Abstract
The epidemic of insulin resistance, obesity, and metabolic syndrome has led to the emergence of nonalcoholic steatohepatitis (NASH) as the most common cause of liver disease in the US. Patients with NASH are at an increased risk for hepatic disease-related morbidity and death, and chronic inflammation in NASH patients can lead to hepatocellular carcinoma (HCC). The prevalence of HCC is higher in males than in females, and genetic studies have identified androgen and androgen receptors (ARs) as partially responsible for the gender disparity in the development of liver disease and HCC. Although many factors are known to play important roles in the progression of inflammation in NASH patients, the role of androgen and AR in the progression of NASH to HCC has been understudied. This review summarizes the evidence for a potential role of androgen and the AR pathway in the development of NASH-related HCC and in the treatment of HCC. It has been proposed that AR plays a role in the progression of HCC: inhibitory roles in early stages of hepatocarcinogenesis and tumor-promoting roles in advanced stages. AR can be activated by several pathways, even in the absence of androgen. While AR has been explored as a potential therapeutic target in HCC, several clinical trials have failed to demonstrate a clinical benefit of antiandrogen drugs in HCC. This review discusses the potential reason for these observations and discuss the potential future trials design in this important setting.
Keywords: hepatocellular carcinoma, nonalcoholic steatohepatitis, androgen receptor, flutamide, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is an aggressive neoplasm with a poor prognosis, resulting in a 5-year relative survival rate of only 17.2% and an estimated 24,550 deaths in the US in 2015.1 Over the last three decades, the incidence of HCC in the US has increased from 1.4 per 100,000 to 8.2 per 100,000 per year.2 The incidence of HCC is higher in developing countries, particularly those in the Asian Pacific regions, owing to the high prevalence of chronic hepatitis B and C viral infections. However, the incidence in developed countries has also been increasing owing to the emergence of nonalcoholic steatohepatitis (NASH) as an important risk factor.3,4 In fact, recently, a large retrospective study demonstrated that the proportion of non-virus–related HCC increased from 10% in 1991 to 24.1% in 2010, with most cases related to nonalcoholic fatty liver disease (NAFLD) and diabetes.5–7 In a Japanese study, the 5-year HCC development rates in cirrhosis patients were 11.3% in NAFLD cirrhosis, 12.5% in alcoholic cirrhosis, and 30.5% in hepatitis C virus cirrhosis, showing similar rates of HCC development in both alcoholic and NAFLD-related cirrhosis.8 Although metabolic syndrome had the lowest relative risk among other factors for HCC (1.5%−2.5%), its high prevalence in the general population (30%−40%) led to the highest population-attributable fraction.9
Regardless of the underlying etiology, the incidence of HCC is higher in males, with male-to-female ratios varying between 3:1 and 4:1 depending on geographic location.10,11 Moreover, males have poorer survival despite there being many treatment options.12–14 The increased incidence and disease aggressiveness in males suggest that androgen and androgen receptors (ARs) might promote HCC development and progression and/or that estrogen and estrogen receptors might suppress HCC development.15 This review summarizes the evidence for the potential role of androgen and ARs in NAFLD/NASH-related HCC.
NAFLD pathogenesis
NAFLD is the most common cause of liver dysfunction, with a prevalence of 20%−30% in the general population and up to 57%−74% among obese patients.16 NAFLD was usually considered to be one of the components of the metabolic syndrome and to be strongly linked to central obesity, insulin resistance, dyslipidemia, and hypertension (Table 1).17–22 Recent studies support the association of NAFLD with type 2 diabetes mellitus or metabolic syndrome, suggesting that NAFLD actually precedes the development of both conditions and is considered as a risk factor for development of type 2 diabetes mellitus.23 NAFLD includes disorders ranging from isolated liver steatosis (in which triglycerides accumulate in the hepatocytes), characterized by macrovesicular fatty change with or without nonspecific inflammation in the absence of cellular injury (ballooning), to NASH (characterized by the presence of additional cellular ballooning) and, subsequently, to cirrhosis and even HCC.24–26 Fibrosis is the most important determinant of the outcome.
Table 1.
