Abstract
Biological invasions are governed by spatial processes that tend to be distributed in non-random ways across landscapes. Characterizing the spatial and temporal heterogeneities of the introduction, establishment, and spread of non-native insect species is a key aspect of effectively managing their geographic expansion. The Asian citrus psyllid (Diaphorina citri), a vector of the bacterium associated with huanglongbing (HLB), poses a serious threat to commercial and residential citrus trees. In 2008, D. citri first began expanding northward from Mexico into parts of Southern California. Using georeferenced D. citri occurrence data from 2008–2014, we sought to better understand the extent of the geographic expansion of this invasive vector species. Our objectives were to: 1) describe the spatial and temporal distribution of D. citri in Southern California, 2) identify the locations of statistically significant D. citri hotspots, and 3) quantify the dynamics of anisotropic spread. We found clear evidence that the spatial and temporal distribution of D. citri in Southern California is non-random. Further, we identified the existence of statistically significant hotspots of D. citri occurrence and described the anisotropic dispersion across the Southern California landscape. For example, the dominant hotspot surrounding Los Angeles showed rapid and strongly asymmetric spread to the south and east. Our study demonstrates the feasibility of quantitative invasive insect risk assessment with the application of a spatial epidemiology framework.
Introduction
Human-mediated changes to ecosystems can significantly impact the health and well-being of plant, animal, and human populations [1]. Biological invasions are an important aspect of this global change and are accelerating in many regions where human activities facilitate introduction events [2, 3]. Urban environments play a particularly important role in the establishment of invasive insect species by serving as refuges and, ultimately, sources of invasive species spread [4–7]. This is especially true in regions where extensive urban encroachment may strengthen further linkages with nearby agricultural or natural habitats [8].
California has faced an influx of phytophagous insects that are important pests in agricultural and natural systems, yet whose establishment is centered in urban and suburban areas. Recent examples include the brown marmorated stink bug (Halyomorpha halys), bagrada bug (Bagrada hilaris), red palm weevil (Rhynchophorus ferrugineus), and polyphagous shot hole borer (Euwallacea spp.) [9]. These invaders threaten landscape flora, and put adjacent habitats at risk in the event of a spillover. Of particular concern agriculturally is the detection in 2008 of the Asian citrus psyllid (Diaphorina citri), a vector of the bacterium (Candidatus Liberibacter asiaticus and other spp.) that is associated with huanglongbing (HLB) or citrus greening disease. D. citri is likely native to Southeast Asia or India, but has spread to the Middle East, South America, Central America, and more recently, Mexico and the United States [10, 11]. This psyllid specializes on plants in the family Rutaceae, but feeds broadly within the family on different citrus varieties and many close relatives. HLB disease is characterized by mottled leaves, deformed and off-flavor fruit, defoliation and plant death [12, 13]. No citrus varieties are fully resistant to HLB and, to date, no cure for infected trees is available. As a result, regions where the co-occurrence of D. citri and HLB have become widespread (i.e. Florida) have suffered substantial economic losses [14]. Therefore, it is critical to limit pathogen spread by controlling D. citri populations, particularly in California and other regions where the disease is not yet widespread.
The Asian citrus psyllid was first documented in California in a residential setting in San Diego County in 2008. In response, a range of measures were implemented in an attempt to contain its spread [15]. These included establishment of an extensive monitoring program, the establishment of quarantines and insecticide treatments to limit human-assisted movement of the insect via nurseries and harvested fruit, as apparently occurred in Florida [16], protective structures to house nursery stock, residential and commercial insecticide treatment programs, and starting in 2011 a biological control program employing the nymphal parasitoid Tamarixia radiata for residential areas [17]. Despite these management efforts, D. citri continued its spread throughout Southern California. In 2012, the first case of HLB was documented in a residential area of Los Angeles County [15, 18].
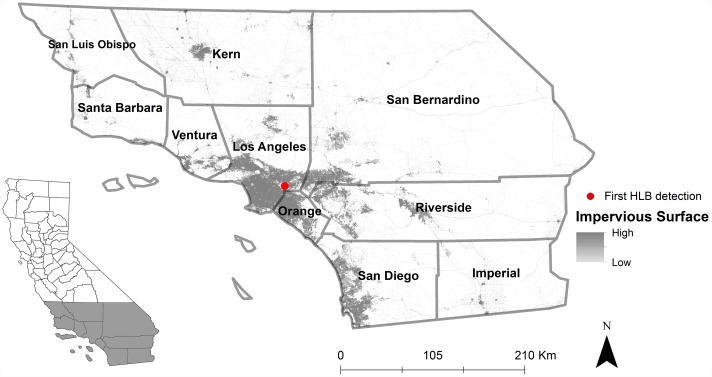
The progressive stages of biological invasions (introduction, establishment, spread, and impact) are governed by spatial and temporal processes that tend to be distributed in non-random ways across landscapes [19–21]. In California, a convergence of social and ecological factors appear to be contributing to the establishment of D. citri [22], yet little is published about the dynamic spread of D. citri in this region. Using georeferenced D. citri occurrence data from an area-wide network of trapping from 2008–2014, we sought to characterize the extent and features of the geographic expansion of this invasive vector species as a step towards understanding and mitigating the factors driving its invasion. Leveraging a suite of spatial statistics, our objectives were to: (1) describe the spatial distribution and temporal dynamics of D. citri in Southern California; (2) identify the locations of statistically significant D. citri hotspots; and (3) quantify the extent of anisotropic (i.e. directionally-dependent) spread throughout the Southern California study area (Fig 1).
Fig 1. Study area including ten Southern California counties.
Materials and methods
D. citri monitoring data source
We obtained a database of georeferenced D. citri collection sites throughout Southern California from 2008 through 2014 from the California Department of Food and Agriculture (CDFA). These include upwards of 78,000 D. citri detection records from 14 x 23 cm double-sided yellow panel traps that were deployed throughout ten counties in the Southern part of California (i.e. San Luis Obispo, Santa Barbara, Ventura, Los Angeles, Kern, San Bernardino, Riverside, Imperial, San Diego, and Orange) (Fig 1). Records come from multiple D. citri monitoring programs being conducted in parallel in these counties, totaling collectively up to more than 5,000 unique trapping locations per county over the year, with traps checked on average once per month, but up to three times per month.
