Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by chronic obstruction of major pulmonary arteries by organized thromboembolic material. Untreated CTEPH can result in pulmonary hypertension and eventually right heart failure, yet it is the only form of pulmonary hypertension that is potentially curable with surgical or catheter-based intervention. While early diagnosis is key to increasing the likelihood of successful treatment, CTEPH remains largely underdiagnosed. This article reviews the role of echocardiogram, ventilation/perfusion scan, and other available modalities in the diagnosis of CTEPH.
Keywords: pulmonary hypertension, chronic thromboemboli, pulmonary hypertension, pulmonary angiography, ventilation/perfusion scan
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by occlusion of pulmonary arteries by organized thrombi. This causes an increase in pulmonary vascular resistance and pulmonary artery pressure (PAP) that eventually leads to pulmonary hypertension (PH), which is defined as mean PAP ≥ 25 mm Hg and pulmonary artery wedge pressure ≤ 15 mm Hg. When left untreated, severe CTEPH can lead to right heart failure as a result of progressive right ventricular dysfunction. Additionally, inoperable severe CTEPH has been associated with an approximate 5-year survival of 30%. 1 Early diagnosis is therefore paramount in these patients.
CTEPH is defined as PH in the presence of mismatched perfusion defects on ventilation/perfusion (V/Q) scan and signs of thromboemboli on computed tomography pulmonary angiogram (CTPA), and/or conventional pulmonary angiography, in a patient who has received at least 3 months of therapeutic anticoagulation.2,3 The detection of CTEPH warrants various diagnostic studies, including pulmonary function tests, V/Q scan, transthoracic echocardiogram (TTE), right heart catheterization, and pulmonary angiography. The use of all these modalities in the diagnosis of CTEPH is discussed in this article.
Pulmonary Function Testing in Chronic Thromboembolic Pulmonary Hypertension
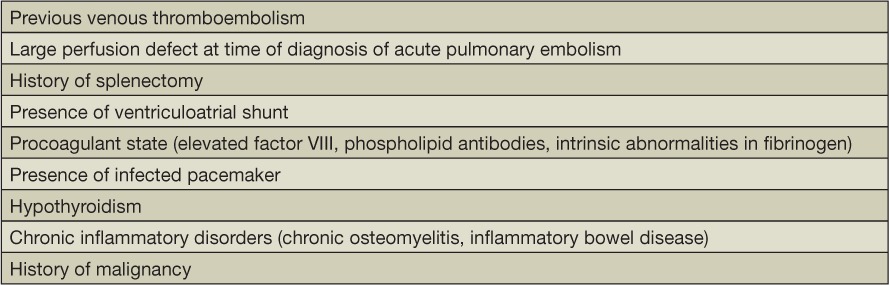
Patients who are diagnosed with CTEPH initially present with nonspecific symptoms that tend to overlap with those of diseases such as chronic obstructive pulmonary disease, asthma, or deconditioning. Hence, to rule out air flow obstruction or other parenchymal lung diseases that can mimic CTEPH, the first step in the diagnosis should include tests such as chest radiography and pulmonary function test (PFT). In CTEPH the PFTs are by and large unremarkable. However, in a few patients who have concurrent parenchymal scarring, the results obtained can demonstrate a mild restrictive pattern and/or reduced diffusing capacity of the lungs for carbon monoxide.4 Therefore, in the presence of relevant risk factors (Table 1), once preliminary testing as described above has ruled out other causes of presenting symptoms, evaluation for CTEPH can then be initiated as detailed below.
Table 1.
Risk factors for chronic thromboembolic pulmonary hypertension.
Echocardiogram in Chronic Thromboembolic Pulmonary Hypertension
Echocardiogram is currently the best screening tool for PH. While an echocardiogram is not specific in diagnosing CTEPH, it can indicate the presence of PH. Furthermore, it can help exclude other causes of PH such as intracardiac shunt and left heart disease, thereby increasing the likelihood of CTEPH being the cause of PH.
Features on an echocardiogram suggestive of PH include an estimated pulmonary artery systolic pressure > 25 mm Hg, dilated right ventricle and atrium, tricuspid velocity > 2.8 m/s,2 tricuspid regurgitation, and flattening or paradoxical motion of the interventricular septum with normal left ventricular function.
