Inguinal lymphadenectomy, or groin dissection, has a key role in the management of patients with penile, vulval, anal, and cutaneous malignancy. About 500 procedures are performed in the United Kingdom each year by general, gynaecological, plastic, and urological surgeons. Groin dissection is associated with high postoperative morbidity, chiefly related to wound healing and lymphoedema. As the preoperative diagnosis and postoperative care of these patients may also involve general practitioners, oncologists, dermatologists, and specialist nurses, this review is aimed at providing a concise yet comprehensive summary of the key aspects of managing inguinal lymph nodes.
Methods
We searched the Cochrane Library and Medline online databases, using the terms “inguinal lymphadenectomy”, “groin dissection”, and “sentinel lymph node biopsy”, combined with “melanoma”, or “carcinoma” and either “vulva”, “penis”, or “anus”. We reviewed abstracts and selected relevant articles.
Pathology
Tumours of the male genital tract
Squamous cell carcinoma is the most common tumour of the penis (table 1, accounting for 95% of primary penile malignancies.w1 Relatively uncommon in developed countries, it accounts for up to 17% of all male malignancies in developing countries.1,2 Penile malignancy affects about 800 men per annum in the United Kingdom.1 The mean age of affected individuals is 64.1 Palpable inguinal lymphadenopathy at presentation may represent metastatic disease or secondary inflammation, so a four to six week course of oral antibiotics is usually prescribed, followed by re-evaluation of the lymphadenopathy. However, studies have shown that up to 20% of patients with no palpable lymphadenopathy will have nodal metastasis.w2
Table 1.
Pathology of tumours commonly metastasising to the inguinal lymph nodes
| Organ system | Tumour |
|---|---|
| Skin | Malignant melanoma |
| Squamous cell carcinoma |
|
| Female genital tract |
Squamous cell carcinoma of vulva |
| Male genital tract |
Squamous cell carcinoma of penis |
| Gastrointestinal tract | Squamous cell carcinoma of anus |
Tumours of the female genital tract
Tumours arising from the vulva and lower third of the vagina metastasise to the inguinal lymph nodes. Vaginal tumours are rare and will not be considered further here. Squamous cell carcinoma is the most common tumour of the vulva (table 1), and 1996 cases of vulval cancer were diagnosed in England in 2003.1 Incidence increases with increasing age. The presence of lymph node metastasis is related to the size and depth of invasion of the primary tumour.3
Summary points
Inguinal lymphadenectomy is a common operation performed by general, gynaecological, plastic, and urological surgeons
The anatomy of the inguinal lymphatics informs a logical approach to the examination of the region that can readily be taught to patients at risk of inguinal metastasis from a range of cancers
Meticulous surgical technique is required to reduce postoperative complications
Complications of inguinal lymphadenectomy are common, and trials are currently seeking ways of reducing morbidity associated with the operation
Sentinel lymph node biopsy is a promising technique which may in future refine the indications for inguinal lymphadenectomy, provide better prognostic information, and reduce morbidity
Cutaneous tumours
Malignant tumours of the skin, most commonly malignant melanoma or squamous cell carcinoma arising on the legs and trunk, may metastasise to the inguinal lymph nodes.
In 2003, 9325 cases of malignant melanoma were diagnosed in England, an increase of 120% since 1992, when 4151 cases were reported.1 Women are affected more commonly than men. The incidence increases markedly over age 30 and peaks in the sixth decade. Tumour thickness is correlated with the probability of lymph node involvement.4
Squamous cell carcinoma is more common than malignant melanoma and has a similar epidemiology and aetiology.1 The leg is an uncommon location for squamous cell carcinoma; it may occur here particularly, however, when associated with immunosupression or chronic ulceration (Marjolin's ulcer). Tumour thickness is again correlated with the probability of lymph node involvement.
Tumours of the gastrointestinal tract
Squamous cell carcinoma of the anal canal is the most common gastrointestinal tumour to metastasise to the inguinal lymph nodes. It is a relatively uncommon tumour, with an incidence in England of 2712 in 2003. Women are affected more often than men.1 Inguinal node metastasis is a poor prognostic indicator, and such patients should be entered into clinical trials of treatment modalities wherever possible in order to determine optimal treatment.