The D. citri trapping program was intended primarily to demarcate the geographic extent of D. citri distribution to inform the establishment of quarantine zones and prioritize management options. Given this intent, there are important implications for the nature of the data that were available for our analyses. First, data on the geographic location of insect presence was limited to D. citri positive sampling sites (i.e. D. citri presences) rather than absences as well. In addition, since the number of D. citri adults collected on a trap was not reported consistently, our data only considers D. citri positive traps rather than counts per trap (i.e. traps with one or more D. citri caught during a given census were considered equivalent presences). Finally, the locations of traps and trapping effort in certain areas shifted over time (Table A in S1 File). Despite these potential limitations with the data, we expect that the sheer volume of D. citri positive records in the database (i.e. more than 78,000 positive traps over 7 years) allows for robust description of the broad dynamics of D. citri in the region.
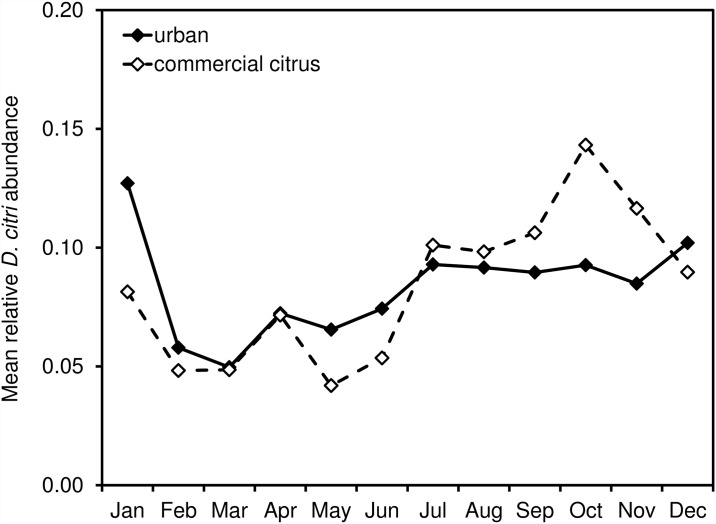
For this study we largely restricted analyses to those data from urban and suburban areas (hereafter referred to collectively as “urban”) in Southern California. This is primarily due to the vast majority of D. citri detections over the first five years of the program occurring in these areas (i.e. > 95%) as opposed to commercial citrus (Fig 2). To better delineate gradients of D. citri abundance and to conduct spatial analyses, we aggregated the number of D. citri positive traps into 5 x 5 km grid cells. Prior to estimating abundance, the data were checked for identical georeferenced records in a given year, and all duplicate records were deleted. Unless otherwise noted the response variable considered in analyses was cumulative count of positive D. citri traps per grid cell per unit time (i.e. D. citri abundance).
Fig 2. Cumulative annual D. citri occurrences in urban areas versus commercial citrus.
Spatial analysis
To assess whether a geographic pattern (i.e. clustered, random, or evenly dispersed) of D. citri positive sites existed during the study period, we utilized the global Moran’s I measure of spatial autocorrelation. Specifically, we computed the incremental Moran’s I statistic for each year to detect the peak distance at which spatial clustering of the D. citri data was maximized (Table B in S1 File). These statistically significant peak distances were then used as distance thresholds for subsequent local spatial statistical tests. We used several locally-derived spatial statistics to assess whether the observed spatial pattern of D. citri during each year was significantly different from a distribution that would be expected if underlying spatial processes were random. Both the local Moran’s I and Getis-Ord Gi* were calculated as complementary measures of the extent to which D. citri counts in neighboring grid cells were similar or dissimilar. To further complement these purely spatial measures, we added a temporal aspect to the analysis by calculating the Kulldorff space-time permutation scan statistic. Clusters of higher than expected D. citri positive sampling sites may be considered ‘hotspots’ of elevated entomological risk [23].
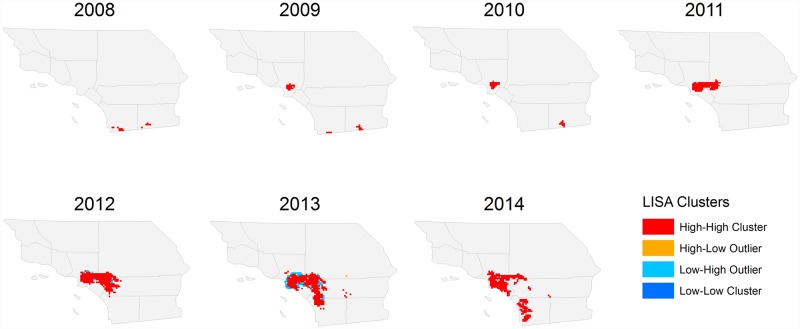
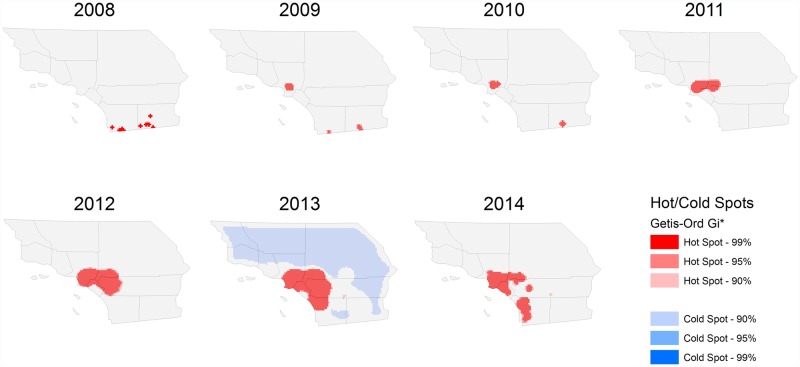
First, we computed the local Moran’s I statistic, which uses a measure of local spatial autocorrelation at each location, as a means of detecting the geographic location of hotspots [24]. Significant local Moran’s I clusters represent areas of similarly high or low numbers of D. citri positive collection sites. We aggregated grid cell clusters as “high-high” hotspots of similarly elevated D. citri abundance (high risk cells next to other high risk cells) or “low-low” cold spots of similarly lower D. citri abundance (low risk cells next to other low risk cells). In addition, the local Moran’s I statistic produces measures of outliers, including indicators of high risk cells next to low risk cells (“high-low” outliers) and low risk cells next to high risk cells (“low-high” outliers). Next, we calculated the Getis-Ord Gi* statistic for each grid cell to identify locations where the magnitude of D. citri abundance, compared to other parts of the study area, was higher than would be expected by chance [25, 26]. Finally, we implemented the Kulldorff space-time permutation statistic using SaTScan spatial software to identify statistically significant space-time clusters (i.e. space-time hotspots) across each year of the study period [27]. Space-time clusters, defined as an excess of D. citri positive sites that are close to one another in both space and time, were identified by a cylindrical scanning window with a circular geographic base and height defined by a 1-year time interval. To test the null hypothesis that all D. citri positive sites are independent of space-time interaction, a test statistic was derived by computing maximum likelihood ratios and corresponding significance values obtained through 999 Monte Carlo simulations. The scan test reports the relative risk (RR) of each significant space-time cluster, which is estimated as the observed number of D. citri positive sites divided by the expected number of sites within the cluster. We used the magnitude of the test statistic to rank each statistically significant cluster. Spatial analysis was conducted using ArcGIS v.10.2.2 [28] and the SaTScan spatial software program [29].