Five parameters of the metabolic syndrome according to the WHO and AACE
| Parameter | WHO (1998) | IDF (2005) | EGIR (1999) | NCEP-ATP III | ATP III (2001) | AHA/NHLBI (2005) |
|---|---|---|---|---|---|---|
| Required for diagnosis | Insulin resistance | Ethnicity-based increased waist circumference | Insulin resistance | – | Any three out of five | Any three out of five |
| + Number of abnormalities | ≥2 | ≥2 | ≥2 | ≥3 | – | – |
| Glucose | DM | ≥100 mg/dL | DM | ≥100 mg/dL | ≥110 mg/dL | ≥100 mg/dL |
| HDL cholesterol | Males <35 mg/dL, females <39 mg/dL | Males <40 mg/dL, females <50 mg/dL | Males and females >39 mg/dL | Males <40 mg/dL, females <50 mg/dL | Males <40 mg/dL, females <50 mg/dL | Males <40 mg/dL, females <50 mg/dL |
| Triglycerides | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/L | ≥150 mg/L |
| Obesity | Waist/hip >0.9 (males) or >0.85 (females); BMI ≥30 kg/m2 | WC: males ≥102 cm, females ≥88 cm | WC: males ≥94 cm, females ≥80 cm | WC ≥94 cm | WC: males ≥102 cm, females ≥88 cm | WC: males ≥102 cm, females ≥88 cm |
| Hypertension | ≥140/90 mmHg or use of antihypertensive drugs | ≥130/85 mmHg or use of antihypertensive drugs | ≥140/90 mmHg or use of antihypertensive drugs | ≥130/85 mmHg or use of antihypertensive drugs | ≥130/85 mmHg or use of antihypertensive drugs | >130/85 mmHg or use of antihypertensive drugs |
Abbreviations: AACE, American Association of Clinical Endocrinologists; BMI, body mass index; DM, diabetes mellitus; HDL, high density lipoprotein; WC, waist circumference; WHO, World Health Organization; IDF, International Diabetes Federation; EGIR, European Group for the Study of Insulin Resistance; NCEP-ATPIII, National Cholesterol Education Program Adult Treatment Panel III; ATP III, Adult Treatment Panel III.
The molecular pathogenesis of liver steatosis and its progression to cirrhosis and eventually HCC is not clearly understood. Previously, a two-hit theory had been proposed to explain the molecular changes through which fatty liver leads to lipid peroxidation, cytokine production, and Fas ligand induction.27 However, more recently, a multiple-hit theory which better explains NAFLD development and progression to HCC has gained ground. The first hit is caused by insulin resistance and leads to fat accumulation in the hepatocytes, induced by both lipolysis and hyperinsulinemia. This is followed by multiple hits with many factors playing different roles, including genetic predisposition, obesity, oxidative stress and mitochondrial dysfunction, inflammation, adipokines, small intestinal microbacteria, and others. Hyperinsulinemia could also lead to increased levels of insulin growth factor-1, which causes stimulation of insulin growth factor receptors and promotion of proliferative and antiapoptotic effects, and vascular endothelial growth factor–mediated promotion of angiogenesis.28
Thus, in this setting, the liver is more susceptible to oxidative stress and inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which promote progression to NASH and fibrosis. IL-6 activates signal transducer and activator of transcription 3, which induces cell proliferation and antiapoptotic mechanisms. TNF-α activates pro-oncogenic pathways, including c-Jun N-terminal kinase, nuclear factor kappa-light-chain-enhancer of activated B cells, mammalian target of rapamycin, and the extracellular signal-regulated kinases.29–32 interestingly, several studies indicated that both dietary and genetic obesity could promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF-α expression.33–36
Finally, the high circulating levels of leptin in NAFLD have been recently shown to exert proinflammatory and profibrogenic effects.37,38 In addition, there is evidence that lipid peroxides and free radicals are elevated in metabolic syndrome; these may cause oxidative injury, endoplasmic reticulum stress, mitochondrial dysfunction, and apoptosis.15
Role of gender differences in NAFLD
Several studies have shown that gender differences play a role in various liver disorders. Earlier beliefs that NAFLD/NASH was a female-predominant condition have been dispelled; recent studies have shown higher prevalence in males.39–46 Interestingly, in a preclinical study, a high-fat, high-cholesterol diet induced NASH and hepatic ballooning in ovariectomized mice, which showed that estrogen deficiency promoted NASH progression, while estrogen treatment reversed it.47 Furthermore, many studies have shown that NAFLD patients have significantly lower levels of sex hormone binding globulin, a glycoprotein that binds to sex hormones (both estrogen and androgen).48,49 Increased prevalence of NAFLD has also been reported in patients with polycystic ovary syndrome (PCOS).50,51 Diethylnitrosamine administration caused greater increase in serum IL-6 in male than in female mice. Also, ablation of IL-6 abolished the gender differences in hepatocarcinogenesis in mice.52,53