Clinically relevant anatomy
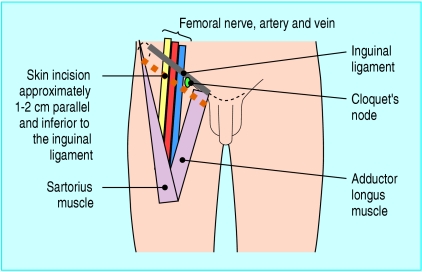
The lymphatic system is a physiological continuum, yet the inguinal lymph nodes are traditionally divided into two anatomical groups. The superficial inguinal nodes are found superficial to the fascia lata within the boundaries of the femoral triangle (fig 1). They receive afferent superficial lymphatics from the lower extremity, the scrotum, penis, vulva, clitoris, anus, and the infra-umbilical region of the anterior abdominal wall. The femoral triangle is bounded superiorly by the inguinal ligament, medially by adductor longus, and laterally by sartorius. The roof of the femoral triangle is formed by fascia lata, and the floor is comprised of the iliopsoas and pectineus muscles and contains the femoral neurovascular structures as they pass beneath the inguinal ligament.
Fig 1.
Representation of the right groin, showing the landmarks of the femoral triangle
Deep to the fascia lata, medial to the femoral vein, reside six to eight deep inguinal nodes, including Cloquet's node, which is sited at the apex of the femoral canal. The deep nodes receive afferents from the superficial inguinal nodes and the deep lymphatic trunks associated with the femoral vessels, which in turn drain the popliteal nodes. The deep inguinal nodes drain into the external iliac nodes, which also receive direct afferents from the superficial inguinal group.
Clinical assessment
After a comprehensive medical history has been obtained, the patient should be thoroughly clinically examined, including the site of the primary tumour. For examination of the inguinal nodes, the patient should be positioned supine and appropriately exposed from umbilicus to the mid thigh. Both femoral triangles should be systematically palpated firmly but gently, to detect any underlying lymphadenopathy; the affected lymph nodes often have a firm, bean-like consistency. Patients at risk of developing inguinal metastases can be taught to use this technique to examine themselves.
General examination to exclude generalised lymphadenopathy or metastatic disease should then be undertaken. It is relatively uncommon to have an unknown primary lesion manifest as a palpable metastatic inguinal node.5 Detection of palpable lymphadenopathy in a patient with possible malignancy should trigger an urgent referral to an appropriate specialist.
Investigations
Outpatient pathological sampling of a palpable lymph node is performed by fine needle aspiration cytology. In the context of melanoma, adequate sampling is achieved in 89% of aspirations, with a subsequent sensitivity and specificity approaching 100%.6 Open biopsy of enlarged lymph nodes should be undertaken by specialist surgeons only, as an inappropriately placed incision may compromise subsequent surgery. Computed tomography or magnetic resonance imaging are undertaken to stage the disease accurately.
Prophylactic versus therapeutic inguinal lymphadenectomy
For penile and vulval squamous cell carcinoma, prophylactic inguinal lymphadenectomy has been recommended.2,7 A prophylactic inguinal lymphadenectomy aims to remove clinically undetectable micrometastases in order to prevent further dissemination of disease. The benefits of this approach must be weighed against the morbidity of the procedure. Therapeutic inguinal lymphadenectomy is undertaken when lymph node involvement is confirmed pathologically.