Quantifying anisotropic spread
To quantify the extent of geographic dispersion of D. citri across time, we modeled the spread of D. citri positive sites, as well as the spread of the statistically significant hotspots. This consisted of two complimentary analyses.
First, kernel density raster maps were developed using D. citri abundance within grid cells for 7 incremental time periods: 2008, 2008–09, 2008–10, 2008–11, 2008–12, 2008–13 and 2008–14. Kernel density estimation is a smoothing technique used to capture the intensity or magnitude of a given spatial event within a study region. To identify the appropriate search radius needed for smoothing, we first analyzed the D. citri abundance data for each of the incremental time periods for spatial autocorrelation. The resulting kernel density rasters were then classified using the standard deviation scheme into 7 bands of varying density range values for the dominant Los Angeles hotspot. The kernel density raster maps (S1 Fig) were then used to capture the rate of spread in each of the cardinal and inter-cardinal directions (i.e. N, E, W, S, NE, SE, NW, and SW). To quantify spread rate, the distance covered by D. citri in a given time period was estimated relative to the earliest known occurrence of D. citri in Los Angeles County. For each direction the rate of spread was quantified as the slope of the regression between cumulative number of years of D. citri invasion since 2009 and distance covered by D. citri density raster from the hypothetical point of origin in each incremental time period.
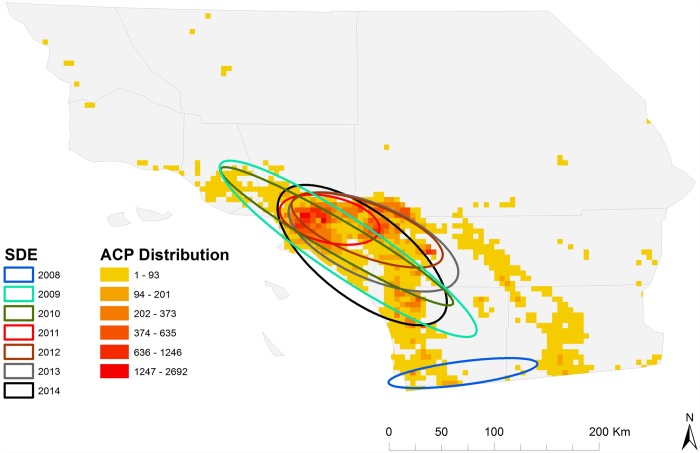
To further quantify the extent of directionally-dependent spread, standard deviational ellipses (SDE) were calculated to summarize the central tendency, dispersion and directional trends for both D. citri abundance per grid cell and hotspots identified with the Getis-Ord Gi* statistic. Each SDE represent areas of higher concentrations of D. citri positive sampling sites or presence of hotspots and provide measures of the extent of asymmetrical distribution of the data across the landscape [30]. We calculated SDE for each year in the study period using yearly D. citri distribution data throughout Southern California.