Echocardiogram in these patients can also serve as an important tool to gauge RV function; RV function is a proven prognostic factor in patients with PH.5 However, given its oblique contraction and complex geometry, direct assessment of RV function can be difficult. For this reason, various surrogates of RV function on an echocardiogram such as tricuspid annular plane systolic excursion (TAPSE) and Tei index have been validated. TAPSE is a measure of the longitudinal displacement of the lateral tricuspid annulus and has been correlated with right ventricular ejection fraction (RVEF) determined by radionuclide angiography.6 TAPSE can also be followed to objectively determine improvement in patients with CTEPH who undergo endarterectomy.7 On the other hand, Tei, which is the myocardial performance index, assesses global myocardial function. As its measurement relies on Doppler echocardiography and is thus independent of geometric shape and size, it is an excellent means of assessing RV function and has been shown to correlate with mPAP, response to therapy, and clinical status of patients with CTEPH.8
Hence, patients who present with symptoms suspicious for PH such as persistent dyspnea, fatigue, exercise intolerance, and especially risk factors for CTEPH should undergo TTE screening. It should be noted that a prior diagnosis of venous thromboembolism (VTE) carries low sensitivity, as many patients diagnosed with CTEPH often never experience a documented pulmonary embolism (PE) or deep vein thrombosis (DVT).9,10 In fact, European Respiratory Society (ERS) and European Society of Cardiology (ESC) guidelines do not recommend that all patients undergo screening after an episode of DVT or PE. Instead, they recommend a follow-up echocardiogram in 3 to 6 months in patients who demonstrate PH or right ventricular dysfunction at the time VTE is diagnosed. Following diagnosis of PH, patients with no other explainable cause should then undergo further diagnostic workup for CTEPH.
Role of Ventilation/Perfusion Scintigraphy in Chronic Thromboembolic Pulmonary Hypertension
According to the European Association of Nuclear Medicine and 2009 European Guidelines on the diagnosis and treatment of PH, any patient with unexplained PH on an echocardiogram, especially with a history of PE or DVT, should undergo a V/Q scan for CTEPH.11,12 Contrary to the approach taken to diagnose acute PE, a V/Q scan is recommended over a CTPA in diagnosing CTEPH.13 In a single-center retrospective study comparing V/Q scan with CTPA, Tunaria et al. demonstrated that the V/Q scan was more sensitive (97.4% vs 51%) in diagnosing chronic thromboemboli when compared to CTPA.13
Along with providing increased sensitivity, V/Q scan also allows one to distinguish CTEPH from other forms of PH. V/Q scans of patients with CTEPH tend to be remarkable for one or more segmental or larger mismatched perfusion defects (Figure 2) while other forms of PH—such as PAH, which involves smaller vessels—either have normal scans or demonstrate subsegmental defects (i.e., “mottled” perfusion scans).14,15 Compared to CTPA, V/Q scans use less radiation, avoid complications secondary to administration of intravenous contrast, and are more cost effective.16 In addition, changes associated with CTEPH tend to be more subtle on CTPA and therefore require rare technical expertise and experience to detect these findings, further favoring V/Q scan as a screening modality for CTEPH.
Figure 2.
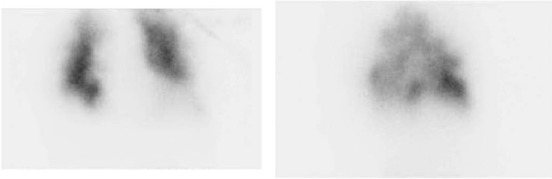
Ventilation/perfusion scans with marked heterogeneity of lung perfusion bilaterally, highly suspicious of CTEPH.
Figure 1.
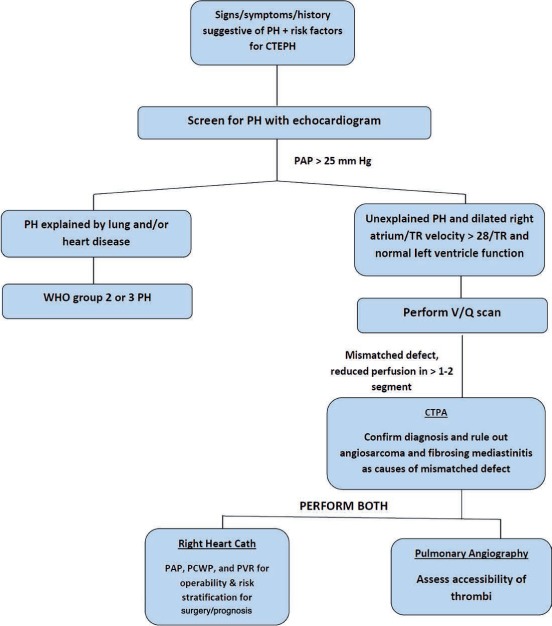
Diagnostic algorithm for chronic thromboembolic pulmonary hypertension. PH: pulmonary hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; TR: tricuspid regurgitation; WHO: World Health Organization; V/Q: ventilation/perfusion; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; CTPA: computed tomography pulmonary angiogram.
