Abstract
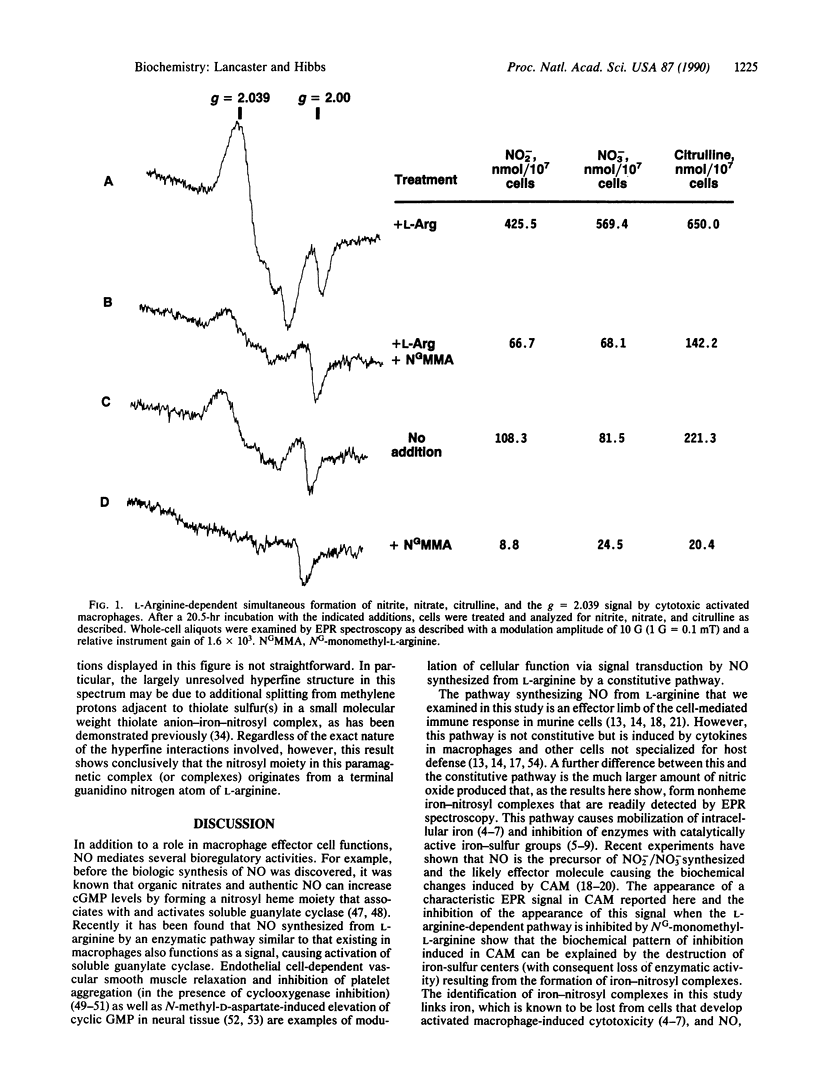
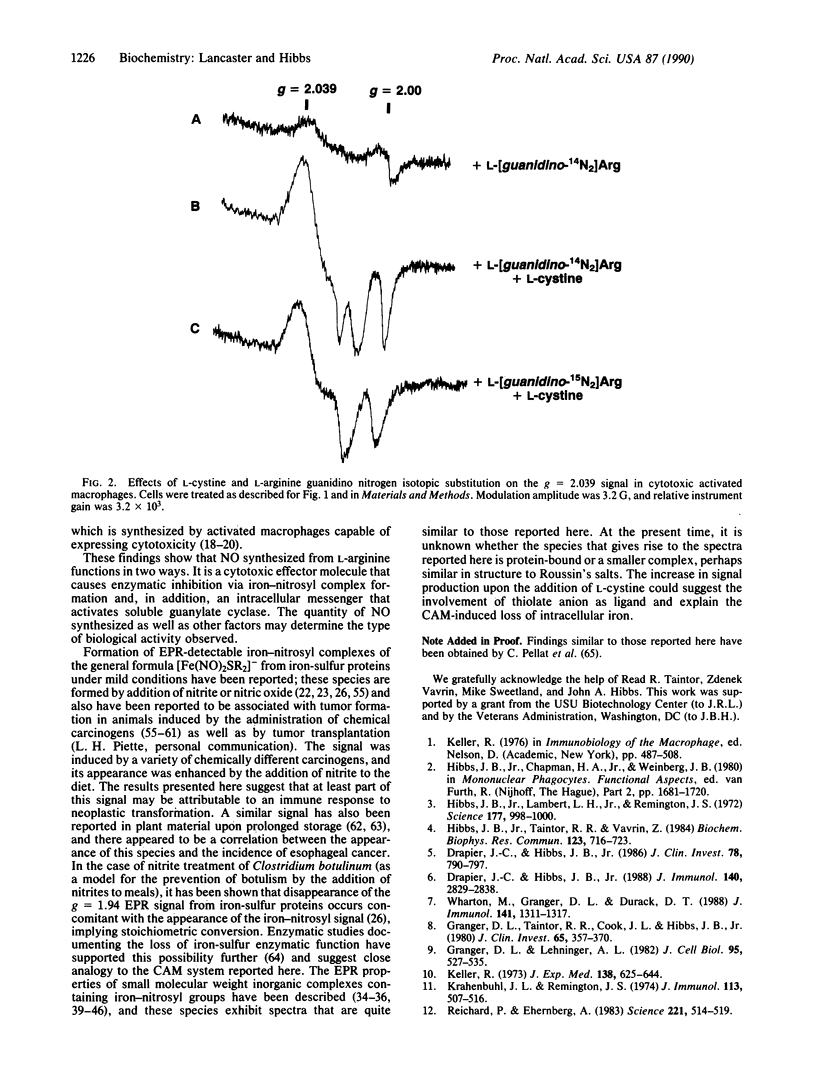
Activated macrophage cytotoxicity is characterized by loss of intracellular iron and inhibition of certain enzymes that have catalytically active nonheme-iron coordinated to sulfur. This phenomenon involves the oxidation of one of the terminal guanidino nitrogen atoms of L-arginine, which results in the production of citrulline and inorganic nitrogen oxides (NO2-, NO3-, and NO). We report here the results of an electron paramagnetic resonance spectroscopic study performed on cytotoxic activated macrophage (CAM) effector cells, which develop the same pattern of metabolic inhibition as their targets. Examination of activated macrophages from mice infected with Mycobacterium bovis (strain bacillus Calmette-Guérin) that were cultured in medium with lipopolysaccharide and L-arginine showed the presence of an axial signal at g = 2.039, which is similar to previously described iron-nitrosyl complexes formed from the destruction of iron-sulfur centers by nitric oxide (NO). Inhibition of the L-arginine-dependent pathway by addition of NG-monomethyl-L-arginine (methyl group on a terminal guanidino nitrogen) inhibits the production of nitrite, nitrate, citrulline, and the g = 2.039 signal. Comparison of the hyperfine structure of the signal from cells treated with L-arginine with terminal guanidino nitrogen atoms of natural abundance N14 atoms or labeled with N15 atoms showed that the nitrosyl group in this paramagnetic species arises from one of these two atoms. These results show that loss of iron-containing enzyme function in CAM is a result of the formation of iron-nitrosyl complexes induced by the synthesis of nitric oxide from the oxidation of a terminal guanidino nitrogen atom of L-arginine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Cytokines induce an L-arginine-dependent effector system in nonmacrophage cells. J Leukoc Biol. 1988 Jul;44(1):58–65. doi: 10.1002/jlb.44.1.58. [DOI] [PubMed] [Google Scholar]
- Bartholomew B. A rapid method for the assay of nitrate in urine using the nitrate reductase enzyme of Escherichia coli. Food Chem Toxicol. 1984 Jul;22(7):541–543. doi: 10.1016/0278-6915(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Carpenter C. E., Reddy D. S., Cornforth D. P. Inactivation of clostridial ferredoxin and pyruvate-ferredoxin oxidoreductase by sodium nitrite. Appl Environ Microbiol. 