Abstract
Background
The risk of developing tuberculosis (TB) in allogeneic hematopoietic stem cell transplantation (HSCT) recipients is expected to be relatively high in an intermediate TB burden country. This single-center retrospective study was conducted to investigate risk factors and the incidence of TB after allogeneic HSCT.
Methods
From January 2004 to March 2011, 845 adult patients were enrolled. Starting April 2009, patients were given isoniazid (INH) prophylaxis based on interferon-γ release assay results. The incidence of TB was analyzed before and after April 2009, and compared it with that of the general population in Korea.
Results
TB was diagnosed in 21 (2.49%) of the 845 allogeneic HSCT patients. The median time to the development of TB was 386 days after transplantation (range, 49–886). Compared with the general population, the standardized incidence ratio of TB was 9.10 (95% CI; 5.59–14.79). Extensive chronic graft-versus-host disease (GVHD) was associated with the development of TB (P = 0.003). Acute GVHD, conditioning regimen with total body irradiation and conditioning intensity were not significantly related. INH prophylaxis did not reduce the incidence of TB (P = 0.548). Among 21 TB patients, one patient had INH prophylaxis.
Conclusion
Allogeneic HSCT recipients especially those who suffer from extensive chronic GVHD are at a high risk of developing TB. INH prophylaxis did not statistically change the incidence of TB, however, further well-designed prospective studies are needed.
Introduction
Mycobacterium tuberculosis is an acid-fast, aerobic bacillus, which is one of the leading infectious diseases around the world [1, 2]. Tuberculosis (TB) is also well known for a significant opportunistic infection among hematopoietic stem cell transplantation (HSCT) recipients, although it is 10 times less frequent than in solid organ transplant patients [2, 3]. The immune system of HSCT recipients are suppressed as a result of their hematologic diseases, chemotherapy, radiation, immunosuppressive therapy, and graft-versus-host disease (GVHD) [1]. Thus, the incidence of TB among allogeneic HSCT recipients is reportedly 2–40 times higher than that of the general population [1, 4–6]. In particular, diagnosis of TB is frequent in Korea, with an estimated TB incidence rate of 97 cases per 100 000 persons in the general population in 2013 according to World Health Organization report [7].
Isoniazid (INH) prophylaxis in patients with latent TB infection (LTBI) is recommended for the prevention of active TB disease [2]. However, INH prophylaxis guidelines have not yet been adopted by many centers in Korea. There are a few studies on TB and INH prophylaxis in HSCT recipients.
The purpose of this study was to examine the incidence of TB disease after allogeneic HSCT as compared with the general population, identify risk factors for TB disease, and analyze the efficacy of INH prophylaxis.
Materials and methods
Study design and subjects
We retrospectively reviewed the medical records of all consecutive adult patients (age ≥18 years) who underwent allogeneic HSCT for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), multiple myeloma (MM), or myelodysplastic syndrome (MDS), from January 2004 to March 2011, at the Catholic Blood and Marrow Transplantation Center, Seoul, Korea. Patients undergoing a second transplantation, or with human immunodeficiency virus infection were excluded from the study. Those who were taking anti-TB medication before or during the HSCT were excluded as well. The demographic features, laboratory and radiologic data of study participants were carefully examined (For details see S1 Data). The end of this study was set at December 2014 or time of death or the date of loss to follow-up.
As of April 2009, patients who planned to undergo allogeneic HSCT were given INH prophylaxis (300 mg/day) based on interferon-γ release assay (IGRA) results. Korea Centers for Disease Control and Prevention has recommended INH prophylaxis for latent TB since 2008 [8, 9]. In accordance with the national policy for latent TB, the Catholic Blood and Marrow Transplantation Center in Korea changed guidelines for TB prevention in April 2009. INH prophylaxis was started on the day that the IGRA results were made available, and continued for 9 months after HSCT. The IGRA results were evaluated using QuantiFERON®-TB Gold In-Tube (QFT-GIT; Cellestis Ltd., Victoria, Australia). The incidence of TB in each study group was analyzed (group A, allogeneic HSCT between January 2004 and March 2009, n = 550; group B, allogeneic HSCT between April 2009 and March 2011, n = 295).
This study obtained approval from the Institutional Review Board of Seoul and Yeouido St. Mary’s Hospital with a waiver of informed consent. (No. KC15RIMI0162, SC10RISI0023)
Definitions
Definitions of TB and assessment of treatment outcome
The diagnosis of TB was considered proven if M.tuberculosis was cultured from any of the clinical specimens. The diagnosis of a probable case of active TB required at least one of the following criteria in patients with clinical symptoms and signs suggestive of TB disease: 1) acid-fast bacilli from clinical specimen (sputum, body fluid, etc.) present; 2) tissue histology suggestive of TB such as caseous necrosis with or without granuloma or positive Ziehl-Neelsen staining; or 3) fluid cytology (pleural or pericardial effusion or ascites or cerebrospinal fluid [CSF]) showing exudates with lymphocyte predominance and high adenosine deaminase (>40 IU/L in the body fluid, or >10 IU/L in case of CSF), when the case had no evidence of malignancy. A possible case was defined when a patient with clinical symptoms and signs, had radiological findings suggestive of TB at the time of diagnosis without definite evidence of another infectious cause, and the patient’s condition improved with anti-TB treatment when other antibacterial and antifungal agents were ineffective [6, 10–16]. Miliary TB was defined as described in a previous study [17]. The radiological findings were confirmed by two highly-experienced radiologists. Polymerase chain reaction for TB was used only to distinguish TB and non-tuberculous mycobacterial infection, not for TB diagnosis. The IGRA results had no influence on the diagnosis of TB. Clinical response of TB treatment was classified as cure, improvement, or failure [16]. The definition of Immune reconstitution inflammatory syndrome was given as previously [18].