Despite several advantages, however, V/Q scans are not specific for CTEPH. For this reason, some experts argue that a “low probability” V/Q scan cannot suffice as a normal result in patients with a high suspicion for CTEPH. This is mainly attributed to false negative results seen in such patients due to the recanalization of chronic thrombi.17 Recanalization refers to the formation of channels either through or around these obstructive thrombi, allowing the administered agent to reach the periphery of the lung, thereby making them appear normal.18 In contrast, false positive results can be seen on V/Q scan in pathologies such as pulmonary artery sarcoma,19 fibrosing mediastinitis,20 and extrinsic compression of the pulmonary vasculature, as they too produce large segmental perfusion defects.
Given their high sensitivity, however, V/Q scans are still preferred when screening patients with PH for CTEPH. A normal or low-probability V/Q scan can effectively rule out CTEPH while a high-probability and indeterminate scan requires further diagnostic workup.
Role of Computed Tomography Pulmonary Angiogram in CTEPH
Computed tomography pulmonary angiogram plays an important role during the initial evaluation of a patient suspected of having CTEPH. Any patient with perfusion defects or ambivalent results on V/Q scan should undergo a CTPA.21 Findings on CTPA consistent with chronic thromboemboli include intraluminal webs or bands, abrupt vessel narrowing, and complete and partial vascular obstruction by organized lining thrombi that mimic thickening of the vascular wall. Mosaic attenuation and lung parenchymal changes such as linear or wedge-shaped opacities due to infarction can further help to strengthen the diagnosis of CTEPH (Figure 3). These findings assist not only in confirming the diagnosis of CTEPH but also in assessing the anatomical extent of the disease, i.e., whether it is main branch, lobar, or subsegmental in nature. This in turn allows clinicians to make a decision regarding suitability of interventions such as pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), or medical treatment.20,22–25 Of note, multidetector CTPA has been deemed essential in performing the aforementioned function. Studies have proven this form of CTPA to be equivalent and at times even superior to conventional pulmonary angiography for detecting central clot burden and segmental and subsegmental vessel occlusion.22,26,27 Additionally, enlarged bronchial arteries and collateral arteries from systemic circulation may also be seen on the CTPA of a patient with CTEPH and can further help to risk stratify patients undergoing PEA surgery.25,26,28–35
Figure 3.
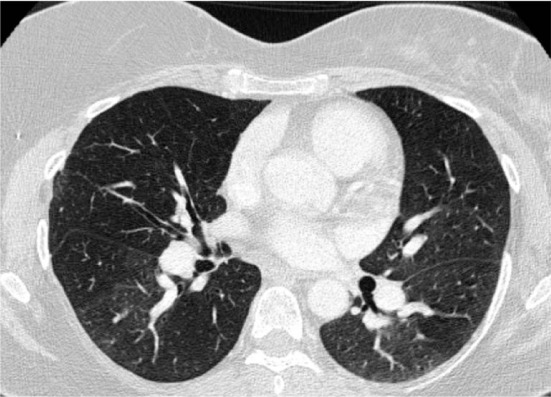
Chest CT angiogram demonstrating marked mosaic attenuation in bilateral lung fields as well as pruning of distal pulmonary arteries and enlargement of proximal pulmonary arteries.
In a case series comparing CTPA to pulmonary angiography (the gold standard for diagnosing chronic thromboemboli), it was found that both imaging modalities were equally effective in the determination of disease amenable to resection.26 Despite such findings, evaluation of patients for CTEPH still entails undergoing a pulmonary angiography. This is because a normal CTPA does not exclude the possibility of CTEPH.21 In a recent meta-analysis exploring the use of CTPA in diagnosing CTEPH, adequate sensitivity and specificity were observed only when the disease was limited to the proximal vessels, i.e., the main and lobar pulmonary arteries.36 Thus, regardless of recent innovations such as the 320-slice CT, one of the biggest disadvantages of using CTPA to diagnose chronic thromboemboli is that it may not detect clots presenting within the distal segmental or subsegmental vessels.37 However, a more promising development is the availability of dual-energy CT that is showing the potential for increased sensitivity in detecting distal clots.36
Role of Conventional Pulmonary Angiography
Before the availability of CTPA, pulmonary angiography was the mainstay of confirming a diagnosis of CTEPH. Despite advancements such as CTPA and magnetic resonance angiography (MRA), conventional pulmonary angiography to this day remains the gold standard for confirming the diagnosis of chronic thromboemboli.2,38 In addition to confirmation, it helps isolate the sites of the pulmonary vasculature involved and determines their accessibility for surgery. Therefore, it is important to obtain a conventional pulmonary angiogram for anyone with significant findings on V/Q scan, CTPA, and/or MRA.21,39
The procedure for the initial diagnostic pulmonary angiography entails injecting iodinated contrast into the proximal pulmonary vasculature through a catheter in the proximal left or right pulmonary artery. Concurrently, a biplane acquisition is often used as it determines the extent and distribution of the disease in greater detail compared to anterior-posterior and lateral projections individually. Mapping out the extent and site of disease in this manner facilitates the decision for surgery and the procedure of PEA itself.
