Abstract
Background
Histological chorioamnionitis (HCA) is related to perinatal morbidity. However, there is no definite diagnostic method for detecting chorioamnionitis before delivery.
Methods
We evaluated whether the delta neutrophil index (DNI) was an effective early marker of HCA in patients with preterm premature rupture of membranes (PPROM). We retrospectively evaluated 149 women diagnosed with PPROM (gestational age, 20+0 to 36+6 weeks) at Severance Hospital from January 2013 to December 2014. The women were categorized into the following two groups: (a) PPROM without HCA and (b) PPROM with HCA. The maternal white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) level, and DNI were measured at admission. The DNI has been reported to reflect the fraction of circulating immature granulocytes associated with infection.
Results
Of the 149 patients, 87 were included in the PPROM without HCA group and 62 were included in the PPROM with HCA group. The interval between admission and delivery was significantly shorter in the PPROM with HCA group than in the PPROM without HCA group. There was no significant difference in the maternal WBC count. The serum CRP level, NLR, and DNI were significantly lower in the PPROM without HCA group than in the PPROM with HCA group, while the lymphocyte count was significantly lower in the PPROM with HCA group than in the PPROM without HCA group. A predictive equation was generated by combining the DNI, lymphocyte count, and CRP level, and the sensitivity and specificity for predicting a placental inflammatory response were 69.1% and 70.5%, respectively.
Conclusions
The DNI could be a predictive marker for HCA in patients with PPROM. Our predictive equation involving the DNI, lymphocyte count, and CRP level may be helpful for predicting the placental inflammatory response in patients with PPROM.
Introduction
Preterm premature rupture of membranes (PPROM) is one of the leading causes of preterm birth [1], and approximately 30–40% of pregnant women with PPROM show intra-amniotic infection [2, 3]. Pregnant women with PPROM combined with intra-amniotic infection are at increased risk of adverse maternal and neonatal outcomes, such as preterm delivery, neonatal intraventricular hemorrhage, cerebral palsy, bronchopulmonary dysplasia, neonatal sepsis, and death [4, 5]. Intrauterine infection is one of the important risk factors for subsequent spontaneous preterm labor [6], and considering placental pathologic findings, the presence of intra-amniotic infection represents histological chorioamnionitis (HCA). A fetal inflammatory response occurs when there is infection of the umbilical cord, including the umbilical veins, umbilical arteries, and Wharton’s jelly [7]. It can be defined as funisitis and is related to a more advanced stage of intrauterine infection. However, intra-amniotic infection could be subclinical, and a microbiological study is required to confirm infection in the amniotic fluid. Gram staining and measurements of the white blood cell (WBC) count and glucose level in the amniotic fluid are the most sensitive and specific methods for detecting intra-amniotic infection. Despite the advantage of amniocentesis, it is an invasive procedure that can result in complications; thus, selection of this procedure needs careful consideration. Moreover, amniocentesis is impossible or difficult to perform in pregnant women with PPROM because of their decreased amniotic fluid volume. Previous studies reported that the success rate for obtaining amniotic fluid was 45–97% in patients with PPROM, and the results were primarily dependent on the condition of the accessible amniotic fluid pocket [8].
Recent studies have shown that the serum delta neutrophil index (DNI) is associated with diverse infectious conditions, such as bacterial peritonitis, acute appendicitis, and sepsis [9–11]. The DNI is a new inflammatory marker, but no recent studies have reported its predictive value in patients with PPROM with HCA.
Therefore, we conducted this study to determine whether the DNI is an effective early marker of HCA in patients with PPROM and to develop a non-invasive, easy, and prompt predictive equation that combined maternal serum markers and the DNI.
Materials and methods
A total of 149 pregnant women with PPROM between 20+0 and 36+6 gestational weeks from January 2013 to December 2014 at Severance Hospital in Seoul, Korea were enrolled in this study. This study was approved by the Institutional Review Board of the Yonsei University Health System. We excluded women with multiple pregnancies, fetal anomalies, missing placental pathologic results, and medically indicated preterm birth. Additionally, pregnant women with hematologic or any other autoimmune disease affecting hematologic parameters were excluded.
