | 3 (1.3%) | 3 (2.5%) | 0 (0.0%) | |
|
Tumour size group | ||||
| <30 mm | 49 (21.3%) | 31 (25.6%) | 18 (16.5%) | 0.07 |
| >30 mm | 105 (45.7%) | 47 (38.8%) | 58 (53.2%) | |
| Unknown | 76 (33.0%) | 43 (35.5%) | 33 (30.3%) | |
|
Residual mass post resection | ||||
| Yes | 67 (29.1%) | 33 (27.3%) | 34 (31.2%) | 0.09 |
| No | 151 (65.7%) | 78 (64.5%) | 73 (67.0%) | |
| Unknown | 12 (5.2%) | 10 (8.3%) | 2 (1.8%) | |
|
Haemoglobin, g dl−1 | ||||
| N | 230 | 121 | 109 | 0.20 |
| Median (Q25–Q75) | 12.9 (11.9–14.1) | 12.8 (11.8–13.7) | 13.3 (11.9–14.2) | |
| Min–Max | 8–16.7 | 8.5–16.7 | 8–16.4 | |
|
WBC, × 109 per l | ||||
| N | 230 | 121 | 109 | 0.90 |
| Median (Q25–Q75) | 7 (5.6–8.8) | 7 (5.4–8.8) | 7.1 (5.7–8.8) | |
| Min–Max | 1.9–24 | 1.9–23.2 | 1.9–24 | |
|
Glomerular filtration rate (ml min−1 per 1.73 m2) | ||||
| N | 215 | 118 | 97 | 0.28 |
| Median (Q25–Q75) | 64 (49–80) | 64.5 (52–80) | 63 (48–77) | |
| Min–Max | 28–156 | 29–140 | 28–156 | |
|
Radiotherapy randomisation | ||||
| sTRT | 43 (18.7%) | 20 (16.5%) | 23 (21.1%) | 0.50 |
| RHDVRT | 35 (15.2%) | 21 (17.4%) | 14 (12.8%) | |
| Elective stRT | 152 (66.1%) | 80 (66.1%) | 72 (66.1%) | |
|
Radiotherapy treatment | ||||
| 55 Gy/20F | 74 (32.2%) | 39 (32.2%) | 35 (32.1%) | 0.98 |
| 64 Gy/32F | 156 (67.8%) | 82 (67.8%) | 74 (67.9%) | |
|
Neoadjuvant therapy received | ||||
| No | 159 (69.1%) | 84 (69.4%) | 75 (68.8%) | 0.92 |
| Yes | 71 (30.9%) | 37 (30.6%) | 34 (31.2%) | |
|
Necrosis | ||||
| Absent | 142 (61.7%) | 73 (60.3%) | 69 (63.3%) | 0.64 |
| Present | 88 (38.3%) | 48 (39.7%) | 40 (36.7%) | |
|
Muscle invasive BC (as per central review) | ||||
| Noc | 70 (30.4%) | 36 (29.8%) | 34 (31.2%) | 0.81 |
| Yes | 160 (69.6%) | 85 (70.2%) | 75 (68.8%) | |
|
Concurrent Cis (as per central review) | ||||
| No | 185 (80.4%) | 98 (81.0%) | 87 (79.8%) | 0.82 |
| Yes | 45 (19.6%) | 23 (19.0%) | 22 (20.2%) | |
Q1: Lower quartile, 25% percentile, Q3: Upper quartile, 75% percentile.
P-value: for categorical variables, χ2-test; for continuous variables, non-parametric Kruskall–Wallis rank test, except for Haemoglobin, t-test.
This tumour was deemed to be pathological stage T1, but radiologic staging confirmed the tumour as T3. Therefore, the patient was not considered to be ineligible for the trial. Analysed as T3.
All patients were confirmed as MIBC within the BC2001 study, however, not all tissue blocks were available for central review.