1987 Mar;53(3):549–552. doi: 10.1128/aem.53.3.549-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang R. W., Woolum J. C., Commoner B. Further study on the properties of the rat liver protein involved in a paramagnetic complex in the livers of carcinogen-treated rats. Biochim Biophys Acta. 1972 Feb 29;257(2):452–460. doi: 10.1016/0005-2795(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Commoner B., Woolum J. C., Senturia B. H., Jr, Ternberg J. L. The effects of 2-acetylaminofluorene and nitrite on free radicals and carcinogenesis in rat liver. Cancer Res. 1970 Aug;30(8):2091–2097. [PubMed] [Google Scholar]
- Dervartanian D. V., Albracht S. P., Berden J. A., van Gelder B. F., Slater E. C. The EPR spectrum of isolated complex 3. Biochim Biophys Acta. 1973 Feb 22;292(2):496–501. doi: 10.1016/0005-2728(73)90055-8. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel N. M., Saprin A. N., Shabalkin V. A., Kozlova L. E., Krugljakova K. E. Detection and investigation of a new type of ESR signal characteristic of some tumour tissues. Nature. 1969 Apr 12;222(5189):165–167. doi: 10.1038/222165a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Taintor R. R., Cook J. L., Hibbs J. B., Jr Injury of neoplastic cells by murine macrophages leads to inhibition of mitochondrial respiration. J Clin Invest. 1980 Feb;65(2):357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthöhrlein G., Knappe J. Modified determination of citrulline. Anal Biochem. 1968 Oct 10;26(1):188–191. doi: 10.1016/0003-2697(68)90045-6. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R. Activated macrophage-mediated cytotoxicity: use of the in vitro cytotoxicity assay for study of bioenergetic and biochemical changes that develop in tumor target cells. Methods Enzymol. 1986;132:508–520. doi: 10.1016/s0076-6879(86)32036-6. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Ignarro L. J., Gruetter C. A. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980 Aug 13;631(2):221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon H. Paramagnetic resonance study of Nitric Oxide hemoglobin. J Biol Chem. 1968 Aug 25;243(16):4350–4357. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. The role of activated macrophages in specific and nonspecific cytostasis of tumor cells. J Immunol. 1974 Aug;113(2):507–516. [PubMed] [Google Scholar]
- Lu S. H., Camus A. M., Tomatis L., Bartsch H. Mutagenicity of extracts of pickled vegetables collected in Linhsien County, a high-incidence area for esophageal cancer in Northern China. J Natl Cancer Inst. 1981 Jan;66(1):33–36. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Kataoka N., Nagase S., Nakada H., Sato H., Sasaki H. Identification of three-line electron spin resonance signal and its relationship to ascites tumors. Cancer Res. 1971 Feb;31(2):179–184. [PubMed] [Google Scholar]