Clinical data included demographic variables, such as maternal age; body mass index (BMI); preterm birth history; gestational age at diagnosis; and maternal WBC count, neutrophil count, lymphocyte count, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) level, and DNI. The diagnosis of PROM was based on clinical visual examination of amniotic fluid pooling in the vaginal cavity during sterile speculum examination or a positive nitrazine test. If the nitrazine test result was inconclusive, we performed the placental alpha microglobulin-1 test (AmniSure test). Sterile cotton swabs were used to obtain vaginal secretion samples for culture in order to assess group B streptococcus (GBS), Ureaplasma urealyticum, and Mycoplasma hominis. The serum CRP levels were determined using an automated nephelometer (Beckman Coulter Image, Fullerton, CA, USA) according to the manufacturer’s instructions. The detection limit of this analysis was 0.01 mg/dL, and the normal serum CRP level was considered to be <0.8 mg/dL [12, 13]. The serum DNI value was obtained using an automated cell analyzer (ADVIA 2120 Hematology System, Siemens, Healthcare Diagnostics, Forchheim, Germany). This is a flow cytometry-based hematologic analyzer, which uses two independent WBC counting methods, including a myeloperoxidase (MPO) channel and a lobularity/nuclear density channel. The DNI value was calculated using the following formula: DNI (%) = (the leukocyte subfraction assayed in the MPO channel by cytochemical reactions)–(the leukocyte subfraction assayed in the nuclear lobularity channel by reflected light beam measurements) [9, 14, 15]. Determination of the serum CRP level requires additional laboratory processes and expense, whereas the serum DNI value can be obtained during routine complete blood counts at our institution, without additional time or expense.
Placentas were sent to the pathologic department for detection of infection. Histopathologic chorioamnionitis was defined as microscopic evidence of infection on any part of the placenta, and deciduitis was defined as infection of the decidua. Funisitis was defined as infection of the connective tissue of the umbilical cord [16].
All pregnant women with PPROM included in this study were divided into the following two groups according to their pathologic results: (a) patients with PPROM without HCA and (b) patients with PPROM with HCA. All patients with PPROM received prophylactic intravenous antibiotics and 12 mg intramuscular betamethasone injection twice at a 24-hour interval for lung maturation. Tocolysis was used if patients had preterm labor. All pregnant women were treated until 34 gestational weeks if they showed no signs of clinical chorioamnionitis. If clinical chorioamnionitis occurred, delivery was performed.
Continuous variables are expressed as means and standard deviations (SDs) or medians and ranges. The chi-squared test or Fisher’s exact test was used for categorical variables, and the two-sample t-test or Wilcoxon rank sum test was used for continuous variables. Multiple logistic regression analysis was performed to estimate the odds ratio of HCA prediction with adjustment for confounders. The combination of significant parameters was used to generate a predictive equation. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). Statistical significance was set at a p-value <0.05.
Results
The study included 149 pregnant women who had PPROM between 20+0 and 36+6 gestational weeks. Of the 149 patients, 87 were included in the PPROM without HCA group and 62 were included in the PPROM with HCA group, and we compared the clinical characteristics and perinatal outcomes between these groups (Table 1). There were significant differences in gestational age at admission, gestational age at birth, interval between admission and birth, and cervix length at admission between the two groups. The cervix length at admission was significantly longer, while the interval between admission and birth was significantly shorter in the PPROM with HCA group than in the PPROM without HCA group (p = 0.004 and p = 0.005, respectively).
Table 1. Clinical characteristics and pregnancy outcomes in the PPROM without HCA and PPROM with HCA groups.