Results
General spatial and temporal patterns
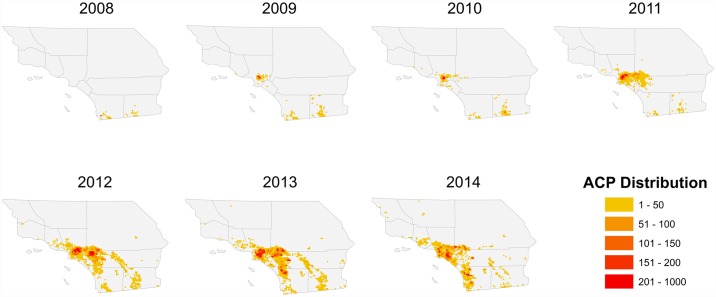
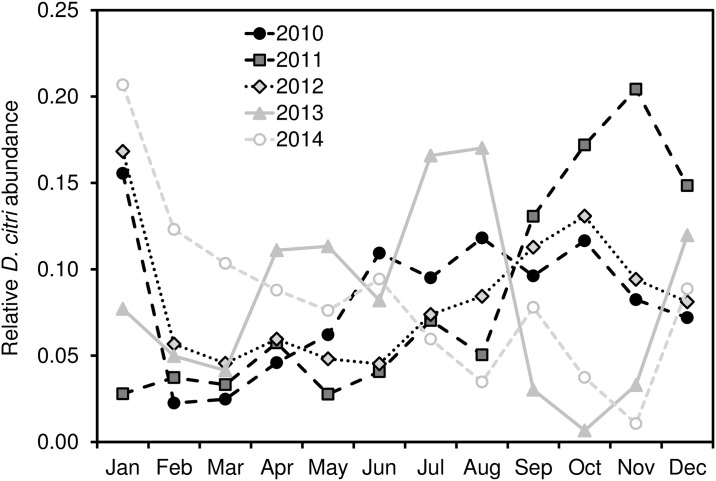
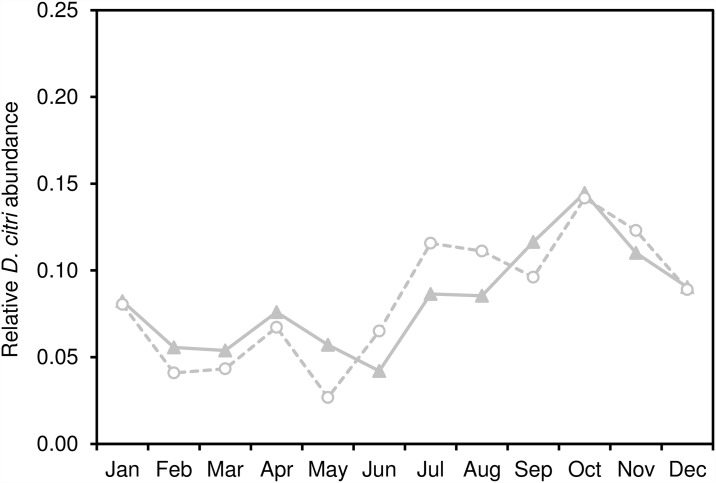
Since 2008, the cumulative abundance of D. citri in Southern California increased dramatically, particularly in the urban environment (Fig 2). After first appearing on residential citrus in San Diego County, D. citri positive sites were subsequently identified in parts of Imperial, Orange, Los Angeles, Riverside, San Bernardino, Ventura, and eventually Santa Barbara, San Luis Obispo and Kern Counties (Fig 3). Within these areas, the geographic focus of D. citri was primarily urban, especially in the first 5 years of the invasion, with the first detections in commercial citrus groves in 2011. For urban areas, we found equivocal evidence of seasonality over the study period, with a general increase in the frequency of D. citri detections during the fall months; however, the year to year distribution was variable and largely asynchronous (Fig 4). In the two years for which the frequency of commercial psyllid finds was sufficient to analyze (i.e. 2013, 2014), these areas demonstrated a more pronounced increase in the fall months, likely attributable to onset of fall citrus flush (Fig 5). Neither urban nor commercial citrus showed clear evidence of a spring peak in D. citri positive traps, a time of year that is typically associated with the largest peak in citrus flushing in California.
Fig 3. Cumulative D. citri occurrences in Southern California, 2008–2014.
Fig 4. Proportion of annual urban D. citri occurrences in a given month.
Only those years shown for which there were adequate occurrences each month of the year (2010–2014 for urban, 2013–2014 for commercial citrus).
Fig 5. Proportion of annual commercial D. citri occurrences in a given month.
Only those years shown for which there were adequate occurrences each month of the year (2010–2014 for urban, 2013–2014 for commercial citrus).
Hotspots and space-time clusters
We found evidence that the spatial distribution of D. citri in Southern California during the 7 year time period was non-random. Significant measures of spatial autocorrelation in each year indicated clustering of D. citri positive sites. We identified the location of these clusters and documented their spread using several local statistics. In 2008, both local Moran’s I (Fig 6) and Getis-Ord Gi* (Fig 7) statistics indicated that D. citri hotspots were located near the United States-Mexico ports of entry in San Diego and Imperial Counties. In 2009, the first D. citri hotspot was detected within an urbanized area of Los Angeles County. Subsequently, the dominant hotspot surrounding Los Angeles showed rapid and strongly asymmetric spread to the south and east. Following the establishment of the Los Angeles hotspot, clusters of high D. citri-abundance appeared to the south and east in Orange, San Bernardino, and Riverside Counties. Although D. citri detections in San Diego and Imperial counties continued to occur throughout the study period (Fig 3), the statistical significance of hotspots in this region appears to have been weakened relative to the magnitude of the Los Angeles hotspots in subsequent years.
Fig 6. Local Moran’s I clusters of D. citri abundance, 2008–2014.
Fig 7. Hot and cold spots of D. citri abundance, 2008–2014.
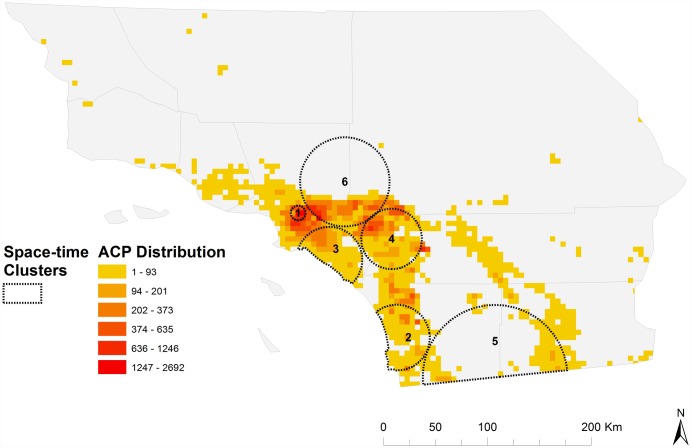
We identified six distinct space-time clusters of elevated D. citri abundance that were ranked from 1 to 6 based on decreasing relative risk measures (Table C in S1 File). Four probable clusters (clusters 1, 3, 4, 6) were located in Los Angeles, Orange, San Bernardino, and Riverside Counties and two (clusters 2, 5) in San Diego and Imperial Counties (Fig 8). The first, and most likely, cluster to be detected during the study period (cluster 5) was located on the San Diego-Mexico border in San Diego and Imperial County in 2008. Los Angeles space-time clusters were detected in 2009, with a small geographic cluster of elevated risk (RR = 4.08, p<0.001) occurring in the downtown region from 2009–2011.
Fig 8. Space-time clusters depicting anisotropic spread of D. citri hotspots.