However, the roles of androgen in the development of NAFLD remain unclear, as the results of experimental and clinical studies have been inconsistent.54,55 For example, Jones et al found a significantly higher degree of hepatic steatosis in high-androgen-expressed PCOS patients as compared to low-androgen-expressed PCOS patients or controls.56 Another study observed that androgenic steroid usage by bodybuilders could be a possible risk factor for NAFLD.57 In contrast, Haider et al showed that normalizing serum testosterone levels in obese hypogonadal males could improve their weight loss and metabolic state and suppress the development of NASH.58
The role of ARs in the pathogenesis of NAFLD is also unclear. An experimental study showed that whole-body AR-knockout mice fed a high-fat diet developed liver steatosis and insulin resistance, possibly through either 1) suppression of fatty acid synthesis by decreased sterol regulatory element-binding protein 1 (SREBP1) expression or 2) increased insulin sensitivity by suppression of phosphoenolpyruvate carboxykinase and protein tyrosine phosphatase 1B.59 Notably, other studies showed that activation of AR in orchidectomized mice led to obesity and altered lipid metabolism, and progression of NASH to HCC.60 This may have been mediated either through downregulation of liver X receptor (which complements SREBP2 activation and increases cellular cholesterol levels) or through upregulation of AR messenger RNA levels and increased activity of CYP27A1 (an enzyme that plays a significant role in cholesterol homeostasis and vitamin D3 metabolism through activation of the c-Jun N-terminal kinase pathway).61,62
Prevention of NASH/metabolic syndrome-related HCC using existing drugs
Statins (3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors) are commonly prescribed for prevention of cardiovascular disease and act by decreasing the biosynthesis of cholesterol. Statins may play a role in the prevention of cancer development by inducing apoptosis and inhibiting cellular proliferation, angiogenesis, inflammation, and immu-nomodulation.63 In multiple observational studies,64–67 the use of statins has been associated with a decreased risk of HCC in patients with viral hepatitis and those with diabetes, and statins may also decrease the risk of HCC recurrence after surgical treatment.68 However, a randomized controlled trial did not demonstrate a significant difference in HCC incidence between the statin and placebo groups.69
In diabetic patients, the use of both metformin and thiazolidinediones (peroxisome proliferator-activated receptor gamma agonists) was associated with a decreased cancer risk, whereas sulfonylurea was correlated with an increased overall risk of cancer.70 Also, the use of combined statin and metformin in diabetic patients showed no benefit to reduce the risk of HCC in Asian population.71 Metformin has been shown to inhibit hepatocellular proliferation and leads to arrest of the cell cycle at the G0/G1 phase by downregulation of cyclin D1,72 while thiazolidinediones were reported to decrease the risk of HCC by accumulation of p27, inhibition of ubiquitin-proteasome, MEK-ERK signaling, and induction of apoptosis.70,73,74 Also, drugs of proven antifibrogenic efficacy may potentially decrease the risk of developing HCC.75
Mechanistic pathway of androgen and ARs in hepatocarcinogenesis
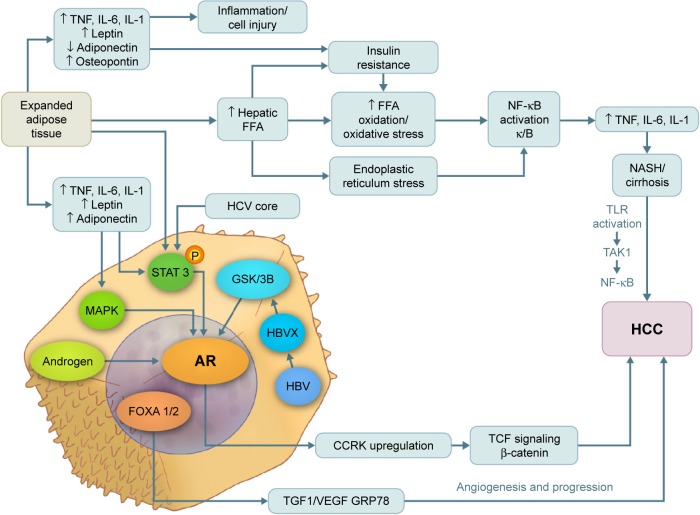
Both estrogen and androgen are steroid hormones that mediate their action by binding to nuclear receptors and acting as transcription factors to regulate the expression of multiple genes. Progression from hyperplasia to HCC is associated with suppression of estrogen receptors and elevated AR expression.76–80 In addition, several studies81 have shown that AR messenger RNA protein is expressed at higher levels in hepatic tumor tissue than in normal hepatic tissue. The AR gene is located on the X chromosome with a single copy in males. The AR molecule is a ligand-activated transcriptional factor with three domains – the N-terminal domain, the DNA-binding domain, and the ligand-binding domain.82 ARs can be activated directly by androgen, inducing cell cycle-relatd kinase (CCRK) transcription through promoter binding; CCRK then upregulates β-catenin/T-cell factor signaling, leading to promotion of hepatocarcinogenesis.83 ARs can also be activated directly in the absence of androgen by many alternative pathways such as the mitogen-activated protein kinase, AKt, and signal transducer and activator of transcription pathways, which are also involved in hepatocarcinogenesis (Figure 1).84,85 In an experimental study, adding functional ARs to HCC cells promoted cell growth and increased cellular oxidative stress, DNA damage, and suppression of the p53-mediated sensing/repairing system and of cell apoptosis.86 Furthermore, several studies have shown that AR is expressed in both normal liver and malignant tissues, but at higher levels in HCC tumor tissue.56,61,86
Figure 1.
