Abstract
The ventral tegmental area (VTA) and the rostromedial tegmental nucleus (RMTg) each contribute to opiate reward and each receive inputs from the laterodorsal tegmental and pedunculopontine tegmental nuclei, the two principle brainstem cholinergic cell groups. We compared the contributions of VTA or RMTg muscarinic cholinergic receptors to locomotion induced by morphine infusions into the same sites. VTA co-infusion of atropine completely blocked VTA morphine-induced locomotion providing additional support for the important role of VTA muscarinic cholinergic receptors in the stimulant effects of opiates. By contrast, RMTg co-infusion of atropine increased RMTg morphine-induced locomotion. Furthermore, RMTg co-infusion of the M3-selective antagonist 4-DAMP, but not the M4-selective antagonist Tropicamide, strongly increased RMTg morphine-induced locomotion. RMTg infusions of 4-DAMP, but not of Tropicamide, by themselves strongly increased drug-free locomotion. Muscarinic cholinergic receptors in the RMTg thus also contribute to the stimulant effects of morphine, but in a way opposite to those in VTA. We suggest that the net effect of endogenous cholinergic input to the RMTg on drug-free and on RMTg morphine-induced locomotion is inhibitory.
Keywords: laterodorsal tegmental nucleus, pedunculopontine tegmental nucleus, opiates, dopamine, acetylcholine
Muscarinic cholinergic receptors in the ventral tegmental area (VTA) contribute to the stimulant effects [1, 2] and to the rewarding effects of systemic morphine [3, 4], as well as to nucleus accumbens (NAcc) dopamine increases induced by systemic [3, 5] or by intra-VTA [6] morphine. Rats or mice learn to self-administer opiates into either the VTA [7, 8] and into a region just caudal to the VTA identified by some as the rostromedial tegmental nucleus (RMTg [9, 10]) and by others as the “tail” of the VTA [11]. Cholinergic input to the RMTg also contributes to the stimulant effects of systemic morphine [12].
The VTA receives cholinergic inputs from the pedunculopontine tegmental nucleus (PPTg) and the laterodorsal tegmental nucleus (LDTg) [13, 14]. The RMTg also receives input from each of the LDTg and PPTg [9, 15] and recent evidence shows that at least some of the LDTg/PPTg input to the RMTg input is cholinergic with the same LDT/PPTg cholinergic neurons sending collaterals to both RMTg and VTA [12]. Given that the VTA and the RMTg each receive mesopontine cholinergic inputs and that muscarinic cholinergic receptors in VTA and RMTg each contribute to the stimulant effects of systemic morphine, we wanted to 1) compare locomotion induced by morphine infusions into either the VTA or the RMTg, and 2) determine the contributions of muscarinic cholinergic receptors in VTA or RMTg to locomotion induced by morphine infused into the same sites. To accomplish this C57BL/6 mice (Charles River), maintained on a 12-hour light/dark cycle (lights on at 7:00 AM) with food and water available ad libitum throughout, were implanted with guide cannulae (26 ga; Plastics One Inc.) aimed at either the VTA (A-P -3.4, M-L ± 0.5, D-V – 4.2, n = 6) or the RMTg (A-P -4.0, M-L ± 0.3, D-V – 4.3, n = 20) as previously described [1]. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were tested individually in opaque open-field enclosures (40×40×35 cm) equipped with designated digital cameras connected to a video-tracking system (ANY-maze; Stoelting Inc.) that quantified the amount of forward locomotion. All intra- cranial infusions (33 ga injector cannulae 2 mm longer than guides) were made at a volume of 0.3 µl and at rate of 0.2 µl/min. Following a 3 hr drug-free habituation session each mouse received each of four intracranial treatments across days 2–5: a) 5nM morphine (generously provided by the National Institute on Drug Abuse), b) antagonist alone (3 µg Atropine [Sigma- Aldrich] or 2 µg 1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide [4-DAMP; Tocris Bioscience] or1.3 µg Tropicamide [Tocris Bioscience], c) 5nM morphine co-infused with antagonist, and d) vehicle (saline for atropine studies, 0.05% DMSO for 4-DAMP and Tropicamide studies). The order of treatments across mice followed a Latin-square design. Following completion of behavioral testing mice were transcardially perfused and coronal cryosections throughout the extent of the RMTg and VTA were stained with cresyl violet to verify injection sites.