Anisotropic spread
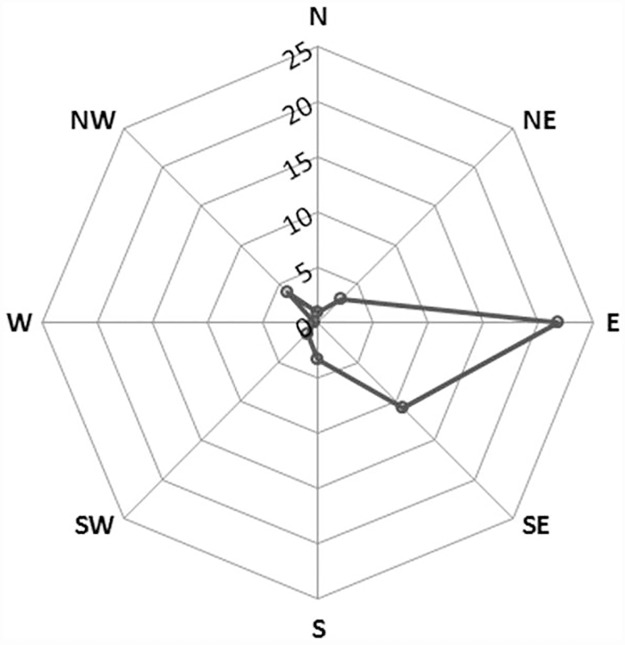
Kernel density raster maps showed a clear anisotropic pattern of D. citri spread through the incremental time periods (S1 Fig). Of particular note is the observed initial hotspot of D. citri in San Diego, followed by the presence of the next hotspot for the year 2008–09 in urban Los Angeles. However, from 2008–09 onwards the spread of D. citri was continuous in both space and time as is evident from the strong eastward spread of the Los Angeles D. citri hotspot. The eastward spread of D. citri then shifts direction toward the south from 2012 onwards; thus indicating an overall east and south east spread of D. citri density from its initial strong presence in urban Los Angeles. A spider plot for the Los Angeles hotspot clarifies further that the maximal spread rate is in the eastern direction followed by the south east (Fig 9).
Fig 9. Spider plot of the anisotropic spread of the Los Angeles D. citri hotspot from 2009 to 2014.
Concentric rings indicate the rate of spread in increments of 5 km per year.
The orientation of the yearly SDE further revealed the directional bias of D. citri distribution from 2008 to 2014 (Table D in S1 File). D. citri positive sites had an ellipsoid distribution during each year, with a strong east-to-west direction during 2008 in San Diego and Imperial Counties, and a stronger northwest-to-southeast directional bias during subsequent time periods in Los Angeles, Orange, Ventura, San Bernardino and Riverside Counties (Fig 10). The spatial concentration (i.e. area of each ellipse) and extent of directional dispersion (i.e. long axis of each ellipse) exhibited significant differences across years for both the distribution of D. citri positive sites and hotspots. The highest level of spatial concentration occurred in 2011 and centered on downtown Los Angeles, whereas the 2009 SDE exhibited the largest directional (northwest-to-southeast) dispersion (Fig 10). We calculated the eccentricity of each SDE (i.e. polarity of the distribution within the ellipse), which indicated significant differences in how uniformly D. citri positive sites were distributed around the mean center of each SDE. Certain years (2008, 2011) demonstrated a more polar distribution (i.e. more points distributed at each end of the ellipse with fewer in the middle). We also found significant differences between the angle of rotation (i.e. angle between north and y-axis rotated clockwise) for each year, providing further evidence of anisotropic spread of D. citri positive sites and hotspots across Southern California.
Fig 10. Standard deviational ellipses depicting anisotropic spread of D. citri hotspots.
Discussion
The impacts of invasive vector-borne diseases on ecosystem services are gaining increased attention as the far-reaching effects of global change begin to crystalize [31]. Recent increases in trade globalization have led to a rapid growth in the cross-border movement of agricultural products [32]. An unintended consequence of this trend has been the introduction of non-native insect species into a growing number of new environments [33]. An example of this emerging global threat is the recent introduction, presumptively via human transportation of infested plant material, of D. citri into Southern California in 2008 [10]. Using a spatial framework, we describe the geographic and temporal dynamics of the Southern California D. citri invasion from its presumed beginning.
We found clear evidence that the spatial distribution of D. citri in Southern California during the study period was non-random and is characterized by the presence of statistically significant hotspots of elevated entomological risk. These hotspots of D. citri abundance were strongly associated with certain urbanized regions and suggest more frequent introduction events in these areas, perhaps due to the transportation of plant material, equipment and fruit via road networks [22, 33, 34]. In addition to the transportation-mediated introduction events of invasive insects, urbanization may also be correlated with the amount of suitable habitat available for establishment and spread [35]. Subsequent spread of D. citri was likely the result of natural dispersal of the psyllid throughout areas with a high density of residential citrus trees, coupled with some continuing longer distance movement via unregulated or illegal movement of plant material [22, 33–36].
Many of the reasons behind the introduction and spread of D. citri in Southern California likely have similarities in other regions where invasion has been studied in greater detail. In Florida, where the economic burden of the D. citri invasion in the United States is greatest [14], long distance dispersal is thought to be a combination of both natural adult psyllid movement and human-mediated transportation events [16, 37]. Several studies in Florida have suggested that adult psyllids may be capable of flight distances of several kilometers and have allowed the insect to become widespread throughout the state [38–40]. Similar capabilities have also been demonstrated in Japan [41], included the purported movement of the psyllid between islands [42]. In addition to long distance migration potential, D. citri dispersal across large portions of Florida may have been further facilitated by the distribution of abandoned citrus groves [40]. Proximity to road networks and the unregulated movement of bulk citrus and infested nursery stock also contributed to the rapid distribution of D. citri in Florida [16].