Pathways of androgen and AR in the pathogenesis of NASH, cirrhosis, and hepatocellular carcinoma.
Note: Visual Art: © 2015 The University of Texas MD Anderson Cancer Center.
Abbreviations: AR, androgen receptor; CCRK, cycle cycle-related kinase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IL, interleukin; MAPK, mitogen-activated protein kinase; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; STAT 3, signal transducer and activator of transcription 3; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; GSK3b, glycogen synthase kinase 3 beta; FFA, free fatty acid; HBVX, hepatitis B virus protein X; TGF1, tumor growth factor 1.
Clinical evidence of androgen’s role in promoting HCC recurrence and metastasis
Several clinical studies have reported a strong correlation between AR expression and the rate of HCC recurrence (summarized in Table 2).87–89 However, there is controversy regarding the relationship between AR and tumor characteristics such as size, another factor that affects tumor recurrence.57,70
Table 2.
Clinical studies that evaluated the role of androgen and its receptors in the recurrence of surgically treated HCC
| Author | Year | Country | Sample size | HCC riskfactor | Sex (M/F) | AR expression | Recurrence rate | Survival rate |
|---|---|---|---|---|---|---|---|---|
| Nagasue et al87 | 1989 | Japan | 45 | Alcoholic 15 | 31/12 | 31 AR+ (25 M/4 F) | AR+ 67.9% | AR+ 1 year: 84%; 5 years: 17.3% |
| HBsAg 24 | 14 AR− (6 M/8 F) | AR− 33.3% | AR− 1 year: 100%; 5 years: 62.2% | |||||
| AntiHbc 32 | ||||||||
| AntiHBs 15 | ||||||||
| Boix et al88 | 1995 | Spain | 43 | Cirrhosis 40 | 30/13 | 30 AR+ | AR+ 1 year: 34%; 2 years: 51% | Not reported |
| Normal 3 | 13 AR− | AR− 1 year: 0%; 2 years: 20% |
Abbreviations: AntiHbc, Anti Hepatitis B core antibodies; antiHBs, antihepatitis B surface antibodies; AR, androgen receptor; F, female; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; M, male.
Moreover, the role of AR in promoting metastasis in HCC has been understudied. A few studies have reported a role for AR in metastatic HCC lesions, which may include the following: 1) AR activation by its ligand leads to increased expression of ID1 (a metastasis-promoting gene), which causes HCC cell migration and invasion90 or 2) tighter and faster adhesion of the cancer cells to collagen IV and various extracellular membranes occurs through β1 integrin expression induced by activated ARs.91 The latter effect of AR on cell adhesion was shown to be mediated by the β1 integrin-phosphoinositide-3-kinase (PI3K)/Akt pathway. In contrast, another study found that AR expression was significantly reduced in advanced metastatic lesions compared with early primary HCC lesions, with the AR upregulated in tumors <3 cm.92
Effect of antiandrogen treatment on HCC outcome
Despite the mounting evidence suggesting the potential role of the AR pathway as a therapeutic target in HCC, data on the use of antiandrogens are limited to a few clinical trials and no definite activity has been reported using this strategy (Table 3).87,93–99 One reason for the failure of these clinical trials could be the fact that these studies included mostly advanced and metastatic HCC patients, and the role of AR in late-stage HCC – whether it promotes or suppresses invasion and metastasis – remains unclear. Also, AR expression might be more critical than androgen concentration in HCC92 and no correlative studies have tested the predictive value of expression of AR in the tissue of HCC patients. Finally, most trials were performed on viral hepatitis–induced HCC, which may not have the same histologic characteristics or pathogenesis as non-hepatitis–induced HCC. The biological heterogeneity of HCC makes the prognosis of tumor growth, survival of patients, and treatment outcomes difficult.100–102
Table 3.