Transplantation procedure and diagnosis of GVHD
All patients received oral ciprofloxacin (250–500 mg twice a day) as antibacterial prophylaxis. Itraconazole oral solution (2.5 mg/kg twice a day) or micafungin (50 mg daily) were used as primary antifungal prophylaxis, from the first day of conditioning until engraftment. Fluconazole (400 mg daily) prophylaxis after engraftment was administered according to GVHD status and the national guideline [19]. Trimethoprim-sulfamethoxazole (one single-strength tablet daily) was administered as prophylaxis for Pneumocystis jirovecii infection after engraftment until discontinuation of the immunosuppressants. The patients also received a herpes simplex virus prophylaxis of acyclovir (400–800 mg orally twice a day or 5 mg/kg intravenously two or three times a day) from day 7 to engraftment for transplants of matched related donors. Patients who underwent allogeneic HSCT from mismatched related donors, unrelated donors, or umbilical cord blood received a higher dose of intravenous acyclovir (10 mg/kg three times a day) until engraftment. To prevent cytomegalovirus disease after engraftment, the preemptive strategy was conducted in a CMV DNA load-guided, risk-adapted manner [13]. The myeloablative conditioning regimen was used when at least one of the following criteria was met: 1) total body irradiation ≥ 500 cGy as a single fraction or ≥ 800 cGy as fractionated; 2) busulfan 4 mg kg/day administered for 4 days, combined with cyclophosphamide 120 mg/kg; 3) total busulfan of ≥ 9 mg/kg; and 4) melphalan of ≥ 140 mg/m2. The reduced intensity conditioning regimen was used when meeting any of the following criteria: 1) total body irradiation < 500 cGy as a single fraction or < 800 cGy as fractionated; 2) total busulfan of < 9 mg/kg; 3) melphalan of < 140 mg/m2; 4) thiotepa of < 10 mg/kg; and 5) the carmustine, etoposide, cytarabine, and melphalan regimen [20]. Diagnoses and grades of acute GVHD were made based on the consensus criteria [21]. The definition of chronic GVHD was given by using the revised Seattle classification [20]. The patients concomitant treated with cyclosporine and rifampicin were frequently monitored for therapeutic drug levels of cyclosporine.
Statistical analysis
Overall survival (OS), disease-free survival (DFS), and the cumulative incidences of TB disease were considered the main end points of this study. OS and DFS results were plotted using the Kaplan-Meier method and compared using log-rank test. The cumulative incidences of TB disease under which method non-TB disease was used as a competing risk event of TB disease, were plotted and compared using the Gray’s test. To evaluate the potential factors for TB disease, categorical variables were analyzed using a χ2 or Fisher’s exact test. For multivariate analysis, variables with P-value of < 0.30, on univariate analysis were entered into the model selection procedure on the basis of Cox proportional hazard modeling.
Two-sided P values < 0.05 were considered statistically significant. The standardized incidence ratios (SIR) of TB disease were calculated to compare the incidence of TB disease in our cohort with that of the general population in Korea, whose data were collected from the annual reports of the Korea Centers for Disease Control and Prevention between 2004 to 2011 [22]. SIR with corresponding 95% confidence intervals (CI) were calculated using Poisson regression. Statistical studies were performed with the Statistical Package for the Social Sciences version 13.0 (SPSS, Inc., Chicago, IL, USA) and the cumulative incidence analyses were performed using with R (freely distributed on the web, http://cran.r-project.org/).
Results
Overall HSCT recipient characteristics and risk factors for the development of TB
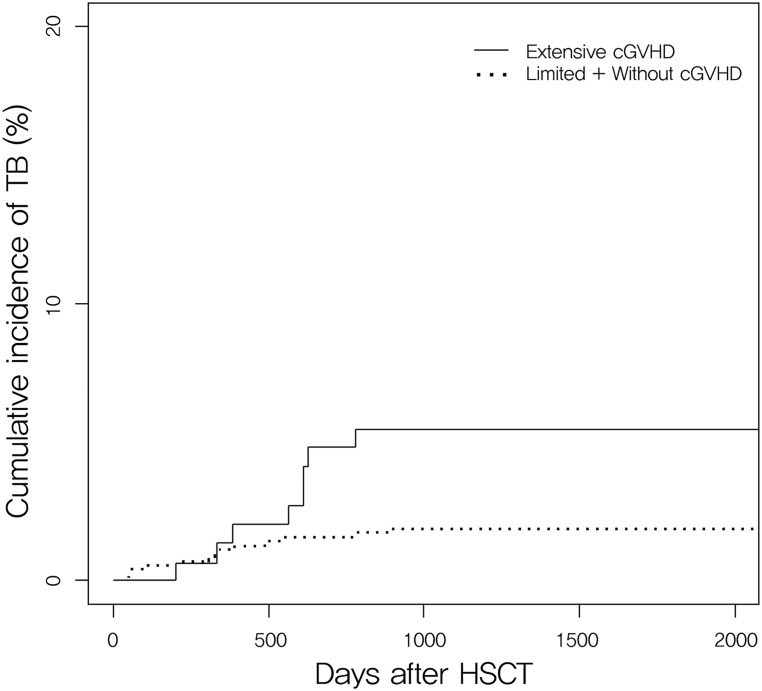
From January 2004 to March 2011, a total of 861 patients with acute leukemia, CML, MDS, and MM underwent allogeneic HSCT. During the study period, there were 9 cases of second transplantation and 7 patients were taking anti-TB medication prior to the HSCT. After these patients were excluded, a total of 845 patients were enrolled in this study. None of the patients was human immunodeficiency virus-positive. Table 1 shows the general characteristics of the transplantations and the clinical information of the study population. Post-HSCT TB occurred in 21 (2.49%) of the 845 patients in the study. The median follow-up duration of the survivors was 2,044 days (range, 108–3,916). On multivariate analysis (Table 2), risk factors for post-HSCT TB revealed that extensive chronic GVHD was only associated with TB in the study population (P = 0.008). Acute GVHD, conditioning regimen with total body irradiation, and conditioning intensity were not significantly related. According to Gray’s test (Fig 1), there was a 4.89 ± 1.32% probability of having TB disease 1000 days after allogeneic HSCT for extensive chronic GVHD and 1.42 ± 0.50% for limited or without chronic GVHD (P = 0.003). There was no significant influence of chronic GVHD site on TB development by logistic regression. Chronic GVHD occurred in the skin (TB patients vs. Non-TB patients; 47.6% vs. 40.7%, P = 0.523), in the mouth (TB patients vs. Non-TB patients; 19.0% vs. 30.5%, P = 0.268), in the liver (TB patients vs. Non-TB patients; 33.3% vs. 19.8%, P = 0.134), in the eye (TB patients vs. Non-TB patients; 14.3% vs. 12.5%, P = 0.807), in the lung (TB patients vs. Non-TB patients; 19.0% vs. 9.7%, P = 0.168), in the gastro-intestinal tract (TB patients vs. Non-TB patients; 14.3% vs. 5.8%, P = 0.122) in that order. The outcome of chronic GVHD treatment showed also no association with TB development (P = 0.206). Grade of chronic GVHD was associated with TB development (P = 0.006). Severe chronic GVHD was 19.0% in TB patients and 13.8% in non-TB patients while moderate chronic GVHD was 47.6% and 18.1%, and mild chronic GVHD was 14.3% and 17.2%, respectively.