Biological invasions are known to exhibit strong gradients of anisotropic spread through susceptible landscapes [43, 44]. The initial detection of D. citri in urban Los Angeles in 2009 was followed by rapid spread and infestation of urban areas in a southern and easterly direction across Los Angeles and adjoining counties of Riverside, San Bernardino and Orange, thus providing an ideal scenario to explore the observed anisotropic spread pattern. The dynamic nature of D. citri geographic distribution and evidence of anisotropic spread suggests that hotspots of entomological risk are likely a function of not only anthropogenic factors (e.g., transportation associated with trade and commerce), but also differences in environmental suitability (e.g., resource availability, temperature, wind speed, elevation) that exist across urbanization gradients [22, 33–36, 40, 41]. One explanation for the strong directional spread of the Los Angeles hot spot to the Southwest, and slower invasion to the Northwest, is that it reflects the prevailing wind direction in the area. However caution should be used in drawing this conclusion, as multiple studies from urban areas and commercial citrus in Florida have found little to no evidence that wind affects D. citri activity or dispersal ability [38, 39]. Rather, D. citri’s non-random, asymmetric spread in Southern California is likely to be primarily attributable to other aspects of habitat suitability, including warmer conditions [45–47] and more favorable landscape contexts (i.e. greater prevalence of suburban residential citrus trees) towards the interior of the region [34].
In addition to the spatial patterns observed from year-to-year, we detected modest temporal variation within years across both urban and commercial citrus sites. Seasonal variation is important to both D. citri detection and spread because the psyllid’s lifecycle and performance is tightly linked to periods of new shoot production (i.e. “flush”) that is most prominent in many California citrus groves during the spring and fall [11]. New flush production is a critical aspect of D. citri nymphal development and represents a significant component of HLB infection risk in citrus trees [48, 49]. The higher D. citri detection rate in fall months suggests that the flush of young leaves among host plants as the nights cool are key contributors to the observed seasonality pattern. This pattern was more predictable in commercial citrus sites, perhaps reflecting more substantial variability in horticultural practices among residences as compared to commercial groves. Yet, neither urban nor commercial citrus showed clear evidence of a spring peak in adult D. citri trapping frequency. This pattern is contrary to what typically occurs in Florida and Brazil [50, 51]. However, it is consistent with what has been found in other studies in California [52] and is also similar to Fall-dominance in D. citri adult trap counts that has been reported from Texas [53]. The lack of an observed spring peak is likely attributable to the impact of overwintering mortality on D. citri populations, which appears to significantly decrease D. citri population growth [54]. Colder winter temperatures may reduce D. citri numbers to below a population threshold that can adequately recover in time to fully benefit from spring flush [45–47, 55]. In addition to climatic factors, early season D. citri dynamics may be further constrained by the activity of the parasitoid Tamarixia radiata [17] and other resident generalist predators [54].
HLB, associated with the bacterium transmitted by D. citri, has been documented in regions throughout the world, including a recent emergence in the Western Hemisphere [10, 56, 57]. In 2012, the first HLB positive test in a hybrid-lemon citrus tree was identified in a residential neighborhood in Los Angeles County [18], confirming the relative importance of residential areas for the likelihood of HLB-related damage resulting from D. citri spread. Given the long latent period, coupled with the ability of D. citri to acquire the pathogen several months before detection in trees is possible [56, 57], it is likely that other cases exist but have yet to manifest. In regions where HLB is more widespread (i.e. China, Brazil, Florida), the spatial distribution of the disease appears clustered at the regional level and new foci of infection can be widely spaced [10]. Here we show a similar regional clustering of D. citri, suggesting that the dynamic dispersion of hotspots over time may contribute to the spread of HLB infection foci into new regions in a predictable manner.
Quantifying the spatial dynamics of entomological risk over time is a critical component of understanding invasive insect spread in human-dominated ecosystems and for prioritizing disease management objectives [58–60]. We detected hotspots of D. citri that showed clear directionally-dependent movement to the south and east after first appearing in an urbanized region of Los Angeles. This information may be useful for predicting the risk of HLB disease transmission and for further prioritizing prevention and control efforts in certain areas [60]. Specifically, disease surveillance efforts may be more effective if targeted towards the interior areas of hotspots where conditions for the establishment of D. citri are presumptively favorable and less in western areas. Further, differences in certain landscape and abiotic factors (e.g., temperature, elevation) across the region may be contributing to the observed asymmetric pattern of spread. Surveillance may also be improved by following the leading edge of hotspots along a southeastern trajectory. Given the relative stability of the Los Angeles hotspot under existing management efforts, information on the distribution of D. citri may ultimately be more useful for enhanced HLB risk prediction and less for psyllid eradication.
Currently D. citri is predominantly found in urban and suburban areas of Southern California; however, spillover into commercial citrus and areas further north has begun. The spread of D. citri and HLB in Southern California represents a significant threat to citrus in the region. To better understand this threat, we quantified the spatial distribution of entomological risk using spatial statistics to identify hot spots of D. citri abundance. Using information on the location and directional spread of hotspots as a proxy for HLB risk may be of great use towards refining disease management efforts.
Supporting information
(TIF)
Table A. Yearly Diaphorina citri trapping statistics, by Southern California county, from two sources. Records from the California Department of Food & Agriculture (CDFA) database most closely represents the maximum number of traps deployed at any given time over the year. Records from the United States Department of Agriculture Integrated Plant Health Information System (USDA) represent reported total number of trap deployments over the year. Table B. Global test of spatial autocorrelation with incremental Moran’s I statistic. Table C. Six probable clusters of positive sites defined by the Kulldorff space-time permutation scan statistic, 2008–2014. Table D. Standard deviational ellipses of hotspot geographic center dispersion.