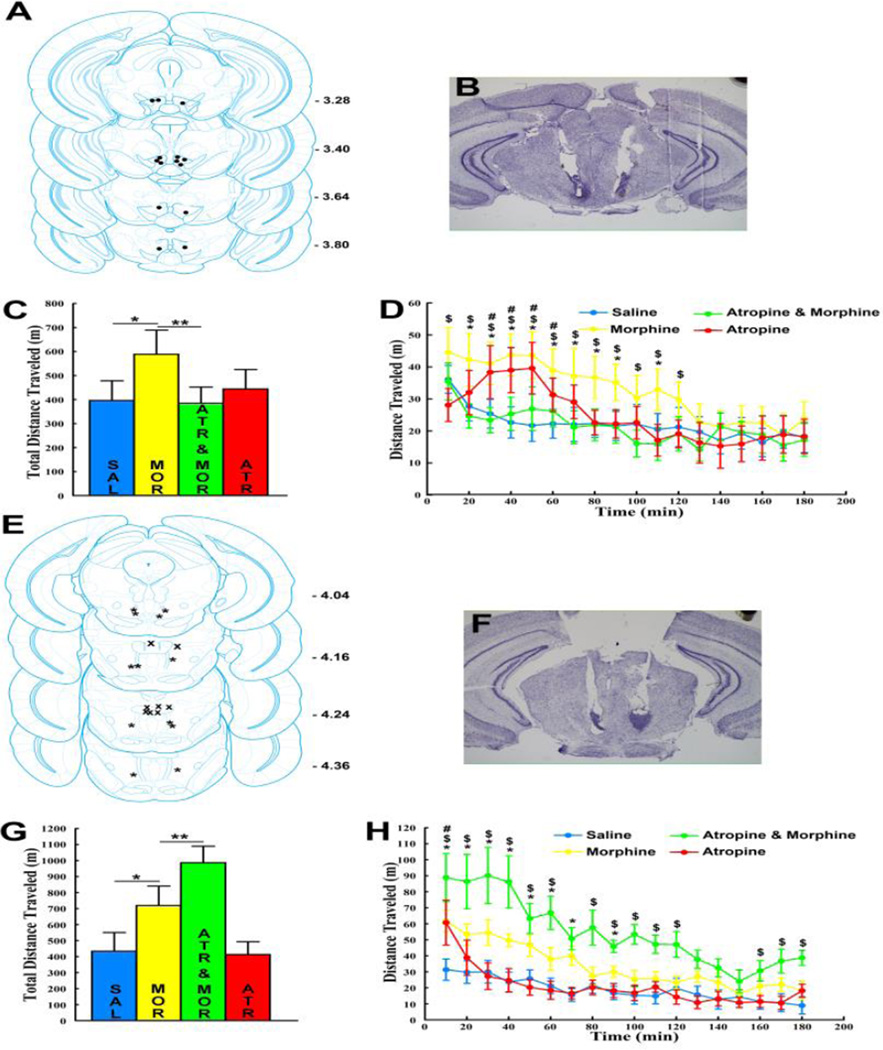
Morphine infusions into VTA sites (between Bregma -3.28 to – 3.80 [Fig 1A and 1B]) or into RMTg sites (between Bregma -4.04 and -4.36 [Fig 1E and 1F]) increased total locomotion relative to saline infusions (Fig 1C and Fig 1D). VTA atropine co-infusions reduced total VTA- morphine induced locomotion while RMTg atropine co-infusions increased total RMTg- morphine induced locomotion (ANOVA main effects of TREATMENT: VTA [F3, 18 = 4.12, p < 0.05] and RMTg [F3, 15 = 12.99, p < 0.01]. Fisher’s LSD post-hoc test confirmed greater locomotion following morphine compared to saline for each site [p’s < 0.05], decreased locomotion following VTA atropine co-infusion relative to VTA morphine alone [p < 0.01] and increased locomotion following RMTg atropine co-infusion relative to RMTg morphine alone [p < 0.01]). Time course analysis showed that VTA atropine co-infusion reduced VTA morphine- induced locomotion to saline levels throughout the testing period (Fig 1D; TREATMENT×TIME interaction [F51, 306 = 1.66, p < 0.01]) while RMTg atropine co-infusion increased RMTg morphine-induced locomotion throughout most of the 3 hr testing period, beyond the 10–90 min time period when RMTg morphine by itself maximally increased locomotion (Fig 1H; TREATMENT×TIME interaction [F51, 255 = 3.63, p < 0.00001]). VTA atropine infusions by themselves did not significantly affect total locomotion relative to saline (Fig 1C [p > .1]), but the time course analysis showed that locomotion was increased relative to saline between 30–50 min (Fig 1D [p’s between < 0.0001 and < 0.05]). In a previous report [1] the same dose of VTA atropine was also shown to cause a small and delayed, albeit statistically not significant, increase in locomotion in C57BL/6 mice. RMTg atropine infusions by themselves also did not significantly affect total locomotion relative to saline (Fig 1G, p > 0.1]), but the time course analysis showed an immediate onset short lasting locomotion increase in the first 10 min (Fig 1H, p < 0.00001]).