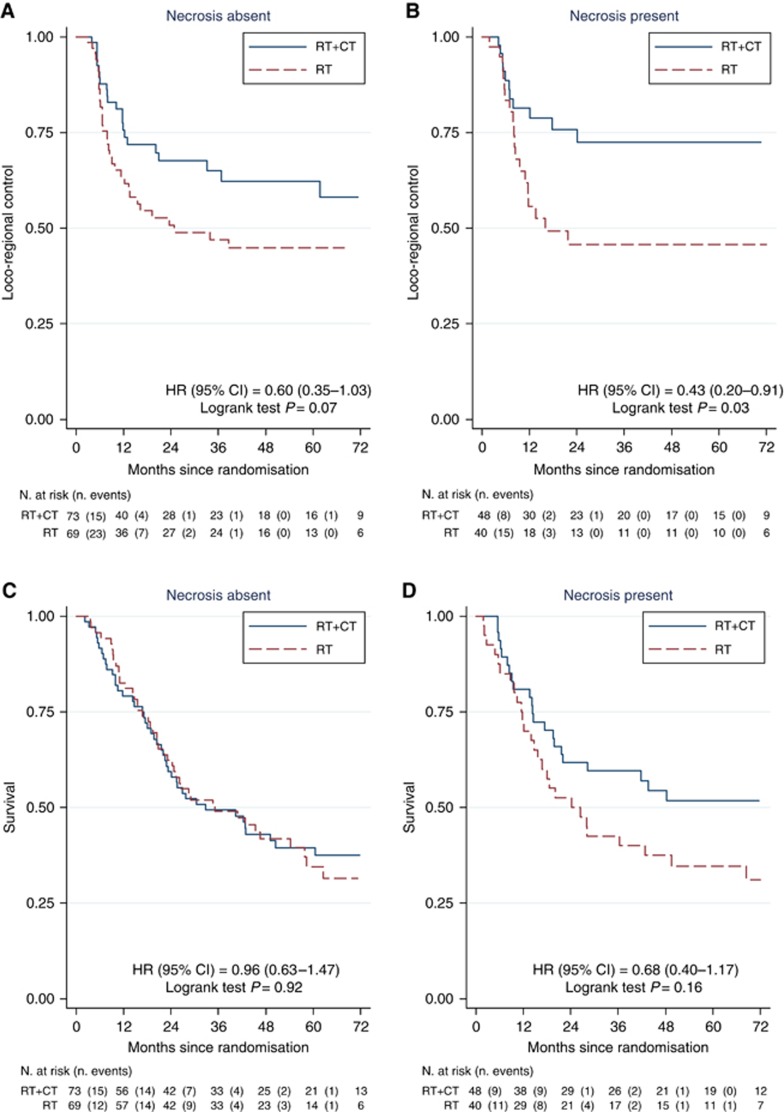
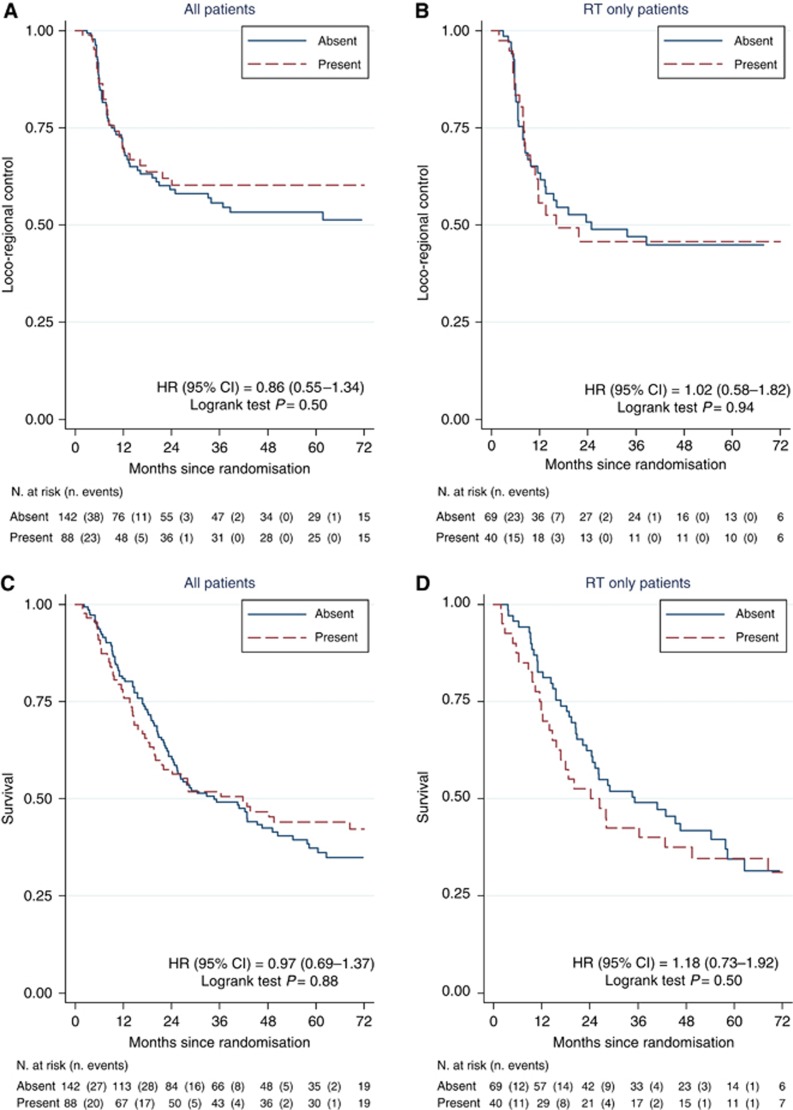
Two-year LRC estimates were 71% (95% CI 61–79%) for the chemoradiotherapy group and 49% (95% CI 38–59%) for the radiotherapy group. The presence of necrosis did not predict benefit from the addition of chemotherapy to radiotherapy. Figure 2A and B shows that concomitant chemotherapy improved LRC in patients with or without tumour necrosis. In 142 patients where necrosis was absent, 2-year LRC was 67.6% for the chemoradiotherapy group and 50.8% for radiotherapy alone (HR=0.60, 95% CI 0.35–1.03, log-rank P=0.07). In 88 patients with tumour necrosis, two-year LRC was 75.7% for the chemoradiotherapy group and 45.8% for radiotherapy alone (HR=0.43, 95% CI 0.20–0.91, log-rank P=0.03). There was no interaction between treatment and necrosis in either univariable (P=0.47 unadjusted) or multivariable (Table 2, predictive model, P=0.71 adjusted) analyses. Table 2 also summarises the results of the univariable and multivariable analyses of possible prognostic factors for LRC in the 230 patients. Necrosis had no prognostic significance for LRC in either univariable (P=0.50) or multivariable (P=0.65) analyses (Table 2, Figure 3A). Significant adverse prognostic factors in the combined cohort were advanced stage, a residual mass after resection and lack of chemotherapy. The presence of necrosis also had no prognostic significance when the analysis was restricted to patients receiving radiotherapy alone (Figure 3B).
Figure 2.
Kaplan–Meier curves for locoregional control (A, B) and overall survival (C, D) after radiotherapy (RT) or chemoradiotherapy (RT+CT) and stratified according to absence (A, C) or presence (B, D) of necrosis. Test for interaction between necrosis and treatment yielded unadjusted P-value of 0.467 (locoregional control) and 0.0323 (overall survival).
Table 2. Prognostic and predictive value of necrosis for locoregional control.