The findings on angiography in patients with chronic thrombi are a reflection of the remodeling and recanalization that these clots undergo. Therefore, these findings vary considerably from the intraluminal filling defects found on pulmonary angiograms in acute PE. Various patterns have been identified for CTEPH on pulmonary angiograms. The most common include pouch defects, intimal irregularities, abrupt narrowing of the vessel, and pulmonary artery webs/bands.4,40 When two or more are found on an angiogram, CTEPH can be diagnosed with certainty.
Role of Right Heart Catheterization
In making a diagnosis of CTEPH, the final critical step that is imperative for determining the appropriate treatment and patient prognosis is to perform a right heart catheterization (RHC).39 Since obtaining a pulmonary angiogram is vital for diagnosing CTEPH, an RHC can be carried out concurrently by using the same introducer sheath in these patients. During the RHC, various hemodynamic parameters such as PAP, right atrial pressure, PWCP, and right ventricular pressure can be obtained; these along with superior vena cava, PA, and systemic arterial blood oxygen saturations help to calculate PVR, which in turn is used to stratify the severity of PH and secondary cardiac dysfunction. This process of correlating the hemodynamic disturbances with radiographic evidence of the clot burden is essential in deciphering the operability as well as surgical risk before PEA.41 For example, a PVR that is excessively high compared to the level of obstruction indicates the possible presence of additional distal small vessel disease, which is not amenable to surgical resection. Furthermore, a high measured PVR is also associated with both high procedural risk as well as increased general mortality.42 In patients with a PVR > 1,200 dynes·sec·cm−5, the in-hospital and 1-year mortality rates have been reported as high as 11% and 13%, respectively.43 Additionally, the PAP and PVR obtained via RHC prior to PEA can be used for follow-up and patient prognostication after surgery and discharge from the hospital. One study showed that persistent PAP elevation after surgery or residual PH after PEA is the most important predictor of death in patients with CTEPH.44
Role of Magnetic Resonance Imaging in CTEPH
The role of magnetic resonance imaging (MRI) in the diagnosis of CTEPH is evolving. The promise of MRI lies in the fact that it allows diagnosis of both acute and chronic thromboemboli while assessing the presence and severity of PH and right ventricular dysfunction at the same time. However, the major advantage of MRI is that it does all of this without exposing the patient to radiation or nephrotoxic contrast. Additionally, when combined with cine techniques, MRI allows the qualitative and quantitative determination of right and left ventricular function. Common findings on cine MRI in these patients include right ventricular hypertrophy and dilatation, decreased right ventricular ejection fraction without impairment of left ventricular function, and paradoxical interventricular septal wall motion.45,46
In this manner, aforementioned information obtained through MRI in patients with CTEPH can then be used for preoperative and functional assessment of pulmonary circulation as well as the heart. Despite these benefits, use of MRI in the evaluation of patients with suspected CTEPH is still not common practice. For one thing, data regarding the accuracy of MRI in diagnosing CTEPH is conflicting. In one study comparing CTPA with conventional angiography and MRA, the latter was found to be less accurate than both CT and pulmonary angiography in revealing central disease.26 By contrast, another study comparing the use of MRA and digital subtraction angiography (DSA) in patients with CTEPH found both of these imaging modalities to be equally accurate in evaluating for disease in proximal pulmonary vasculature (main, lobar, and segmental pulmonary vessels). However, the same study proved MRA to be inferior to DSA (Figures 4–6) at the subsegmental level.
Figure 4.