Figure 1. VTA atropine decreases VTA morphine-induced locomotion while RMTg atropine increases RMTg morphine-induced locomotion.
A. Bilateral VTA injection sites (n =7). B. Representative image of a cresyl violet- stained section showing VTA injection sites. C. VTA morphine (MOR) increased total locomotion (3 hr) relative to VTA saline (SAL). VTA co-infusion of 3µg atropine (ATR & MOR) reduced VTA morphine-induced locomotion to saline levels. VTA infusion of atropine alone (ATR) had no significant effect on locomotion (n =7; * p < 0.05; ** p < 0.01). D. Locomotion time course: VTA morphine increased locomotion relative to VTA saline between 20 and 110 minutes (* p’s between < .00001 and < .01). VTA co-infusion of 3µg atropine reduced VTA morphine-induced locomotion to saline levels throughout the 3-hr testing period ($ p’s between < 0.0001 and < 0.05 [Atropine & Morphine vs. Morphine]). VTA atropine alone increased locomotion relative to VTA saline between 30–60 min (# p’s between < 0.0001 and < 0.05). E. Bilateral RMTg injection sites (n = 6; stars) and injections sites that were medial or dorsal to the RMTg (n = 4; X symbol; morphine infusion into these sites did not induce locomotion and atropine by itself or co- infused with morphine also was without any effect). F. Representative image of a cresyl violet-stained section showing RMTg injection sites. G. RMTg morphine (MOR) increased total locomotion (3 hr) relative to RMTg saline (SAL). RMTg co-infusion of 3 µg atropine (ATR & MOR) increased RMTg morphine-induced locomotion relative to RMTg morphine alone (MOR). RMTg infusion with atropine alone (ATR) had no significant effect on locomotion (n = 6; * p < 0.05; ** p < 0.01). H. Locomotion time course: RMTg morphine increased locomotion relative to RMTg saline between 10 and 90 minutes (* p’s between < 0.000001 and < 0.01). RMTg co-infusion of 3 µg atropine increased RMTg morphine-induced locomotion ($ p’s between < 0.000001 and < 0.01 [Atropine & Morphine vs. Morphine]). RMTg atropine alone increased locomotion relative to RMTg saline in the first 10 min (# p < .00001). All error bars represent ±SEM.