| Variables | PPROM without HCA (n = 87) | PPROM with HCA (n = 62) | p-value |
|---|---|---|---|
| Maternal age (yrs) | 33.4 ± 3.4 | 32.8 ± 3.3 | 0.299 |
| Primiparous | 44 (50.6%) | 21 (33.9%) | 0.221 |
| Prior abortion | 29 (33.3%) | 26 (41.9%) | 0.571 |
| Previous PTB history | 3 (3.5%) | 5 (8.1%) | 0.278 |
| BMI at admission (kg/m2) | 25.3 ± 3.6 | 24.7 ± 3.4 | 0.244 |
| Gestational age at admission (wks) | 33+2 (20+3–36+6) | 31+4(20+0–36+3) | 0.038 |
| Gestational age at birth (wks) | 33+4 (20+4–36+6) | 31+5(20+1–39+3) | 0.017 |
| Interval of admission to birth (days) | 4.4 ± 8.4 | 3.0 ± 8.5 | 0.005 |
| Clinical chorioamnionitis | 2 (2.3%) | 3 (4.8%) | 0.650 |
| Cervix length (cm) | 1.6 ± 1.3 | 2.4 ± 1.2 | 0.004 |
| Delivery mode | 0.006 | ||
| Vaginal delivery | 24 (27.6%) | 31 (50%) | |
| Cesarean section | 63 (72.4%) | 31 (50%) | |
| Neonatal birth weight (g) | 1879 ± 521 | 1735 ± 678 | 0.164 |
| APGAR score at 1min | 5 (0–8) | 4 (0–7) | 0.382 |
| APGAR score at 5min | 7 (0–9) | 6 (0–9) | 0.702 |
| NICU admission (%) | 82 (94.3%) | 55 (88.7%) | 0.238 |
PPROM, preterm premature rupture of membranes; HCA, histological chorioamnionitis; PTB, preterm birth; BMI, body mass index; NICU, neonatal intensive care unit
The distribution of the placental pathologic findings among the study patients is shown in Table 2. Among the 149 pregnant women, 62 (41.6%) had HCA, including chorioamnionitis, chorionitis, amnionitis, choriodeciduitis, and funisitis. The most common pathologic result was chorioamnionitis (39/62, 62.9%).
Table 2. Distribution of placental pathologic results.
| Pathologic results | |
| Chorionitis | 7 (11.3%) |
| Chorionitis + deciduitis | 1 (1.6%) |
| Deciduitis | 7 (11.3%) |
| Amnionitis + deciduitis | 2 (3.2%) |
| Chorioamnionitis | 39 (62.9%) |
| Chorioamnionitis + funisitis | 3 (4.9%) |
| Chorionitis + deciduitis + funisitis | 2 (3.2%) |
| Chorionitis + funisitis | 1 (1.6%) |
| 62 (100%) |
To evaluate the differences in laboratory variables between the PPROM without HCA and PPROM with HCA groups, we compared the laboratory results, including vaginal culture findings, between the two groups (Table 3). There were significant differences in the hemoglobin (Hb) levels, hematocrit (Hct) values, lymphocyte counts, NLRs, DNI values, and CRP levels between the two groups. The median NLRs were 4.7 (range, 2.3–17.9) and 6.2 (2.0–48.8) (p = 0.001), DNI values were 0 (0–2.6) and 0.25 (0–4.7) (p = 0.006), and CRP levels were 3.3 (0.6–32.0) and 7.5 (0.6–65.2) (p = 0.008) in the PPROM with HCA group and PPROM without HCA group, respectively. The NLR, DNI, and CRP levels were significantly higher, while the Hb levels, Hct values, and lymphocyte counts were significantly lower in the PPROM with HCA group than in the PPROM without HCA group. The median neutrophil count was higher in the PPROM with HCA group than in the PPROM without HCA group; however, the difference was not statistically significant. Among the study population, the proportion of patients with positive culture results for Ureaplasma urealyticum was higher in the PPROM with HCA group than in the PPROM without HCA group (37.9% [33/87] vs. 56.5% [35/62]; p = 0.034). However, there was no difference in the proportion of patients with positive culture results for Mycoplasma hominis between the two groups (p = 0.102).
Table 3. Laboratory characteristics of the PPROM without HCA and PPROM with HCA groups.