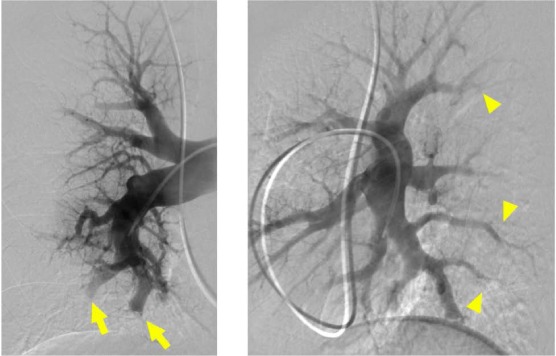
Digital subtraction pulmonary angiography performed in anterior-posterior and lateral views of the right pulmonary artery demonstrating severe subsegmental disease. Multiple sites of abrupt narrowing (arrowhead) and pouch defect and subtotal occlusions (arrow) are visible.
Figure 6.
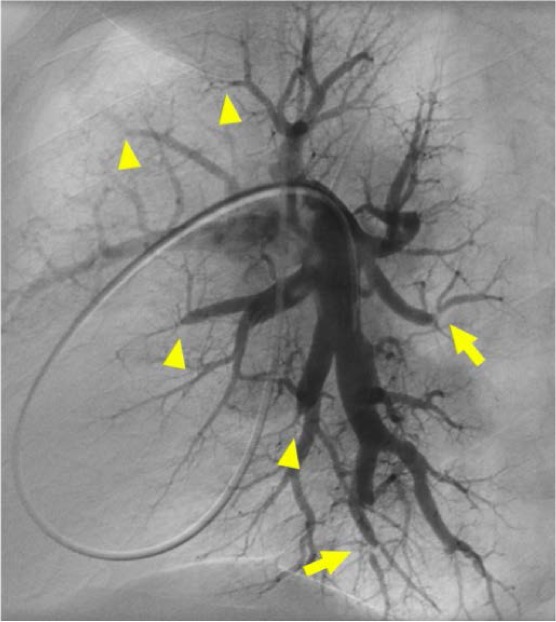
Digital subtraction pulmonary angiography, lateral view of the left pulmonary artery demonstrating severe subsegmental disease. Multiple sites of subtotal and total occlusions (arrowhead) are visible. There are also multiple severe ring lesions (arrow).
Figure 5.
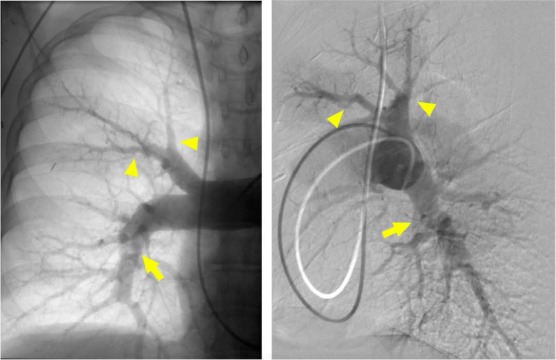
Digital subtraction and native pulmonary angiography performed in anterior-posterior and lateral views of the right pulmonary artery demonstrating severe segmental disease. There are multiple sites of pruning and abrupt narrowing (arrowhead). The hazy band across the right interlobar artery is suggestive of a web lesion (arrow).
In a recent prospective study, 132 patients with unexplained PH who were recruited from the ASPIRE registry underwent detailed diagnostic workup for CTEPH that included 3-dimensional contrast-enhanced lung perfusion MRI, RHC, CTPA, and V/Q scan. The study analyzed the utility of all these imaging modalities in diagnosing chronic clots within the pulmonary vasculature. Lung perfusion MRI was found to have a sensitivity and specificity of 97% and 92%, respectively, making it equally efficacious as CTPA.47
In light of these variable results, the role of both MRI and angiography is still evolving and holds the promise of being a “one-stop shop” in the diagnosis and evaluation of CTEPH.
Functional Assessments of Patients with CTEPH
It is imperative that patients who are diagnosed with CTEPH undergo assessment for functional capacity. Apart from questioning the patients about the subjective limitations brought on by the disease, objective assessment of the same can be achieved by certain validated tools such as 6-minute walk test (6MWT) and cardiopulmonary exercise testing (CPET). The 6MWT is a method of assessing the integrated multisystem response to physical activity in patients with cardiopulmonary diseases. It does so by measuring the distance that the patient can cover in 6 minutes by walking quickly on a hard flat surface. It is not only a fairly simple and quick way of assessment but has also been proven to be reproducible and to correlate with other parameters that reflect the severity of disease in patients with CTEPH.48 Moreover, as demonstrated by Reesink et al., changes in the 6MWT closely parallel the changes in hemodynamic parameters after treatment and interventions such as PEA in CTEPH patients.48
CPET is considered to be a more accurate estimate of the functional capacity of patients with CTEPH, as it is solely a reflection of the cardiopulmonary system. CPET usually entails exercising on a treadmill or cycle ergometer while minute ventilation (VE) and expiratory gas concentrations are measured for oxygen uptake (VO2) and carbon dioxide output (VCO2) on a breath-by-breath basis throughout the respiratory cycle. It is known that CTEPH is associated with decreased ventilator efficiency and this is then reflected by a change in the various parameters measured during CPET. Among these different measurements, the VE/VCO2 slope has been proven to be the best determinant for CTEPH.49 Xi et al. demonstrated this parameter to also be a sensitive indicator for disease monitoring and prognosis.49 Thus, once the diagnosis of CTEPH has been established, CPET or 6MWT can establish baseline functional capacity for patients. Then physicians can monitor any changes secondary to worsening of the underlying disease or improvement brought on by treatment.