|
Univariable models |
Multivariable model (prognostic) |
Multivariable model (prognostic) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P-value | N | HR | 95% CI | P-value | N | HR | 95% CI | P-value | ||||
| Sex | |||||||||||||||
| Male (ref) | 183 | 1.00 | 172 | 1.00 | 172 | 1.00 | |||||||||
| Female | 47 | 1.06 | 0.63 | 1.79 | 0.83 | 46 | 1.21 | 0.70 | 2.08 | 0.50 | 46 | 1.22 | 0.71 | 2.10 | 0.48 |
| Age (years) | 230 | 1.00 | 0.97 | 1.03 | 0.79 | 218 | 1.00 | 0.97 | 1.03 | 0.80 | 218 | 1.00 | 0.97 | 1.03 | 0.80 |
| WHO performance status | |||||||||||||||
| 0 (ref) | 146 | 1.00 | 142 | 1.00 | 142 | 1.00 | |||||||||
| 1–2 | 84 | 1.27 | 0.82 | 1.97 | 0.28 | 76 | 1.37 | 0.86 | 2.19 | 0.19 | 76 | 1.36 | 0.85 | 2.17 | 0.21 |
| Stage | |||||||||||||||
| 2 (ref) | 202 | 1.00 | 191 | 1.00 | 191 | 1.00 | |||||||||
| 3–4 | 28 | 2.08 | 1.20 | 3.58 | 0.009 | 27 | 1.86 | 1.00 | 3.47 | 0.05 | 27 | 1.86 | 1.00 | 3.45 | 0.05 |
| Tumour size | |||||||||||||||
| <30 mm (ref) | 49 | 1.00 | 46 | 1.00 | 46 | 1.00 | |||||||||
| ⩾30 mm | 105 | 1.59 | 0.86 | 2.92 | 0.14 | 102 | 1.30 | 0.67 | 2.52 | 0.44 | 102 | 1.30 | 0.67 | 2.52 | 0.44 |
| Unknown | 76 | 1.69 | 0.90 | 3.19 | 0.10 | 70 | 1.67 | 0.85 | 3.28 | 0.14 | 70 | 1.69 | 0.85 | 3.34 | 0.13 |
| Residual mass after resection* | |||||||||||||||
| No (ref) | 151 | 1.00 | 151 | 1.00 | 151 | 1.00 | |||||||||
| Yes | 67 | 2.32 | 1.49 | 3.60 | <0.001 | 67 | 1.96 | 1.19 | 3.22 | 0.008 | 67 | 1.94 | 1.18 | 3.20 | 0.01 |
| Cis | |||||||||||||||
| Absent (ref) | 185 | 1.00 | 175 | 1.00 | 175 | 1.00 | |||||||||
| Present | 45 | 0.80 | 0.45 | 1.42 | 0.45 | 43 | 0.87 | 0.48 | 1.59 | 0.65 | 43 | 0.87 | 0.48 | 1.58 | 0.64 |
| Necrosis | |||||||||||||||
| Absent (ref) | 142 | 1.00 | 134 | 1.00 | Included in interaction below | ||||||||||
| Present | 88 | 0.86 | 0.55 | 1.34 | 0.50 | 84 | 0.89 | 0.55 | 1.44 | 0.65 | |
||||
| Treatment | |||||||||||||||
| Radiotherapy (ref) | 109 | 1.00 | 107 | 1.00 | Included in interaction below | ||||||||||
| Chemoradiotherapy | 121 | 0.53 | 0.34 | 0.82 | 0.005 | 111 | 0.51 | 0.32 | 0.81 | 0.004 | |
||||
| Necrosis absent: treatment | |||||||||||||||
| Radiotherapy | Not applicable, model without interaction | 68 | 1.00 | ||||||||||||
| Chemoradiotherapy | |
66 |
0.55 |
0.31 |
0.98 |
0.04 |
|||||||||
| Necrosis present: treatment | |||||||||||||||
| Radiotherapy | Not applicable, model without interaction | 39 | 1.00 | ||||||||||||
| Chemoradiotherapy | 45 | 0.46 | 0.21 | 0.99 | 0.05 | ||||||||||
Hazard ratios for all covariates in the models are included. Univariable models present unadjusted estimates for necrosis treatment, and all other prognostic factors; Multivariable model (prognostic) provides the prognostic value for necrosis adjusted by other relevant factors; Multivariable model (predictive) model provides predictive value for necrosis adjusted for other important factors (test for interaction necrosis:treatment, adjusted P-value 0.71).
Figure 3.
Investigation of necrosis as a prognostic factor for locoregional control in all 230 patients (A) and in 109 patients receiving radiotherapy only (B), and for overall survival in all 230 patients (C) and in 109 patients receiving radiotherapy alone (D).