- Meyer J. Comparison of carbon monoxide, nitric oxide, and nitrite as inhibitors of the nitrogenase from Clostridium pasteurianum. Arch Biochem Biophys. 1981 Aug;210(1):246–256. doi: 10.1016/0003-9861(81)90186-7. [DOI] [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Nagata C., Ioki Y., Kodama M., Tagashira Y., Nakadate M. Free radical induced in rat liver by a chemical carcinogen, N-methyl-N'-nitro-N-nitrosoguanidine. Ann N Y Acad Sci. 1973 Dec 31;222:1031–1047. doi: 10.1111/j.1749-6632.1973.tb15322.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D., Lancaster J. R., Jr, Cornforth D. P. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983 Aug 19;221(4612):769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Russell S. W., Doe W. F., McIntosh A. T. Functional characterization of a stable, noncytolytic stage of macrophage activation in tumors. J Exp Med. 1977 Dec 1;146(6):1511–1520. doi: 10.1084/jem.146.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Ohnishi T. Tetranuclear and binuclear iron-sulfur clusters in succinate dehydrogenase: a method of iron quantitation by formation of paramagnetic complexes. Biochem Biophys Res Commun. 1976 Dec 6;73(3):833–840. doi: 10.1016/0006-291x(76)90884-6. [DOI] [PubMed] [Google Scholar]
- Stevens T. H., Bocian D. F., Chan S. I. EPR studies of 15NO-ferrocytochrome alpha3 in cytochrome c oxidase. FEBS Lett. 1979 Jan 15;97(2):314–316. doi: 10.1016/0014-5793(79)80110-6. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithayathil A. J., Ternberg J. L., Commoner B. Changes in electron spin resonance signals of rat liver during chemical carcinogenesis. Nature. 1965 Sep 18;207(5003):1246–1249. doi: 10.1038/2071246a0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]
- Woods L. F., Wood J. M., Gibbs P. A. the involvement of Nitric Oxide in the inhibition of the phosphoroclastic system in Clostridium sporogenes by sodium nitrite. J Gen Microbiol. 1981 Aug;125(2):399–406. doi: 10.1099/00221287-125-2-399. [DOI] [PubMed] [Google Scholar]
- Woolum J. C., Commoner B. Isolation and identification of a paramagnetic complex from the livers of carcinogen-treated rats. Biochim Biophys Acta. 1970 Jan 27;201(1):131–140. doi: 10.1016/0304-4165(70)90018-8. [DOI] [PubMed] [Google Scholar]
- Woolum J. C., Tiezzi E., Commoner B. Electron spin resonane of iron-nitric oxide complexes with amino acids, peptides and proteins. Biochim Biophys Acta. 1968 Aug 13;160(3):311–320. doi: 10.1016/0005-2795(68)90204-3. [DOI] [PubMed] [Google Scholar]
- Zhang W. X., Xu M. S., Wang G. H., Wang M. Y. Quantitative analysis of Roussin red methyl ester in pickled vegetables. Cancer Res. 1983 Jan;43(1):339–341. [PubMed] [Google Scholar]