Conclusion
The diagnosis of CTEPH should be considered in all patients who present with PH as it is the only form of PH that is curable. The evaluation for CTEPH includes a detailed diagnostic workup that requires sequential acquisition of multiple imaging modalities followed by assessment of the patients' functional and exercise capacity. Furthermore, early diagnosis is important to maximize the potential of successful treatment and minimize the surgical risk for patients who can ultimately undergo PEA.
Key Points
A diagnosis of CTEPH should be considered in patients with unexplained pulmonary hypertension and in those who have evidence of ongoing RV dysfunction on an echocardiogram despite being on anticoagulation for 3 to 6 months after an episode of DVT/PE.
Despite recent developments that have resulted in the use of CTPA and MRA in established centers, V/Q scan and pulmonary angiography still remain the best screening tool and gold standard for diagnosing CTEPH respectively.
Right heart catheterization can determine operability and procedural risk for pulmonary endarterectomy as well as overall prognostication for the patient.
Conflict of Interest Disclosure
The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Nishimura R, Tanabe N, Sugiura T, . et al. Improved survival in medically treated chronic thromboembolic pulmonary hypertension. Circ J. 2013; 77( 8): 2110– 7. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Hoeper MM, Humbert M, . et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009; 30: 2493– 2537. [DOI] [PubMed] [Google Scholar]
- 3. Guidelines for the diagnosis and treatment of pulmonary hypertension. Task force for diagnosis and treatment of pulmonary hypertension of European Society of Cardiology (ESC) European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Galiè N, Hoeper MM, Humbert M, . et al. Eur Respir J. 2009. December; 34( 6): 1219– 63. [DOI] [PubMed] [Google Scholar]
- 4. Auger WR, Kerr KM, Kim NH, Fedullo PF.. Evaluation of patients with chronic thromboembolic pulmonary hypertension for pulmonary endarterectomy. Pulm Circ. 2012. Apr-Jun; 2( 2): 155– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chin KM, Nick HS, Rubin LJ.. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005. February; 16( 1): 13– 8. [DOI] [PubMed] [Google Scholar]
- 6. Kaul S, Tei C, Hopkins JM, Shah PM.. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984. March; 107( 3): 526– 31. [DOI] [PubMed] [Google Scholar]
- 7. Li YD, Zhai ZG, Wu YF, . et al. Improvement of right ventricular dysfunction after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of echocardiography to demonstrate restoration of the right ventricle during 2-year follow-up. Thromb Res. 2013. February 27; 131( 5): e196– e201. [DOI] [PubMed] [Google Scholar]
- 8. Blanchard DG, Malouf PJ, Gurudevan SV, . et al. Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. JACC Cardiovasc Imaging. 2009. February; 2( 2): 143– 9. [DOI] [PubMed] [Google Scholar]
- 9. Bonderman D, Jakowitsch J, Adlbrecht C, . et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005. March; 93( 3): 512– 6. [DOI] [PubMed] [Google Scholar]
- 10. Dartevelle P, Fadel E, Mussot S, . et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004. April; 23( 4): 637– 48. [DOI] [PubMed] [Google Scholar]
- 11. Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B; EANM Committee. . EANM guidelines for ventilation/perfusion scintigraphy: Part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. Eur J Nucl Med Mol Imaging. 2009. August; 36( 8): 1356– 70. [DOI] [PubMed] [Google Scholar]
- 12. Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B.. EANM guidelines for ventilation/perfusion scintigraphy: Part 2. Algorithms and clinical considerations for diagnosis of pulmonary emboli with V/P(SPECT) and MDCT. Eur J Nucl Med Mol Imaging. 2009. September; 36( 9): 1528– 38. [DOI] [PubMed] [Google Scholar]