Five-year OS rates were 44.4% (95% CI 35–53.3%) for the chemoradiotherapy group and 35.1% (95% CI 25.7–44.5%) for the radiotherapy group (log-rank test P=0.32). There was a trend to a benefit in OS for adding chemotherapy to radiotherapy in patients with rather than without tumour necrosis (Figure 2C and D) though this did not reach statistical significance. In 142 patients where necrosis was absent, 5-year OS was 39.5% for the chemoradiotherapy group and 34.6% for radiotherapy alone (HR=0.96, 95% CI 0.63–1.47, log-rank P=0.92). In 88 patients with tumour necrosis, five-year OS was 51.8% for the chemoradiotherapy group and 34.6% for radiotherapy alone (HR=0.68, 95% CI 0.40–1.17, log-rank P=0.16). However, there was no interaction between treatment and necrosis in either univariable (P=0.32) or multivariable (Table 3, predictive model, P=0.56) analyses. As for LRC, necrosis had no prognostic significance for OS in either univariable or multivariable analyses (Table 3, univariable and prognostic models; Figure 3C). Significant adverse prognostic factors in the 230 patient cohort were poor performance status, residual mass after resection and low haemoglobin. The presence of necrosis also had no prognostic significance when the radiotherapy only arm was analysed for OS (Figure 3D).
Table 3. Prognostic and redictive value of necrosis for overall survival.
|
Univariable models |
Multivariable model (prognostic) |
Multivariable model (predictive) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P-value | N | HR | 95% CI | P-value | N | HR | 95% CI | P-value | ||||
| Sex | |||||||||||||||
| Male (ref) | 183 | 1.00 | 172 | 1.00 | 172 | 1.00 | |||||||||
| Female | 47 | 0.84 | 0.55 | 1.29 | 0.43 | 46 | 0.81 | 0.51 | 1.27 | 0.36 | 46 | 0.82 | 0.52 | 1.29 | 0.38 |
| Age (years) | 230 | 1.02 | 1.00 | 1.05 | 0.05 | 218 | 1.02 | 1.00 | 1.05 | 0.07 | 218 | 1.02 | 1 | 1.05 | 0.07 |
| WHO performance status | |||||||||||||||
| 0 (ref) | 146 | 1.00 | 142 | 1.00 | 142 | 1.00 | |||||||||
| 1–2 | 84 | 1.79 | 1.28 | 2.50 | 0.001 | 76 | 1.55 | 1.08 | 2.24 | 0.02 | 76 | 1.54 | 1.06 | 2.21 | 0.02 |
| Stage | |||||||||||||||
| 2 (ref) | 202 | 1.00 | 191 | 1.00 | 191 | 1.00 | |||||||||
| 3–4 | 28 | 1.34 | 0.83 | 2.15 | 0.23 | 27 | 1.00 | 0.58 | 1.71 | 0.99 | 27 | 0.99 | 0.58 | 1.7 | 0.98 |
| Residual mass after resection* | |||||||||||||||
| No (ref) | 151 | 1.00 | 151 | 1.00 | 151 | 1.00 | |||||||||
| Yes | 67 | 2.10 | 1.48 | 3.00 | <0.001 | 67 | 2.04 | 1.38 | 3.02 | <0.001 | 67 | 2.02 | 1.37 | 3 | <0.001 |
| Haemoglobin, g dl−1 | 230 | 0.89 | 0.81 | 0.98 | 0.02 | 218 | 0.89 | 0.80 | 0.99 | 0.03 | 218 | 0.89 | 0.8 | 0.98 | 0.03 |
| Cis | |||||||||||||||
| Absent (ref) | 185 | 1.00 | 175 | 1.00 | 175 | 1.00 | |||||||||
| Present | 45 | 1.08 | 0.72 | 1.63 | 0.71 | 43 | 1.26 | 0.80 | 1.98 | 0.32 | 43 | 1.26 | 0.8 | 1.98 | 0.32 |
| Necrosis | |||||||||||||||
| Absent (ref) | 142 | 1.00 | 134 | 1.00 | Included in interaction below | ||||||||||
| Present | 88 | 0.97 | 0.69 | 1.37 | 0.88 | 84 | 0.86 | 0.59 | 1.25 | 0.44 | |
||||
| Treatment | |||||||||||||||
| Radiotherapy (ref) | 109 | 1.00 | 107 | 1.00 | Included in interaction below | ||||||||||
| Chemoradiotherapy | 121 | 0.84 | 0.61 | 1.18 | 0.32 | 111 | 0.81 | 0.56 | 1.15 | 0.24 | |
||||
| Necrosis absent: treatment | |||||||||||||||
| Radiotherapy (ref) | Not applicable, model without interaction | 68 | 1.00 | ||||||||||||
| Chemoradiotherapy | |
66 |
0.88 |
0.56 |
1.37 |
0.56 |
|||||||||
| Necrosis present: treatment | |||||||||||||||
| Radiotherapy (ref) | Not applicable, model without interaction | 39 | 1.00 | ||||||||||||
| Chemoradiotherapy | 45 | 0.71 | 0.4 | 1.26 | 0.24 | ||||||||||
Hazard ratios for all covariates in the models are included. Univariable models present unadjusted estimates for necrosis treatment, and all other prognostic factors; Multivariable model (prognostic) provides the prognostic value for necrosis adjusted by other relevant factors; Multivariable model (predictive) model provides predictive value for necrosis adjusted for other important factors (test for interaction necrosis:treatment, P-value 0.56).