Table 1. Overall characteristics of allogeneic hematopoietic stem cell transplantation recipients with or without tuberculosis.
| Characteristics | TB (n = 21) | No TB (n = 824) | P |
|---|---|---|---|
| Age (median, range) | 41 (18–60) | 40 (18–72) | 0.960 |
| Sex (M,%) | 10 (47.6) | 473 (57.4) | 0.371 |
| Transplantation year (%) | 0.280 | ||
| • Jan. 2004-Mar. 2009 | 16 (76.2) | 534 (64.8) | |
| • Apr. 2009-Mar. 2011 | 5 (23.8) | 290 (35.2) | |
| Diagnosis (%) | 0.569 | ||
| • AML | 12 (57.1) | 399 (48.4) | |
| • ALL | 4 (19.4) | 227 (27.5) | |
| • MDS | 4 (19.4) | 118 (14.3) | |
| • CML | 0 (0) | 53 (6.4) | |
| • MM | 1 (4.8) | 27 (3.3) | |
| Donor type (%) | 0.870 | ||
| • Matched sibling | 12 (57.1) | 456 (55.3) | |
| • Alternativea | 9 (42.9) | 368 (44.7) | |
| Stem cell source (%) | 0.163 | ||
| • BM | 11 (52.4) | 388 (47.1) | |
| • PB | 8 (38.1) | 406 (49.3) | |
| • BM+PB | 2 (9.5) | 19 (2.3) | |
| • Cord blood | 0 (0) | 11 (1.3) | |
| Conditioning regimen (%) | 0.918 | ||
| • TBI based | 15 (71.4) | 597 (72.5) | |
| • no-TBI based | 6 (28.6) | 227 (27.5) | |
| ATG given as conditioning (%) | 0.662 | ||
| • Yes | 5 (23.8) | 232 (28.2) | |
| • No | 16 (76.2) | 592 (71.8) | |
| Conditioning intensity (%) | 0.779 | ||
| • MAC | 13 (61.9) | 485 (58.9) | |
| • RIC | 8 (38.1) | 339 (41.1) | |
| GVHD prophylaxis (%) | 0.693 | ||
| • CS based | 12 (57.1) | 435 (52.8) | |
| • FK based | 9 (42.9) | 389 (47.2) | |
| Acute GVHD (%) | 0.983 | ||
| • Grade 0-I | 13 (61.9) | 512 (62.1) | |
| • Grade II-IV | 8 (38.1) | 312 (37.9) | |
| Chronic GVHD (%) | 0.003 | ||
| • None + Limited | 8 (38.1) | 569 (69.1) | |
| • Extensive | 13 (61.9) | 255 (30.9) | |
| Relapse | 0.597 | ||
| • Yes | 4 (19.0) | 198 (24.0) | |
| • No | 17 (81.0) | 626 (76.0) | |
| Follow-up duration, median days (range) | 0.268 | ||
| • Patients who died | 343 (121–799) | 259 (2-2959) | |
| • Patients alive at last follow-up | 2353 (258–3217) | 2037 (108–3916) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ATG, anti-thymocyte globulin; BM, bone marrow stem cell; CML, chronic myelogenous leukemia; CS, cyclosporine; FK, tacrolimus; GVHD, graft-versus-host disease; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; PB, peripheral blood stem cell; RIC, reduced intensity conditioning; TB, tuberculosis; TBI, total body irradiation.
a alternative donor included unrelated donors and mismatched relatives.
Table 2. Risk factors for tuberculosis in patients after allogeneic hematopoietic stem cell transplantation.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age >40 years | 1.29 (0.55–3.03) | 0.565 | ||
| Male sex | 0.72 (0.31–1.70) | 0.452 | ||
| Transplantation year (%) | ||||
| • Jan. 2004-Mar. 2009 | 1 | 1 | ||
| • Apr. 2009-Mar. 2011 | 0.57 (0.21–1.57) | 0.278 | 0.55 (0.20–1.51) | 0.242 |
| Donor type | ||||
| • Matched sibling | 1 | |||
| • Alternative | 1.00 (0.42–2.38) | 0.997 | ||
| Stem cell source | ||||
| • BM | 1 | 1 | ||
| • PB | 0.72 (0.29–1.80) | 0.485 | 0.62 (0.24–1.57) | 0.312 |
| • Others | 2.46 (0.55–11.09) | 0.242 | 2.45 (0.54–11.06) | 0.244 |
| TBI based Conditioning regimen | 0.92 (0.36–2.36) | 0.857 | ||
| ATG given as conditioning | 0.88 (0.32–2.40) | 0.798 | ||
| Conditioning intensity | ||||
| • MAC | 1 | |||
| • RIC | 1.13 (0.47–2.72) | 0.792 | ||
| GVHD prophylaxis | ||||
| • CS based | 1 | |||
| • FK based | 1.15 (0.75–2.73) | 0.753 | ||
| Acute GVHD (grade ≥ II) | 1.01 (0.42–2.43) | 0.989 | ||
| Extensive chronic GVHD | 2.84 (1.18–6.86) | 0.020 | 3.38 (1.37–8.32) | 0.008 |
All variables with values of P < 0.300 on univariate analysis were included in the multivariate analysis on the basis of Cox proportional hazard modeling.
ATG, anti-thymocyte globulin; BM, bone marrow stem cell; CI, confidence interval; CS, cyclosporine; FK, tacrolimus; GVHD, graft-versus-host disease; HR, hazard ratio; MAC, myeloablative conditioning; PB, peripheral blood stem cell; RIC, reduced intensity conditioning; TBI, total body irradiation.
Fig 1. Cumulative incidence of tuberculosis in allogeneic hematopoietic stem cell transplantation recipients with and without chronic graft-versus-host-disease.
Extensive chronic GVHD was associated with the development of TB (P = 0.003) There is a 4.89 ± 1.32% probability of having TB disease at 1000 days after allogeneic HSCT for extensive chronic GVHD and 1.42 ± 0.50% for limited or without chronic GVHD. cGVHD, chronic graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; TB, tuberculosis.