- 13. Tunariu N, Gibbs SJ, Win Z, . et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007. May; 48( 5): 680– 4. [DOI] [PubMed] [Google Scholar]
- 14. Lisbona R, Kreisman H, Novales-Diaz J, Derbekyan V.. Perfusion lung scanning: differentiation of primary from thromboembolic pulmonary hypertension. AJR Am J Roentgenol. 1985. January; 144( 1): 27– 30. [DOI] [PubMed] [Google Scholar]
- 15. Powe JE, Palevsky HI, McCarthy KE, Alavi A.. Pulmonary arterial hypertension: value of perfusion scintigraphy. Radiology. 1987. September; 164( 3): 727– 30. [DOI] [PubMed] [Google Scholar]
- 16. Freeman JM. Don't bury the V/Q scan: It's as good as multidetector CT angiograms with a lot less radiation exposure. J Nucl Med. 2007. December 12; 49( 1): 5– 8. [DOI] [PubMed] [Google Scholar]
- 17. Ryan KL, Fedullo PF, Davis GB, Vasquez TE, Moser KM.. Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest. 1988. June; 93( 6): 1180– 5. [DOI] [PubMed] [Google Scholar]
- 18. Bailey CL, Channick RN, Auger WR, . et al. “High probability” perfusion lung scans in pulmonary venoocclusive disease. Am J Respir Crit Care Med. 2000. November; 162( 5): 1974– 8. [DOI] [PubMed] [Google Scholar]
- 19. Kerr KM. Pulmonary artery sarcoma masquerading as chronic thromboembolic pulmonary hypertension. Nat Clin Pract Cardiovasc Med. 2005. February; 2( 2): 108– 12. [DOI] [PubMed] [Google Scholar]
- 20. Rossi SE, McAdams HP, Rosado-de-Christenson ML, Franks TJ, Galvin JR.. Fibrosing mediastinitis. Radiographics. 2001. May-Jun; 21( 3): 737– 57. [DOI] [PubMed] [Google Scholar]
- 21. Hoeper MM, Andreas S, Bastian A, . et al. Pulmonary hypertension due to chronic lung disease: updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol. 2011. December; 154 Suppl 1: S45– 53. [DOI] [PubMed] [Google Scholar]
- 22. Tardivon AA, Musset D, Maitre S, . et al. Role of CT in chronic pulmonary embolism: comparison with pulmonary angiography. J Comput Assist Tomogr. 1993. May-Jun; 17( 3): 345– 51. [DOI] [PubMed] [Google Scholar]
- 23. Reichelt A, Hoeper MM, Galanski M, Keberle M.. Chronic thromboembolic pulmonary hypertension: evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009. July; 71( 1): 49– 54. [DOI] [PubMed] [Google Scholar]
- 24. Garg K, Welsh CH, Feyerabend AJ, . et al. Pulmonary embolism: diagnosis with spiral CT and ventilation-perfusion scanning—correlation with pulmonary angiographic results or clinical outcome. Radiology. 1998. July; 208( 1): 201– 8. [DOI] [PubMed] [Google Scholar]
- 25. Falaschi F, Palla A, Formichi B, . et al. CT evaluation of chronic thromboembolic pulmonary hypertension. J Comput Assist Tomogr. 1992. Nov-Dec; 16( 6): 897– 903. [DOI] [PubMed] [Google Scholar]
- 26. Bergin CJ, Sirlin CB, Hauschildt JP, . et al. Chronic thromboembolism: diagnosis with helical CT and MR imaging with angiographic and surgical correlation. Radiology. 1997. September; 204( 3): 695– 702. [DOI] [PubMed] [Google Scholar]
- 27. Pitton MB, Kemmerich G, Herber S, Schweden F, Mayer E, Thelen M.. [Chronic thromboembolic pulmonary hypertension: diagnostic impact of Multislice-CT and selective pulmonary DSA]. Rofo. 2002. April; 174( 4): 474– 9. [DOI] [PubMed] [Google Scholar]
- 28. Coulden R. State-of-the-art imaging techniques in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006. September; 3( 7): 577– 83. [DOI] [PubMed] [Google Scholar]
- 29. Remy-Jardin M, Remy J, Mayo JR, Müller NL.. CT angiography of the chest. Philadelphia: Lippincott Williams & Wilkins; 2001; p 67– 81. [Google Scholar]
- 30. Wittram C, Kalra MK, Maher MM, Greenfield A, McLoud TC, Shepard JA.. Acute and chronic pulmonary emboli: angiography-CT correlation. AJR Am J Roentgenol. 2006. June; 186( 6 Suppl 2): S421– 9. [DOI] [PubMed] [Google Scholar]