Discussion
Necrosis was not associated with survival or predictive of outcome following chemoradiotherapy with MMC and 5-FU. Previous studies of lower and upper tract transitional cell carcinoma showed necrosis to be strongly associated with prognosis (Ord et al, 2007; Zigeuner et al, 2010; Eustace et al, 2013; Soave et al, 2015). This discrepancy in findings could be accounted for by tumour heterogeneity with a single section taken at random not being indicative of the whole transurethral resection of bladder tumour specimen. Compared with the BCON study where 121 out of 231 (52%) had necrosis, fewer patients in the BC2001 study (38%) had necrosis present as defined by a specialist uro-pathologist. This possible explanation is supported by differences in the stage distribution between the two studies where the BCON cohort had a greater proportion of T3 and T4 tumours than seen in the BC2001 cohort. These results may suggest that necrosis is an important prognostic factor in patients with more advanced disease where hypoxia could be more prevalent. A further explanation as to why necrosis may not be prognostic in this study could be that TURBT or the use of neoadjuvant chemotherapy may have had an impact on the results compared with those of previous studies in the literature. For example, in the BC2001 necrosis cohort 11% were not resected (Table 1) in comparison with 27% in the BCON necrosis cohort (Eustace et al, 2013). The higher level of complete resection in BC2001 (54 vs 39% for BCON) might reduce the impact of necrosis as an adverse prognostic factor for radiotherapy outcomes. Similarly, 31% of BC2001 (Table 1) vs 0% of BCON (Eustace et al, 2013) patients had neoadjuvant chemotherapy following TURPT and prior to radiotherapy. It is worth noting that the same experienced pathologist scored both the BCON and BC2001 cohorts for necrosis, which reduces possible observer bias.
Although hypoxia has been shown to be an important prognostic factor in MIBC (Hoskin et al, 2003; Palit et al, 2005; Ord et al, 2007; Hunter et al, 2014), it is difficult to measure directly using Eppendorf electrodes due to issues of access. Our group (Eustace et al, 2013) has previously shown that coagulative tumour necrosis, thought to be specific to tumour hypoxia, is an easily measurable histopathological parameter which can be used to predict benefit from hypoxia-modifying treatment using CON. We have also shown that the predictive power of necrosis is not improved by the addition of immunohistochemical scoring with hypoxia-associated proteins CAIX, GLUT-1 or HIF-1α (Hunter et al, 2014).
Although BCON is an accepted standard of care for bladder preserving treatment for MIBC, concurrent chemotherapy is more commonly used as a radiosensitiser. The BC2001 trial randomised patients with MIBC between radiotherapy alone and radiotherapy with MMC and 5-FU resulting in an improvement in locoregional control at 2 years from 54 to 67% (James et al, 2012). MMC is a weak bioreductive agent resulting in DNA crosslinks once inside the cell. 5-FU is a thymidylate synthase (TS) inhibitor. Inhibition of this enzyme blocks synthesis of the pyrimidine thymidine, which is a nucleoside essential for DNA replication. These drugs have a different primary mechanism of action compared with CON, which act by improving the oxygenation of tissues by increasing the oxygen tension and improving vasodilatation. The absence of a significant association between necrosis and response to MMC and 5-FU confirms our hypothesis that necrosis may only be suitable for selecting MIBC patients with significantly hypoxic tumours, which would benefit from hypoxia modification. Future work will involve looking at potential biomarkers associated with the mode of action of MMC and 5-FU to try and select patient groups who specifically benefit from this chemoradiotherapy regimen.