Clinical characteristics of patients with TB
Of the 21 patients with TB after allogeneic HSCT, 10 patients (47.6%) had a previous history of active TB prior to allogeneic HSCT. There were 9 proven, 6 probable, and 6 possible cases in TB diagnosis. Median time from transplantation to TB diagnosis was 386 days (range, 49–886). Eleven of the 21 cases were pulmonary TB, while the other 10 were extrapulmonary TB (47.6%). There were two patients with miliary TB. Median time from onset of symptoms and signs to TB diagnosis was 23 days (range, 0–126). No differences in the outcome were observed for the cases diagnosed with tuberculosis before or after 100 days of HSCT.
17 patients (81.0%) received first-line anti-TB treatment (INH, rifampicin, ethambutol, and pyrazinamide) and 4 patients received second-line therapy. Among 9 cases of proven TB, 6 were subjected to an antimicrobial susceptibility test. Five of these cases had susceptible results to anti-TB medication, while only 1 case had INH resistance. The reasons for the administration of second-line therapy were drug resistance (patient No. 6), first line treatment failure (patient No. 18), and drug intolerance (patient No. 7 and 14). The median time of anti-TB treatment duration was 12 months (range, 1–18 months). Two patients (9.5%) showed anti-TB treatment failure. Patient No. 8 died of variceal bleeding within the esophagus due to liver cirrhosis and didn’t show any improvement despite acid-fast bacilli cultures all being susceptible to anti-TB medications. Patient No. 14 died of pneumonia. This patient was administered INH, ethambutol, pyrazinamide, and levofloxacin. Another 5 patients died for various reasons despite successful anti-TB treatment. Three patients (No 4, 8, and 15) developed immune reconstitution inflammatory syndrome. There wasn’t any case of relapse after anti-TB treatment administered during the study period. The clinical characteristics and diagnostic and treatment data of the patients with TB after HSCT are summarized in Table 3.
Table 3. Clinical characteristics of patients with tuberculosis after allogeneic hematopoietic stem cell transplantation.
| Patient No. | Age/Sex | Dx | The year of HSCT | Acute GVHD, grade | Chronic GVHD (site, grade) | Therapeutic approach of chronic GVHD | Outcome of chronic GVHD | Relapse of hematologic disease, Y/N | Previous history of TB | Category of TB | Time to Dx of TB after HSCT, day | Site of infection | Treatment | Treatment duration, month | Treatment outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20/F | AML | 2004 | 0 | None | N | N | Possible | 311 | Lung | HERZa | 2 | Improved | ||
| 2 | 40/M | AML | 2004 | 0 | Extensive (mouth, liver, severe) | Steroid pulse therapy, cyclosporine, and tacrolimus | Aggravation | N | Y | Possible | 536 | Spine | HERZa | 18 | Cure |
| 3 | 38/F | AML | 2004 | II | Extensive (BOOP, liver, skin, GI, moderate) | Steroid pulse therapy | Aggravation | N | Y | Possible | 207 | Lung | HERZa | 4 | Improved |
| 4 | 33/M | AML | 2004 | III | Extensive (skin, moderate) | Tacrolimus | Improvement | N | Y | Probable | 612 | Pleura | HERZa | 12 | Cure |
| 5 | 18/M | ALL | 2004 | II | Limited (liver, moderate) | Steroid and cyclosporine | Improvement | Y | N | Probable | 491 | Lymph node | HERZa | 12 | Cure |
| 6 | 52/F | AML | 2005 | 0 | None | N | N | Proven | 50 | Lymph node | 2nd lineb | 13 | Improved | ||
| 7 | 39/M | AML | 2005 | 0 | Extensive (liver, moderate) | Steroid pulse therapy and tacrolimus | Improvement | N | N | Probable | 328 | Lung (miliary) | 2nd linec | 12 | Cure |
| 8 | 50/M | MDS | 2005 | 0 | None | N | Y | Proven | 217 | Lung (miliary) | HERZa | 1 | Failed | ||
| 9 | 43/M | AML | 2005 | 0 | Extensive (mouth, skin, 10liver, severe) | Steroid and tacrolimus | Improvement | N | Y | Proven | 340 | Lung | HERZa | 14 | Cure |
| 10 | 41/M | AML | 2006 | 0 | Limited (skin, mild) | Tacrolimus | Improvement | N | Y | Possible | 49 | Lung | HERZa | 10 | Improved |
| 11 | 44/F | MM | 2006 | 0 | Extensive (mouth, skin, moderate) | Steroid and tacrolimus | Improvement | Y | Y | Possible | 57 | Lymph node | HERZa | 10 | Improved |
| 12 | 24/F | AML | 2006 | II | Extensive (skin, liver, moderate) | Steroid and tacrolimus | Improvement | N | N | Proven | 631 | Lung | HERZa | 12 | Cure |
| 13 | 34/F | ALL | 2007 | 0 | Extensive (mouth, GI, severe) | Steroid and mycophenolate Mofetil | Improvement | N | N | Probable | 568 | Pericardium, Pleura | HERZa | 12 | Improved |
| 14 | 60/F | ALL | 2007 | 0 | None | N | N | Proven | 93 | Pleura | 2nd linec | 1 | Failed | ||
| 15 | 29/M | ALL | 2008 | II | Extensive (GI, moderate) | Steroid and tacrolimus | Improvement | N | N | Proven | 386 | Lung | HERZa | 12 | Cure |
| 16 | 40/M | AML | 2008 | III | Extensive (skin, BO, severe) | Steroid, tacrolimus, and mycophenolate Mofetil | Improvement | N | N | Probable | 331 | Pericardium | HERZa | 10 | Improved |
| 17 | 50/F | MDS | 2009 | 0 | Extensive (BOOP, moderate) | Steroid, tacrolimus, and mycophenolate Mofetil | Improvement | Y | N | Proven | 393 | Lung | HERZa | 8 | Cure |
| 18 | 59/F | AML | 2009 | II | Extensive (eye, skin, liver, moderate) | Cyclosporine | Aggravation | N | Y | Proven | 617 | Lung | 2nd lined | 17 | Cure |
| 19 | 55/F | MDS | 2009 | 0 | Limited (eye, mild) | Steroid and cyclosporine | Improvement | N | Y | Possible | 770 | Spine | HERZa | 12 | Cure |
| 20 | 55/M | AML | 2010 | II | Extensive (skin, eye, BO, moderate) | Steroid, tacrolimus, and mycophenolate Mofetil | Improvement | N | Y | Proven | 782 | Lung | HERZa | 9 | Improved |
| 21 | 52/F | AML | 2010 | 0 | Limited (skin, mild) | Tacrolimus | Improvement | N | N | Probable | 886 | Lymph node | HERZa | 12 | Cure |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BO, bronchiolitis obliterans; BOOP, bronchiolitis obliterans organizing pneumonia; Dx, diagnosis; GI, gastrointestinal; GVHD, graft-versus-host disease; HERZ, treatment regimen of isoniazid, ethambutol, rifampicin, pyrazinamide; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MM, multiple myeloma; TB, tuberculosis.
a HERZ was replaced by HER after 2 months.
b 2nd line therapy included ethambutol, cycloserine, levofloxacin, and prothionamide
c 2nd line therapy included levofloxacin, isoniazid, and ethambutol.
d 2nd line therapy included levofloxacin, p-aminosalicylic acid, pyrazinamide, and streptomycin.