Results from VTA atropine studies confirm the critical role of VTA muscarinic receptors in the stimulant effects of morphine [1, 2]. Previously it has been shown that VTA morphine-induced dopamine increases are absent in M5 knockout mice and are similarly blocked by VTA scopolamine pre-treatment in wild-type mice [6]. Here we show that VTA atropine completely blocks VTA morphine-induced locomotion, suggesting that the ability of VTA morphine to increase locomotion, like systemic morphine [1], depends on M5-mediated cholinergic excitation of mesolimbic dopamine signaling. Results from RMTg atropine studies reveal two novel findings. First, to our knowledge, this is the first demonstration that morphine infusions into the RMTg induce locomotion in mice, which is consistent with RMTg opioids inducing place preference and supporting opioid self-administration [9]. Second, RMTg muscarinic receptors also contribute to the stimulant effects of RMTg morphine. However, the effects of RMTg and VTA co-infusion of atropine on locomotion induced by morphine infusion into the same site are opposite.
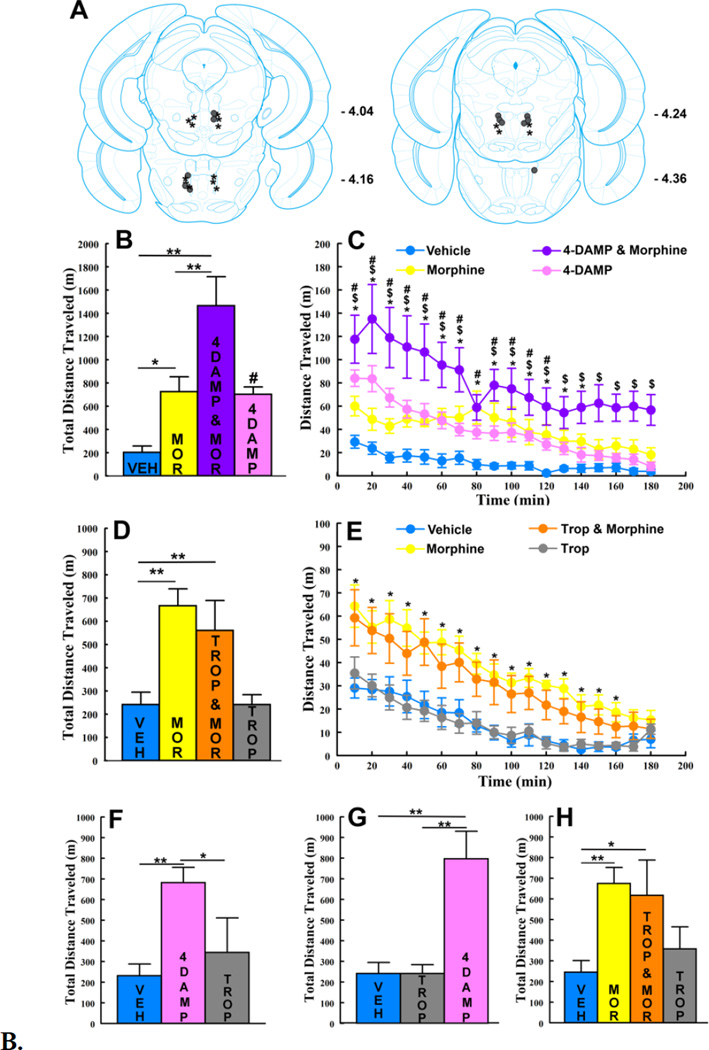
As it recently has been shown that both muscarinic M3 and M4 receptors are found on and surrounding RMTg µ-opioid receptor-positive cells [12], each of which would be blocked by atropine, we next tested the contributions of RMTg M3 and M4 muscarinic receptors to RMTg morphine-induced locomotion (Fig 2A) in two additional groups of mice. RMTg morphine infusions increased total locomotion relative to RMTg saline in all mice tested to the same extent as seen in atropine studies (Fig 2B and 2D). RMTg co-infusions of the M3-selective antagonist 4-DAMP increased total RMTg morphine-induced locomotion relative to RMTg morphine alone (Fig 2B; main effect of TREATMENT [F3, 15 = 15.143, p < 0.0001]). By contrast, RMTg co-infusions of the M4-selective antagonist Tropicamide did not significantly affect total RMTg morphine- induced locomotion relative to RMTg morphine alone (Fig 2D; main effect of TREATMENT [F1.58, 11.08 = 14.47, p < 0.01 with Hunyh-Feldt adjusted degrees of freedom]. Fisher’s post-hoc test confirmed greater total locomotion following morphine relative to saline for each antagonist group (p’s between < 0.05 and < 0.01), increased locomotion following RMTg 4-DAMP co-infusion relative to morphine alone (p < 0.01) and no change in locomotion following RMTg Tropicamide co-infusion (p > 0.1). Time course analysis showed that RMTg morphine-induced locomotion was increased by 4- DAMP co-infusion throughout the 3 hr testing period, beyond the time period during which RMTg morphine by itself maximally increased locomotion (Fig 2C; TREATMENT×TIME interaction [F51, 255 = 1.85, p < 0.01]). RMTg infusions of 4- DAMP by themselves also increased total locomotion relative to RMTg saline (Fig 2B, p < .05]) and did so between 10 and 120 min (Fig 2C, p’s between < .00001 and < .05]).