A limitation of the study was that samples were only available for 64% of the patients enroled in the BC2001 trial. Although there were no statistically significant differences in outcomes for patients for whom samples were or were not available (Supplementary Figure S2), there was a trend for better OS in patients with samples. A second limitation, related to the first, was that the study may be underpowered and weakened by small sample sizes for sub-groups. For example, in Figure 2 the benefit of giving chemotherapy with radiotherapy appears stronger in patients with necrosis present.
Further work to validate our results is required with the issue of necrosis being a prognostic factor for MIBC still not resolved. If necrosis is validated as a predictive factor for response to hypoxia modification and radical radiotherapy for MIBC, then it could be used to select patients who are likely to benefit from CON. The conclusion from the study reported here is that tumour necrosis is neither predictive nor prognostic, and therefore MMC/5-FU is an appropriate radiotherapy-sensitising treatment independent of necrosis status.
Acknowledgments
BC2001 was supported by grants (C547/A2606, C547/A6845, C9764/A9904, and C1491/A9895) from Cancer Research UK (trial reference number, CRUK/01/004). The current study was supported by Cancer Research UK (C2094/A11365) and Experimental Cancer Medicine Centre funding (C1467/A7286).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Booth CM, Siemens DR, Li G, Peng Y, Kong W, Berman DM, Mackillop WJ (2014) Curative therapy for bladder cancer in routine clinical practice: a population-based outcomes study. Clin Oncol (R Coll Radiol) 26: 506–514. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, Symonds P, Wylie JP, Ramani V, Sangar V, Lyons J, Bottomley I, McCaul D, Clarke NW, Kiltie AE, Cowan RA (2011) Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol 29: 733–738. [DOI] [PubMed] [Google Scholar]
- Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A, Ryder D, Ord JJ, Harris AL, Rojas AM, Hoskin PJ, West CM (2013) Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol 108: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI (2010) Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 28: 4912–4918. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Sibtain A, Daley FM, Wilson GD (2003) GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer 89: 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart RA, Hall E, Hussain SA, Jenkins P, Rawlings C, Tremlett J, Crundwell M, Adab FA, Sheehan D, Syndikus I, Hendron C, Lewis R, Waters R, James ND (2013) Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: results of the BC2001 trial (CRUK/01/004). Int J Radiat Oncol Biol Phys 87: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BA, Eustace A, Irlam JJ, Valentine HR, Denley H, Oguejiofor KK, Swindell R, Hoskin PJ, Choudhury A, West CM (2014) Expression of hypoxia-inducible factor-1alpha predicts benefit from hypoxia modification in invasive bladder cancer. Br J Cancer 111: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA BC2001 Investigators (2012) Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366: 1477–1488. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ (2007) Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol) 19: 427–442. [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- Ord JJ, Agrawal S, Thamboo TP, Roberts I, Campo L, Turley H, Han C, Fawcett DW, Kulkarni RP, Cranston D, Harris AL (2007) An investigation into the prognostic significance of necrosis and hypoxia in high grade and invasive bladder cancer. J Urol 178: 677–682. [DOI] [PubMed] [Google Scholar]
- Palit V, Phillips RM, Puri R, Shah T, Bibby MC (2005) Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol Rep 14: 909–913. [DOI] [PubMed] [Google Scholar]
- Soave A, John LM, Dahlem R, Minner S, Engel O, Schmidt S, Kluth LA, Fisch M, Rink M (2015) The Impact of tumor diameter and tumor necrosis on oncologic outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. Urology 86: 92–98. [DOI] [PubMed] [Google Scholar]
- Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A, Kikuchi E, Remzi M, Raman JD, Bolenz C, Bensalah K, Capitanio U, Koppie TM, Kassouf W, Sircar K, Patard JJ, Fernández MI, Wood CG, Montorsi F, Ströbel P, Wheat JC, Haitel A, Oya M, Guo CC, Ng C, Chade DC, Sagalowsky A, Langner C (2010) Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 57: 575–581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.