(DOCX)
Acknowledgments
Thanks to R. Johnson and R. Dunn for providing the D. citri geodatabase, T. Galindo, M. Luque-Williams, D. Tanoue, and R. Broadway for ACP trapping summary statistics, and J. Morse and R. Stouthamer for helpful discussion. This work was supported by funding from USDA-APHIS-PPQ and NIFA-AFRI grant #2012–01803 to Mathew P Daugherty.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funding from USDA-APHIS-PPQ and NIFA-AFRI grant #2012-01803 to M.P.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group, Island Press; 2005 [Google Scholar]
- 2.Vitousek PM, Mooney H A, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems.Science. 1997; 277:494–499. [Google Scholar]
- 3.Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, et al. Impacts of biological invasions: what's what and the way forward.Trends Ecol. Evol. 2013; 28:58–66. 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 4.Alston KP, Richardson DM. The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol. Cons. 2006; 132:183–198. [Google Scholar]
- 5.Poland TM, McCullough DG. Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource.J. Forest. 2006;104:118–124. [Google Scholar]
- 6.Gavier-Pizarro GI, Radeloff VC, Stewart SI, Huebner CD, Keuler NS. Housing is positively associated with invasive exotic plant species richness in New England, USA.Ecol. Appl. 2010;20:1913–1925. [DOI] [PubMed] [Google Scholar]
- 7.Säumel I, Kowarik I. Urban rivers as dispersal corridors for primarily wind-dispersed invasive tree species.Landsc. Urban Plan.. 2010;94:244–249. [Google Scholar]
- 8.Pereira-Peixoto MH, Pufal G, Martins CF, Klein AM. Spillover of trap-nesting bees and wasps in an urban–rural interface. J. Insect Conserv. 2014; 18:815–826. [Google Scholar]
- 9.University of California, Riverside. Center for Invasive Species Research. 2016. http://cisr.ucr.edu/invasive_species.html.
- 10.Gottwald TR. Current epidemiological understanding of citrus huanglongbing.Annu. Rev. Phytopathol. 2010;48:119–139. 10.1146/annurev-phyto-073009-114418 [DOI] [PubMed] [Google Scholar]
- 11.Grafton-Cardwell EE, Stekinski LL, Stansly PA. Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annu. Rev. Entomol. 2013; 58:413–432. 10.1146/annurev-ento-120811-153542 [DOI] [PubMed] [Google Scholar]
- 12.Bové JM. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006; 88:7–37. [Google Scholar]
- 13.Dagulo L, Danyluk MD, Spann TM, Valim MF, Goodrich‐Schneider R, Sims C, Rouseff R. Chemical characterization of orange juice from trees infected with citrus greening (Huanglongbing).J. Food Sci. 2010; 75:C199–C207. 10.1111/j.1750-3841.2009.01495.x [DOI] [PubMed] [Google Scholar]
- 14.Spreen TH, Baldwin JP, Futch SH. An economic assessment of the impact of Huanglongbing on citrus tree plantings in Florida. HortScience. 2014; 49:1052–1055. [Google Scholar]
- 15.Daugherty M, Zeilinger A, Byrne F, Grafton-Cardwell B. Challenges with implementing IPM for urban infestations of the Asian citrus psyllid in California: Current status and recommendations. CAPCA Adviser Magazine. 2013; 16:14–16. [Google Scholar]
- 16.Halbert S, Manjunath K, Ramadugu C, Brodie M, Webb S, Lee R. Trailers transporting oranges to processing plants move Asian citrus psyllids. Fla. Entomol. 2010; 93:33–38. [Google Scholar]
- 17.Hoddle M.S. 2012. Biological control of Asian citrus psyllid takes big steps forward in Southern California. CAPCA Adviser 15:18–20. [Google Scholar]
- 18.California Department of Food and Agriculture (CDFA).. News release—Citrus disease huanglongbing detected in Hacienda Heights area of Los Angeles County. 2015. https://www.cdfa.ca.gov/egov/Press_Releases/Press_Release.asp?PRnum=12-012.
- 19.Shaver GR. Spatial heterogeneity: past, present, and future In: Ecosystem Function in Heterogeneous Landscapes. Springer, New York, USA, 2005. pp. 443–449. [Google Scholar]
- 20.Liebhold AM, Tobin PC. Population ecology of insect invasion and their management. Annu. Rev. Entomol. 2008; 53:387–408. 10.1146/annurev.ento.52.110405.091401 [DOI] [PubMed] [Google Scholar]
- 21.Vinatier F, Tixier P, Duyck P, Lescourret F. Factors and mechanisms explaining spatial heterogeneity: a review of methods for insect populations. Methods Ecol. Evol. 2011; 2:11–22. [Google Scholar]
- 22.Richards TJ, Shanafelt DW, Fenichel EP. Foreclosures and invasive insect spread: the case of Asian citrus psyllid. Am. J. Agric. Econ. 2014; 96:615–630. [Google Scholar]
- 23.Ostfeld RS, Glass GE, Kessing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol. Evolut. 2005; 20:328–336. [DOI] [PubMed] [Google Scholar]
- 24.Anselin L. Local indicators of spatial association—LISA. Geogr. Anal.1995; 27:93–115. [Google Scholar]
- 25.Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992; 24:189–206. [Google Scholar]
- 26.Ord J. K., and Getis A. 1995. Local spatial autocorrelation statistics: Distributional issues and an application. Geographical Analysis 27:286–306. [Google Scholar]
- 27.Kulldorff M, Heffernan R, Hartman J, Assunção R, Mostashari F. A space–time permutation scan statistic for disease outbreak detection. PLoS Med. 2005; 2:e59 10.1371/journal.pmed.0020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ESRI 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- 29.Kulldorff M. A spatial scan statistic. Commun. Stat. Theory Methods.1997; 26:1481–1496. [Google Scholar]
- 30.Lefever DW. Measuring geographic concentration by means of the standard deviational ellipse. Am. J. Sociol.. 1926; 32:88–94. [Google Scholar]
- 31.Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ. The consequence of tree pests and diseases for ecosystem services.Science. 2013; 342:1235773 10.1126/science.1235773 [DOI] [PubMed] [Google Scholar]
- 32.Anderson K.. Globalization's effects on world agricultural trade, 1960–2050. Phil. Trans. R. Soc. B. 2010; 365:3007–3021. 10.1098/rstb.2010.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulme PE. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009; 46:10–18. [Google Scholar]
- 34.Thomas SM, Simmons GS, Daugherty MP. Using regulatory data to characterizing introduction, establishment and impact of an invasive insect: application to Asian Citrus Psyllid and its management. In Review.