Clinical studies that evaluated the role of antiandrogen drugs in the treatment of HCC
| Author | Year | Country | Sample size (N) | HCC risk factor | Sex (M/F) | Antiandrogen type | Stage of disease | Changes in testosterone level | Overall survival rate | Responses |
|---|---|---|---|---|---|---|---|---|---|---|
| Forbes et al93 | 1987 | UK | 25 | Alcohol 10 HBV 8 Cryptogenic 3 PBC 3 Autoimmune 1 |
23/2 | Cyproterone acetate | Unresectable (five metastatic) | ↓ | 3 months | 3 (12%) |
| Gupta et al94 | 1988 | USA | 8 | Alcohol 7 | 7/1 | Ketoconazole | Unresectable | ↓ | Not significant | NR |
| Nagasue et al87 | 1989 | Japan | 16 | NR | 16/0 | Cyproterone acetate | Unresectable/recurrent | – | Not significant | 3 (19%) |
| Chao et al95 | 1996 | People’s Republic of China | 32 | Alcohol 1 Hepatitis 28 Cryptogenic 3 |
31/1 | Flutamide | Unresectable | <3 months | NR | |
| Grimaldi et al96 | 1998 | France | 238 | Alcohol 89 HBV 46 Hemochromatosis 10 |
202/35 | Nilutamide versus placebo Triptorelin versus placebo |
Unresectable | Nilutamide 3.9 versus 5.5 months |
NR | |
| Other 12 Missing 37 |
Triptorelin 3.4 versus 5.5 months | |||||||||
| Manesis et al97 | 1995 | Greece | 85 | Alcohol 21 HBV 53 HCV 11 HDV 10 |
66/19 | Tamoxifen + triptorelin versus flutamide + triptorelin versus placebo | Unresectable | ↓ | 4, 3, and 4 months, respectively | NR |
| GRETCH98 | 2004 | France | 375 | Alcohol 242 HCV 54 HBV 23 Other 15 |
375/0 | Leuprorelin + flutamide + tamoxifen versus tamoxifen | Unresectable | ↓ | 135.5 and 176 days, respectively | Not significant |
Note: ↓ represents decrease in testosterone level.
Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; NR, not reported; PBC, primary biliary cirrhosis.
Current systemic therapy in HCC: potential role of AR
Currently, single-agent sorafenib, a putative multitargeted kinase inhibitor, is the only systemic therapy approved by the US Food and Drug Administration to treat patients with advanced HCC. Sorafenib prolongs the overall survival by approximately 3 months in this population13 Phase II studies of other targeted drugs such as sunitinib, linifanib, erlotinib, ramucirumab, and everolimus demonstrated promising results in the management of late-stage HCC, but Phase III studies of these agents did not show overall survival benefit in unselected patient populations.103–105 Notably, subgroup analyses of the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial suggested that survival outcomes varied with patient demographics, geographic location, and risk factors. Interestingly, recent Phase II studies of other agents, including two studies in the first-line treatment setting conducted by the group using the combination of bevacizumab and erlotinib, showed activity in HCC,103,106–111 which also suggested differential outcomes based on patients’ demographics, geographic locations, and risk factors.
Another important factor that affects the outcome in HCC patient population is their tolerance to therapies, given the coexistence of HCC tumors and underlying liver disease in most of the cases. Although the adverse effects of targeted therapy are usually tolerable, serious complications can develop, especially with higher doses or in combination with another angiogenic agent or with chemotherapy. Fatigue, diarrhea, and hand/foot skin reaction are the most common dose-limiting side effects, with bleeding, arterial thromboembolism, and other fatal complications also possible.13 Therefore, increasing the efficiency of sorafenib and other targeted therapies by combination with other drugs that increase their efficacy while maintaining a lower dose is essential.
AR was found to suppress HCC metastasis by modulating p38. Also, the addition of functional AR in SKhep1 and HepG2 HCC cells was found to decrease p38, leading to enhanced effectiveness of sorafenib against HCC cells.92 In preclinical studies, sorafenib treatment had greater anti-metastatic effects against AR-positive than AR-negative HCC (66.7% vs 0%, respectively; P=0.0109). Another study showed that sorafenib with or without AR inhibitors induced significant apoptosis in HCC cells with knocked out AR when compared with unmanipulated HCC cells; the authors concluded that inhibition of AR in combination with sorafenib may be beneficial for the treatment of HCC.90,111 These data suggest that AR may be a target of combination therapy, and that AR expression may be a potential biomarker of response to sorafenib in HCC.
Conclusion
Androgen and ARs may play a critical role in hepatocarcinogenesis and could mediate, in part, the mechanisms responsible for the gender disparity in HCC incidence. ARs can be activated both by androgen and, in the absence of androgen, by alternative pathways. Liver steatosis can progress into NASH, which can lead to cirrhosis and HCC. The high prevalence of NASH in the general population led to recent significant increase in incidence of NASH-related HCC cases. Gender disparity appears to play a significant role in the development of NASH, as NASH is more common in males than in females and also contributes to higher risk of HCC development. Nevertheless, the clinical trials conducted so far have failed to demonstrate a significant benefit of antiandrogen drugs in the treatment of HCC patients. This failure could be explained, at least partially, by 1) the marked heterogeneity of HCC, with most trials performed on virus-induced HCC; 2) the dual, yet opposite effects of AR during early and late HCC, with most trials performed on advanced HCC only; and 3) the direct stimulation of AR by pathways other than androgen. Therefore, it is critical to understand the molecular mechanisms associated with HCC in males with NASH to design successful targeted therapy studies focusing on AR pathway.