Surgical management of vulval squamous cell carcinoma comprises resection of the primary tumour and bilateral inguinal lymphadenectomy. In patients who are unfit to have radical surgery, radiotherapy may be administered to the inguinal lymph nodes. This results in less morbidity but a higher incidence of groin recurrence and poorer survival than surgery.7
In penile squamous cell carcinoma, timing of the inguinal lymphadenectomy is controversial. Proponents of prophylactic lymphadenectomy note that patients without clinical lymphadenopathy have a 20% rate of occult lymph node metastasis.w2 In contrast, proponents of therapeutic lymphadenectomy have proposed that close observation with bimonthly clinical examination for three years allows detection of disease and subsequent lymphadenectomy at a curable stage.w3 In patients with a unilateral recurrence bilateral inguinal lymphadenectomy should be undertaken, as the incidence of occult contralateral metastases is high owing to crossover lymphatics at the base of the penis.w3
Prophylactic lymphadenectomy in melanoma has not been shown to improve survival, although the procedure may be beneficial in certain population subgroups.8,9 The UK guidelines for the management of cutaneous melanoma do not currently support routine prophylactic lymphadenectomy.10
In anal squamous cell carcinoma, inguinal lymphadenectomy is generally reserved for those patients with clinically evident disease in the inguinal nodes after the course of chemoradiotherapy has been completed.w4
Sentinel lymph node biopsy
As a potential solution to the high morbidity associated with prophylactic inguinal lymphadenectomy, sentinel lymph node biopsy can be performed in order to identify those patients with micrometastatic nodal disease, therefore avoiding major surgery in patients without metastatic disease.
Theoretically, lymphatics from defined areas of the body follow a predetermined pattern of drainage, and consistently drain to a “sentinel” lymph node(s) in a nodal basin. The sentinel node will therefore be the first to contain metastatic tumour cells. If tumour cells are absent from the sentinel node, the remainder of the nodal basin is assumed to be tumour free.
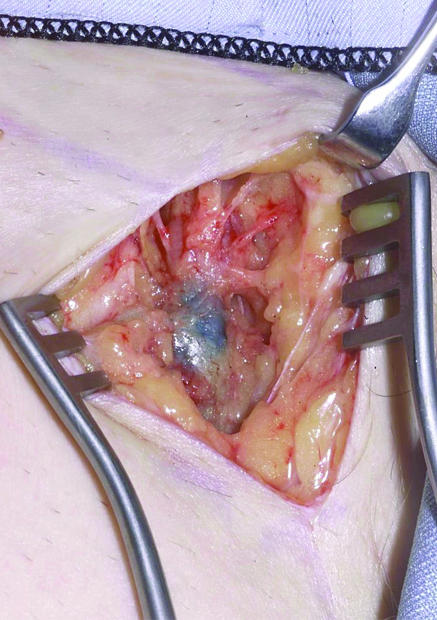
On the day of surgery, technetium-99m radiolabelled nanocolloid (which has a half life of six hours) is injected at the site of the primary tumour or excision biopsy scar, and a lymphoscintogram is obtained that details the site and number of sentinel lymph nodes. At operation, blue dye is injected around the primary tumour or excision biopsy scar. The incision is made over the radioactive “hot spot” detected by the handheld gamma camera. The location of the sentinel node in the groin is determined by using the camera and by visualisation of blue dye in the node (fig 2). When this combined approach is used, 98% of sentinel nodes are successfully identified in our department—which corresponds favourably with the published literature.11
Fig 2.
Intraoperative appearance of a left groin sentinel lymph node stained with blue dye
Sentinel lymph node biopsy has been studied most extensively in the context of malignant melanoma.12 The American Joint Committee on Cancer staging system for melanoma includes micrometastasis in the regional lymph nodes—sentinel lymph node biopsy positive.13 A recent report proposes a survival advantage for patients having lymphadenectomy for micrometastatic disease.14 However, the results of prospective randomised controlled trials dealing with this important issue are currently under way and are due to report in the near future.
Sentinel lymph node biopsy is now also being used to provide accurate staging information in other solid tumours, including squamous cell carcinoma of the vulva, penis, and anus.15 Experience of sentinel lymph node biopsy in these cancers is at an early stage, and large, multicentre, randomised controlled trials are required to define the role of the procedure in these and other solid tumours.16 w5 w6 However, sentinel lymph node biopsy may render prophylactic lymphadenectomy obsolete.