| Variables | PPROM without HCA (n = 87) | PPROM with HCA (n = 62) | p-value |
|---|---|---|---|
| Laboratory variables | |||
| Hemoglobin (g/dl) | 12.08 ± 1.38 | 11.62 ± 1.13 | 0.034 |
| Hematocrit | 35.77 ± 4.23 | 34.33 ± 3.78 | 0.033 |
| Platelet (x103/L) | 221.94 ± 66.20 | 226.3 ± 71.6 | 0.704 |
| WBC (cells/L) | 10.68 ± 3.91 | 11.30 ± 3.74 | 0.333 |
| Neutrophil (cells/L) | 7.57 (3.05–22.62) | 8.67 (3.42–23.41) | 0.114 |
| Lymphocyte (cells/L) | 1.6 (0.5–5.2) | 1.4 (0.4–2.6) | 0.016 |
| Monocyte (cells/L) | 0.49 (0.06–1.15) | 0.48 (0.12–0.81) | 0.508 |
| Eosinophil (cells/L) | 0.09 (0.01–0.45) | 0.09 (0.01–0.39) | 0.940 |
| Basophil (cells/L) | 0.03 (0–0.10) | 0.03 (0–0.08) | 0.334 |
| PT (INR) | 0.86 ± 0.06 | 0.88± 0.06 | 0.017 |
| PTT (sec) | 28.54 ± 2.91 | 28.30 ± 2.40 | 0.596 |
| NLR | 4.7 (2.3–17.9) | 6.2 (2.0–48.8) | 0.001 |
| DNI (%) | 0 (0–2.6) | 0.25 (0–4.7) | 0.006 |
| CRP (mg/dl) | 3.3 (0.6–32.0) | 7.5 (0.6–65.2) | 0.008 |
| Vaginal cultures | |||
| Ureaplasma urealyticum | 33 (37.9%) | 35 (56.5%) | 0.034 |
| Mycoplasma hominis | 1 (1.2%) | 1 (1.6%) | 0.102 |
PPROM, preterm premature rupture of membranes; HCA, histological chorioamnionitis; WBC, white blood cell; PT (INR), prothrombin time (international normalized ratio); PTT, partial thromboplastin time; NLR, neutrophil-to-lymphocyte ratio; DNI, delta neutrophil index; CRP, C-reactive protein
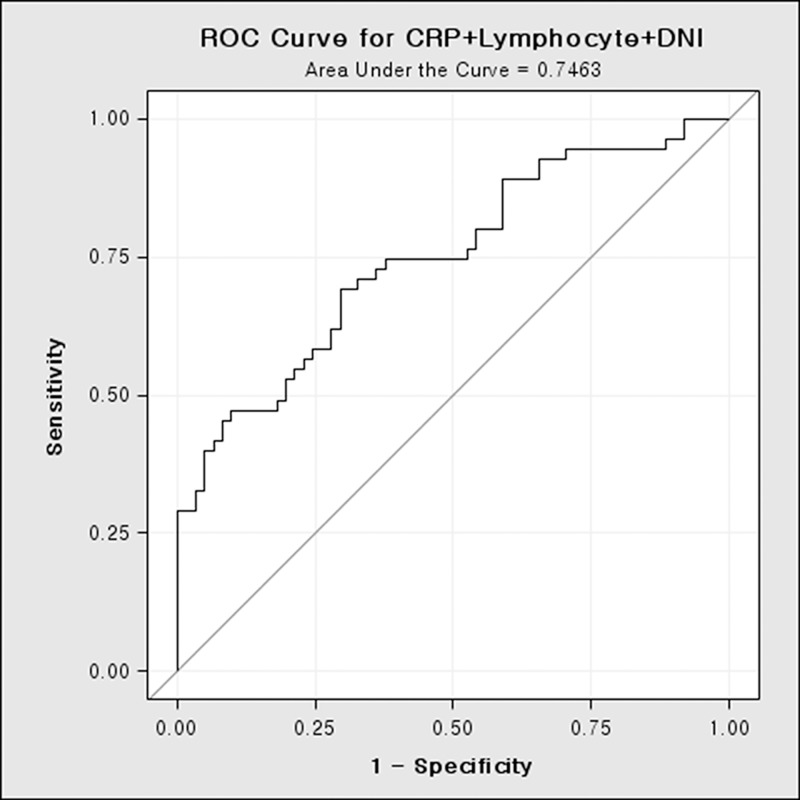
In order to identify the value of the CRP level, lymphocyte count, and DNI that predicted HCA, we determined the odds ratios after adjusting for confounding factors (Table 4). The odds ratios for predicting HCA were 1.069 (95% CI: 1.017–1.125, p = 0.009) for the CRP level, 0.348 (95% CI: 0.157–0.773, p = 0.009) for the lymphocyte count, and 1.818 (95% CI: 1.104–2.991, p = 0.018) for the DNI. Increases in the CRP level and DNI were significantly associated with HCA, while decreases in the lymphocyte count were significantly associated with HCA. The combination of the CRP level, DNI, and lymphocyte count yielded the greatest area under the receiver operating characteristic curve (0.746) (Fig 1).