Incidence ratio of TB in HSCT recipients compared with general population
The incidence ratio of post-HSCT TB compared with the general population is shown in Table 4. The overall incidence of TB in allogeneic HSCT recipients was significantly higher than that in the general population in Korea (SIR, 9.10, 95% CI, 5.59–14.79; P < 0.001). The incidence of TB in allogeneic HSCT recipients was 654.2 per 100 000 patients per year. There was no statistically significant difference in post-HSCT TB incidence ratio before and after applying INH prophylaxis practice. (P = 0.280).
Table 4. The incidence of tuberculosis in allogeneic hematopoietic stem cell transplantation recipients compared with the general population in Korea.
| Transplant perioda | Duration of observation, patient-years | TB patients | SIR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Observed n (%) | Expected n | |||||
| Total (n = 845) | 3210.26 | 21 (2.49) | 2.30 | 9.10 | 5.59–14.79 | < 0.001 |
| Jan. 2004-Mar. 2009 (n = 550) | 2341.64 | 16 (2.91) | 1.65 | 9.58 | 5.57–16.48 | < 0.001 |
| Apr. 2009-Mar. 2011 (n = 295) | 868.62 | 5 (1.69) | 0.64 | 7.73 | 3.13–19.13 | < 0.001 |
TB, tuberculosis; SIR, standardized incidence ratio; CI, confidence interval.
a Starting April 2009, patients were given isoniazid prophylaxis based on interferon-γ release assay results.
INH prophylaxis in latent TB
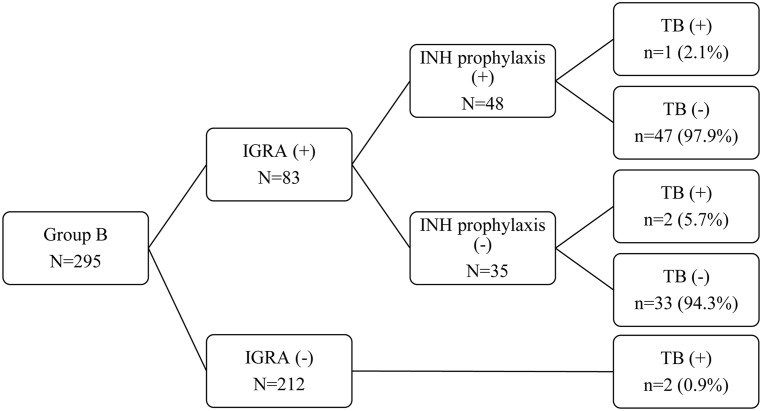
Fig 2 shows the rates of INH prophylaxis use and TB disease in group B (allogeneic HSCT between April 2009 and March 2011). Among the 83 IGRA positive patients, 35 patients (42%) were not administered INH prophylaxis or had to stop the prophylaxis. Twenty-seven patients did not start INH prophylaxis due to poor compliance among doctors (8 cases), GVHD (8), hepatotoxicity (8), poor compliance among recipients (2), and esophageal candidiasis (1). Eight patients had to stop INH prophylaxis due to hepatotoxicity (2 cases), GVHD (1), peripheral neuropathy (1), drug eruption (1), drug-induced diarrhea (1), relapses of underlying diseases (1), and poor compliance among recipients (1). The reasons for poor compliance among doctors were the regulation of clinical studies, missed orders, or the ignorance of results. Five patients had TB disease, and 2 patients had an IGRA negative result and underwent allogeneic HSCT without INH prophylaxis. Another 2 patients had IGRA positive results, but they did not take INH prophylaxis. One patient taking INH prophylaxis had TB; however, the INH was taken for only 3 months due to poor compliance. IGRA result (P = 0.341) and INH prophylaxis (P = 0.548) did not significantly alter the incidence of TB.
Fig 2. Isoniazid prophylaxis and tuberculosis.
INH prophylaxis did not significantly reduce the incidence of TB (P = 0.548). Among 5 TB patients in group B, one patient had INH prophylaxis. IGRA negative included indeterminate results. IGRA, interferon-γ release assays; INH, isoniazid; TB, tuberculosis.
Discussion
The objective of this study was to investigate the incidence of TB in allogeneic HSCT recipients compared with that in the general population in Korea. We also examined the risk factors for the development of TB and the efficacy of INH prophylaxis.
The TB incidence of HSCT recipients is affected by geographical prevalence. Studies on TB development after HSCT published from 1990 to January 2015 are summarized in Table 5. The annual TB incidence of the general population in Korea is about 70–100 cases per 100 000 patients [7, 13]. In our study, active TB after allogeneic HSCT occurred in 2.49% of patients, which is higher than that reported in many western countries, even though study periods and cohorts varied. Taiwan, India, and Australia showed a similar TB incidence (2.3–3.1%). However, Hong Kong and Pakistan had higher incidences than that reported here. Previous studies of TB disease after allogeneic HSCT in Korea demonstrated a diverse range of incidences of 3.3–4.5% (Table 5).
Table 5. Description of studies of tuberculosis after hematopoietic stem cell transplantation published from 1990 to January 2015.