- 35.McKinney ML. Urbanization as a major cause of biotic homogenization. Biol. Cons. 2006; 127: 247–260. [Google Scholar]
- 36.Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evolut. 2002; 17:170–176. [Google Scholar]
- 37.Halbert SE, Manjunath KL, Ramadugu C, Lee RF. Incidence of huanglongbing-associated ‘Candidatus Liberibacter asiaticus’ in Diaphorina citri (Hemiptera: Psyllidae) collected from plants for sale in Florida. Fla. Entomol. 2012; 95: 620–627. [Google Scholar]
- 38.Lewis-Rosenblum H, Martini X, Tiwari S. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econ. Entomol. 2015; 208: 3–10. [DOI] [PubMed] [Google Scholar]
- 39.Martini X, Hoyte A, Stelinski L. Abdominal color of the Asian citrus psyllid (Hemiptera: Liviidae) is associated with flight capabilities. Ann. Entomol. Soc. Am. 2014; 842–847. [Google Scholar]
- 40.Boina DR, Meyer WL, Onagbola EO, Stelinski LL. Quantifying dispersal of Diaphorina citri (Hemiptera: Psyllidae) by immunomarking and potential impact of unmanaged groves on commercial citrus management. Environ. Entomol. 2009; 38:1250–58 [DOI] [PubMed] [Google Scholar]
- 41.Arakawa K, Miyamoto K. Flight ability of Asiatic citrus psyllid, Diaphorina citri Kuwayama (Homoptera; Psyllidae), measured by a flight mill. Res. B. Plant Prot. Serv. Japan. 2007; 43: 23–26. [Google Scholar]
- 42.Sakamaki Y. Possible migration of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae) between and within islands. Occas. Pap. Kagoshima Univ. Res. Cent. 2005; 42: 121–125. [Google Scholar]
- 43.Morin RS, Liebhold AM, Gottschalk KW. Anisotropic spread of hemlock woolly adelgid in the eastern United States. Biol. Invasions. 2009; 11:2341–2350. [Google Scholar]
- 44.Rigot T, van Halder I, Jactel H. Landscape diversity slows the spread of an invasive forest pest species. Ecography. 2014; 37:648–658. [Google Scholar]
- 45.Nakata T. Temperature-dependent development of the citrus psyllid, Diaphorina citri (Homoptera: Psylloidea), and the predicted limit of its spread based on overwintering in the nymphal stage in temperature regions of Japan. Appl. Entomol. Zool. 2006; 41:383–387. [Google Scholar]
- 46.Liu YH, Tsai JH. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. Appl. Biol. 2000; 137:201–206. [Google Scholar]
- 47.Hall DG, Wenninger EJ, Hentz MG. Temperature studies with the Asian citrus psyllid, Diaphorina citri: Cold hardiness and temperature thresholds for oviposition. J. Insect Sci. 2011; 11:83 10.1673/031.011.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiyaka C, Singer BH, Halbert SE, Morris JG, van Bruggen AH. Modeling huanglongbing transmission within a citrus tree. Proc. Natl. Acad. Sci. U.S.A. 2012; 109: 12213–12218. 10.1073/pnas.1208326109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbert S, Manjunath K. Asian citrus psyllids (Sternorrhyncha: psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Fla. Entomol. 2004; 87:330–353. [Google Scholar]
- 50.Beloti VH, Rugno GR, Felippe MR, do Carmo-Uehara A, Garbim LF, Godoy WAC, et al. Population dynamics of Diaphorina citri Kuwayama (Hempotera: Liviidae) in orchards of ‘Valencia’ orange, ‘Ponkin’ mandarin and ‘Murcott’ Tangor. Fla. Entomol. 2013; 96:173–179 [Google Scholar]
- 51.Qureshi JA, Rogers ME, Hall DG, Stansly PA. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol. 2009; 102: 247–256. [DOI] [PubMed] [Google Scholar]
- 52.Kistner EJ, Amrich R, Castillo M, Strode V, Hoddle MS. Phenology of Asian citrus psyllid (Hemiptera: Liviidae), with special reference to biological control by Tamarixia radiata, in the residential landscape of Southern California. J. Econ. Entomol. 2016; 109:1047–1057. [DOI] [PubMed] [Google Scholar]
- 53.Setamou M, Bartels DW. Living on the edges: Spatial niche occupation of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in citrus groves. PLoS ONE. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kistner EJ, Melham N, Carpenter E, Castillo M, Hoddle MS. Abiotic and biotic mortality factors affecting Asian citrus psyllid (Hemiptera: Liviidae) demographics in Southern California. Ann. Entomol. Soc. Am. 2016; 10.1093/aesa/saw053 [DOI] [Google Scholar]
- 55.Tsai JH, Wang JJ, Liu YH. Seasonal abundance of the Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) in southern Florida. Fla. Entomol. 2002; 85:446–451. [Google Scholar]
- 56.Lee JA, Halbert SE, Dawson WO, Robertson CJ, Keesling JE, Singer BH. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:7605–7610. 10.1073/pnas.1508253112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coletta-Filho H, Daugherty MP, Ferreira C, Lopes J. Temporal progression of Candidatus Liberibacter asiaticus infection in citrus and acquisition efficiency by Diaphorina citri. Phytopathology. 2014; 104:416–421 10.1094/PHYTO-06-13-0157-R [DOI] [PubMed] [Google Scholar]
- 58.Kaplan H, van Niekerk A, Le Roux JJ, Richardson DM, Wilson JR. Incorporating risk mapping at multiple spatial scales into eradication management plans. Biol. Invasions. 2014; 16:691–703. [Google Scholar]
- 59.Pyšek P, Richardson DM. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010; 35:25–55. [Google Scholar]
- 60.Gottwald T, Luo W, McRoberts N. Risk-based residential HLB/ACP survey for California, Texas and Arizona. J. Citrus Pathol. 2014; 1:121–125 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Table A. Yearly Diaphorina citri trapping statistics, by Southern California county, from two sources. Records from the California Department of Food & Agriculture (CDFA) database most closely represents the maximum number of traps deployed at any given time over the year. Records from the United States Department of Agriculture Integrated Plant Health Information System (USDA) represent reported total number of trap deployments over the year. Table B. Global test of spatial autocorrelation with incremental Moran’s I statistic. Table C. Six probable clusters of positive sites defined by the Kulldorff space-time permutation scan statistic, 2008–2014. Table D. Standard deviational ellipses of hotspot geographic center dispersion.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.