Table 4. Predictors of HCA as determined by logistic regression analysis.
| Outcomes | OR | 95% CI | p-value |
|---|---|---|---|
| CRP | 1.069 | 1.017–1.125 | 0.009 |
| Lymphocyte | 0.348 | 0.157–0.773 | 0.009 |
| DNI | 1.818 | 1.104–2.991 | 0.018 |
HCA, histological chorioamnionitis; CRP, C-reactive protein; DNI, delta neutrophil index
Fig 1. Receiver operating characteristic curve.
Receiver operating characteristic curve for the prediction of chorioamnionitis using the DNI, CRP level, and lymphocyte count DNI, delta neutrophil index; CRP, C-reactive protein
A predictive equation was generated using these three parameters through logistic regression modeling. The estimated regression equation is defined as p = ef / (1 + ef), where f is a linear function of the selected parameters and e is the base associated with the natural logarithm. Integration of the parameters yields the following equation:
When the predictive probability cutoff was 0.447 according to the Youden index, the sensitivity and specificity for predicting HCA were 69.1% and 70.5%, respectively.
Discussion
To our knowledge, this is the first study to investigate the usefulness of the DNI as a predictive marker for HCA in patients with PPROM. We found that the DNI was a useful non-invasive marker for detecting HCA in pregnant women with PPROM. Moreover, we identified a predictive equation for HCA derived from the combination of the CRP level, lymphocyte count, and DNI, which was more accurate than was the use of each factor alone. The predictive equation for HCA can be used for prenatal counseling and management planning in patients with PPROM.
Intrauterine infection can be diagnosed based on amniocentesis; however, this approach is invasive and can result in complications. Recent studies have indicated that sterile intra-amniotic inflammation, which does not involve microorganisms, is highly associated with HCA [17, 18]. Roberts et al. [19] demonstrated that acute chorioamnionitis can occur without demonstrable intra-amniotic infection. In short, the diagnosis of intra-amniotic infection can be overlooked despite amniocentesis. On the other hand, HCA can be diagnosed through histologic examination of the placenta during the postpartum period. Therefore, these methods are inappropriate for detecting intrauterine inflammation in the prenatal period.
Many researchers have suggested different combinations of biomarkers for predicting intrauterine infection. Ryu et al. [20] showed that the combination of IL-6 in cervicovaginal fluid and gestational age at sampling was a useful predictive marker for intra-amniotic inflammation. However, this finding is difficult to apply in clinical settings because the measurement of IL-6 is time-consuming and requires a special detection kit. Other previous studies suggested the combination of the NLR and cervix length as a more sensitive marker for the prediction of preterm birth [21]. In this study, increased CRP levels and DNI values and decreased lymphocyte counts showed significant associations with HCA. A non-invasive predictive equation model was created using these three markers, and this model can be easily used to diagnose HCA during the prenatal period. Its sensitivity and specificity for predicting HCA were 69.1% and 70.5%, respectively. Regardless of the type of inflammation-causing microorganisms in HCA, the proinflammatory cytokines that are secreted in the early stage of intrauterine inflammation, such as interleukin (IL)-6, IL-10, IL-1β, and tumor necrosis factor-α (TNF-α), flow into the bloodstream of pregnant women. These changes affect the number of leukocyte subtypes and eventually cause neutrophilia [22]. Moreover, it has been reported that the TNF family that goes into action during the early stage of inflammatory response increases lymphocyte apoptosis, and the production of immunosuppressive factors significantly decreases the number of T-helper lymphocytes [23, 24].