Surgical approach
First described by Basset in 1912, the aim of the surgical procedure is to remove en bloc the deep and superficial lymph nodes of the inguinal region.17 Additionally, an ilioinguinal, or radical, groin dissection may be performed, where the inguinal, iliac, and obturator nodes are removed in continuity.18
The skin incision is made parallel to the inguinal ligament, and thick skin flaps are raised so as to cause minimal disruption to the subcutaneous vascular plexus and hence reduce the risk of skin flap necrosis and consequent postoperative wound breakdown.19,20 This straight oblique incision is superior to the straight vertical and S-shaped (“hockey stick”) incisions, which are associated with an increased rate of postoperative complications.17,21
Superficial inguinal lymph nodes in the previously described distribution are removed en bloc. Traditionally, the long saphenous vein is sacrificed, although recent evidence shows that its preservation may reduce the risk of postoperative lymphoedema without compromising the risk of local recurrence.22 Exposure of areolar tissue in the proximity of the femoral vessels deep to fascia lata allows access to the deep inguinal nodes.
Before wound closure, a sartorius transposition is usually performed—the muscle is divided at its proximal insertion, and the muscle belly is reflected medially and secured to the inguinal ligament, hence protecting the underlying femoral vessels in the event of a postoperative wound breakdown.23 After meticulous haemostasis has been obtained and a suction drain placed, the skin is closed in a tension free manner.
A preoperative opinion from a plastic surgeon is warranted if problems with primary wound closure are anticipated. Examples of specific indications for plastic surgery referral include previous groin surgery, lymph nodes fixed to skin, or postoperative skin flap necrosis. Soft tissue coverage may be achieved by means of a musculocutaneous flap.
Postoperative course
No robust evidence exists for the optimal period for maintaining post-operative suction drainage. Some authorities advocate early drain removal at 24 hours after surgery, whereas others recommend removal once drainage falls beneath a specific threshold (30-50 ml over 24 hours)—which may take some weeks. In England, the mean inpatient stay is 12.8 days, although many surgeons advocate early discharge, often with suction drains in situ.1 Early ambulation is encouraged to minimise the risk of deep vein thrombosis, although mobilisation accelerates lymph flow from the lower extremity and may augment lymph drainage. Patients are unlikely to be fit to drive for at least four to six weeks after surgery.
Additional educational resources
Roberts DLL, Anstey AV, Barlow RJ, Cox NH, Newton Bishop JA, Corrie PG, et al. UK guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146: 7-17—guidelines for the management of malignant melanoma
www.netanatomy.com—an excellent free anatomy website which covers gross anatomy, radiographic anatomy, and cross sectional anatomy. The images are first class, and the format allows self testing
www.emedicine.com—this US based website provides up to date, peer reviewed information on topics across the whole of medicine including informative reviews on malignant melanoma, penile cancer, and vulval cancer
Information for patients
www.cancerhelp.org.uk—CancerHelp UK is a free information service about cancer and cancer care for people with cancer and their families provided by Cancer Research UK. The philosophy is that information about cancer should be freely available to all and written in a way that is easily understood. There are sections on individual cancers, treatments, and links to support organisations. The site is approved by the Plain English Campaign
http://cancerresearchuk.org/sunsmart—this website has activity ideas to help children learn about the sun, information on skin cancer, and practical tips on preventing sun damage
Ongoing research
www.cancerbacup.org.uk/cgi-bin/clinicaltrials/searchtrials.pl?d=26&t=&s=a&c=10&Submit=±search±—summary of ongoing trials of melanoma treatment, including further investigation into the role of sentinel lymph node biopsy
www.ncrn.org.uk/portfolio/summary.asp?DiseaseID=45&Status=34&type=0&GroupID=6—details of a trial evaluating chemotherapy for the treatment of locally advanced or metastatic or recurrent vulva cancer
www.cancerbacup.org.uk/cgi-bin/clinicaltrials/searchtrials.pl?d=1—information on current trials investigating the role of chemotherapy and radiation therapy in patients with anal cancer
Postoperative complications
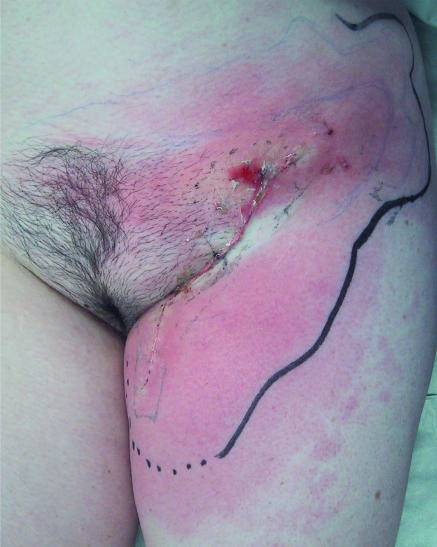
Although rarely fatal, postoperative complications after inguinal surgery are extremely debilitating and harbour considerable socioeconomic costs (table 2).24 Wound infection (fig 3) or dehiscence is more likely in elderly or obese patients.25 In addition, smoking, poor nutrition, and treatment with immunosuppressant drugs represent independent risk factors for impaired wound healing.