- 31. Hasegawa I, Boiselle PM, Hatabu H.. Bronchial artery dilatation on MDCT scans of patients with acute pulmonary embolism: comparison with chronic or recurrent pulmonary embolism. AJR Am J Roentgenol. 2004. January; 182( 1): 67– 72. [DOI] [PubMed] [Google Scholar]
- 32. Castañer E, Gallardo X, Ballesteros E, . et al. CT diagnosis of chronic pulmonary thromboembolism. RadioGraphics. 2009. Jan-Feb; 29( 1): 31– 53. [DOI] [PubMed] [Google Scholar]
- 33. Heinrich M, Uder M, Tscholl D, Grgic A, Kramann B, Schafers HJ.. CT scan findings in chronic thromboembolic pulmonary hypertension: predictors of hemodynamic improvement after pulmonary thromboendarterectomy. Chest. 2005. May; 127( 5): 1606– 13. [DOI] [PubMed] [Google Scholar]
- 34. King MA, Ysrael M, Bergin CJ.. Chronic thromboembolic pulmonary hypertension: CT findings. AJR Am J Roentgenol. 1998. April; 170( 4): 955– 60. [DOI] [PubMed] [Google Scholar]
- 35. Kauczor HU, Schwickert HC, Mayer E, Schweden F, Schild HH, Thelen M.. Spiral CT of bronchial arteries in chronic thromboembolism. J Comput Assist Tomogr. 1994. Nov-Dec; 18( 6): 855– 61. [DOI] [PubMed] [Google Scholar]
- 36. Dong C, Zhou M, Liu D, . et al. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. PLoS One. 2015. April 29; 10( 4): e0126985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugiura T, Tanabe N, Matsuura Y, . et al. Role of 320-slice CT imaging in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest. 2013. April; 143( 4): 1070– 7. [DOI] [PubMed] [Google Scholar]
- 38. Fedullo P, Kerr KM, Kim NH, Auger WR.. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011. June 15; 183( 12): 1605– 13. [DOI] [PubMed] [Google Scholar]
- 39. Fedullo PF, Auger WR, Kerr KM, Rubin LJ.. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001. November; 345( 20): 1465– 72. [DOI] [PubMed] [Google Scholar]
- 40. Auger WR, Fedullo PF, Moser KM, Buchbinder M, Peterson KL.. Chronic major-vessel chronic thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology. 1992. February; 182( 2): 393– 8. [DOI] [PubMed] [Google Scholar]
- 41. Auger WR, Kim NH, Trow TK.. Chronic thromboembolic pulmonary hypertension. Clin Chest Med. 2010. December; 31( 4): 741– 58. [DOI] [PubMed] [Google Scholar]
- 42. Mayer E, Jenkins D, Lindner J, . et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011. March; 141( 3): 702– 10. [DOI] [PubMed] [Google Scholar]
- 43. Guth S, Weidenroth CB, Kramm T, Mayer E.. Pulmonary endarterectomy for the treatment of chronic thromboembolic pulmonary hypertension. Expert Rev Respir Med. 2016. June; 10( 6): 673– 84. [DOI] [PubMed] [Google Scholar]
- 44. Jamieson SW, Kapelanski DP, Sakakibara N, . et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003. November; 76( 5): 1457– 62. [DOI] [PubMed] [Google Scholar]
- 45. Kunz RP, Oellig F, Krummenauer F, . et al. Assessment of left ventricular function by breath-hold cine MR imaging: Comparison of different steady state free precession sequences. J Magn Reson Imaging. 2005; 21( 2): 140– 8. [DOI] [PubMed] [Google Scholar]
- 46. Ley S, Kramm T, Kauczor HU, . et al. Pre- and postoperative assessment of hemodynamics in patients with chronic thromboembolic pulmonary hypertension by MR techniques. Rofo. 2003; 175( 12): 1647– 54. [DOI] [PubMed] [Google Scholar]
- 47. Rajaram S, Swift AJ, Telfer A, . et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: results from the ASPIRE Registry. Thorax. 2013. July; 68( 7): 677– 8. [DOI] [PubMed] [Google Scholar]
- 48. Reesink HJ, van der Plas MN, Verhey NE, van Steenwijk RP, Kloek JJ, Bresser P.. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2007. February; 133( 2): 510– 6. [DOI] [PubMed] [Google Scholar]
- 49. Xi Q, Zhao Z, Liu Z, Ma X, Luo O, Liu W.. The lowest VE/VCO2 ratio best identifies chronic thromboembolic pulmonary hypertension. Thromb Res. 2014. December; 134( 6): 1208– 13. [DOI] [PubMed] [Google Scholar]