In several studies, immature granulocytes have been investigated as predictors of sepsis because the number of immature granulocytes is known to increase in inflammatory conditions [25, 26]. However, there is no method to measure immature granulocytes accurately, and it is difficult to use conventional detection tests in infectious conditions. Nahm et al. [27] reported a study on the DNI based on automated immature granulocyte counts for determining the severity of sepsis to overcome this limitation. The DNI was the difference between the leukocyte differentials assayed in the MPO channel and that assayed in the nuclear lobularity channel. The complete blood cell (CBC) count is frequently and routinely evaluated in pregnant women with PPROM [9]. Therefore, DNI values can be easily calculated without any additional evaluations. In this study, the DNI in the PPROM without HCA group showed a value of 0 (range, 0–2.6) and the DNI in the PPROM with HCA group showed a higher value of 0.25 (0–4.7) (p = 0.006). In addition, we evaluated the DNI value of those pregnant women without PPROM or chorioamnionitis who delivered healthy neonates after 37 gestational weeks. The DNI value in the normal pregnant group was 0 (range, 0–2). In the logistic regression analysis, the odds ratio of the DNI was 1.818 (95% CI: 1.104–2.991, p = 0.018) and the odds ratio of the CRP level was 1.069 (95% CI: 1.017–1.125, p = 0.009). The odds ratio was higher for the DNI than for the prevalently used CRP level. The DNI can also increase in various infectious conditions [9, 10, 28]. Therefore, it shows low sensitivity and a positive predictive value for diagnosing HCA in patients with PPROM. Thus, instead of using each individual marker alone, we established an equation model to improve the predictive value of HCA.
Many researchers have shown that the NLR is an independent diagnostic parameter for systemic inflammation and stress conditions, owing to the fact that leukocytes play a major role in these conditions through an increase in neutrophils and decrease in lymphocytes [29, 30]. In this study, the neutrophil count in the PPROM with HCA group increased to 7.57 × 109/L (range, 3.05–22.62 × 109/L) and that in the PPROM without HCA group increased to 8.67 × 109/L (3.42–23.41 × 109/L); however, the difference was not significant (p = 0.114). The lymphocyte count slightly decreased in the PPROM with HCA group, and this result is similar to that reported previously. The NLR is being increasingly used as a predictive marker for spontaneous preterm birth and intrauterine infection in preterm delivery [21, 31]. The NLR increased in the PPROM with HCA group, but only the lymphocyte count showed a relatively high difference between the two groups. Therefore, only the lymphocyte count was included in the predictive equation. Multiple previous studies have reported that corticosteroid treatment during pregnancy can cause a transient increase in the total WBC count, including neutrophils [32–34]. In our study, 31.0% (27/87) of patients in the PPROM without HCA group and 40.3% (25/62) of patients in the PPROM with HCA group had undergone corticosteroid treatment between PPROM and birth. In the PPROM with HCA group, only one patient had undergone corticosteroid treatment once before being transferred to our hospital. Excluding this one patient, corticosteroid treatment was administered after blood sampling for the DNI assessment in the remaining patients. Thus, there is a low possibility of corticosteroid treatment affecting the WBC and neutrophil counts.
CRP is an acute-phase protein produced by the liver, and it is widely used as an objective marker for detecting tissue damage and infection. As the CRP level increases in chorioamnionitis or neonatal sepsis conditions, it is used in the obstetrical field to detect infectious conditions. Previous studies reported that an increasing CRP level was highly associated with preterm delivery and poor perinatal outcomes in pregnant women with preterm labor or PPROM [35–37].
The present study has some limitations. The DNI can only be calculated by measuring immature granulocytes using the ADIVA 2120 Hematology System. However, there are several hematology analyzers that can measure immature granulocytes, such as the Sysmex XE-series hematology analyzer (Sysmex Corporation, Kobe, Kansia, Japan) and Cell-Dyn series hematology analyzer (Abbot Diagnostics, Abbott Park, III). Although the measurement methods for immature granulocytes differ depending on the analyzer used, we could not identify any differences in the immature granulocyte values. Additionally, we conducted this study using only the DNI values obtained at the time when the patients with PPROM were admitted to the hospital. Therefore, changes in the DNI values are still unclear according to the course of PPROM. In addition, we were not able to prove the correlation between the severity of HCA and the predictive equation due to the unclear recording of the HCA stages in the pathologic results. Thus, a well-designed prospective study is needed in order to evaluate the usefulness of the DNI as a predictive marker of HCA.