Table 2.
Postoperative complications after inguinal lymphadenectomy
| Complication | Rate of occurrence (%) | References |
|---|---|---|
| Seroma or lymphocele |
6-40 |
Tonouchi et al, 200421; Paley et al, 199723; Urist et al, 198325;w11 w12† w13 |
| Haematoma |
2-4 |
Tonouchi et al, 200421; w7 w13 |
| Wound dehiscence |
17-65 |
Paley et al, 199723; w7 w11 w14 |
| Wound infection |
6-20 |
Urist et al, 198325; w7 w12† w13 w15 |
| Lymphoedema | 22-80 | Tonouchi et al, 200421; Paley et al, 199723; w12† w16† |
For ilioinguinal lymphadenectomy.
Fig 3.
Two weeks after left inguinal lymphadenectomy: groin abscess with cellulitis (limit marked with black pen on admission) requiring surgical drainage and intravenous antibiotic therapy
Other common complications include seroma formation (a collection of serous tissue fluid), development of lymphocele (a collection of lymphatic fluid), and lymphoedema of the lower limb. Wound cellulitis requiring readmission for treatment with intravenous antibiotics is not uncommon, and occasionally an abscess requires drainage under general anaesthesia.
The rate of lymphocele formation is reduced by meticulous suture or clip ligation of divided lymphatics and use of suction drainage.w7 Lymphoceles or seromata may be treated in the outpatient clinic by regular percutaneous aspiration, or the instillation of sclerosants such as povidone iodine, talcum powder, or doxycycline.w8 w9 w10
Conclusions
Surgical management of inguinal lymph nodes forms a key element in the treatment algorithm for several malignancies. Clinicians should be aware of the indications for surgery, the high postoperative morbidity, and the need for further randomised controlled trials to ascertain the role of sentinel lymph node biopsy in the management of nodal disease.
Supplementary Material
 Additional references w1-w16 are on bmj.com
Additional references w1-w16 are on bmj.com
Acknowledgements: We thank the patients who consented to involvement with this review, and Nick White from Oxford Medical Illustration for his assistance with the clinical photographs.
Contributors: MCS and DF wrote the commentary, which was critically revised by OCSC. OCSC is guarantor.
Funding: None.
Competing interests: MCS and OCSC are investigators for the Oxford Tisseel (Baxter Healthcare, Newbury, United Kingdom) trial; a prospective randomised controlled trial to determine whether fibrin sealant can reduce post-operative complications following axillary and inguinal lymphadenectomy. They receive no financial remuneration for their work.
Ethical approval: None required.
References
- 1.Office for National Statistics. Cancer registration statistics, England 2003. London: ONS, 2003.
- 2.Ornellas AA, Seixas AL, Marota A, Wisnescky A, Campos F, de Moraes JR. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J Urol 1994;151: 1244-9. [DOI] [PubMed] [Google Scholar]
- 3.Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994-1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet 1999;65: 243-9. [DOI] [PubMed] [Google Scholar]
- 4.Roses DF, Harris MN, Hidalgo D, Valensi QJ, Dubin N. Primary melanoma thickness correlated with regional lymph node metastases. Arch Surg 1982;117: 921-3. [DOI] [PubMed] [Google Scholar]
- 5.Guarischi A, Keane TJ, Elhakim T. Metastatic inguinal nodes from an unknown primary neoplasm. A review of 56 cases. Cancer 1987;59: 572-7. [DOI] [PubMed] [Google Scholar]
- 6.Basler GC, Fader DJ, Yahanda A, Sondak VK, Johnson TM. The utility of fine needle aspiration in the diagnosis of melanoma metastatic to lymph nodes. J Am Acad Dermatol 1997;36: 403-8. [DOI] [PubMed] [Google Scholar]
- 7.Van der Velden J, Ansink A. Primary groin irradiation vs primary groin surgery for early vulvar cancer. Cochrane Library, Issue 2, 2004. Chichester: John Wiley.