| Authors/ publishing year | Country (study period) | TB/ No. of HSCT | TB incidence after HSCT | INH prophylaxis | TB of general population | Onset of TB from HSCT (range) | Pulmonary/ Extrapulmonary TB | TB related death |
|---|---|---|---|---|---|---|---|---|
| Fan et al./2015[1] | Taiwan (1997–2006) | 32/1368(Allo) 7/672 (Auto) | 2.3% (Allo) 1.0% (Auto) | ND | 0.38% | 0.06 to 4.63 y | 34/5 | ND |
| Sester et al./2014[23] | Europe (Jun. 2008-May. 2011) | 0/103 | 0% | 18 (17.5%) | 0%a | ND | ND | ND |
| Moon et al./2013[24] | Korea (Apr. 2009-Jul. 2011) | 2/244 | 0.8% | None | ND | ND | ND | ND |
| Kumar et al./2009[25] | India (Jul. 2004-Nov. 2007) | 1/40 (Allo) | 2.5% (Allo) | ND | ND | ND | 0/1 | 1 |
| Ullah et al./2007[26] | Pakistan (Jul. 2001-Sep. 2006) | 4/154 (Allo) | 2.6% (Allo) | Done | ND | ND | 0/1 | 1 |
| Al-Anazi et al./2007[27] | Saudi Arabia (1991–2002) | 3/103 | 2.9% | Partially done | ND | 1 to 12 m | 2/1 | ND |
| Park et al./2006[28] | Korea (2001–2002) | 8/379 | 2.1% | None | ND | ND | ND | 2 |
| Garces-Ambrossi et al./2005[29] | USA (1993–2001) | 4/577 (Allo) | 0.7% (Allo) | ND | 0.01–0.03% | 60 to 300 d | 3/1 | ND |
| Ahmed et al./2005[30] | Pakistan (Jul. 2001- Oct. 2003) | 4/50 (Allo) | 8% (Allo) | Partially done | ND | 42 to 525 d | 1/3 | ND |
| Yoo et al./2004[31] | Korea (1998–1999) | 8/242 (Allo) | 3.3% (Allo) | None | ND | 176 to 734 d | ND | 0 |
| Lee et al./2004[32] | Korea (Feb. 1996 -Jul.2003) | 7/156 (Allo) 2/139 (Auto) | 4.5% (Allo) 1.4% (Auto) | Partially done | ND | 30 to 165 d | 7 /2 | 0 |
| George et al./2004[33] | India (1986–2001) | 9/304 (Allo) | 3.0% (Allo) | ND | ND | ND | 1/8 | 0 |
| Erdstein et al./2004[34] | Australia (1999–2001) | 4/127 (Allo) | 3.1% (Allo) | ND | ND | 3.3 to 15 m | 3/1 | 2 |
| Ku et al./2001[5] | Taiwan (Mar.1994-Mar. 2000) | 8/255 (Allo) 0/95 (Auto)b | 3.1% (Allo) 0% (Auto) | ND | 13.1 times greater than general population | 1 to 33.5 m | 8/0 | 1 |
| de la Camara et al./2000[6] | Spain (1976–1998) | 12/2866 (Allo) 8/5147 (Auto) | 0.4% (Allo) 0.2% (Auto) | Partially done | 2.21 times greater than general population | 11 to 3337 d | 15/5 | 3 |
| Budak-Alpdogan et al./2000[35] | Turkey (Jan. 1988-Aug. 1998) | 5/351 (Allo) | 1.4% (Allo) | 77 (21.9%) | 35.4 per 100 000 PY | 10 to 47 m | 4/1 | ND |
| Aljurf et al./1999[36] | Saudi Arabia (1986–1997) | 4/641 (Allo) | 0.6% (Allo) | ND | ND | 120 to 600 d | 2/2 | 2 |
| Ip et al./1998[10] | Hong Kong (1991–1994) | 10/183b | 5.5% | 5 (2.7%) | ND | 23 to 550 d | 10 | ND |
| Roy et al./1997[37] | USA (1974–1994) | 1/1486(Allo) 0/755 (Auto) | 0.1% (Allo) 0% (Auto) | ND | 0.01% | 7 to 100 d | 0/1 | ND |
| Lee et al./(this study) | Korea (2004–2011) | 21/824 (Allo) | 2.5% (Allo) | Partially done | 9.1 times greater than general population | 49 to 886 d | 11/10 | 1 |
Allo, allogeneic; Auto, autologous; HSCT, hematopoietic stem cell transplantation; INH, isoniazid; ND, Not described; PY, patient-year; TB, tuberculosis.
a Of 211 cases in a non-immunocompromised control group, the TB incidence was 0%.
b Only pulmonary tuberculosis was included.
The incidence of TB following HSCT is found to be 10–40 times higher than that of the general population [2]. The cumulative incidence of TB disease in our study was 9.1 times higher than that of the general population in Korea. In Taiwan, pulmonary TB in allogeneic HSCT was 13.1-fold higher [5]. In Spain, de la Camara et al. reported that allogeneic HSCT recipients showed 2.95 times higher relative risk of TB incidence compared with the general population [6]. It seems that geographic location and follow-up duration may influence the differences in the incidence of TB disease.
Several risk factors for the development of TB after HSCT have been reported, including AML, CML, MDS, the use of busulfan, cyclophosphamide, TBI, corticosteroid therapy, mismatched allografts, GVHD, or history of previous TB infection [2]. Our study shows that extensive chronic GVHD is associated with the development of TB. Donor type, underlying hematological disease, conditioning regimen, and acute GVHD were not risk factors according to our study. A TBI-based conditioning regimen was given to >70% of the recipients in our center, which seems to affect the result. The other center in Korea previously reported that HSCT patients with TBI-based conditioning are likely to have TB disease, while TBI-based conditioning was given to 45% of patients with HSCT [32, 38].
The extrapulmonary TB rate tends to be higher than that of the general population since decreased cell-mediated immune responses cannot restrict TB infection [10]. The rate of extrapulmonary TB is reportedly 15–20% in the Korean general population [39]. Our result was also much higher (47.6%) than that in the general population. Table 5 shows a diverse range of extrapulmonary TB rates. The reasons behind the wide range of extrapulmonary TB rates are due to various degrees of practices of close examinations, a high index of suspicion, and anti-TB treatment timing [10].
In this study, there was no association with the IGRA results and progression of active TB disease, which corresponds well with those found in the earlier studies. Sester et al. also reported that the IGRA or tuberculin skin test poorly predicted the progression of active TB in immunocompromised hosts [23]. The immunosuppression state of HSCT recipients would influence IGRA test accuracy [40].
INH prophylaxis has been used to prevent active TB in LTBI patients [2]. However, INH prophylaxis has several problems, including hepatotoxicity, drug interaction with immunosuppressants, and manifestation of resistance to INH [2, 41, 42]. Therefore, the efficacy of INH prophylaxis in HSCT recipients needs to be determined. The result of the present study is that INH prophylaxis did not significantly alter the incidence of TB disease, which is similar to the results of earlier studies [32, 41]. However, these findings differ from suggestions of other reports, which recommend INH prophylaxis for HSCT recipients [3, 30]. Despite the long follow-up period, here we reported TB disease in 21 patients in the whole group and only five patients in group B (INH prophylaxis group) due to TB being a relatively rare disease. The small number of patients may not be sufficient for detecting significant differences for evaluating the effect of INH prophylaxis. However, among the 21 TB patients, only one took INH prophylaxis for 3 months due to poor compliance. Well-designed prospective clinical trials are needed to estimate the efficacy of INH prophylaxis. Also, the ideal INH prophylaxis duration is debatable because TB disease usually occurs in the late post-HSCT period [31, 35]. In our report, patients developed TB at a median of 1 year after HSCT.