In conclusion, the serum DNI, a new inflammation parameter, could be a predictive marker for chorioamnionitis or funisitis in patients with PPROM. Our predictive equation involving maternal serum markers could be used as a non-invasive, inexpensive, and simple method for detecting the placental inflammatory response in women with PPROM.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Henderson JJ, McWilliam OA, Newnham JP, Pennell CE. Preterm birth aetiology 2004–2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(6):642–7. Epub 2011/08/11. [DOI] [PubMed] [Google Scholar]
- 2.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Mental retardation and developmental disabilities research reviews. 2002;8(1):3–13. Epub 2002/03/29. 10.1002/mrdd.10008 [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. American journal of obstetrics and gynecology. 1993;169(4):839–51. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 4.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. Journal of perinatal medicine. 2009;37(1):72–8. Epub 2008/11/04. 10.1515/JPM.2009.016 [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition reviews. 2007;65(12 Pt 2):S194–202. Epub 2008/02/05. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG: an international journal of obstetrics and gynaecology. 2006;113 Suppl 3:17–42. Epub 2007/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2003;6(5):435–48. Epub 2004/01/08. [DOI] [PubMed] [Google Scholar]
- 8.Beazley D, Lewis R. The evaluation of infection and pulmonary maturity in women with premature rupture of the membranes. Seminars in perinatology. 1996;20(5):409–17. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 9.Seok Y, Choi JR, Kim J, Kim YK, Lee J, Song J, et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock (Augusta, Ga). 2012;37(3):242–6. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 10.Lim TS, Kim BK, Lee JW, Lee YK, Chang S, Kim SU, et al. Use of the delta neutrophil index as a prognostic factor of mortality in patients with spontaneous bacterial peritonitis: implications of a simple and useful marker. PloS one. 2014;9(1):e86884 Epub 2014/01/28. PubMed Central PMCID: PMCPMC3900662. 10.1371/journal.pone.0086884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim OH, Cha YS, Hwang SO, Jang JY, Choi EH, Kim HI, et al. The Use of Delta Neutrophil Index and Myeloperoxidase Index for Predicting Acute Complicated Appendicitis in Children. PloS one. 2016;11(2):e0148799 Epub 2016/02/10. PubMed Central PMCID: PMCPMC4747520. 10.1371/journal.pone.0148799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurki T, Teramo K, Ylikorkala O, Paavonen J. C-reactive protein in preterm premature rupture of the membranes. Archives of gynecology and obstetrics. 1990;247(1):31–7. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Park KU. Relationship between maternal serum C-reactive protein, funisitis and early-onset neonatal sepsis. Journal of Korean medical science. 2012;27(6):674–80. Epub 2012/06/13. PubMed Central PMCID: PMCPMC3369455. 10.3346/jkms.2012.27.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris N, Kunicka J, Kratz A. The ADVIA 2120 hematology system: flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Laboratory hematology: official publication of the International Society for Laboratory Hematology. 2005;11(1):47–61. Epub 2005/03/26. [DOI] [PubMed] [Google Scholar]
- 15.Yune HY, Chung SP, Park YS, Chung HS, Lee HS, Lee JW, et al. Delta neutrophil index as a promising prognostic marker in out of hospital cardiac arrest. PloS one. 2015;10(3):e0120677 Epub 2015/03/24. PubMed Central PMCID: PMCPMC4370748. 10.1371/journal.pone.0120677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S29–52. Epub 2015/10/03. PubMed Central PMCID: PMCPMC4774647. 10.1016/j.ajog.2015.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. American journal of reproductive immunology (New York, NY: 1989). 2014;71(4):330–58. Epub 2014/01/15. PubMed Central PMCID: PMCPMC3954440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine. 2015;43(1):19–36. Epub 2015/02/27. 10.1515/jpm-2014-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PloS one. 2012;7(3):e31819 Epub 2012/03/14. PubMed Central PMCID: PMCPMC3296706. 10.1371/journal.pone.0031819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu A, Park KH, Oh KJ, Lee SY, Jeong EH, Park JW. Predictive value of combined cervicovaginal cytokines and gestational age at sampling for intra-amniotic infection in preterm premature rupture of membranes. Acta obstetricia et gynecologica Scandinavica. 2013;92(5):517–24. Epub 2013/01/18. 10.1111/aogs.12073 [DOI] [PubMed] [Google Scholar]