Figure 2. RMTg co-infusion of the M3-selective muscarinic receptor antagonist 4- DAMP, but not the M4-selective muscarinic receptor antagonist Tropicamide, increases RMTg morphine-induced locomotion.
A. RMTg injection sites for mice in which the effects of co-infusing the M3-selective muscarinic receptor antagonist 4-DAMP (n = 6; solid circles) or the M4-selective muscarinic receptor antagonist Tropicamide (n = 8; stars) on RMTg morphine- induced locomotion were tested. B. RMTg 4-DAMP increased total locomotion relative to RMTg vehicle (VEH, [# p < .05]). RMTg co-infusion of 2 µg 4- DAMP (4DAMP & MOR) increased RMTg morphine-induced locomotion relative to RMTg morphine (* p < 0.05; ** p < 0.01). C. RMTg 4-DAMP - locomotion time course: RMTg morphine increased locomotion relative to RMTg vehicle between 10 and 140 minutes (* p’s between < 0.0001 and < 0.05). RMTg co-infusion of 2 µg 4DAMP increased RMTg morphine-induced locomotion relative to RMTg morphine alone throughout the 3-hr testing period ($ p’s between < 0.00001 and < 0.05). RMTg infusions of 4-DAMP alone increased locomotion relative to RMTg saline between 10 and 120 min (# p’s between < 0.05 and < 0.00001). D. RMTg co-infusion of 1.3 µg Tropicamide (TROP & MOR) did not significantly affect total (3 hr) RMTg morphine-induced locomotion. RMTg morphine (MOR) increased locomotion relative to RMTg vehicle (VEH, [** p < 0.01]). RMTg infusions of Tropicamide alone (TROP) did not significantly affect locomotion relative to vehicle. E. RMTg Tropicamide - locomotion time course: RMTg morphine increased locomotion relative to RMTg vehicle between 10 and 160 minutes (* p’s between < 0.00001 and < 0.05). RMTg co-infusion of 1.3 µg Tropicamide did not affect RMTg morphine-induced locomotion. RMTg infusions of Tropicamide alone did not significantly affect locomotion relative to RMTg vehicle. F. The effects of RMTg infusions of 1.3 µg Tropicamide were tested in 5 of 6 mice used for initial 4-DAMP testing (1of 6 mice lost its intracranial implant following completion of 4-DAMP testing). RMTg infusion of Tropicamide did not significantly affect total locomotion. Vehicle and 4-DAMP locomotion data for this subset of mice are included from panel B (* p < 0.05; ** p < 0.01). G. The effects of RMTg infusions of 2 µg 4- DAMP were tested in mice used for initial Tropicamide testing (n =8). RMTg infusions of 4-DAMP significantly increased total locomotion. Vehicle and Tropicamide locomotion data are included from panel D (** p < 0.01). H. Eight mice (4 from each of the initial 4-DAMP and Tropicamide testing groups) underwent two additional testing sessions during which the effects of 10.3 µg RMTg Tropicamide alone or in combination with RMTg morphine were tested. RMTg co-infusion of 10.3 µg Tropicamide did not affect total RMTg morphine- induced locomotion relative to RMTg morphine alone. RMTg infusion of 10.3 µg Tropicamide alone did not significantly affect total locomotion relative to RMTg vehicle. RMTg morphine significantly increased total locomotion relative to vehicle (* p < 0.01; ** p < 0.001). Vehicle and morphine locomotion data for this subset of mice are included from panels B & D. All error bars represent ±SEM.