Acknowledgments
Editorial assistance was provided by Michael Worley and the Department of Scientific Publications at MD Anderson Cancer Center. This work was supported by MD Anderson Cancer Center (to AOK).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in hispanics in the United States. Arch Intern Med. 2007;167(18):1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology (Baltimore, Md) 2011;54(2):463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50(3):350–360. doi: 10.1007/s00535-014-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10(12):1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr Diabetes Rev. 2013;9(5):382–386. doi: 10.2174/15733998113099990068. [DOI] [PubMed] [Google Scholar]
- 8.Tokushige K, Hashimoto E, Kodama K. Hepatocarcinogenesis in nonalcoholic fatty liver disease in Japan. J Gastroenterol Hepatol. 2013;28(Suppl 4):88–92. doi: 10.1111/jgh.12239. [DOI] [PubMed] [Google Scholar]
- 9.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hefaiedh R, Ennaifer R, Romdhane H, et al. Gender difference in patients with hepatocellular carcinoma. Tunis Med. 2013;91(8–9):505–508. [PubMed] [Google Scholar]
- 11.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita YI, Yoshida Y, Kurihara T, et al. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: repeat hepatectomy vs. salvage living donor liver transplantation. Liver Transpl. 2015;21(7):961–968. doi: 10.1002/lt.24111. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovascu Intervent Radiol. 2014;37(3):705–715. doi: 10.1007/s00270-013-0708-x. [DOI] [PubMed] [Google Scholar]
- 15.Cazanave SC, Mott JL, Elmi NA, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284(39):26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new worldwide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Essah PA, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. J Endocrinol Invest. 2006;29(3):270–280. doi: 10.1007/BF03345554. [DOI] [PubMed] [Google Scholar]
- 23.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47(3):181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 2009;116(7):539–564. doi: 10.1042/CS20080253. [DOI] [PubMed] [Google Scholar]
- 25.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 26.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Wiegand J, Mossner J, Tillmann HL. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Der Internist. 2007;48(2):154–163. doi: 10.1007/s00108-006-1796-3. German. [DOI] [PubMed] [Google Scholar]
- 28.Tomimaru Y, Koga H, Yano H, de la Monte S, Wands JR, Kim M. Upregulation of T-cell factor-4 isoform-responsive target genes in hepatocellular carcinoma. Liver Int. 2013;33(7):1100–1112. doi: 10.1111/liv.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian Z, Ma X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol. 2012;3:248. doi: 10.3389/fphys.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Zhang J, Shen B, et al. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2015;34:116. doi: 10.1186/s13046-015-0229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung IH, Choi JH, Chung YY, Lim GL, Park YN, Park SW. Predominant activation of JAK/STAT3 pathway by Interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia. 2015;17(7):586–597. doi: 10.1016/j.neo.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bugianesi E, Pagotto U, Manini R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90(6):3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 34.Musso G, Gambino R, Durazzo M, et al. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42(5):1175–1183. doi: 10.1002/hep.20896. [DOI] [PubMed] [Google Scholar]
- 35.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166(6):1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awazawa M, Ueki K, Inabe K, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382(1):51–56. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- 38.Ikejima K, Okumura K, Kon K, Takei Y, Sato N. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S87–S92. doi: 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 39.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 40.Baig S. Gender disparity in infections of hepatitis B virus. J Coll Physicians Surg Pak. 2009;19(9):598–600. [PubMed] [Google Scholar]
- 41.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 42.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107(4):1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 43.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 44.Angelico F, Del Ben M, Conti R, et al. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18(5):588–594. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 45.Kral JG, Schaffner F, Pierson RN, Jr, Wang J. Body fat topography as an independent predictor of fatty liver. Metabolism. 1993;42(5):548–551. doi: 10.1016/0026-0495(93)90210-f. [DOI] [PubMed] [Google Scholar]
- 46.Omagari K, Kadokawa Y, Masuda J, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17(10):1098–1105. doi: 10.1046/j.1440-1746.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 47.Kamada Y, Kiso S, Yoshida Y, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301(6):G1031–G1043. doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- 48.Polyzos SA, Kountouras J, Tsatsoulis A, et al. Sex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver disease. Hormones. 2013;12(3):405–416. doi: 10.1007/BF03401306. [DOI] [PubMed] [Google Scholar]