- 21.Kim MA, Lee BS, Park YW, Seo K. Serum markers for prediction of spontaneous preterm delivery in preterm labour. European journal of clinical investigation. 2011;41(7):773–80. Epub 2011/02/09. 10.1111/j.1365-2362.2011.02469.x [DOI] [PubMed] [Google Scholar]
- 22.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer letters. 2002;183(1):43–51. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 23.Croci DO, Zacarias Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer immunology, immunotherapy: CII. 2007;56(11):1687–700. Epub 2007/06/16. 10.1007/s00262-007-0343-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp K, Bruunsgaard H, Skinhoj P, Klarlund Pedersen B. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infection and immunity. 2002;70(9):5019–25. Epub 2002/08/17. PubMed Central PMCID: PMCPMC128234. 10.1128/IAI.70.9.5019-5025.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nigro KG, O'Riordan M, Molloy EJ, Walsh MC, Sandhaus LM. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. American journal of clinical pathology. 2005;123(4):618–24. Epub 2005/03/04. 10.1309/73H7-K7UB-W816-PBJJ [DOI] [PubMed] [Google Scholar]
- 26.Ansari-Lari MA, Kickler TS, Borowitz MJ. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. American journal of clinical pathology. 2003;120(5):795–9. Epub 2003/11/12. 10.1309/LT30-BV9U-JJV9-CFHQ [DOI] [PubMed] [Google Scholar]
- 27.Nahm CH, Choi JW, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Annals of clinical and laboratory science. 2008;38(3):241–6. Epub 2008/08/22. [PubMed] [Google Scholar]
- 28.Kim H, Kim Y, Lee HK, Kim KH, Yeo CD. Comparison of the delta neutrophil index with procalcitonin and C-reactive protein in sepsis. Clinical laboratory. 2014;60(12):2015–21. Epub 2015/02/06. [DOI] [PubMed] [Google Scholar]
- 29.Muhmmed Suliman MA, Bahnacy Juma AA, Ali Almadhani AA, Pathare AV, Alkindi SS, Uwe Werner F. Predictive value of neutrophil to lymphocyte ratio in outcomes of patients with acute coronary syndrome. Archives of medical research. 2010;41(8):618–22. Epub 2011/01/05. 10.1016/j.arcmed.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 30.Nunez J, Nunez E, Bodi V, Sanchis J, Minana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. The American journal of cardiology. 2008;101(6):747–52. Epub 2008/03/11. 10.1016/j.amjcard.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 31.Kim MA, Lee YS, Seo K. Assessment of predictive markers for placental inflammatory response in preterm births. PloS one. 2014;9(10):e107880 Epub 2014/10/08. PubMed Central PMCID: PMCPMC4188518. 10.1371/journal.pone.0107880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadanali S, Ingec M, Kucukozkan T, Borekci B, Kumtepe Y. Changes in leukocyte, granulocyte and lymphocyte counts following antenatal betamethasone administration to pregnant women. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1997;58(3):269–74. Epub 1997/09/01. [DOI] [PubMed] [Google Scholar]
- 33.Denison FC, Elliott CL, Wallace EM. Dexamethasone-induced leucocytosis in pregnancy. British journal of obstetrics and gynaecology. 1997;104(7):851–3. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 34.Vaisbuch E, Levy R, Hagay Z. The effect of betamethasone administration to pregnant women on maternal serum indicators of infection. Journal of perinatal medicine. 2002;30(4):287–91. Epub 2002/09/19. 10.1515/JPM.2002.041 [DOI] [PubMed] [Google Scholar]
- 35.Potkul RK, Moawad AH, Ponto KL. The association of subclinical infection with preterm labor: the role of C-reactive protein. American journal of obstetrics and gynecology. 1985;153(6):642–5. Epub 1985/11/15. [DOI] [PubMed] [Google Scholar]
- 36.Watts DH, Krohn MA, Hillier SL, Wener MH, Kiviat NB, Eschenbach DA. Characteristics of women in preterm labor associated with elevated C-reactive protein levels. Obstetrics and gynecology. 1993;82(4 Pt 1):509–14. Epub 1993/10/01. [PubMed] [Google Scholar]
- 37.Mazor M, Kassis A, Horowitz S, Wiznitzer A, Kuperman O, Meril C, et al. Relationship between C-reactive protein levels and intraamniotic infection in women with preterm labor. The Journal of reproductive medicine. 1993;38(10):799–803. Epub 1993/10/01. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.