- 8.Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet 1998;351: 793-6. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Soong SJ, Bartolucci AA, Urist MM, Karakousis CP, Smith TJ, et al. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg 1996;224: 255-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DL, Anstey AV, Barlow RJ, Cox NH, Newton Bishop JA, Corrie PG, et al. U.K. guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146: 7-17. [DOI] [PubMed] [Google Scholar]
- 11.Hettiaratchy SP, Kang N, O'Toole G, Allan R, Cook MG, Powell BWEM. Sentinel lymph node biopsy in malignant melanoma: a series of 100 consecutive cases. Br J Plast Surg 2000;53: 559-62. [DOI] [PubMed] [Google Scholar]
- 12.Cascinelli N, Belli F, Santinami M, Fait V, Testori A, Ruka W, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO melanoma program experience. Ann Surg Oncol 2000;7: 469-74. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19: 3635-48. [DOI] [PubMed] [Google Scholar]
- 14.Kretschmer L, Hilgers R, Mohrle M, Balda BR, Breuniger H, Konz B, et al. Patients with lymphatic metastasis of cutaneous malignant melanoma benefit from sentinel lymphanodectomy and early excision of their nodal disease. Eur J Cancer 2004;40: 212-8. [DOI] [PubMed] [Google Scholar]
- 15.Gipponi M, Solari N, Di Somma FC, Bertoglio S, Cafiero F. New fields of application of the sentinel lymph node biopsy in the pathologic staging of solid neoplasms: review of the literature and surgical perspectives. J Surg Oncol 2004;85: 171-9. [DOI] [PubMed] [Google Scholar]
- 16.Moore RG, DePasquale SE, Steinhoff MM, Gajewski W, Steller M, Noto R, et al. Sentinel node identification and the ability to detect metastatic tumour to inguinal lymph nodes in squamous cell cancer of the vulva. Gynaecol Oncol 2003;89: 475-9. [DOI] [PubMed] [Google Scholar]
- 17.Spratt J. Groin dissection. J Surg Oncol 2000;73: 243-62 [DOI] [PubMed] [Google Scholar]
- 18.Karakousis CP. Ilioinguinal lymph node dissection. Am J Surg 1983;141: 299-303. [DOI] [PubMed] [Google Scholar]
- 19.Baronofsky ID. Technique of inguinal node dissection. Surgery 1948;24: 555-67. [PubMed] [Google Scholar]
- 20.Woodhall JP. Radical groin surgery with particular reference to postoperative healing. Surgery 1953;33: 886-95. [PubMed] [Google Scholar]
- 21.Tonouchi H, Ohmori Y, Kobayashi M, Konishi N, Tanaka K, Mohri Y, et al. Operative morbidity associated with groin dissections. Surg Today 2004;34: 413-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SH, Sood AK, Sorosky JI, Anderson B, Buller RE. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer 2000;89: 1520-5. [PubMed] [Google Scholar]
- 23.Paley PJ, Johnson PR, Adcock LL, Cosin JA, Chen MD, Fowler JM, et al. The effect of sartorius transposition on wound morbidity following inguinal-femoral lymphadenectomy. Gynecol Oncol 1997;64: 237-41. [DOI] [PubMed] [Google Scholar]
- 24.Bland KI, Klamer TW, Polk HC Jr, Knutson CO. Isolated regional lymph node dissection: morbidity, mortality and economic considerations. Ann Surg 1981;193: 372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urist MM, Maddox WA, Kennedy JE, Balch CM. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer 1983;51: 2152-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.