- 49.Shin JY, Kim SK, Lee MY, et al. Serum sex hormone-binding globulin levels are independently associated with nonalcoholic fatty liver disease in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(1):156–162. doi: 10.1016/j.diabres.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 50.Vassilatou E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J Gastroenterol. 2014;20(26):8351–8363. doi: 10.3748/wjg.v20.i26.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciotta L, Pagano I, Stracquadanio M, Formuso C. Polycystic ovarian syndrome incidence in young women with non-alcoholic fatty liver disease. Minerva Ginecol. 2011;63(5):429–437. Italian. [PubMed] [Google Scholar]
- 52.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 53.Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48(2):380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Liu Y, Wang L, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54(2):345–357. doi: 10.1194/jlr.M028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chow JD, Jones ME, Prelle K, Simpson ER, Boon WC. A selective estrogen receptor alpha agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J Endocrinol. 2011;210(3):323–334. doi: 10.1530/JOE-10-0462. [DOI] [PubMed] [Google Scholar]
- 56.Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(10):3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 57.Schwingel PA, Cotrim HP, Salles BR, et al. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011;31(3):348–353. doi: 10.1111/j.1478-3231.2010.02346.x. [DOI] [PubMed] [Google Scholar]
- 58.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014;8(4):e339–e349. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Lin HY, Yu IC, Wang RS, et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology (Baltimore, Md) 2008;47(6):1924–1935. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- 60.Moverare-Skrtic S, Venken K, Andersson N, et al. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring, Md) 2006;14(4):662–672. doi: 10.1038/oby.2006.75. [DOI] [PubMed] [Google Scholar]
- 61.Norlin M, Pettersson H, Tang W, Wikvall K. Androgen receptor-mediated regulation of the anti-atherogenic enzyme CYP27A1 involves the JNK/c-jun pathway. Arch Biochem Biophys. 2011;506(2):236–641. doi: 10.1016/j.abb.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. J Biol Chem. 2011;286(23):20637–20647. doi: 10.1074/jbc.M111.227082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27(11):1654–1664. doi: 10.1111/j.1440-1746.2012.07232.x. [DOI] [PubMed] [Google Scholar]
- 64.Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol. 2013;28(6):485–492. doi: 10.1007/s10654-013-9806-y. [DOI] [PubMed] [Google Scholar]
- 65.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 66.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136(5):1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 69.Emberson JR, Kearney PM, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7(1):e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(7):E1170–E1175. doi: 10.1210/jc.2012-1162. [DOI] [PubMed] [Google Scholar]
- 71.Chen HH, Lin MC, Muo CH, Yeh SY, Sung FC, Kao CH. Combination therapy of mtetformin and statin may decrease hepatocellular carcinoma among diabetic patients in Asia. Medicine. 2015;94(24):e1013. doi: 10.1097/MD.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 73.Okumura T. Mechanisms by which thiazolidinediones induce anticancer effects in cancers in digestive organs. J Gastroenterol. 2010;45(11):1097–1102. doi: 10.1007/s00535-010-0310-9. [DOI] [PubMed] [Google Scholar]
- 74.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35(1):119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh S, Khera R, Murad MH, Loomba R. Reply. Hepatology (Baltimore, Md) 2016;64(2):694. doi: 10.1002/hep.28412. [DOI] [PubMed] [Google Scholar]
- 76.Eagon PK, Elm MS, Epley MJ, Shinozuka H, Rao KN. Sex steroid metabolism and receptor status in hepatic hyperplasia and cancer in rats. Gastroenterology. 1996;110(4):1199–1207. doi: 10.1053/gast.1996.v110.pm8613010. [DOI] [PubMed] [Google Scholar]
- 77.Ostrowski JL, Ingleton PM, Underwood JC, Parsons MA. Increased hepatic androgen receptor expression in female rats during diethylnitrosamine liver carcinogenesis. A possible correlation with liver tumor development. Gastroenterology. 1988;94(5 Pt 1):1193–1200. doi: 10.1016/0016-5085(88)90012-1. [DOI] [PubMed] [Google Scholar]
- 78.Tejura S, Rodgers GR, Dunion MH, Parsons MA, Underwood JC, Ingleton PM. Sex-steroid receptors in the diethylnitrosamine model of hepatocarcinogenesis: modifications by gonadal ablation and steroid replacement therapy. J Mol Endocrinol. 1989;3(3):229–237. doi: 10.1677/jme.0.0030229. [DOI] [PubMed] [Google Scholar]
- 79.Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemo-prevention by leuprorelin. Jpn J Cancer Res. 2001;92(3):249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor- dependent hepatocarcinogenesis. J Clin Invest. 2011;121(8):3159–3175. doi: 10.1172/JCI45967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian Y, Wong VW, Chan HL, Cheng AS. Epigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty liver disease. Semin Cancer Biol. 2013;23(6 Pt B):471–482. doi: 10.1016/j.semcancer.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20(13):3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 83.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Culig Z. Androgen receptor coactivators in regulation of growth and differentiation in prostate cancer. J Cell Physiol. 2016;231(2):270–274. doi: 10.1002/jcp.25099. [DOI] [PubMed] [Google Scholar]