One recent study reported that Korea shows a low rate of multidrug-resistant TB (<2000 cases in 2011) [43]. In 2004, the proportion of INH resistance among the total number of notified new TB cases was 9.9% in Korea [44]. Our study population showed only one patient with INH resistance among 6 antimicrobial susceptibility test-proven TB cases, and this patient improved when underwent second-line anti-TB treatment. However, Korea is one of the countries in which multidrug-resistant TB has been increasing [45]. Therefore, when patients with TB are treated, resistance to anti-TB medications needs to be taken into consideration. Also, the value of INH prophylaxis needs to be assessed carefully.
The present study has some limitations. First, it was retrospective, which caused some problems in that specific information about the history of previous TB infection and data of therapeutic approach of chronic GVHD in patients without current TB disease were missing from the medical records. Second, it included a small number of patients with TB. Third, in group B, INH prophylaxis was not given to all IGRA positive patients. Regardless of these limitations, major strengths of our study include a long follow-up period (11 years) and the study population, a relatively homologous large number of allogeneic HSCT patients with only AML, CML, MDS, or MM. Also, the cumulative incidence calculated by Gray’s test considered the competing risks and we used a standardized incidence ratio when making comparisons with the general population.
In conclusion, our study provides a useful evaluation for allogeneic HSCT patient group who is at high risk of contracting TB disease, compared to the general population. TB should be considered when evaluating allogeneic HSCT patients, especially those with extensive chronic GVHD. INH prophylaxis did not statistically change the incidence of TB; however, there is a tendency of decreased TB incidence among the INH prophylaxis group. More research is warranted on the efficacy of INH prophylaxis in allogeneic HSCT recipients in an intermediate TB burden country.
Supporting information
(PDF)
Acknowledgments
The statistical consultation was supported by Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea.
Data Availability
All relevant data are within the paper or its Supporting Information files.
Funding Statement
The statistical consultation was supported by Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fan WC, Liu CJ, Hong YC, Feng JY, Su WJ, Chien SH, et al. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int J Tuberc Lung Dis. 2015;19: 58–64. 10.5588/ijtld.14.0301 [DOI] [PubMed] [Google Scholar]
- 2.Al-Anazi KA, Al-Jasser AM, Alsaleh K. Infections Caused by Mycobacterium tuberculosis in Recipients of Hematopoietic Stem Cell Transplantation. Front Oncol. 2014;4: 231 10.3389/fonc.2014.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40: 990–1013. 10.1183/09031936.00000712 [DOI] [PubMed] [Google Scholar]
- 4.Arslan O, Gurman G, Dilek I, Ozcan M, Koc H, Ilhan O, et al. Incidence of tuberculosis after bone marrow transplantation in a single center from Turkey. Haematologia (Budap). 1998;29: 59–62 [PubMed] [Google Scholar]
- 5.Ku SC, Tang JL, Hsueh PR, Luh KT, Yu CJ, Yang PC. Pulmonary tuberculosis in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27: 1293–1297. [DOI] [PubMed] [Google Scholar]
- 6.de la Camara R, Martino R, Granados E, Rodriguez-Salvanes FJ, Rovira M, Cabrera R, et al. Tuberculosis after hematopoietic stem cell transplantation: incidence, clinical characteristics and outcome. Spanish Group on Infectious Complications in Hematopoietic Transplantation. Bone Marrow Transplant. 2000;26: 291–298. 10.1038/sj.bmt.1702506 [DOI] [PubMed] [Google Scholar]
- 7.World Health Oraganization. Estimates of TB and MDR-TB burden are produced by WHO in consultation with countries. 4 Oct 2016. https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=KR&LAN=EN&outtype=html. Cited 26 February 2015.
- 8.Shim TS, Koh WJ, Yim JJ, Lew WJ. Treatment of latent tuberculosis infection in Korea. Tuberculosis and Respiratory Diseases. 2008;65: 79–90. [Google Scholar]
- 9.Korea Centers for Disease Control and Prevention. Revised national tuberculosis control programme in Korea. 25 Aug 2012. http://cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0037-MNU1380&cid=12254. Cited 3 October 2016.
- 10.Ip MS, Yuen KY, Woo PC, Luk WK, Tsang KW, Lam WK, et al. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158: 1173–1177. 10.1164/ajrccm.158.4.9712072 [DOI] [PubMed] [Google Scholar]
- 11.Tuon FF, Litvoc MN, Lopes MI. Adenosine deaminase and tuberculous pericarditis—a systematic review with meta-analysis. Acta Trop. 2006;99: 67–74. 10.1016/j.actatropica.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Jung JI, Lee DG, Kim YJ, Yoon HK, Kim CC, Park SH. Pulmonary tuberculosis after hematopoietic stem cell transplantation: radiologic findings. J Thorac Imaging. 2009;24: 10–16. 10.1097/RTI.0b013e31818c6b97 [DOI] [PubMed] [Google Scholar]
- 13.Eom KS, Lee DG, Lee HJ, Cho SY, Choi SM, Choi JK, et al. Tuberculosis before hematopoietic stem cell transplantation in patients with hematologic diseases: report of a single-center experience. Transpl Infect Dis. 2015;17: 73–79. 10.1111/tid.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364: 2196–2203. 10.1016/S0140-6736(04)17592-2 [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Choi SJ, Kim HB, Kim NJ, Oh MD, Choe KW. Diagnostic usefulness of a T-cell based assay for extrapulmonary tuberculosis. Arch Intern Med. 2007;167: 2255–2259. 10.1001/archinte.167.20.2255 [DOI] [PubMed] [Google Scholar]
- 16.Kwon JC, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, et al. Clinical characteristics and the usefulness of the QuantiFERON-TB Gold In-Tube test in hematologic patients with hepatic or splenic lesions. Korean J Intern Med. 2013;28: 187–196. 10.3904/kjim.2013.28.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1): 1376–1395. 10.1164/ajrccm.161.4.16141 [DOI] [PubMed] [Google Scholar]
- 18.Sun HY, Munoz P, Torre-Cisneros J, Aguado JM, Lattes R, Montejo M, et al. Mycobacterium tuberculosis-associated immune reconstitution syndrome in solid-organ transplant recipients. Transplantation. 2013;95: 1173–1181. 10.1097/TP.0b013e31828719c8 [DOI] [PubMed] [Google Scholar]
- 19.Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011;26: 220–252. 10.3904/kjim.2011.26.2.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Kee SY, Lee DG, Choi SM, Park SH, Kwon JC, et al. Infectious complications following allogeneic stem cell transplantation: reduced-intensity vs. myeloablative conditioning regimens. Transpl Infect Dis. 2013;15: 49–59. d 10.1111/tid.12003 [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15: 825–828 [PubMed] [Google Scholar]
- 22.Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea 2012. 6 Dec 2013. http://www.cdc.go.kr/CDC/info/CdcKrInfo0302.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0038&fid=32&q_type=&q_value=&cid=22011&pageNum=. Cited 26 February 2015.