To further evaluate the observed dissociation between RMTg M3 and M4 receptors we tested the effects of RMTg Tropicamide infusions in a subset of mice in which the effects of 4-DAMP had been tested first. RMTg Tropicamide infusions also did not significantly affected total locomotion in these mice (Fig 2F, ANOVA main effect of TREATMENT [F2, 8 = 8.80, p < 0.01], Fisher’s LSD post hoc test confirmed no significant difference in locomotion between RMTg Tropicamide relative to RMTg saline [p > 0.1]). By contrast, RMTg 4-DAMP infusions in mice in which the effects of Tropicamide had been tested first, increased total locomotion relative to RMTg saline (Fig 2G, ANOVA main effect of TREATMENT [F1.14, 7.99 = 19.52, p < 0.01 with Huynh- Feldt adjusted degrees of freedom], Fisher’s LSD posthoc test showed that locomotion was significantly increased following RMTg 4-DAMP relative to RMTg saline [p < 0.01]). The extent to which RMTg 4-DAMP increased locomotion in these mice was comparable to levels observed in mice used for initial 4-DAMP studies (compare pink bars in Fig 2G and 2B). Thus, in the same RMTg sites in which Tropicamide infusions had no effect, 4-DAMP infusions significantly increased locomotion. Conversely, in the same RMTg sites in which 4-DAMP infusions significantly increased locomotion, Tropicamide infusions had no effect.
To test whether the lack of Tropicamide effects were unique to the dose used (1.3 µg Tropicamide was chosen to be approximately equi-molar to 3 µg atropine) 4 mice from each of the 4-DAMP and Tropicamide groups underwent two additional testing days on which the effects of 10.3 µg RMTg Tropicamide alone or co-infused with morphine on locomotion were evaluated. In these mice RMTg co-infusions of 10.3 µg Tropicamide with morphine did not significantly affect total locomotion relative to morphine alone. RMTg infusions of 10.3 µg Tropicamide by themselves appeared to slightly increase total locomotion relative to RMTg saline but not to a statistically significant extent (Fig 2H; main effect of TREATMENT [F1.59, 11.17 = 6.93, p < 0.05 with Huynh-Feldt adjusted degrees of freedom] and Fisher’s post-hoc test confirmed that locomotion was significantly increased following RMTg morphine relative to RMTg saline [p < 0.01]).
Our data support the importance of cholinergic input to the RMTg in modulating open-field locomotion [2, 12]. We show for the first time that endogenous cholinergic input to the RMTg, mediated through M3 receptors, contributes to drug-free locomotion and to RMTg morphine-induced locomotion. Cholinergic excitation of M3 receptors, associated with RMTg GABA neurons [12], is expected to increase RMTg GABAergic input to VTA dopamine neurons. Accordingly, RMTg M3 receptor blockade strongly increased drug-free locomotion. It has been suggested that opiate inhibition of RMTg GABA neurons, similar to what has been previously suggested for opiate inhibition of VTA GABA neurons [1, 6], results in decreased GABAergic input to the PPTg and/or LDTg, in turn resulting in increased cholinergic input to the RMTg [2, 12]. Our data suggest that such increased cholinergic input to the RMTg must excite RMTg GABA neurons via M3 receptors. This, unlike cholinergic feedback to the VTA, results in inhibition of morphine-induced locomotion. In support of this concurrent blockade of M3 receptors strongly increased locomotion induced by RMTg morphine. The net effect of blocking an excitatory cholinergic input to the RMTg may be inhibition of RMTg GABA neurons. Consistent with this direct chemogenetic inhibition of RMTg GABA neurons has also been shown to increase locomotion induced by systemic morphine [12].