- 85.Agoulnik IU, Weigel NL. Coactivator selective regulation of androgen receptor activity. Steroids. 2009;74(8):669–674. doi: 10.1016/j.steroids.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14(39):5945–5961. doi: 10.3748/wjg.14.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagasue N, Kohno H, Chang YC, et al. Androgen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in women. Cancer. 1989;63(1):112–116. doi: 10.1002/1097-0142(19890101)63:1<112::aid-cncr2820630118>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 88.Boix L, Castells A, Bruix J, et al. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995;22(6):616–622. doi: 10.1016/0168-8278(95)80217-7. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X, He L, Lu Y, Liu M, Huang X. Androgen receptor in primary hepatocellular carcinoma and its clinical significance. Chin Med J. 1998;111(12):1083–1086. [PubMed] [Google Scholar]
- 90.Ao J, Meng J, Zhu L, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6(5):507–515. doi: 10.1016/j.molonc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma WL, Jeng LB, Lai HC, Liao PY, Chang C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating beta1-integrin-AKT signaling in hepatocellular carcinoma cells. Cancer Lett. 2014;351(1):64–71. doi: 10.1016/j.canlet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 92.Ma WL, Hsu CL, Yeh CC, et al. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology (Baltimore, Md) 2012;56(1):176–185. doi: 10.1002/hep.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forbes A, Wilkinson ML, Iqbal MJ, Johnson PJ, Williams R. Response to cyproterone acetate treatment in primary hepatocellular carcinoma is related to fall in free 5 alpha-dihydrotestosterone. Eur J Cancer Clin Oncol. 1987;23(11):1659–1664. doi: 10.1016/0277-5379(87)90446-9. [DOI] [PubMed] [Google Scholar]
- 94.Gupta S, Korula J. Failure of ketoconazole as anti-androgen therapy in nonresectable primary hepatocellular carcinoma. J Clin Gastroenterol. 1988;10(6):651–654. doi: 10.1097/00004836-198812000-00016. [DOI] [PubMed] [Google Scholar]
- 95.Chao Y, Chan WK, Huang YS, et al. Phase II study of flutamide in the treatment of hepatocellular carcinoma. Cancer. 1996;77(4):635–639. [PubMed] [Google Scholar]
- 96.Grimaldi C, Bleiberg H, Gay F, et al. Evaluation of antiandrogen therapy in unresectable hepatocellular carcinoma: results of a European Organization for Research and Treatment of Cancer multicentric double-blind trial. J Clin Oncol. 1998;16(2):411–417. doi: 10.1200/JCO.1998.16.2.411. [DOI] [PubMed] [Google Scholar]
- 97.Manesis EK, Giannoulis G, Zoumboulis P, Vafiadou I, Hadziyannis SJ. Treatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: a randomized, controlled trial. Hepatology (Baltimore, Md) 1995;21(6):1535–1542. [PubMed] [Google Scholar]
- 98.Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology (Baltimore, Md) 2004;40(6):1361–1369. doi: 10.1002/hep.20474. [DOI] [PubMed] [Google Scholar]
- 99.Di Maio M, De Maio E, Morabito A, et al. Hormonal treatment of human hepatocellular carcinoma. Ann N Y Acad Sci. 2006;1089:252–261. doi: 10.1196/annals.1386.007. [DOI] [PubMed] [Google Scholar]
- 100.Villa E, Critelli R, Lei B, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65(5):861–869. doi: 10.1136/gutjnl-2014-308483. [DOI] [PubMed] [Google Scholar]
- 101.Weiskirchen R. Intratumor heterogeneity, variability and plasticity: questioning the current concepts in classification and treatment of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2016;5(2):183–187. doi: 10.3978/j.issn.2304-3881.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2015;379(2):191–197. doi: 10.1016/j.canlet.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 103.Hsu CH, Kang YK, Yang TS, et al. Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: a multicenter phase II study. Oncology. 2013;85(1):44–52. doi: 10.1159/000350841. [DOI] [PubMed] [Google Scholar]
- 104.Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Welker MW, Trojan J. Antiangiogenic treatment in hepatocellular carcinoma: the balance of efficacy and safety. Cancer Manag Res. 2013;5:337–347. doi: 10.2147/CMAR.S35029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Govindarajan R, Siegel E, Makhoul I, Williamson S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol. 2013;36(3):254–257. doi: 10.1097/COC.0b013e318248d83f. [DOI] [PubMed] [Google Scholar]
- 107.Kaseb AO, Garrett-Mayer E, Morris JS, et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology. 2012;82(2):67–74. doi: 10.1159/000335963. [DOI] [PubMed] [Google Scholar]
- 108.Philip PA, Mahoney MR, Holen KD, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer. 2012;118(9):2424–2430. doi: 10.1002/cncr.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yau T, Wong H, Chan P, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs. 2012;30(6):2384–2390. doi: 10.1007/s10637-012-9808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27(6):843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 111.Jiang X, Kanda T, Nakamoto S, Miyamura T, Wu S, Yokosuka O. Involvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesis. Exp Cell Res. 2014;323(2):326–336. doi: 10.1016/j.yexcr.2014.02.017. [DOI] [PubMed] [Google Scholar]