- 23.Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, et al. Risk Assessment of Tuberculosis in Immunocompromised Patients. A TBNET Study. Am J Respir Crit Care Med. 2014;190: 1168–1176. 10.1164/rccm.201405-0967OC [DOI] [PubMed] [Google Scholar]
- 24.Moon SM, Lee SO, Choi SH, Kim YS, Woo JH, Yoon DH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis. 2013;15: 104–109. 10.1111/j.1399-3062.2012.00765.x [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Naithani R, Mishra P, Mahapatra M, Seth T, Dolai TK, et al. Allogeneic hematopoietic SCT performed in non-HEPA filter rooms: initial experience from a single center in India. Bone Marrow Transplant. 2009;43: 115–119. 10.1038/bmt.2008.307 [DOI] [PubMed] [Google Scholar]
- 26.Ullah K, Ahmed P, Raza S, Satti T, Nisa Q, Mirza S, et al. Allogeneic stem cell transplantation in hematological disorders: single center experience from Pakistan. Transplant Proc. 2007;39: 3347–3357. 10.1016/j.transproceed.2007.08.099 [DOI] [PubMed] [Google Scholar]
- 27.Al-Anazi KA, Al-Jasser AM, Evans DA. Infections caused by mycobacterium tuberculosis in patients with hematological disorders and in recipients of hematopoietic stem cell transplant, a twelve year retrospective study. Ann Clin Microbiol Antimicrob. 2007;6: 16 10.1186/1476-0711-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Lee JW, et al. Current trends of infectious complications following hematopoietic stem cell transplantation in a single center. J Korean Med Sci. 2006;21: 199–207. 10.3346/jkms.2006.21.2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garces Ambrossi G, Jakubowski A, Feinstein MB, Weinstock DM. Active tuberculosis limited to foreign-born patients after allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2005;36: 741–743. 10.1038/sj.bmt.1705129 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed P, Anwar M, Khan B, Altaf C, Ullah K, Raza S, et al. Role of isoniazid prophylaxis for prevention of tuberculosis in haemopoietic stem cell transplant recipients. J Pak Med Assoc. 2005;55: 378–381 [PubMed] [Google Scholar]
- 31.Yoo JH, Lee DG, Choi SM, Choi JH, Park YH, Kim YJ, et al. Infectious complications and outcomes after allogeneic hematopoietic stem cell transplantation in Korea. Bone Marrow Transplant. 2004;34: 497–504. 10.1038/sj.bmt.1704636 [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Lee MH, Kim WS, Kim K, Park SH, Lee SH, et al. Tuberculosis in hematopoietic stem cell transplant recipients in Korea. Int J Hematol. 2004;79: 185–188. [DOI] [PubMed] [Google Scholar]
- 33.George B, Mathews V, Srivastava A, Chandy M. Infections among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplant. 2004;33: 311–315. 10.1038/sj.bmt.1704347 [DOI] [PubMed] [Google Scholar]
- 34.Erdstein AA, Daas P, Bradstock KF, Robinson T, Hertzberg MS. Tuberculosis in allogeneic stem cell transplant recipients: still a problem in the 21st century. Transpl Infect Dis. 2004;6: 142–146. 10.1111/j.1399-3062.2004.00068.x [DOI] [PubMed] [Google Scholar]
- 35.Budak-Alpdogan T, Tangun Y, Kalayoglu-Besisik S, Ratip S, Akan H, Baslar Z, et al. The frequency of tuberculosis in adult allogeneic stem cell transplant recipients in Turkey. Biol Blood Marrow Transplant. 2000;6: 370–374. [DOI] [PubMed] [Google Scholar]
- 36.Aljurf M, Gyger M, Alrajhi A, Sahovic E, Chaudhri N, Musa M, et al. Mycobacterium tuberculosis infection in allogeneic bone marrow transplantation patients. Bone Marrow Transplant. 1999;24: 551–554. 10.1038/sj.bmt.1701930 [DOI] [PubMed] [Google Scholar]
- 37.Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: a 20 year retrospective review. Bone Marrow Transplant. 1997;19: 467–470. 10.1038/sj.bmt.1700686 [DOI] [PubMed] [Google Scholar]
- 38.Won YW, Yi SY, Jang JH, Kim K, Kim SJ, Kim WS, et al. Retrospective analysis of paranasal sinusitis in patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2011;93: 383–388. 10.1007/s12185-011-0797-8 [DOI] [PubMed] [Google Scholar]
- 39.Lee DG. Common infectious diseases in hematopoietic stem cell transplant Recipients. Korean J Med. 2013;84: 158. [Google Scholar]
- 40.Lee YM, Kim SM, Park SJ, Lee SO, Choi SH, Kim YS, et al. Factors associated with a strong response to the T-SPOT.TB in patients with extrapulmonary tuberculosis. Infect Chemother. 2014;46: 248–252. 10.3947/ic.2014.46.4.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akan H, Arslan O, Akan OA. Tuberculosis in stem cell transplant patients. J Hosp Infect. 2006;62: 421–426. 10.1016/j.jhin.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 42.Cobelens FG. For whom the bell tolls: isoniazid preventive therapy and tuberculosis drug resistance. Sci Transl Med. 2013;5: 180fs112. [DOI] [PubMed] [Google Scholar]
- 43.Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis. 2013;13: 690–697. 10.1016/S1473-3099(13)70130-0 [DOI] [PubMed] [Google Scholar]
- 44.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373: 1861–1873. 10.1016/S0140-6736(09)60331-7 [DOI] [PubMed] [Google Scholar]
- 45.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ. 2012;90: 111–119d. 10.2471/BLT.11.092585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper or its Supporting Information files.