The experimental design employed here allowed us to compare the contributions of muscarinic cholinergic receptors to local VTA or RMTg opiate effects. Systemic morphine by contrast will act in the VTA and RMTg simultaneously, in turn activating each proposed cholinergic feedback loop. An outstanding question is thus whether both pathways will necessarily contribute equally to the effects of systemic morphine. While the importance of VTA muscarinic receptors to the locomotor, rewarding and dopamine release-inducing effects of systemic morphine have been well documented, the effects of muscarinic antagonists in RMTg to each of these have not been systematically studied.
In conclusion, we show that locomotor effects are elicited by either VTA or RMTg morphine in mice. Cholinergic receptors in VTA and in RMTg each contribute to the locomotor effects of VTA and RMTg morphine, respectively, but do so in opposite ways.
Highlights.
VTA or RMTg morphine each increase locomotion
VTA atropine blocks VTA morphine locomotion
RMTg atropine increases RMTg morphine locomotion
RMTg M3 but not M4 receptors are critical for RMTg morphine locomotion
Acknowledgments
We thank Rawan Harb for assistance with tissue sectioning. This work was funded by start-up funds provided to SS by Loyola University Chicago, US National Institutes of Health grant R15 DA041694 to SS, a Loyola University Mulcahy Undergraduate Fellowship to ED. We thank John Yeomans and David Wasserman for helpful discussion of the manuscript. We thank the National Institute on Drug Abuse for generously providing morphine for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steidl S, Yeomans JS. M5 muscarinic receptor knockout mice show reduced morphine-induced locomotion but increased locomotion after cholinergic antagonism in the ventral tegemental area. J. Pharmacol. Exp. Ther. 2009;329:263–275. doi: 10.1124/jpet.108.144824. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman DI, Wang HG, Rashid AJ, Josselyn SA, Yeomans JS JS. Cholinergic control of morphine-induced locomotion in rostromedial tegmental nucleus versus ventral tegmental area sites. Eur. J. Neurosci. 2013;38:2774–2785. doi: 10.1111/ejn.12279. [DOI] [PubMed] [Google Scholar]
- 3.Basile A, Fedorova I, Zapata A, Liu X, Shippenberg T, Duttaro A, Yamada M, Wess J. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc. Natl. Acad. Sci. USA. 2002;99:11452–11457. doi: 10.1073/pnas.162371899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezayof A, Nazari-Serenjeh F, Zarrindast MR, Sepehri H, Delphi L. Morphine-induced place preference: involvement of cholinergic receptors of the ventral tegmental area. Eur. J. Pharmacol. 2007;562:562–592. doi: 10.1016/j.ejphar.2007.01.081. [DOI] [PubMed] [Google Scholar]
- 5.Miller AD, Forster GL, Yeomans JS, Blaha CD. Midbrain muscarinic receptors modulate morphine-induced accumbal and striatal dopamine efflux in the rat. Neuroscience. 2005;136:531–538. doi: 10.1016/j.neuroscience.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Steidl S, Miller AD, Blaha CD, Yeomans JS. M5 muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PLoS ONE. 2011;6:e27538. doi: 10.1371/journal.pone.0027538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- 8.David V, Cazala P. A comparative study of self-administration of morphine into the amygdala and the ventral tegmental area in mice. Behav. Brain. Res. 1994;15:205–211. doi: 10.1016/0166-4328(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 9.Jhou TC, Xu SP, Lee MR, Gallen CL, Ikemoto S. Mapping of reinforcing and analgesic effects of the mu opioid agonist endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology. 2012;224:303–312. doi: 10.1007/s00213-012-2753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- 12.Wasserman DI, Tan JM, Kim JC, Yeomans JS. Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion, . Eur. J. Neurosci. 2016;44:1761–1770. doi: 10.1111/ejn.13237. [DOI] [PubMed] [Google Scholar]
- 13.Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J. Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 15.Yetnikoff L, Cheng AY, Lavezzi HN, Parsley KP, Zahm DS. Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: A study in rat. J. Comp. Neurol. 2015;523:2426–2456. doi: 10.1002/cne.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]